Abstract
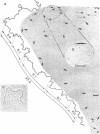
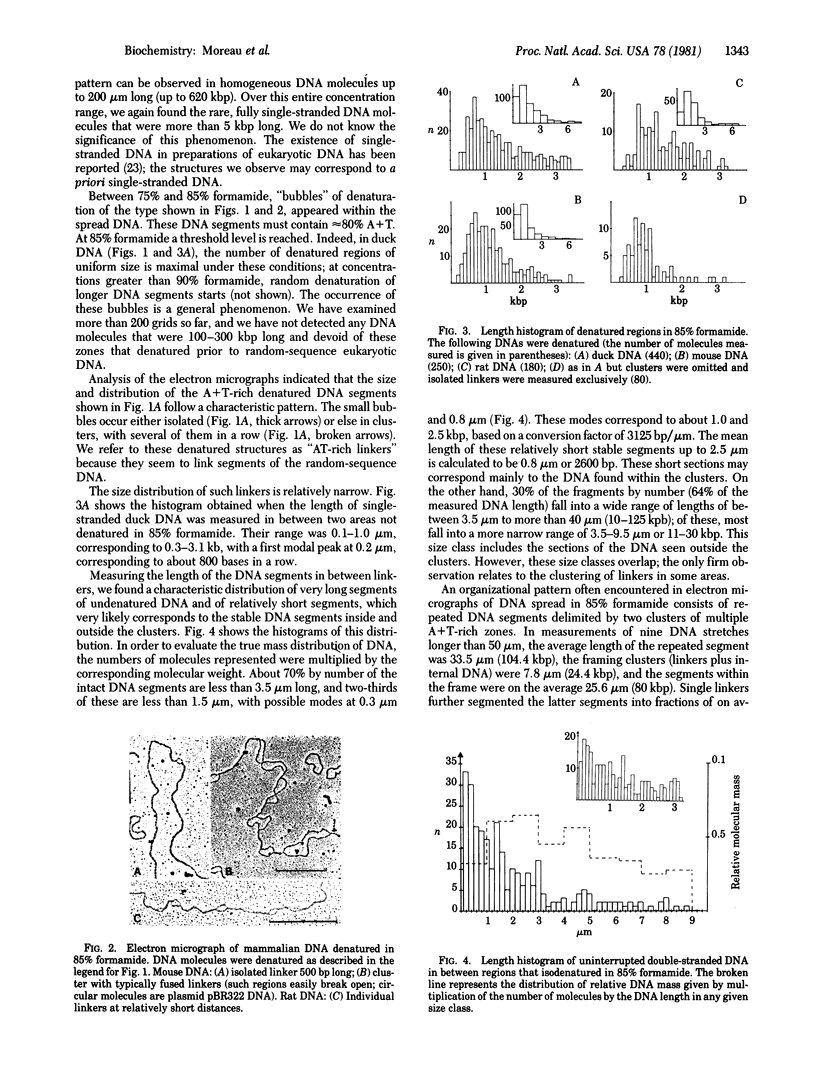
Isodenaturation of avian and mammalian DNA of Mr greater than 2.5 X 10(8) in 85% (vol/vol) formamide led to the observation by electron microscopy of A+T-rich zones ("AT-rich linkers") of 300-3000 base pairs which are distributed over the entire genome in a characteristic pattern. Linkers of mean length 800 base pairs are found either isolated or in clusters of about four to six linkers of more heterogeneous size separated on average by 2500 base pairs (in duck DNA). In between clusters, single linkers segment the DNA at distances of 10 to more than 100 kilobase pairs, with a majority in the range of 10-30 kilobase pairs. An analogous organization of linkers is found in rat and mouse DNA. The internal organization of the clusters varies, however, in a fashion that might be related to the large amount of light satellite DNA in the mouse and its apparent absence in rat and avian DNA. It is possible to fragment the DNA under appropriate conditions by the single-strand-specific nuclease S1 at the site of these A+T-rich zones and to obtain, on alkaline sucrose gradients, a bimodal pattern of DNA fragments of the size corresponding to the pattern observed by electron microscopy. The implications of this observation for DNA organization, chromatin structure, units of transcription and replication, and possible targets of A+T-specific drugs are discussed.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BRUNS G. P., FISCHER S., LOWY B. A. A STUDY OF THE SYNTHESIS AND INTERRELATIONSHIPS OF RIBONUCLEIC ACIDS IN DUCK ERYTHROCYTES. Biochim Biophys Acta. 1965 Feb 8;95:280–290. doi: 10.1016/0005-2787(65)90492-2. [DOI] [PubMed] [Google Scholar]
- Davis R. W., Hyman R. W. A study in evolution: the DNA base sequence homology between coliphages T7 and T3. J Mol Biol. 1971 Dec 14;62(2):287–301. doi: 10.1016/0022-2836(71)90428-1. [DOI] [PubMed] [Google Scholar]
- Fedoroff N. V., Brown D. D. The nucleotide sequence of oocyte 5S DNA in Xenopus laevis. I. The AT-rich spacer. Cell. 1978 Apr;13(4):701–716. doi: 10.1016/0092-8674(78)90220-9. [DOI] [PubMed] [Google Scholar]
- Firtel R. A., Timm R., Kimmel A. R., McKeown M. Unusual nucleotide sequences at the 5' end of actin genes in Dictyostelium discoideum. Proc Natl Acad Sci U S A. 1979 Dec;76(12):6206–6210. doi: 10.1073/pnas.76.12.6206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flamm W. G., Walker P. M., McCallum M. Some properties of the single strands isolated from the DNA of the nuclear satellite of the mouse (Mus musculus). J Mol Biol. 1969 Mar 28;40(3):423–443. doi: 10.1016/0022-2836(69)90163-6. [DOI] [PubMed] [Google Scholar]
