Abstract
Inflammatory cytokine levels predict a wide range of human diseases, including depression, cardiovascular disease, type 2 diabetes, autoimmune disease, general morbidity, and mortality. Stress and social experiences throughout the lifecourse have been associated with inflammatory processes. We conducted studies in humans and laboratory rats to examine the effect of early life experience and adult social position in predicting IL-6 levels. Human participants reported family homeownership during their childhood and current subjective social status. Interleukin-6 (IL-6) was measured from oral mucosal transudate. Rats were housed in groups of three, matched for quality of maternal care received. Social status was assessed via competition for resources, and plasma IL-6 was assessed in adulthood. In both humans and rats, we identified an interaction effect; early social experience moderated the effect of adult social status on IL-6 levels. Rats that experienced low levels of maternal care and people with low childhood socioeconomic status represented both the highest and lowest levels of IL-6 in adulthood, depending on their social status as young adults. The predicted interaction held for non-Hispanic people, but did not occur among Hispanic individuals. Adversity early in life may not have a monotonically negative effect on adult health, but may alter biological sensitivity to later social experiences.
Keywords: stress, adversity, socioeconomic status, rat, cytokine, lifecourse
Introduction
Growing evidence links inflammatory processes to a wide range of human diseases, including depression, cardiovascular disease, type 2 diabetes, and autoimmune disease, as well as general morbidity and mortality. The inflammatory cytokine interleukin 6 (IL-6) has been identified as an important marker of disease risk; elevated levels of IL-6 predict onset of disease, disease progression, and functional decline (Cesari et al. 2003; Ferrucci et al. 1999; Hohensinner et al. 2011; Volpato et al. 2001). Increased levels of salivary IL-6 have been associated with children's mental health problems, suggesting that even small differences in inflammation early in life can have important health effects (Keller et al. 2010).
Social experiences correlate with inflammatory processes in both human populations and laboratory animals. Inflammatory cytokines, including IL-6, increase in response to acute psychosocial stress (Steptoe et al. 2007), and appear to be elevated under conditions of chronic stress, including low socioeconomic status (SES) (Gimeno et al. 2007; Friedman and Herd 2010; Loucks et al. 2010), caring for a spouse with dementia (Kiecolt-Glaser et al. 2003), and unemployment (Hintikka et al. 2009). Positive psychosocial resources such as coping and self-esteem inversely correlate with IL-6 levels in both serum and saliva (Sjogren et al. 2006). In mice, social stress can alter immune system functioning (Bartolomucci 2007), may increase proinflammatory cytokine (IL-6 and TNF-α) production, and can lead to glucocorticoid resistance (Kinsey et al. 2008; Powell et al. 2009). Psychological stress has been found to increase plasma IL-6 in rats (LeMay et al. 1990; Takaki et al. 1994), accompanied by up-regulation of IL-6 gene expression in the brain (Shizuya et al. 1997; Jankord et al. 2010). Stress-induced disruptions in neuroendocrine-immune signaling may lead to increased levels of circulating inflammatory mediators, independent of an acute inflammatory response.
Early-life experience during sensitive periods has the potential to shape developmental trajectories and may have wide-ranging impacts on physiology and behavior. Parental care is a potent source of variability in experience during mammalian development that affects multiple outcomes from stress reactivity to sexual behavior. Aspects of maternal care and offspring characteristics have been studied extensively in rodents, primates, and humans (Levine et al. 1957; Harlow and Zimmermann 1959; Denenberg et al. 1962; Francis et al. 1999b; Fleming et al. 2002; Cameron et al. 2008).
The laboratory rat model studying natural variations in maternal care is an extension of the neonatal separation/handling paradigm pioneered by Seymour Levine (and employed by many others) and the primate work of Harry Harlow originating in the 1950's and 1960's. This model has helped elucidate a pathway by which variations in maternal care predict differences in behavioral and endocrine responses to stress in the offspring. Data from the rat maternal care paradigm have identified a role for epigenetic processes as mechanistic mediators linking the quality of maternal care received and observed postnatal programming effects. Maternal licking/grooming appears to influence glucocorticoid receptor (GR) density in the hippocampus, at least in part, by differential methylation of the GR promoter in the days after birth (Weaver et al. 2004). These findings suggest that offspring reared in the high-maternal condition possess more tightly regulated stress-axes. This model has broadened understanding of the potential for epigenetic mechanisms, such as DNA methylation, to allow for the biological encoding of experience in ways that affect long-term gene expression (see (Meaney 2010) for a general review). Relevant to the current paper, no studies have been conducted that explicitly examine the role of early-experience and differential regulation of IL-6 in rats or humans.
The stress-diathesis model of disease would suggest that existing biologic vulnerabilities interact with life experiences or stressors to produce disease or pathology. Recent data across multiple disciplines suggest that early life stressful experiences are actually contributing to the creation or instantiation of biologic vulnerability. For instance, a recent review suggests that the effects of stress on health in adulthood appear more consistent among populations with underlying vulnerability, perhaps induced in response to early life environment (Champagne 2010). Miller and Chen (2010) found such an interactive effect when examining the relationship between harsh parenting experiences and life stress among adolescent girls. The combination of the two exposures predicted the emergence of a proinflammatory phenotype. Similarly, severe early life stress, such as childhood maltreatment, has been associated with increased inflammatory response to acute psychosocial stress in adulthood (Carpenter et al. 2010).
Animal models have also demonstrated an interactive effect between juvenile and adult environment in predicting inflammation. In an animal model of colitis, neuroendocrine changes and exacerbated symptoms appeared strongest among mice that had also been stressed as juveniles (Veenema et al. 2008). Evidence from animal models employing rhesus macaques and laboratory rats suggest that stress during gestation (Coe et al. 2002) and lipopolysaccharide (LPS, bacterial endotoxin) exposure in the neonatal period (Walker et al. 2009; Spencer et al. 2010; Walker et al. 2010) can lead to long-lasting alterations in cytokine biology and neuroendocrine stress responses. These studies reinforce the paradigm that adult experiences are most potent among those already vulnerable due to early life circumstances. While these examples highlight the interactions of stressful experiences over the lifecourse, they do not directly examine the potential beneficial effects of a positive environment for the more biologically sensitive group.
In the current study, we examine the interaction of social experiences over the lifecourse, characterized across species, using an approach that bears similarities to Boyce and Ellis' theory of biological sensitivity to context (BSC) and Belsky's concept of differential susceptibility. Boyce and Ellis propose in a series of elegant papers that, in humans, different early life experiences can prime biological reactivity to later environments. Ultimate health outcomes for an individual depend on both biological sensitivity to a context and the quality of later environments, with the highly reactive individuals experiencing both the best and worst outcomes (Ellis and Boyce 2011; Boyce and Ellis 2005; Ellis et al. 2005; Ellis et al. 2011). Belsky's model describes temperament, endophenotypes, and genotypes as factors that can make individuals more responsive to environmental circumstances (Belsky and Pluess 2009).
Although our questions arise from the field of social epidemiology, human studies face inherent limitations in addressing questions of social experiences over the lifespan. Human studies cannot fully control for all social environments encountered over a lifetime, especially when childhood risks are measured retrospectively. In addition, many risk factors for inflammation are correlated, such as social class, material resources, neighborhood environment, and personal health behaviors. The potential for confounding, both measured and unmeasured, is quite high. When attempting to separate the effects of early life environment and later social status in human studies, researchers face the challenge of including study participants in all combinations of exposure categories and risk violating the assumption of positivity (Westreich and Cole 2010).
