Abstract
Background
Cigarette smoking is the leading cause of preventable death in the world, and long-term abstinence rates remain modest. Mindfulness Training (MT) has begun to show benefits in a number of psychiatric disorders, including depression, anxiety and more recently, in addictions. However MT has not been evaluated for smoking cessation through randomized clinical trials.
Methods
88 treatment-seeking, nicotine-dependent adults who were smoking an average of 20 cigarettes/day were randomly assigned to receive MT or the American Lung Association’s Freedom From Smoking (FFS) treatment. Both treatments were delivered twice weekly over four weeks (eight sessions total) in a group format. The primary outcomes were expired-air carbon monoxide-confirmed 7-day point prevalence abstinence and number of cigarettes/day at the end of the 4-week treatment and at a follow-up interview at week 17.
Results
88% of individuals who received MT and 84% of individuals who received FFS completed treatment. Compared to those randomized to the FFS intervention, individuals who received MT showed a greater rate of reduction in cigarette use during treatment and maintained these gains during follow-up (F=11.11, p = .001). They also exhibited a trend toward greater point prevalence abstinence rate at the end of treatment (36% vs. 15%, p = .063), which was significant at the 17-week follow-up (31% vs. 6%, p = .012).
Conclusions
This initial trial of Mindfulness Training may confer benefits greater than those associated with current standard treatments for smoking cessation.
Keywords: Tobacco, Nicotine Dependence, Mindfulness, behavioral treatment, addiction
1. Introduction
Cigarette smoking along with other tobacco use is the leading cause of preventable death in the world, associated with approximately five million people annually, and accounting for 10% of all deaths (Jha et al., 2006). In the US, smoking costs more than $193 billion in health care and lost productivity per year (Center for Disease Control, 2007). Although over 70% of smokers want to quit, fewer than 5% achieve this goal annually (Center for Disease Control, 2007).
As outlined in models previously (Baker et al., 2004; Curtin et al., 2006), acquisition and maintenance of nicotine dependence is a complex process, developed by associative learning mechanisms and perpetuated through positive and negative reinforcement. Habitual smoking begins in part from the formation of associative memories between smoking and both positive (e.g., after a good meal), and negative (e.g., when “stressed”) affective states (Bevins and Palmatier, 2004; Brown et al., 1996; Kandel and Davies, 1986; Leknes and Tracey, 2008; Piasecki et al., 1997). Subsequently, cues that are judged to be positive or negative can induce positive or negative affective states, which can then trigger craving to smoke (Baker et al., 2004; Brandon, 1994; Carter and Tiffany, 1999; Cox et al., 2001; Hall et al., 1993; Huston-Lyons and Kornetsky, 1992; Kassel et al., 2003; Perkins et al., 2010; Shiffman and Waters, 2004; Strong et al., 2009; Zinser et al., 1992). Though the centrality of craving remains controversial (Perkins, 2009; Tiffany, 1990; Tiffany and Carter, 1998; Tiffany and Conklin, 2000), evidence suggests that craving is strongly associated with smoking, which, mainly through the psychophysical properties of nicotine (Imperato et al., 1986), results in the maintenance or improvement of positive, or reduction of negative affective states (Cook et al., 2004; Perkins et al., 2010; Shiffman et al., 1997; Zinser et al., 1992). This sets up positive or negative reinforcement loops respectively, by reinforcing associative memories between these affective states and smoking (Baker et al., 2004; Bevins and Palmatier, 2004; Brandon and Baker, 1991; Carmody et al., 2007; Carter et al., 2008; Carter and Tiffany, 2001; Cook et al., 2004; Hall et al., 1993; Hyman, 2007; Rose and Levin, 1991; Warburton and Mancuso, 1998).
Mainstay behavioral treatments for smoking have focused on teaching individuals to avoid cues, foster positive affective states, develop lifestyle changes that reduce stress (e.g., practice relaxation), divert attention from cravings, substitute other activities for smoking, learn cognitive strategies that reduce negative mood, and develop social support mechanisms (Fiore et al., 2008; Fiore et al., 2000; Lando et al., 1990). These have shown modest success, with abstinence rates hovering between 20–30% over the past three decades (Law and Tang, 1995; Shiffman, 1993). This may be because triggers are often ubiquitous, and diversion of attention requires cognitive reserves, which are often depleted after strong negative affective states (Muraven and Baumeister, 2000). Also, substitutions (e.g., eating candy or carrot sticks) are not always available. The evidence for affective states and craving as perpetuators of smoking, coupled with the modest success of current treatments, highlights the need for innovative treatments (Niaura and Abrams, 2002).