- Gross-Bellard M., Oudet P., Chambon P. Isolation of high-molecular-weight DNA from mammalian cells. Eur J Biochem. 1973 Jul 2;36(1):32–38. doi: 10.1111/j.1432-1033.1973.tb02881.x. [DOI] [PubMed] [Google Scholar]
- Holtzer H., Rubinstein N., Fellini S., Yeoh G., Chi J., Birnbaum J., Okayama M. Lineages, quantal cell cycles, and the generation of cell diversity. Q Rev Biophys. 1975 Nov;8(4):523–557. doi: 10.1017/s0033583500001980. [DOI] [PubMed] [Google Scholar]
- Hotta Y., Chandley A. C., Stern H. Biochemical analysis of meiosis in the male mouse. II. DNA metabolism at pachytene. Chromosoma. 1977 Jul 8;62(3):255–268. doi: 10.1007/BF00286047. [DOI] [PubMed] [Google Scholar]
- Hutton J. R., Wetmur J. G. Activity of endonuclease S1 in denaturing solvents: dimethysulfoxide, dimethylformamide, formamide and formaldehyde. Biochem Biophys Res Commun. 1975 Oct 6;66(3):942–948. doi: 10.1016/0006-291x(75)90731-7. [DOI] [PubMed] [Google Scholar]
- Lazowska J., Jacq C., Slonimski P. P. Sequence of introns and flanking exons in wild-type and box3 mutants of cytochrome b reveals an interlaced splicing protein coded by an intron. Cell. 1980 Nov;22(2 Pt 2):333–348. doi: 10.1016/0092-8674(80)90344-x. [DOI] [PubMed] [Google Scholar]
- Leibovitch S. A., Leibovitch M. P., Kruh J., Harel J. Relationship between single-stranded DNA isolated from cultured muscular cells during differentiation and the transcription of messenger RNA. Eur J Biochem. 1979 Jul;97(2):327–333. doi: 10.1111/j.1432-1033.1979.tb13118.x. [DOI] [PubMed] [Google Scholar]
- Marsden M. P., Laemmli U. K. Metaphase chromosome structure: evidence for a radial loop model. Cell. 1979 Aug;17(4):849–858. doi: 10.1016/0092-8674(79)90325-8. [DOI] [PubMed] [Google Scholar]
- Nakamura K., Inouye M. DNA sequence of the gene for the outer membrane lipoprotein of E. coli: an extremely AT-rich promoter. Cell. 1979 Dec;18(4):1109–1117. doi: 10.1016/0092-8674(79)90224-1. [DOI] [PubMed] [Google Scholar]
- Nevins J. R., Darnell J. E., Jr Steps in the processing of Ad2 mRNA: poly(A)+ nuclear sequences are conserved and poly(A) addition precedes splicing. Cell. 1978 Dec;15(4):1477–1493. doi: 10.1016/0092-8674(78)90071-5. [DOI] [PubMed] [Google Scholar]
- Pardue M. L., Gall J. G. Chromosomal localization of mouse satellite DNA. Science. 1970 Jun 12;168(3937):1356–1358. doi: 10.1126/science.168.3937.1356. [DOI] [PubMed] [Google Scholar]
- Paulson J. R., Laemmli U. K. The structure of histone-depleted metaphase chromosomes. Cell. 1977 Nov;12(3):817–828. doi: 10.1016/0092-8674(77)90280-x. [DOI] [PubMed] [Google Scholar]