Using animal models in combination with human studies provides a novel approach to understanding how social experiences become biologically embedded across the lifecourse. Animal models provide an opportunity to explore the questions raised by the epidemiologic work in humans. They allow for full characterization of social exposures and control of environmental circumstances; results from animal studies can be used to support correlational evidence in human populations. In the current paper, we present data from conceptually similar studies in college students and laboratory rats to address the relationship between early life environment, social status, and IL-6. Our study utilizes a younger human sample than those often studied, in concert with a cohort of laboratory rats fully characterized with respect to their relative social rank. Our innovative approach to interdisciplinary research combines data from studies in humans and an animal model to explore how early life experience interacts with adult social status to influence levels of IL-6. More generally, we investigate how social experiences that occur over the lifecourse interact to influence markers of inflammation.
Methods
Humans
Participants
One hundred and twelve participants (70 females, 42 males) were sampled from the student undergraduate population at the University of California, Berkeley. The study was advertised on the UC Berkeley campus; subjects were recruited through the Psychology Department's Research Participation Program and received partial course credit for participation. Participants ranged from 18 to 33 years of age (mean=19). The sample included 19% Caucasian, 47% Hispanic, 3% African American, and 23% Asian students. Only individuals free of acute illness at the time of the study were allowed to participate. All procedures were carried out in accordance with the standards and practices of the UC Berkeley Committee for Protection of Human Subjects.
Procedures
Assessment of childhood SES, social status, and covariates
Participants reported to a designated room on the UC Berkeley campus during academic school hours. They completed a questionnaire upon arrival. The survey collected general demographic information, including age, gender, and race/ethnicity. Participants were also asked to report the highest level of education achieved by their mother and father (1=elementary/middle school, 2=high school, 3=college, 4=beyond Bachelor's degree), and each parent's current income.
As a marker of childhood SES participants were asked if their parents were owners of their home when the participant was in kindergarten. Home ownership has been repeatedly used to index childhood SES and is a robust measure of economic conditions. It correlates with, but is distinct from, traditional measures such as income, and can be more reliably assessed than such measures when assessed retrospectively (Cohen et al. 2010). Home ownership has been identified as an independently predictor of improved quality of children's physical and emotional environment, decreased stress, and increased stability (Haurin et al. 2002). In addition, several studies have examined parental home ownership in relation to later health, immune function, and inflammation (Cohen et al. 2004; Miller and Chen 2007; Chen et al. 2010).
The MacArthur SES ladder was used as a measure of subjective social status (SSS). Participants were shown a ladder representing where people stand in the United States. They were asked to place an “X” on the ladder indicating where they place themselves on the 10-rung ladder relative to others in society. Subjective social status measures self-perceived placement in the social hierarchy. This measure correlates with, but is not equivalent to, objective measures of SES such as income or education (Adler et al. 2000; Operario et al. 2004). Because all participants were college students, the subjective measure of social status was used to capture finer gradations than an objective measure of SES.
The Beck Depression Inventory (BDI) was used to obtain a marker of depressive symptoms. Participants were also asked to report their body mass index (BMI), self-rated health, and perceived stress levels (PSS).
IL-6 measurement
After completion of the survey, participants provided baseline samples of oral mucosal transudate (OMT) for IL-6 measurement. Although not a proxy for blood levels, OMT has been shown to reflect immune system activity (Nishanian et al. 1998) and correlate with psychosocial variables (Sjogren et al. 2006). To collect the sample, an Orasure collective device (Epitope, Beaverton, OR) was placed between the lower cheek and gum of an individual for two minutes. Immediately following collection, the sample was frozen and stored at -80 C. The samples were thawed and IL-6 concentrations were determined by ELISA using commercially available kits (R&D systems, Minneapolis, MN). All IL-6 samples were run in duplicate. The intra-assay coefficient of variation (cv) was 9.6% and inter-assay cv was 10.1%. Protein in oral fluids was quantified using the BCA Protein Assay with bovine serum albumin as the standard (Thermo Scientific, Rockford, IL), using HEPES as the diluent. All total protein samples were run in triplicate on the same plate according to kit instructions. All IL-6 results are reported using analyte to protein ratios, as this measure has been shown to be more reliable than analyte values alone and controls for individual differences in salivary flow rate (Dickerson et al. 2004). Two samples had concentrations below the limit of detection (LOD) of the assay, so their IL-6 concentrations were replaced with the LOD.
Statistical analysis
All analyses were conducted using Stata v.10 (College Station, TX). The IL-6/total protein ratio was natural-log transformed for all analyses as the untransformed data were skewed and did not meet the diagnostic criteria for linear regression, according to the IQR and Shapiro-Wilk tests. The log-transformed IL-6/total protein measurement became the dependent variable for all analyses. One individual (0.9% of total sample) was excluded as an outlier (log(IL-6/total protein) > 3 standard deviations from the mean).
Linear regression was used to assess the relationship between home ownership, subjective social status, their interaction, and IL-6. Analyses include both unadjusted linear regression models and adjusted models including potential confounders (gender, age, maternal education, depressive symptoms, perceived stress, and BMI). Table 2 presents correlation coefficients for all predictor variables. Collinearity was assessed by examining variance inflation factors (VIFs) for the variables included in the analysis. Although the predictor variables were correlated, VIFs ranged from 1.05 (BMI) to 1.97 (maternal education), indicating that collinearity was not a problem.
Table 2.
Bivariate correlations among study variables (r (p-value)).
Correlations between predictor variables and IL-6 in humans and rats
| Humans | ||||||||
|---|---|---|---|---|---|---|---|---|
| Log(IL-6/tp) | SSS | Home ownership | Maternal education | BDI | BMI | PSS | Age | |
| Log(IL-6/tp) | 1.0 | |||||||
| SSS | -0.40 (<0.001) | 1.0 | ||||||
| Home ownership | -0.12 (0.2) | 0.25 (0.07) | 1.0 | |||||
| Maternal education | -0.49 (<0.001) | 0.45 (<0.001) | 0.40 (<0.001) | 1.0 | ||||
| BDI | 0.16 (0.1) | -0.30 (0.001) | -0.11 (0.2) | -0.36 (<0.001) | 1.0 | |||
| BMI | -0.12 (0.2) | 0.0048 (0.9) | 0.033 (0.7) | -0.081 (0.4) | 0.12 (0.2) | 1.0 | ||
| PSS | 0.062 (0.5) | -0.19 (0.05) | -0.064 (0.5) | -0.08 (0.4) | 0.59 (<0.001) | 0.045 (0.6) | 1.0 | |
| Age | -0.25 (0.007) | 0.25 (0.008) | 0.073 (0.4) | 0.27 (0.004) | -0.11 (0.3) | 0.050 (0.6) | -0.064 (0.5) | 1.0 |
| Rats | ||||||||
| Log(IL-6/tp) | Social status | Maternal care | ||||||
| Log(IL-6/tp) | 1.00 | |||||||
| Social status | -0.28 (0.04) | 1.00 | ||||||
| Maternal care | -0.14 (0.3) | 0.011 (0.9) | 1.00 | |||||
Rats
Participants
Male rats included in the study were born in our colony from outbred Long Evans rats originally purchased from Charles River Breeding Laboratories (Wilmington, MA). Temperature was kept constant at 20 ± 2 °C and relative humidity was maintained at 50 ± 5%. Rats were maintained on a 12-h light–dark cycle (lights on 0700 h to 1900 h) and allowed access to food (Purina Rat Chow, Purina Mills, St. Louis, Missouri) and tap water ad libitum. Housing and care of the rats were carried out in accordance with the standards and practices of the UC Berkeley Animal Care and Use Committee.
Procedures
Maternal behavior and housing conditions
Female rats were bred and permitted to give birth. Day of birth was marked as postnatal day (PND) 0. Maternal observations were performed beginning on PND 1 and continued until PND 5. Each litter was observed for five hours a day at the following times; 0600h-0800h, 1200h-1300h and 1800h-2000h. During each observation session, litters were observed and behaviors recorded every two minutes (each litter was observed 150 times per day: (Liu et al. 1997; Francis et al. 1999a; Champagne et al. 2003)). Behaviors recorded included: mother on/off the nest and maternal licking behaviors directed at self or at pups. A maternal care distribution curve was generated by calculating the frequency with which pup-directed maternal licking was observed. Maternal licking was expressed as a percentage of the total number of observations performed for each litter (750 individual observations for each cage). High and Low licking litters were assessed as those falling above or below the median of all litters.