Thus, recently developed smoking cessation treatments have begun to target components of the addictive process by helping patients tolerate negative affect and craving rather than avoiding cues or substituting other activities (e.g., “urge surfing” techniques in cognitive behavioral therapies) (Carroll, 2005; Marlatt and Donovan, 2005). Recent work has focused on recognition and tolerance of negative affect states. For example, in an uncontrolled trial, 16 participants who underwent distress tolerance training (six individual + nine group sessions + eight weeks of nicotine patch), one-week point prevalence abstinence was 31% at the end of treatment, but 0% at the 26-week follow-up (Brown et al., 2008). Acceptance and Commitment Therapy (ACT), which includes an emphasis on tolerance and “defusion” of aversive states has also has shown preliminary efficacy for smoking cessation (Gifford et al., 2004; Hernandez-Lopez et al., 2009). Gifford and colleagues randomized 76 participants to nicotine replacement or ACT (seven individual + seven group sessions), and found 33% and 35%, respectively, achieved 24-hour smoking abstinence in NRT and ACT after treatment, 11% and 23%, after six months, and 15% and 35% one year later (Gifford et al., 2004). Though preliminary, these studies suggest that targeting affective states may aid smoking cessation.
Thus, treatments that target both affective states and craving, such as Mindfulness Training (MT), may be helpful in smoking cessation (Brewer et al., 2009). Mindfulness approaches have been operationalized to include two components: (1) maintaining attention on the individual’s immediate experience and (2) maintaining an attitude of acceptance toward this experience (Bishop et al., 2004). Through these complementary components, MT has been hypothesized to not only bring habituated behaviors into consciousness such that they can be worked with effectively, but also target the associative learning process with an emphasis on affect and craving as critical components of positive and negative reinforcement loops (Brewer et al., 2010b). For example, similar to treatments such as ACT that place an emphasis on accepting one’s immediate experience, MT may help individuals learn “sit with” negative affect, cravings, and withdrawal without habitually reacting to these unpleasant states by smoking. Further, and perhaps somewhat unique to this practice, MT emphasizes the ability to perceive the selfless quality of affective/mind states in that it teaches individuals to recognize these as transient feelings and sensations in the mind and body rather than something that is happening to ‘them. ’ In doing so, individuals may learn to (literally) not take affective and withdrawal states personally, which also may help them quit smoking (Brewer et al., 2010a; Teasdale et al., 2002). Thus, MT may have the relative advantage of teaching a single technique that may lead to the dampening and eventual dismantling of the complex interrelated associative processes of smoking rather than just removing stimuli that might propagate them.
Treatments that include MT have shown promise for a number of disorders, including anxiety and depression (Hofmann et al., 2010) and have recently been explored in the treatment of addictions (Bowen et al., 2009; Brewer et al., 2009; Zgierska et al., 2008). Data on the efficacy of these approaches remain rare: a recent review reported that of 22 published studies that included mindfulness, only one was a randomized control trial (as an add-on treatment) (Zgierska et al., 2009). Mindfulness approaches have only recently been extended to smoking (Bowen and Marlatt, 2009; Davis et al., 2007). For example, in an uncontrolled pilot study for smoking cessation, Davis and colleagues provided eight weeks of Mindfulness-Based Stress Reduction and found 10 of 18 smokers were abstinent at a six weeks post-quit follow-up visit (Davis et al., 2007). These encouraging findings provide a basis for larger, controlled trials of MT for smoking cessation.
Though MT has been incorporated into other treatments, such as cognitive therapy (Mindfulness-based Cognitive Therapy (Segal, 2002)) and relapse prevention (Mindfulness-based Relapse Prevention (Bowen et al., 2009; Brewer et al., 2009), as well as ACT, to our knowledge, its efficacy as a stand-alone treatment (i.e., not as a component of or combined with another form of treatment) for smoking cessation has not been compared to empirically-based smoking cessation treatments.
In this report, we describe outcomes from a preliminary trial in which we evaluated the efficacy of MT compared to the American Lung Association’s Freedom From Smoking (FFS), a manualized, validated, widely-disseminated treatment for smoking cessation (Addington et al., 1998; Association, 2010; Lando et al., 1990). The primary objective was to assess the efficacy of MT vs. FFS using 1-week point prevalence abstinence and number of cigarettes smoked/day as primary endpoints at treatment completion and a 17-week follow-up. As we have previously found positive relationships between homework completion and substance use outcomes with behavioral treatments, (Carroll et al., 2005), our secondary objective was to assess correlations between the amount of completed home practice in both treatment arms and smoking outcomes. We hypothesized that MT would demonstrate at least similar efficacy as FFS with regards to smoking cessation and would show greater correlations between amount of home practice and these outcomes.