- Pavlakis G. N., Jordan B. R., Wurst R. M., Vournakis J. N. Sequence and secondary structure of Drosophila melanogaster 5.8S and 2S rRNAs and of the processing site between them. Nucleic Acids Res. 1979 Dec 20;7(8):2213–2238. doi: 10.1093/nar/7.8.2213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pech M., Igo-Kemenes T., Zachau H. G. Nucleotide sequence of a highly repetitive component of rat DNA. Nucleic Acids Res. 1979 Sep 25;7(2):417–432. doi: 10.1093/nar/7.2.417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pribnow D. Nucleotide sequence of an RNA polymerase binding site at an early T7 promoter. Proc Natl Acad Sci U S A. 1975 Mar;72(3):784–788. doi: 10.1073/pnas.72.3.784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prunell A., Bernardi G. The mitochondrial genome of wild-type yeast cells. IV. Genes and spacers. J Mol Biol. 1974 Jul 15;86(4):825–841. doi: 10.1016/0022-2836(74)90356-8. [DOI] [PubMed] [Google Scholar]
- Scherrer K., Imaizumi-Scherrer M. T., Reynaud C. A., Therwath A. On pre-messenger RNA and transcriptions. A review. Mol Biol Rep. 1979 May 31;5(1-2):5–28. doi: 10.1007/BF00777484. [DOI] [PubMed] [Google Scholar]
- Scherrer Klaus. Adenosine-rich sequences in rapidly hybridizing messenger-like RNA and their possible significance for reiterated base sequences in eukaryotic DNA. FEBS Lett. 1971 Sep 15;17(1):68–72. doi: 10.1016/0014-5793(71)80565-3. [DOI] [PubMed] [Google Scholar]
- Schildkraut C. Dependence of the melting temperature of DNA on salt concentration. Biopolymers. 1965;3(2):195–208. doi: 10.1002/bip.360030207. [DOI] [PubMed] [Google Scholar]
- Thiery J. P., Macaya G., Bernardi G. An analysis of eukaryotic genomes by density gradient centrifugation. J Mol Biol. 1976 Nov;108(1):219–235. doi: 10.1016/s0022-2836(76)80104-0. [DOI] [PubMed] [Google Scholar]
- Tibbetts C., Johansson K., Philipson L. Hydroxyapatite chromatography and formamide denaturation of adenovirus DNA. J Virol. 1973 Aug;12(2):218–225. doi: 10.1128/jvi.12.2.218-225.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vizard D. L., Rinehart F. P., Rubin C. M., Schmid C. W. Intramolecular base composition heterogeneity of human DNA. Nucleic Acids Res. 1977 Nov;4(11):3753–3768. doi: 10.1093/nar/4.11.3753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmer C. Effects of the antibiotics netropsin and distamycin A on the structure and function of nucleic acids. Prog Nucleic Acid Res Mol Biol. 1975;15(0):285–318. doi: 10.1016/s0079-6603(08)60122-1. [DOI] [PubMed] [Google Scholar]