Naturally occurring variations in maternal licking are associated with the development of individual differences in the hypothalamic-pituitary-adrenal (HPA) endocrine axis and behavioral responses to stress in the offspring. As adults, offspring of high-licking mothers are behaviorally less fearful, exhibit a more modest HPA response to stress, and demonstrate enhanced glucocorticoid feedback sensitivity when compared to offspring reared by low-licking dams (Liu, Diorio et al. 1997) In our laboratory rat model, variations in quality of maternal care received early in life do not influence baseline measures of weight or general activity levels in offspring assessed as adults.
Male pups were weaned at PND 24 and housed in cages of three, with non-littermates matched for weight and maternal care received. Rats were housed in guinea pig cages (20×16×8.5 inches), which are substantially larger than standard rat cages (10.5×19×8 inches).
Assessment of Social Status in Rats; Access to Resources
Food competition task
Several days prior to weaning all rats were exposed to the novel taste of chocolate. On the first day of habituation training a small amount of melted chocolate was smeared onto the inside of each home cage. The next day mini chocolate chips were placed inside each home cage. Rats were provided with mini chocolate chips daily for five days, until all rats quickly approached and ate the chocolate. To assess within cage social rank we administered a brief food competition task. In this task rats within a single home cage competed for access to a small container of chocolate secured vertically to the cage wall. The chocolate was melted and allowed to cool in a small glass dish before testing, so the rats could not remove pieces of the chocolate, but were required to eat at the dish. All rat behavior was video recorded following chocolate provision and a blinded investigator scored the amount of time each rat spent eating chocolate over the first minute of the task. The food competition task was conducted at 109 days of age.
Water competition task
Rats were deprived of access to water bottles for eight hours prior to testing. Upon return of the water bottles all behaviors were recorded and a blinded investigator scored the amount of time each rat spent drinking over the first two minutes of the task. The water competition task took place on postnatal day 111.
Assignment of social status
Social status was determined by averaging the proportion of time each rat spent accessing resources (chocolate or water), relative to cage mates. A social status score of 1 for all rats in a cage would represent equal access to resources. Two of the 24 cages did not form measurable hierarchies; failure to form a hierarchy was defined as <5% difference in social status score between all animals in the cage. These two cages (8% of total sample) were excluded from all analyses. Laboratory rat studies of social dominance often find that up to 50% of groups do not form hierarchies (Hoshaw et al. 2006; Barnum et al. 2008).
IL-6 measurement
At the termination of the study all animals were sacrificed. Blood was collected from each animal and plasma was extracted, frozen immediately and stored at -80° C for future use. At the time of assay all samples were thawed and IL-6 concentrations were determined by ELISA using commercially available kits (R&D systems, Minneapolis, MN). All samples were run in duplicate. Intra-assay coefficient of variability (cv) was 8.7% and inter-assay cv was 9.8%. 25% of samples fell below the manufacturer's reported minimum level of detection (LOD). We replaced the values of those below the LOD with the manufacturer's reported LOD. Total protein levels in plasma were quantified using the BCA Protein Assay with bovine serum albumin as the standard (Thermo Scientific, Rockford, IL) and HEPES as the diluent. All protein samples were run in triplicate on the same plate, according to kit instructions. All IL-6 results are reported using analyte to protein ratios, to parsimoniously compare with human levels. Analyses were also run using log-IL-6 values without adjustment for total protein (data not shown). Results did not change.
Statistical analysis
All analyses were conducted using Stata v.10 (College Station, TX). The IL-6/total protein ratio was natural-log transformed for all analyses, because the untransformed data were skewed and did not meet the diagnostic criteria for linear regression, according to the IQR and Shapiro-Wilk tests. The log-transformed IL-6/total protein measurement became the dependent variable for all analyses. One rat sample (1.7% of total sample) was excluded as an outlier (log(IL-6/total protein) > 3 standard deviations from the mean). Linear regression, controlling for clustering by cage, was used to assess the relationship between explanatory variables and IL-6. The cluster option for linear regression in Stata adjusts variance-covariance matrices to account for correlated observations within groups. The reported standard errors take into account that the observations within cages are non-independent.
Results
In the human study, mean OMT IL-6 concentration was 1.34 pg/mL, as shown in Table 1. Participants were all college students, with an average age of 19.6 years. 37% of the participants were male, and 47% were Hispanic. Bivariate correlations between predictor variables are presented in Table 2. Social status was significantly associated with maternal education, depressive symptoms, perceived stress, and age. Home ownership was significantly correlated with maternal education. In the rat study, mean plasma IL-6 concentration was 18.50 pg/mL (see Table 1). Social status and maternal care were not correlated (see Table 2).
Table 1. Descriptive characteristics of both study populations.
| Humans | All | Non-Hispanic | Hispanic | p-value |
|---|---|---|---|---|
| IL-6 (pg/mL) (mean (SD)) | 1.35 (2.24) | 1.10 (2.45) | 1.63 (1.94) | 0.2 |
| Age (mean (SD)) | 19.6 (2.38) | 20.19 (2.76) | 18.98 (1.69) | 0.007 |
| Gender (% male) | 37% | 45% | 28% | 0.07 |
| Subjective social status (mean (SD)) | 5.51 (1.98) | 6.28 (1.90) | 4.64 (1.70) | <0.001 |
| Home ownership (%) | 52% | 68% | 34% | <0.001 |
| Maternal education (mean(SD)) | 2.15 (0.95) | 2.71 (0.72) | 1.51 (0.75) | <0.001 |
| Beck Depression Index (mean(SD)) | 7.90 (6.16) | 6.85 (6.14) | 9.09 (6.02) | 0.05 |
| Perceived Stress Scale (mean(SD)) | 6.88 (2.71) | 6.62 (2.73) | 7.17 (2.68) | 0.3 |
| Body mass index (mean(SD)) | 24.5 (3.19) | 24.35 (3.16) | 24.68 (3.23) | 0.6 |
|
| ||||
| Rats | ||||
| IL-6 (pg/mL) (mean (SD)) | 18.50 (6.80) | |||
| IL-6/total protein (pg/μg) (mean | 0.018 | |||
| (SD)) | (0.0069) | |||
| Age | 163 days | |||
| Social status | 1.02 (0.38) | |||
| Maternal care (mean (SD)) | 6.8% (2.0) | |||
We report results for both the unadjusted and adjusted linear regression models. Fully adjusted models include the following potential confounders, in addition to the study variables: gender, age, maternal education, depressive symptoms, perceived stress, and body mass index. We conducted analyses for all human participants, and stratified by Hispanic ethnicity, because potential confounders differed significantly by ethnicity (Table 1). Relationships between our exposures of interest and IL-6 differed markedly by ethnicity (see Table 3), although we did not hypothesize differences by ethnicity at the outset.
Table 3.