2. Methods
2.1. Study Design
This study was a randomized, controlled pilot trial with a four-week treatment and post-treatment follow-up interviews at six, 12 and 17 weeks after treatment initiation. It was approved by the Yale University and Veteran’s Administration institutional review boards.
2.2. Study Population
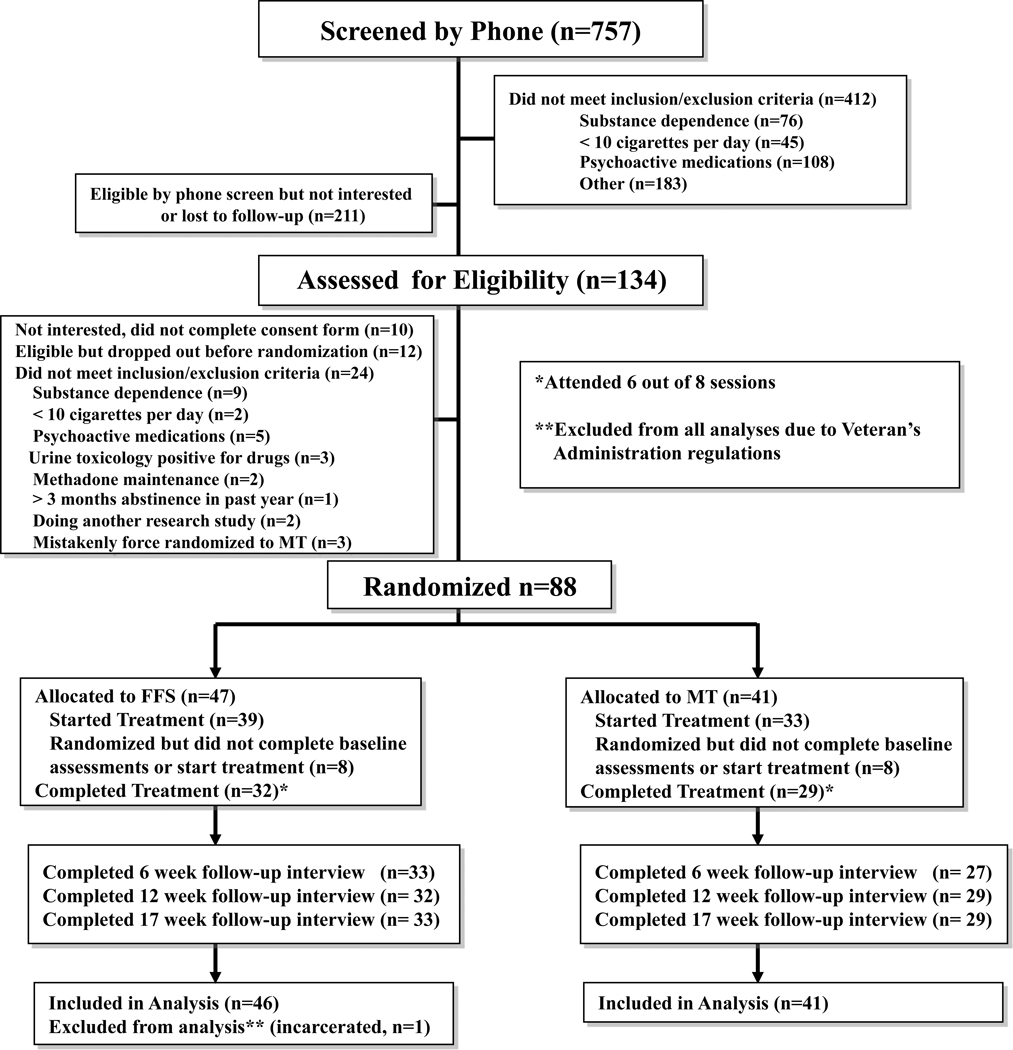
Participants were recruited through flyers and media advertisements offering behavioral treatment for smoking cessation. Those eligible were 18–60 years of age, smoked 10+ cigarettes/day, had fewer than 3 months of abstinence in the past year, and reported interest in quitting smoking. Participants were excluded if they currently used psychoactive medications, had a serious or unstable medical condition in the past six months, or met DSM-IV criteria for other substance dependence in the past year. After complete description of the study to the subjects, written informed consent was obtained. Of the 103 eligible individuals, 88 were randomized (see CONSORT diagram, Fig. 1).
Figure 1. CONSORT diagram.
2.3. Interventions
A computer-generated urn randomization program assigned participants to MT or FFS based on age (> vs. ≤ 40 years old), sex, race (white vs. non-white), and cigarettes smoked/day (> vs. ≤ 20). All participants received twice weekly group sessions (eight total) that were manualized and delivered by instructors experienced in MT (a single therapist with >13 years of training in MT) or certified in FFS respectively (2 therapists with masters (+) level of training in drug counseling/health psychology). FFS was chosen as an active ‘standard treatment’ comparison condition for several reasons: 1) It has demonstrated efficacy (Lando et al., 1990), 2) is manualized and standards for training and certification of therapists are established, 3) is widely available, and 4) includes components that are well-matched with MT, but does not include hypothesized mechanism of MT. For example, both MT and FFS had a quit date at the end of week two (session four), were matched for length (1.5h/session) and delivered on the same days of the week (Monday and Thursday). In addition, home practice materials were matched in a number of ways, including the length (~30 minutes total) and number of tracks (five) on respective CDs. Participants were neither encouraged nor discouraged from using nicotine replacement in either group during active treatment or in the post-treatment follow-up phase.
2.3.1. Mindfulness Training
The MT manual was adapted for active smoking cessation from a previous MT manual for drug relapse prevention (Bowen et al., 2009; Brewer et al., 2009). The overarching theme of momentary awareness and acceptance of cravings and affect (e.g., stress, anxiety etc.) was introduced and reinforced in complementary ways throughout the training (Kabat-Zinn, 1982). The first session introduced participants to the concept of how smoking can become a habituated behavior triggered by an environmental, physical, or mental stimulus through associative learning. It also explored how cravings feel in the body and how MT can help individuals become more aware of these processes. Session two examined how thoughts, emotions and body sensations become triggers for craving and smoking, and introduced a technique to ‘mindfully’ work with cravings (Recognize, Accept, Investigate and Note what cravings feel like as they arise, acronym: RAIN). Session three introduced how difficult emotions perpetuate smoking as well as a standard meditation technique called loving-kindness as a way to work with them (Gunaratana, 2002). Loving-kindness is practiced through directed well-wishing, typically by repetition of phrases such as ‘may X be happy. ’ Session four (quit date) taught participants how cravings thwart long-term goals, and reinforced mindfulness techniques as a way to help individuals disengage from habitual responding and realign with their goals. Session five introduced participants to mindfulness practice in everyday life, including “awareness of breath” meditation and mindful walking (“four modes of walking”, during which individuals practice systematically noting objects that they see, and then objects that they hear, then objects that they smell, and then tactile objects such as the pressure of their feet on the ground). Session six explored the automaticity of thought, and how thoughts can lead to habitual behaviors. Session seven reinforced the concept of acceptance and its role in changing habits. It also explored how both mental and physical actions can “plant seeds” for future actions and habits. Session eight summarized the course tools and explored ways of maintaining these in the future. Home practice was suggested after each session as a combination of formal MT meditations (the “body scan” which teaches individuals to systematically pay attention to different parts of their bodies as a way to reduce habitual mind-wandering and strengthen their attentional capacities, loving-kindness, and awareness of breath, which through focused attention on the breath also is intended to help individuals retrain their minds from habitually engaging in self-related pre-occupations -such as thinking about the past or future, or reacting to stressful stimuli- to more present moment awareness), and informal practices (four modes of walking, mindfulness of daily activities, mindfulness of smoking, RAIN). Each participant received a meditation practice CD.
2.3.2. Freedom From Smoking
FFS was delivered as previously described (Lando et al., 1990), with the exception that sessions were delivered over four weeks (twice weekly) instead of eight weeks. Briefly, the program covered behavior modification, stress reduction, and relapse prevention, and was divided into three stages: preparation, action, and maintenance. In the preparation stage (sessions 1–3), participants examined smoking patterns through self-monitoring, identified triggers, and developed a personalized quit plan. On quit day (session four), participants affirmed their decision to quit and identified specific coping strategies. During the maintenance stage, participants identified ways to remain smoke-free and maintain a healthy lifestyle (e.g., weight management, exercise, relapse prevention), and continued to discuss the importance of social support and relaxation strategies. Home practice was suggested after each session typically as a combination of formal (e.g., practicing guided relaxation techniques) and informal (e.g., “packtracks”) techniques. Each participant received a practice CD of cessation techniques.