Social status and early life environment predict IL-6 levels in humans and rats. Outcome for all regression models is log(pg IL-6/μg total protein).
| Humans (All) | ||||
|---|---|---|---|---|
| Unadjusted interaction model | β | SE | t | p |
| Subjective social status | -0.32 | 0.079 | -4.05 | <0.001 |
| Home ownership | -0.69 | 0.65 | -1.05 | 0.3 |
| Interaction (social status*home ownership) | 0.12 | 0.11 | 1.14 | 0.3 |
| Constant | -5.58 | 0.45 | -12.40 | <0.001 |
|
| ||||
| R2=0.17 | ||||
| F(3, 108)=8.78, p<0.001 | ||||
|
| ||||
| Humans (All) | ||||
| Adjusted interaction model | β | SE | t | p |
|
| ||||
| Subjective social status | -0.22 | 0.078 | -2.76 | 0.007 |
| Home ownership | -0.62 | 0.63 | -0.98 | 0.3 |
| Interaction (social status*home ownership) | 0.18 | 0.10 | 1.81 | 0.07 |
| Gender | 0.17 | 0.19 | 0.88 | 0.4 |
| Age | -0.071 | 0.033 | -2.15 | 0.03 |
| Maternal education | -0.59 | 0.12 | -4.96 | <0.001 |
| Depression symptoms (BDI) | -0.030 | 0.021 | -1.41 | 0.2 |
| Perceived stress (PSS) | 0.042 | 0.042 | 0.99 | 0.3 |
| Body mass index (BMI) | 0.036 | 0.033 | 1.12 | 0.3 |
| Constant | -4.73 | 0.91 | -5.19 | <0.001 |
|
| ||||
| R2=0.34 | ||||
| F(9, 96)=7.04, p<0.001 | ||||
|
| ||||
| Humans (Non-Hispanic) | ||||
| Unadjusted interaction model | β | SE | t | p |
|
| ||||
| Subjective social status | -0.50 | 0.11 | -4.68 | <0.001 |
| Home ownership | -2.50 | 1.05 | -2.37 | 0.02 |
| Interaction (social status*home ownership) | 0.40 | 0.15 | 2.62 | 0.01 |
| Constant | -4.73 | 0.68 | -6.97 | <0.001 |
|
| ||||
| R2=0.24 | ||||
| F(3, 56)=7.84, p<0.001 | ||||
|
| ||||
| Humans (Non-Hispanic) | ||||
| Adjusted interaction model | β | SE | t | p |
|
| ||||
| Subjective social status | -0.36 | 0.096 | -3.73 | 0.001 |
| Home ownership | -1.83 | 1.08 | -1.70 | 0.1 |
| Interaction (social status*home ownership) | 0.33 | 0.15 | 2.11 | 0.04 |
| Gender | 0.21 | 0.26 | 0.81 | 0.4 |
| Age | -0.045 | 0.036 | -1.26 | 0.2 |
| Maternal education | -0.57 | 0.29 | -1.95 | 0.06 |
| Depression symptoms (BDI) | -0.028 | 0.038 | -0.74 | 0.5 |
| Perceived stress (PSS) | 0.055 | 0.059 | 0.93 | 0.4 |
| Body mass index (BMI) | 0.11 | 0.039 | 2.75 | 0.009 |
| Constant | -6.14 | 1.12 | -5.27 | <0.001 |
|
| ||||
| R2=0.45 | ||||
| F(9, 46)=, p<0.001 | ||||
|
| ||||
| Humans (Hispanic) | ||||
| Unadjusted interaction model | β | SE | t | P |
|
| ||||
| Subjective social status | -0.15 | 0.095 | -1.59 | 0.1 |
| Home ownership | -0.41 | 0.77 | -0.53 | 0.6 |
| Interaction (social status*home ownership) | 0.20 | 0.13 | 1.55 | 0.1 |
| Constant | -6.28 | 0.54 | -11.71 | <0.001 |
|
| ||||
| R2=0.094 | ||||
| F(3, 48)=3.93, p=0.01 | ||||
|
| ||||
| Humans (Hispanic) | ||||
| Adjusted interaction model | β | SE | t | P |
|
| ||||
| Subjective social status | -0.12 | 0.11 | -1.15 | 0.3 |
| Home ownership | -0.69 | 1.01 | -0.69 | 0.5 |
| Interaction (social status*home ownership) | 0.27 | 0.18 | 1.47 | 0.1 |
| Age | -0.18 | 0.061 | -2.98 | 0.005 |
| Gender | 0.13 | 0.30 | 0.43 | 0.7 |
| Maternal education | -0.41 | 0.20 | -2.10 | 0.04 |
| Depression symptoms (BDI) | -0.040 | 0.035 | -1.10 | 0.3 |
| Perceived stress (PSS) | 0.042 | 0.077 | 0.54 | 0.6 |
| Body mass index (BMI) | -0.035 | 0.041 | -0.86 | 0.4 |
| Constant | -1.50 | 1.72 | -0.88 | 0.4 |
|
| ||||
| R2=0.24 | ||||
| F(9, 40)=3.35, p=0.004 | ||||
|
| ||||
| Rats | β | SE | t | P |
|
| ||||
| Social status | -0.45 | 0.15 | -3.09 | 0.006 |
| High maternal care | -0.53 | 0.19 | -2.76 | 0.01 |
| Interaction (social status *high maternal care) | 0.43 | 0.18 | 2.42 | 0.03 |
| Constant | -3.58 | 0.16 | -22.54 | <0.001 |
|
| ||||
| R2=0.18 | ||||
| F(3,21)=3.79, p=0.03 | ||||
Independent effects of early life environment and adult social status in humans and rats
In the analysis including all human participants, subjective social status (SSS) was inversely associated with IL-6 levels (β=-0.25, SE=0.053, p<0.001) in the unadjusted model. Homeownership did not predict IL-6. In the fully adjusted model, SSS (β=-0.12, SE=0.054, p=0.03) and maternal education (β=-0.57, SE=0.12, p<0.001) each independently predicted IL-6 levels, while homeownership (β=-0.36, SE=0.22, p=0.1) and age (β=-0.064, SE=0.033, p=0.05) each showed a trend towards significance.
Among non-Hispanic participants, in the unadjusted model, SSS predicted IL-6 levels (β=-0.25, SE=0.088, p=0.007, R2=0.13), but childhood home ownership did not.. In the fully adjusted model, SSS (β=-0.16, SE=0.075, p=0.04), maternal education (β=-0.63, SE=0.25, p=0.02), and BMI (β=0.12, SE=0.42, p=0.008) were independently associated with IL-6.
Among Hispanic participants, neither subjective social status nor childhood home ownership predicted IL-6 in either the unadjusted or adjusted model. When we added potential confounders to the regression model, only age was significantly associated with IL-6 (β=-0.18, SE=0.062, p=0.005).
In rats, using linear regression and adjusting for clustering by cage, we tested whether early life environment (maternal care) and/or adult social status explained IL-6 levels. Social status was inversely associated with IL-6 in plasma, such that rats of higher social status had lower levels of plasma IL-6 (β=-0.22, SE=0.10, p=0.048, R2=0.077). Early environment (maternal licking and grooming) was not independently associated with IL-6.
Interactive effects of early life environment and adult social status in humans and rats
We next examined the interactive effect of early life environment and adult social status on levels of IL-6. The interaction reached statistical significance in both non-Hispanic students and rats; however, the interaction between social status and childhood SES was not significant among Hispanic participants.
In the unadjusted model including all participants, subjective social status (SSS) was inversely associated with IL-6 levels; however, neither childhood home ownership nor its interaction with SSS predicted IL-6 (Table 3). In the fully adjusted model, SSS, maternal education, and age were significantly and inversely associated with IL-6 levels. The interaction between SSS and childhood homeownership showed a trend towards significance.
Among non-Hispanic college students; the effect of subjective social status on IL-6 was much larger for people whose families did not own their home during the participant's childhood. The slope of social status versus IL-6 was significantly steeper among individuals who experienced low SES in childhood (Figure 1, Table 3). These interactions were significant both in the unadjusted model and when covariates were added. Maternal education was significant in the adjusted interaction model, with higher maternal education predicting lower IL-6. BMI was positively associated with IL-6. Parental home ownership during kindergarten seemed to buffer individuals from later life social status, lessening the effect of social status on IL-6 both at high and low status. Regression lines plotted within the range of observed SSS values indicated that students disadvantaged early in life showed both the highest and lowest predicted IL-6 OMT levels.