2.4. Smoking Status
Self-reported smoking was assessed at in-person weekly visits by a research assistant who was not involved in treatment delivery via the Timeline Follow Back method (TLFB) (Sobell and Sobell, 1992). Self-reported abstinence was assessed using TLFB and verified by an exhaled carbon monoxide (CO) measurement of ≤ 10 parts per million at each of the twice-weekly treatment and at follow-up visits. Participants who dropped out of treatment were contacted to provide in-person assessments at follow-up time points (see CONSORT diagram) (Hollis and Campbell, 1999). All participants were financially compensated for assessment visits (10 USD per assessment visit during treatment and 20 USD per assessment visit at follow-up). Of 244 CO measurements taken for point prevalence confirmation, eight (3.3%) were unverified due to participants having moved out of the state or being assessed outside of the study timeframe, two (.8%) were confounded by marijuana use that day (Javors et al., 2005; Wu et al., 1988), and one (.4%) was unverified due to research assistant error. One CO measurement (.4%) was inconsistent with self-report and was considered to be non-abstinent.
2.5. Study End Points
The primary outcomes were one-week point prevalence abstinence and average number of cigarettes smoked/day at four (treatment completion) and 17 weeks after treatment initiation.
2.6. Statistical Analysis
Longitudinal data were analyzed using intent-to-treat mixed effect regression models on the full sample of randomized subjects (minus one individual who was incarcerated after treatment and whose data were not allowed to be analyzed per Veteran’s Administration regulations) to evaluate change over time in cigarette use/week during treatment (1st phase) and during the follow-up period (2nd phase) as previously described (Ball et al., 2007; Singer and Willett, 2003). Longitudinal analyses are based on the continuous dependent variable “average number of cigarettes smoked per day by week.” ANOVA, χ2 analysis and Pearson’s correlations were used where appropriate, using SPSS 18. Incomplete data were handled using casewise deletion, using all available data for parameter estimates (Hedeker et al., 2007). All tests of significance are reported as two-tailed, and error is reported as ± standard deviation. Significance is reported as p ≤ .025 to take into account correction for multiple comparisons.
3. Results
3.1. Participants
Baseline and demographic characteristics were comparable between treatment groups (see Table 1). Overall, 45% of participants were members of ethnic minority groups, and 63% were men. On average, participants were 46 years old, smoked 20 cigarettes/day, started smoking regularly at the age of 16, and had 5.2 previous quit attempts. Sixteen (eight in each group) did not complete baseline paperwork and were not exposed to treatment. χ2 and ANOVA analyses revealed no differences between these individuals and those who started treatment (n=33 in MT, n=38 in FFS). Individuals in MT and FFS who started treatment attended 6.7 ± 1.7 and 6.2 ± 2.2 of eight sessions respectively. The six, 12, and 17-week follow-up completion rates were 27 (82% of treatment-exposed individuals) and 33 (87%), 29 (88%) and 32 (84%), and 29 (88%) and 33 (87%) for MT and FFS respectively. No serious adverse events were reported in either treatment group.
Table 1.
Baseline characteristics of participants
| MTS (n=41) | FFS (n=46) | Total (n=87) | For χ2 | df | p | |
|---|---|---|---|---|---|---|
| Sex | N (%) | N (%) | N (%) | |||
| Male | 27(65.9) | 27(58.7) | 54(62.1) | 0.472 | 1 | .492 |
| Female | 14(34.1) | 19(41.3) | 33(37.9) | |||
| Age | 46.5±8.7 | 45.3+11.4 | 45.9±10.2 | 0.339 | 1 | .562 |
| Race | ||||||
| White | 24(58.5) | 19(41.3) | 43(49.4) | 4.557 | 3 | .207 |
| Black | 15(36.6) | 19(41.3) | 34(39.1) | |||
| Hispanic | 2(4.9) | 7(15.2) | 9(10.3) | |||
| Other | 0 | 1(2.2) | 1(1.1) | |||
| Education level | ||||||
| College grad or more | 12(29.3) | 13(28.3) | 25(28.7) | 1.715 | 3 | .634 |
| Partial college | 10(24.4) | 15(32.6) | 25(28.7) | |||
| High School | 17(41.5) | 14(30.4) | 31(35.6) | |||
| Less than high school | 2(4.9) | 4(8.7) | 6(6.9) | |||
| Marital Status | ||||||
| Never married | 20(48.8) | 25(54.3) | 45(51.7) | 0.376 | 3 | .945 |
| Married/Cohabitating | 8(19.5) | 7(15.2) | 15(17.2) | |||
| Separated/Divorced | 12(29.3) | 13(28.3) | 25(28.7) | |||
| Widowed | 1(2.4) | 1(2.2) | 2(2.3) | |||
| Employment Status | ||||||
| Full time | 15(36.6) | 13(28.3) | 28(32.2) | 0.899 | 2 | .638 |
| Part time | 5(12.2) | 8(17.4) | 13(14.9) | |||