Figure 1.
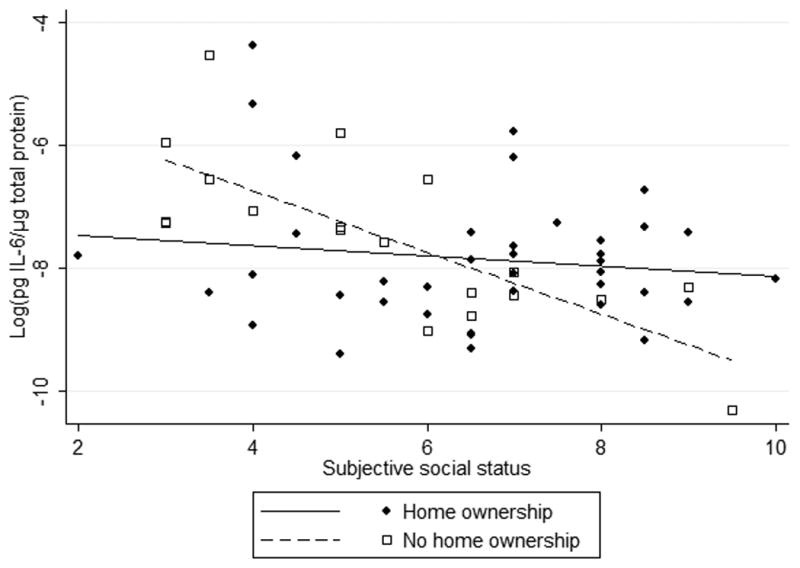
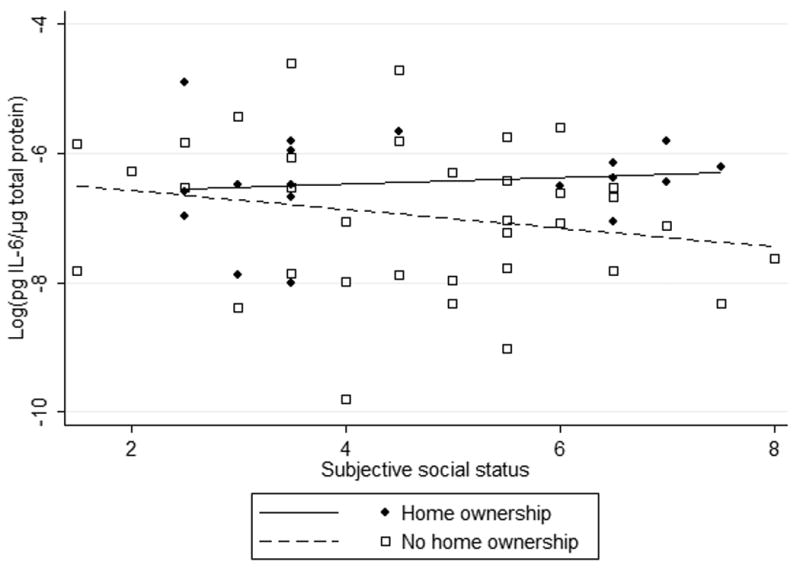
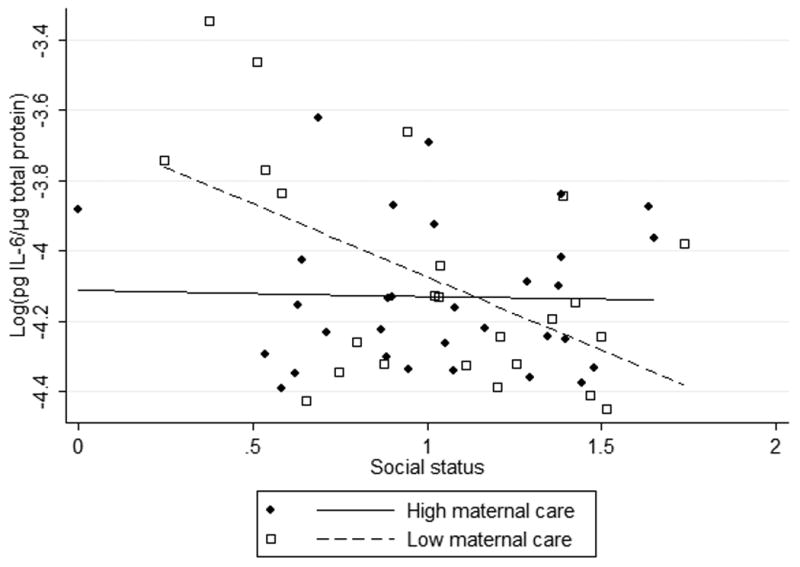
Interaction between early life adversity and adult social status in humans (a,b) and rats (c) predicts IL-6 concentration.
Neither social status, home ownership, nor their interaction predicted IL-6 levels among Hispanic participants in the unadjusted model. In the fully adjusted model, maternal education and age were both inversely associated with IL-6 (Figure 1, Table 3).
We identified an interaction in rats; the effect of social status on IL-6 was stronger among rats that experienced less licking and grooming as pups (Figure 1, Table 3). The slope of social status versus IL-6 is significantly steeper among rats from low licking and grooming litters, compared to those who received higher maternal care. Just as in the human data, the rats that experienced early adversity produced both the highest and lowest levels of IL-6. The low licking and grooming/low status adult rats experienced the highest levels of circulating IL-6, whereas the low licking and grooming/high status adult rats experienced the lowest levels of IL-6.
We then examined the relationship between social status and IL-6 within categories of early life experience (home ownership vs. not, high vs. low licking and grooming). In both non-Hispanic humans and rats, the effect of social status on IL-6 was significant among the early life stress group only. In non-Hispanic participants, subjective social status predicted IL-6 among people whose families did not own their home (β=-0.43, SE=0.14, p=0.01, R2=0.46), but not among those whose families were home owners (β=-0.054, SE=0.11, p=0.6), adjusting for gender, age, maternal education, depressive symptoms, perceived stress, and body mass index. In rats, social status predicted IL-6 levels among pups from low licking and grooming litters (β =-0.45 SE=0.15, p=0.01, R2=0.21), but not high licking and grooming litters (β=-0.015, SE=0.10, p=0.9).
The interaction effects in non-Hispanic humans and rats were strikingly similar. In both species, high quality early life experiences appeared to buffer individuals from later life risks. However, this interaction occurred at both extremes of social status, insulating individuals both from the negative effects of low social status and the beneficial effects of high social status. As a result, the highest and lowest IL-6 levels were predicted among the early life adversity group in both studies.
Discussion
Results from conceptually similar studies in humans and rats demonstrate a strong interaction between early life experience and adult social status in relation to IL-6 levels, in which early adversity increases sensitivity to adult social status. Our findings suggest that adversity in childhood may not have a monotonically negative effect on later life health, but may alter responsiveness to later exposures. Although we did not hypothesize differences by ethnicity at the outset, we found that the interaction occurred only among non-Hispanic participants. Results including all human participants, adjusting for covariates, show a significant effect of subjective social status on IL-6, with the interaction between SSS and homeownership demonstrating a trend towards significance. There are many challenges inherent in garnering a better understanding of biological susceptibility to environment. Our current findings in both people and rats demonstrate that i) an immune parameter, baseline levels of the cytokine IL-6, is sensitive to context, and ii) early developmental contexts (home ownership in people or maternal care in rats) are moderating factors that influence the physiological plasticity of IL-6 levels. The similar data patterns in rats and humans, while correlational, will now allow us to use our animal model in future to experimentally manipulate environmental variables and explore potential moderating factors. The rodent paradigm will allow for a more detailed study of putative mechanistic mediators of observed and identified relationships. For example, borrowing from the stress field, genetic (Caspi et al. 2010) and epigenetic (Champagne and Meaney 2006) sensitivity to the environment has been established. Emerging evidence suggests that genetic variation in the human IL-6 promoter may alter susceptibility to social adversity (Cole et al. 2010). Epigenetic mechanisms have not yet been implicated in differentially regulating IL-6 levels in rats or humans, suggesting other mechanistic processes may be at play.
The current study used family home ownership in humans and maternal care in rats as proxies to capture the quality of early life experiences. These variables have each been examined in relation to programming of the hypothalamic-pituitary-adrenal (HPA) stress axis (Francis, Champagne et al. 1999a; Chen, Cohen et al. 2010). We suggest that early life SES in humans and maternal care in rats contribute to greater responsiveness of the HPA axis, which in turn increases plasticity to later social experiences, as suggested in the biological sensitivity to context model.
There is considerable evidence demonstrating cross-talk between the HPA stress axis and immune responses. Limitations of the current study preclude assessing the role stress and stress-reactivity processes may play in the observed effects of baseline, unstimulated IL-6 levels, however this will be a target for future studies. Glucocorticoids typically modify cytokine levels through regulation of the transcription factor NFκB, while themselves being subject to regulation by various environmental experiences (eg. maternal care, acute stress, chronic stress (Turrin and Rivest 2004)). Using the laboratory rat model characterized in this paper, future research can assess if perturbed levels of IL-6 are directly regulated by stress hormones, indirectly regulated by stress hormones via NFκB or are occurring independently of the stress-axis.
The current study suffers from several important limitations. The human study employs a relatively small sample, which does not reflect US demographics in terms of race, ethnicity, or age. Therefore, the external validity and generalizability of our results are unknown. Further study and replication in other, more representative samples is required to determine the generalizability of our findings.
Although our results for rats and non-Hispanic participants are similar, we found substantially different results by Hispanic ethnicity. Although we did not expect to find differences by ethnicity, our results add to the evidence that cytokine levels differ by ethnic background (Stowe et al. 2010). Similar to our findings, in the Multi Ethnic Study of Atherosclerosis, ethnicity moderated the effect of education on IL-6; higher education was associated with lower levels of IL-6 in whites and blacks, but not in Hispanic participants. The lack of association among Hispanic participants occurred primarily among those who did not speak English at home, suggesting an effect of acculturation (Ranjit et al. 2007). In our study, 74% of Hispanic participants had at least one parent with an occupation characterized by physical labor (e.g. farm labor, factory worker), compared to only 20% of the non-Hispanic participants, and although we did not directly measure immigration status, Hispanic participants may have included many recent immigrants. The differences observed could reflect measurement variation, differences in the subjective meaning of home ownership or social status, or differences in behavioral, psychosocial, and biological correlates of social status by ethnicity and immigration status. The consistency of findings between the non-Hispanic human participants and the laboratory rat model, but lack of comparability to Hispanic participants suggests that while animal studies may provide informative models for some human populations, they do not necessarily generalize to all social, cultural, and ethnic groups. Future studies are needed to replicate our findings and to further explore the ethnic differences identified in the current study.
Our human study risks misclassification of childhood SES by measuring home ownership during a single year. Home ownership has been extensively studied as an independent predictor of children's pyshical, psychological, and social environment (Haurin et al. 2002). However, many other socioeconomic variables influence childhood environment and may not be captured via home ownership (e.g. parental occupation, income, neighborhood, parenting style). Using a single year to indicate childhood SES could lead to misclassification resulting from changing parental circumstances during a child's early life. This misclassification was less of a concern in the rat model employed which was able to more fully measure and control for early life variables (most critically the quality of maternal care experienced). By matching on, and therefore controlling for, quality of maternal care received, we were able to examine the effects of adult social status within levels of early life care.
IL-6 concentrations in our human sample are relatively low (see Table 1); however, our sample consists of young, healthy students. The levels observed in our study are comparable to IL-6 concentrations previously reported in oral fluids (Groer et al. 2010; Slavich et al. 2010). Inflammatory cytokines generally increase with age (Stowe et al. 2010), therefore even small differences at young ages may predict substantial divergence in trajectories later in life.
Nonetheless, measurement of IL-6 in OMT in the human study may limit the interpretation of our results. IL-6 measured in OMT does not directly reflect blood or lymphoid tissue levels and has not been extensively studied in relation to health risks. However, IL-6 in oral fluids has repeatedly been associated with psychosocial variables (Sjogren et al. 2006) and social stress (Slavich et al. 2010). Evidence suggests that cytokine production in oral fluids may reflect activation of the mucosal immune system, such that measurements in serum and saliva reflect related but distinct immune responses, both of which correlate with psychosocial factors (Sjogren et al. 2006). Further research is needed to determine the associations between social experiences, IL-6 in oral fluids, and risk of disease. In the rat study, we adjusted IL-6 measures for total protein levels in the plasma samples. This approach is more common in studies of oral fluids than plasma, and may limit the direct comparability to other IL-6 levels reported in the literature.
IL-6 was assessed at only one timepoint limiting the ability to examine changes in inflammatory processes over time. The similar effects in rats and humans, however, increase our confidence in the validity of the observed effects. In humans, the SES-inflammation relationship may be confounded by unmeasured health behaviors (several studies failed to find associations between SES and IL-6 after adjusting for behavioral risk factors) (Petersen et al. 2008; Ramsay et al. 2008). In the rat model of social hierarchy, the results are not due to differences in material resources or health behaviors as all rats experienced comparable environmental conditions.
It is impossible to fully control for all social environments encountered over a lifetime in adult humans, especially when childhood risks are measured retrospectively, and many risk factors for inflammation are correlated. We believe questions raised by the epidemiologic work in humans could be more thoroughly explored using animal models in combination with human studies, purposefully designing projects to address similar questions to generate converging evidence. Studies with laboratory animals allow for more controlled characterization of social exposures and offer an innovative approach to strengthen evidence in human populations and to address the limitations of human studies.
Viewing early life SES as a predictor of physiological plasticity, rather than simply as a risk factor, suggests a more optimistic perspective for change. If low childhood SES increases biological responsiveness to later life experiences, then interventions targeted in low-SES communities may have significant impacts. Our rat model of social hierarchy provides the opportunity to test interventions in a controlled setting. Continued studies including both laboratory models and human populations may help explain relationships between exposures at different time points and may suggest opportunities to intervene and prevent negative health outcomes.
Highlights.
Early adversity amplifies the effect of adult social status on IL-6 levels in both humans and laboratory rats.