| Unemployed | 21(51.2) | 25(54.3) | 46(52.9) | |||
| mean (±SD) | mean (±SD) | mean (±SD) | ||||
| Started smoking 3×/week (age) | 16.7+4.8 | 15.6+4.0 | 16.1+4.4 | 1.402 | 1, 85 | .240 |
| Number of cigarettes/day | 21.2+10.6 | 19+8.3 | 20.0+9.5 | 1.219 | 1, 85 | .273 |
| Number of smokers in house | .41+.74 | 2.7+11.2 | 1.6+8.2 | 1.7 | 1, 85 | .196 |
| Number of prior quit attempts | 6.0+9.1 | 4.4+4.8 | 5.2+7.2 | 1.037 | 1, 85 | .311 |
| Longest abstinence in life (months) | 14.3+34.3 | 8.9+17.6 | 11.4+26.8 | 0.88 | 1, 85 | .351 |
| Longest abstinence in past year (months) | .07+.35 | .15+.47 | .11+.42 | 0.782 | 1, 85 | .379 |
3.2. Effects of Mindfulness Training on smoking
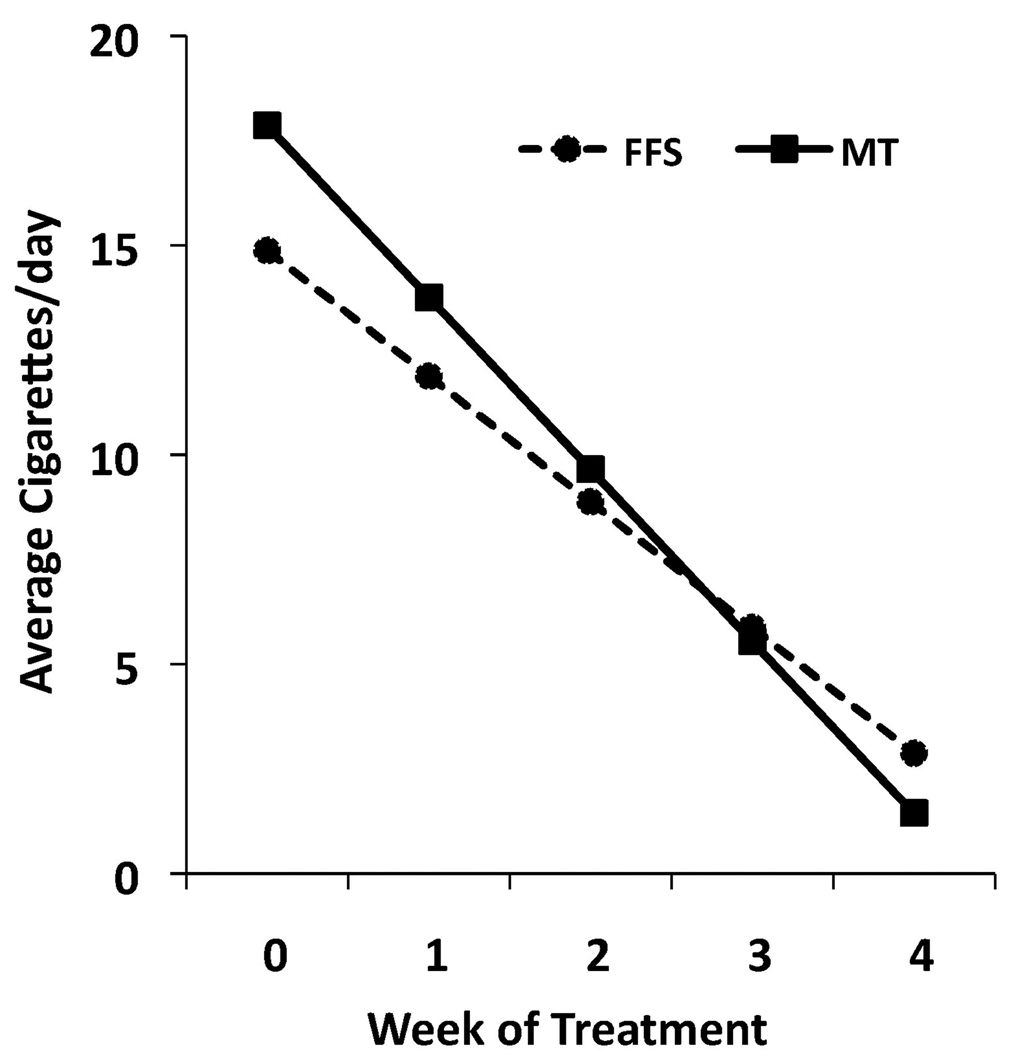
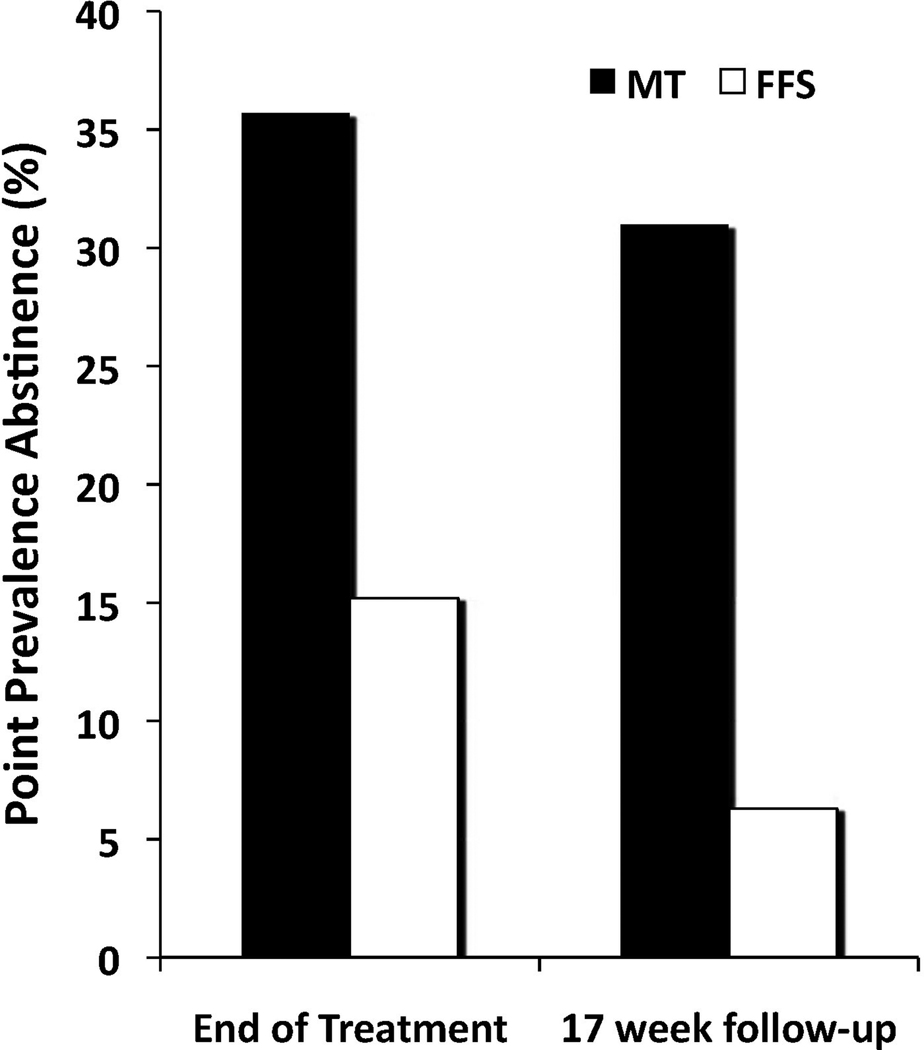
Random effects regression analyses on the full intention to treat sample indicated participants in both groups reduced cigarette use from baseline through the 17-week follow-up (effect for time, F=480.79, df=1,1115, p<.0001). The rate of change during active treatment was significantly greater than the rate of change during post treatment (effect for phase, active versus follow-up, F=579.00, df=1,1115, p<.0001). During active treatment, individuals receiving MT demonstrated a greater reduction in cigarette use than those receiving FFS, and maintained these treatment gains during the follow-up period (treatment group × time, F=7.01, df=1,1115, p=.008). As subject expectancy of receiving treatment can have non-specific effects on smoking, we also analyzed cigarette use using the week before treatment initiation (as compared to baseline) in our regression model. When data from the treatment-exposed sample (n=71) was analyzed using the same model, individuals in the MT group again showed a greater rate of change in smoking compared to those in the FFS group, and maintained these treatment gains during the follow-up period (treatment group × time, F=11.11, df=1,1082, p=.001; estimates from the regression analyses for the treatment-exposed sample are presented in Figure 2). Individuals who received MT showed a trend toward greater one-week point prevalence abstinence at the end of treatment (36% vs. 15%, χ2=3.45, df=1, p=.063, see Figure 3), which was statistically significant at the 17-week follow-up endpoint (31% vs. 6%, χ2=6.32, df=1, p=.012).