Acknowledgments
This research was supported by NIH grant R24 MH081797-01, the Greater Good Science Center, and the Russell M. Grossman Endowment. We are grateful to Jeremy Hamilton for his assistance with the laboratory analyses.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adler NE, Epel ES, et al. Relationship of subjective and objective social status with psychological and physiological functioning: preliminary data in healthy white women. Health Psychol. 2000;19(6):586–92. doi: 10.1037//0278-6133.19.6.586. [DOI] [PubMed] [Google Scholar]
- Barnum CJ, Blandino P, et al. Social status modulates basal IL-1 concentrations in the hypothalamus of pair-housed rats and influences certain features of stress reactivity. Brain, Behavior, and Immunity. 2008;22(4):517–27. doi: 10.1016/j.bbi.2007.10.004. [DOI] [PubMed] [Google Scholar]
- Bartolomucci A. Social stress, immune functions and disease in rodents. Frontiers in Neuroendocrinology. 2007;28(1):28–49. doi: 10.1016/j.yfrne.2007.02.001. [DOI] [PubMed] [Google Scholar]
- Belsky J, Pluess M. Beyond diathesis stress: differential susceptibility to environmental influences. Psychol Bull. 2009;135(6):885–908. doi: 10.1037/a0017376. [DOI] [PubMed] [Google Scholar]
- Boyce WT, Ellis BJ. Biological sensitivity to context: I. An evolutionary-developmental theory of the origins and functions of stress reactivity. Dev Psychopathol. 2005;17(2):271–301. doi: 10.1017/s0954579405050145. [DOI] [PubMed] [Google Scholar]
- Cameron N, Del Corpo A, et al. Maternal programming of sexual behavior and hypothalamic-pituitary-gonadal function in the female rat. PLoS One. 2008;3(5):e2210. doi: 10.1371/journal.pone.0002210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpenter LL, Gawuga CE, et al. Association between Plasma IL-6 Response to Acute Stress and Early-Life Adversity in Healthy Adults. Neuropsychopharmacology. 2010 doi: 10.1038/npp.2010.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caspi A, Hariri AR, et al. Genetic Sensitivity to the Environment: The Case of the Serotonin Transporter Gene and Its Implications for Studying Complex Diseases and Traits. Am J Psychiatry. 2010;167(5):509–527. doi: 10.1176/appi.ajp.2010.09101452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cesari M, Penninx BW, et al. Inflammatory markers and cardiovascular disease (The Health, Aging and Body Composition [Health ABC] Study) Am J Cardiol. 2003;92(5):522–8. doi: 10.1016/s0002-9149(03)00718-5. [DOI] [PubMed] [Google Scholar]
- Champagne FA. Early adversity and developmental outcomes: Interaction between genetics, epigenetics, and social experiences across the lifespan. Perspectives on Psychological Science. 2010;5(5):564–574. doi: 10.1177/1745691610383494. [DOI] [PubMed] [Google Scholar]
- Champagne FA, Francis DD, et al. Variations in maternal care in the rat as a mediating influence for the effects of environment on development. Physiol Behav. 2003;79(3):359–71. doi: 10.1016/s0031-9384(03)00149-5. [DOI] [PubMed] [Google Scholar]
- Champagne FA, Meaney MJ. Stress during gestation alters postpartum maternal care and the development of the offspring in a rodent model. Biol Psychiatry. 2006;59(12):1227–35. doi: 10.1016/j.biopsych.2005.10.016. [DOI] [PubMed] [Google Scholar]
- Chen E, Cohen S, et al. How low socioeconomic status affects 2-year hormonal trajectories in children. Psychol Sci. 2010;21(1):31–7. doi: 10.1177/0956797609355566. [DOI] [PubMed] [Google Scholar]
- Coe CL, Kramer M, et al. Prenatal stress diminishes the cytokine response of leukocytes to endotoxin stimulation in juvenile rhesus monkeys. J Clin Endocrinol Metab. 2002;87(2):675–81. doi: 10.1210/jcem.87.2.8233. [DOI] [PubMed] [Google Scholar]
- Cohen S, Doyle WJ, et al. Childhood socioeconomic status and host resistance to infectious illness in adulthood. Psychosom Med. 2004;66(4):553–8. doi: 10.1097/01.psy.0000126200.05189.d3. [DOI] [PubMed] [Google Scholar]
- Cohen S, Janicki-Deverts D, et al. Childhood socioeconomic status and adult health. Ann N Y Acad Sci. 2010;1186:37–55. doi: 10.1111/j.1749-6632.2009.05334.x. [DOI] [PubMed] [Google Scholar]
- Cole SW, Arevalo JM, et al. Computational identification of gene-social environment interaction at the human IL6 locus. Proc Natl Acad Sci U S A. 2010;107(12):5681–6. doi: 10.1073/pnas.0911515107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denenberg VH, Ottinger DR, et al. Effects of maternal factors upon growth and behavior of the rat. Child Dev. 1962;33:65–71. doi: 10.1111/j.1467-8624.1962.tb05988.x. [DOI] [PubMed] [Google Scholar]
- Dickerson SS, Kemeny ME, et al. Immunological Effects of Induced Shame and Guilt. Psychosom Med. 2004;66(1):124–131. doi: 10.1097/01.psy.0000097338.75454.29. [DOI] [PubMed] [Google Scholar]
- Ellis BJ, Boyce WT. Differential susceptibility to the environment: Toward an understanding of sensitivity to developmental experiences and context. Development and Psychopathology. 2011;23(01):1–5. doi: 10.1017/S095457941000060X. [DOI] [PubMed] [Google Scholar]
- Ellis BJ, Boyce WT, et al. Differential susceptibility to the environment: An evolutionary neurodevelopmental theory. Development and Psychopathology. 2011;23(01):7–28. doi: 10.1017/S0954579410000611. [DOI] [PubMed] [Google Scholar]
- Ellis BJ, Essex MJ, et al. Biological sensitivity to context: II. Empirical explorations of an evolutionary-developmental theory. Dev Psychopathol. 2005;17(2):303–28. doi: 10.1017/s0954579405050157. [DOI] [PubMed] [Google Scholar]
- Ferrucci L, Harris TB, et al. Serum IL-6 level and the development of disability in older persons. J Am Geriatr Soc. 1999;47(6):639–46. doi: 10.1111/j.1532-5415.1999.tb01583.x. [DOI] [PubMed] [Google Scholar]
- Fleming AS, Kraemer GW, et al. Mothering begets mothering: the transmission of behavior and its neurobiology across generations. Pharmacol Biochem Behav. 2002;73(1):61–75. doi: 10.1016/s0091-3057(02)00793-1. [DOI] [PubMed] [Google Scholar]
- Francis DD, Champagne FA, et al. Maternal care, gene expression, and the development of individual differences in stress reactivity. Annals of the New York Academy of Sciences. 1999a;896:66–84. doi: 10.1111/j.1749-6632.1999.tb08106.x. [DOI] [PubMed] [Google Scholar]
- Francis DD, Champagne FA, et al. Maternal care, gene expression, and the development of individual differences in stress reactivity. Ann N Y Acad Sci. 1999b;896:66–84. doi: 10.1111/j.1749-6632.1999.tb08106.x. [DOI] [PubMed] [Google Scholar]
- Friedman EM, Herd P. Income, education, and inflammation: differential associations in a national probability sample (The MIDUS study) Psychosom Med. 2010;72(3):290–300. doi: 10.1097/PSY.0b013e3181cfe4c2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gimeno D, Brunner EJ, et al. Adult socioeconomic position, C-reactive protein and interleukin-6 in the Whitehall II prospective study. Eur J Epidemiol. 2007;22(10):675–83. doi: 10.1007/s10654-007-9171-9. [DOI] [PubMed] [Google Scholar]
- Groer M, Murphy R, et al. Salivary measures of stress and immunity in police officers engaged in simulated critical incident scenarios. J Occup Environ Med. 2010;52(6):595–602. doi: 10.1097/JOM.0b013e3181e129da. [DOI] [PubMed] [Google Scholar]
- Harlow HF, Zimmermann RR. Affectional responses in the infant monkey; orphaned baby monkeys develop a strong and persistent attachment to inanimate surrogate mothers. Science. 1959;130(3373):421–32. doi: 10.1126/science.130.3373.421. [DOI] [PubMed] [Google Scholar]
- Haurin DR, Parcel TL, et al. Does homeownership affect child outcomes? Real Estate Economics. 2002;30(4):635–666. [Google Scholar]
- Hintikka J, Lehto S, et al. Unemployment and ill health: a connection through inflammation? BMC Public Health. 2009;9(1):410. doi: 10.1186/1471-2458-9-410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hohensinner PJ, Niessner A, et al. Inflammation and cardiac outcome. Curr Opin Infect Dis. 2011 doi: 10.1097/QCO.0b013e328344f50f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoshaw BA, Evans JC, et al. Social competition in rats: cell proliferation and behavior. Behav Brain Res. 2006;175(2):343–51. doi: 10.1016/j.bbr.2006.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jankord R, Zhang R, et al. Stress activation of IL-6 neurons in the hypothalamus. Am J Physiol Regul Integr Comp Physiol. 2010;299(1):R343–51. doi: 10.1152/ajpregu.00131.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller PS, El-Sheikh M, et al. Relations between mucosal immunity and children's mental health: the role of child sex. Physiol Behav. 2010;101(5):705–12. doi: 10.1016/j.physbeh.2010.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiecolt-Glaser JK, Preacher KJ, et al. Chronic stress and age-related increases in the proinflammatory cytokine IL-6. Proc Natl Acad Sci U S A. 2003;100(15):9090–5. doi: 10.1073/pnas.1531903100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinsey SG, Bailey MT, et al. The inflammatory response to social defeat is increased in older mice. Physiology & Behavior. 2008;93(3):628–636. doi: 10.1016/j.physbeh.2007.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeMay LG, Vander AJ, et al. The effects of psychological stress on plasma interleukin-6 activity in rats. Physiol Behav. 1990;47(5):957–61. doi: 10.1016/0031-9384(90)90024-x. [DOI] [PubMed] [Google Scholar]
- Levine S, Morton A, et al. Infantile Experience and the Maturation of the Pituitary Adrenal Axis. Science. 1957;126(3287):1347. doi: 10.1126/science.126.3287.1347. [DOI] [PubMed] [Google Scholar]
- Liu D, Diorio J, et al. Maternal care, hippocampal glucocorticoid receptors, and hypothalamic-pituitary-adrenal responses to stress. Science. 1997;277(5332):1659–1662. doi: 10.1126/science.277.5332.1659. [DOI] [PubMed] [Google Scholar]
- Loucks EB, Pilote L, et al. Life course socioeconomic position is associated with inflammatory markers: the Framingham Offspring Study. Soc Sci Med. 2010;71(1):187–95. doi: 10.1016/j.socscimed.2010.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meaney MJ. Epigenetics and the Biological Definition of Gene & Environment Interactions. Child Development. 2010;81(1):41–79. doi: 10.1111/j.1467-8624.2009.01381.x. [DOI] [PubMed] [Google Scholar]
- Miller G, Chen E. Unfavorable Socioeconomic Conditions in Early Life Presage Expression of Proinflammatory Phenotype in Adolescence. Psychosom Med. 2007;69(5):402–409. doi: 10.1097/PSY.0b013e318068fcf9. [DOI] [PubMed] [Google Scholar]
- Miller GE, Chen E. Harsh family climate in early life presages the emergence of a proinflammatory phenotype in adolescence. Psychol Sci. 2010;21(6):848–56. doi: 10.1177/0956797610370161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishanian P, Aziz N, et al. Oral fluids as an alternative to serum for measurement of markers of immune activation. Clin Diagn Lab Immunol. 1998;5(4):507–12. doi: 10.1128/cdli.5.4.507-512.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Operario D, Adler NE, et al. Subjective social status: Reliability and predictive utility for global health. Psychology & Health. 2004;19(2):237–246. [Google Scholar]
- Petersen KL, Marsland AL, et al. Community Socioeconomic Status is Associated With Circulating Interleukin-6 and C-Reactive Protein. Psychosom Med. 2008;70(6):646–652. doi: 10.1097/PSY.0b013e31817b8ee4. [DOI] [PubMed] [Google Scholar]
- Powell ND, Bailey MT, et al. Repeated social defeat activates dendritic cells and enhances Toll-like receptor dependent cytokine secretion. Brain, Behavior, and Immunity. 2009;23(2):225–231. doi: 10.1016/j.bbi.2008.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramsay S, Lowe GD, et al. Relationships of inflammatory and haemostatic markers with social class: results from a population-based study of older men. Atherosclerosis. 2008;197(2):654–61. doi: 10.1016/j.atherosclerosis.2007.02.022. [DOI] [PubMed] [Google Scholar]
- Ranjit N, Diez-Roux AV, et al. Socioeconomic position, race/ethnicity, and inflammation in the multi-ethnic study of atherosclerosis. Circulation. 2007;116(21):2383–90. doi: 10.1161/CIRCULATIONAHA.107.706226. [DOI] [PubMed] [Google Scholar]
- Shizuya K, Komori T, et al. The influence of restraint stress on the expression of mRNAs for IL-6 and the IL-6 receptor in the hypothalamus and midbrain of the rat. Life Sci. 1997;61(10):PL 135–40. doi: 10.1016/s0024-3205(97)00608-5. [DOI] [PubMed] [Google Scholar]
- Sjogren E, Leanderson P, et al. Interleukin-6 levels in relation to psychosocial factors: studies on serum, saliva, and in vitro production by blood mononuclear cells. Brain Behav Immun. 2006;20(3):270–8. doi: 10.1016/j.bbi.2005.08.001. [DOI] [PubMed] [Google Scholar]
- Slavich GM, Way BM, et al. Neural sensitivity to social rejection is associated with inflammatory responses to social stress. Proc Natl Acad Sci U S A. 2010;107(33):14817–22. doi: 10.1073/pnas.1009164107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spencer SJ, Galic MA, et al. Neonatal programming of innate immune function. Am J Physiol Endocrinol Metab. 2010 doi: 10.1152/ajpendo.00516.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steptoe A, Hamer M, et al. The effects of acute psychological stress on circulating inflammatory factors in humans: a review and meta-analysis. Brain Behav Immun. 2007;21(7):901–12. doi: 10.1016/j.bbi.2007.03.011. [DOI] [PubMed] [Google Scholar]
- Stowe RP, Peek MK, et al. Plasma cytokine levels in a population-based study: relation to age and ethnicity. J Gerontol A Biol Sci Med Sci. 2010;65(4):429–33. doi: 10.1093/gerona/glp198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takaki A, Huang QH, et al. Immobilization stress may increase plasma interleukin-6 via central and peripheral catecholamines. Neuroimmunomodulation. 1994;1(6):335–42. doi: 10.1159/000097185. [DOI] [PubMed] [Google Scholar]
- Turrin NP, Rivest S. Unraveling the Molecular Details Involved in the Intimate Link between the Immune and Neuroendocrine Systems. Exp Biol Med. 2004;229(10):996–1006. doi: 10.1177/153537020422901003. [DOI] [PubMed] [Google Scholar]
- Veenema AH, Reber SO, et al. Early life stress enhances the vulnerability to chronic psychosocial stress and experimental colitis in adult mice. Endocrinology. 2008;149(6):2727–36. doi: 10.1210/en.2007-1469. [DOI] [PubMed] [Google Scholar]
- Volpato S, Guralnik JM, et al. Cardiovascular disease, interleukin-6, and risk of mortality in older women: the women's health and aging study. Circulation. 2001;103(7):947–53. doi: 10.1161/01.cir.103.7.947. [DOI] [PubMed] [Google Scholar]
- Walker AK, Nakamura T, et al. Neonatal lipopolysaccharide and adult stress exposure predisposes rats to anxiety-like behaviour and blunted corticosterone responses: implications for the double-hit hypothesis. Psychoneuroendocrinology. 2009;34(10):1515–25. doi: 10.1016/j.psyneuen.2009.05.010. [DOI] [PubMed] [Google Scholar]
- Walker AK, Nakamura T, et al. Neonatal lipopolysaccharide exposure alters central cytokine responses to stress in adulthood in Wistar rats. Stress. 2010;13(6):506–15. doi: 10.3109/10253890.2010.489977. [DOI] [PubMed] [Google Scholar]
- Weaver IC, Cervoni N, et al. Epigenetic programming by maternal behavior. Nat Neurosci. 2004;7(8):847–54. doi: 10.1038/nn1276. [DOI] [PubMed] [Google Scholar]
- Westreich D, Cole SR. Invited commentary: positivity in practice. Am J Epidemiol. 2010;171(6):674–7. doi: 10.1093/aje/kwp436. discussion 678-81. [DOI] [PMC free article] [PubMed] [Google Scholar]