Figure 2. Individuals receiving Mindfulness Training reduce cigarette smoking more than those receiving Freedom From Smoking.
Mixed effect regression model estimates of cigarette smoking in Mindfulness Training (MT, n=33) and Freedom From Smoking (FFS, n=38) during the week before treatment initiation and the four weeks of treatment (F=11.11, df=1,1082, p=.001).
Figure 3. Individuals receiving Mindfulness Training achieve greater point prevalence abstinence rates than those receiving Freedom From Smoking.
One-week point prevalence abstinence rates for Mindfulness Training (MT) and Freedom From Smoking (FFS) at the end of treatment (χ2=3.45, df=1, p = .063) and 17-week follow-up (χ2=6.32, df=1, p=.012), n=33 in MT and n=38 in FFS.
3.3. Medication use during and after treatment
Though participants were neither encouraged nor discouraged from using nicotine replacement, three participants (9%) who received MT and four (11%) who received FFS reported some type of nicotine replacement use during treatment (average of 11.3±6.8 and 19.8±4.8 days respectively, F=3.76, df=1,5, p=.110). During follow-up, three participants (9%) who received MT and eight who received FFS (21%) reported nicotine replacement (average of 17.3±27.4 and 35.4±22.1 days respectively, F=1.30, df=1,9, p=.284). No participants receiving MT reported other cessation medication use, while one participant receiving FFS reported using varenicline (24 days) and one reported using bupropion (12 days).
3.4. Effects of Mindfulness Training Practice on Outcomes
As home practice has been correlated with outcomes in studies of substance use (Carroll et al., 2008) and MT (Carmody and Baer, 2008), we also assessed links between home practice and treatment outcomes. Individuals receiving MT reported formal home practice an average of 18 minutes/day, 4.6 days/week and informal practice an average of 4.8 times/day, 5.1 days/week during treatment. Those receiving FFS reported formal home practice (listening to a 30 minute CD) 1.5 days/week, and informal practice (completing PackTracks®) an average of 3.2 days/week.
Within the MT group, more home practice correlated with less cigarette use for both formal (r= −.442, df=26, p=.019, see Table 2) and informal practice (r= −.479, df=26, p=.010) at the end of treatment. Although not statistically significant, this relationship was also seen for point prevalence abstinence (r= −.342, df=26, p=.075). Post-hoc analysis showed strong correlations between sitting meditation and point prevalence abstinence throughout the follow-up period, and the use of the informal practice RAIN with average number of cigarettes at 4 and 6 weeks (see Table 2). No correlations were found between FFS home practices and outcomes (df=29, all p>.315).
Table 2.
Correlations between MT home practice and treatment outcomes
| Week 4 | Week 6 | Week 12 | Week 17 | |||||
|---|---|---|---|---|---|---|---|---|
| r | p | r | p | r | p | r | p | |
|
Point Prevalence | ||||||||
| Total Formal Practice | −.342 | .075 | −.323 | .088 | −.164 | .395 | −.280 | .141 |
| Body Scan | −.270 | .164 | −.267 | .162 | −.136 | .483 | −.165 | .393 |
| Loving Kindness | −.121 | .546 | −.083 | .673 | .031 | .877 | −.156 | 429 |
| Sitting Meditation | −.584 | .001 | −.582 | .001 | −.400 | .039 | −.520 | .005 |
| Total Informal Practice | −.281 | .147 | −.310 | .101 | −.278 | .144 | −.214 | .265 |
| Setting Aspiration | −.096 | .628 | −.068 | .727 | .026 | .893 | .020 | .917 |
| Daily Activity | −.206 | .294 | −.222 | .246 | −.106 | .584 | −.104 | .592 |
| RAIN | −.283 | .144 | −.332 | .078 | −.406 | .029 | −.292 | .124 |
|
Average Number of Cigarettes/day | ||||||||
| Total Formal Practice | −.442 | .019 | −.267 | .162 | −.190 | .324 | −.197 | .305 |
| Body Scan | −.426 | .024 | −.212 | .271 | −.199 | .300 | −.195 | .311 |
| Loving Kindness | −.296 | .134 | −.218 | .264 | −.193 | .325 | −.206 | .293 |
| Sitting Meditation | −.508 | .007 | −.424 | .027 | −.071 | .724 | −.079 | .696 |
| Total Informal Practice | −.479 | .010 | −.373 | .046 | −.022 | .911 | −.008 | .968 |
| Setting Aspiration | −.225 | .251 | −.085 | .661 | .087 | .652 | .086 | .657 |
| Daily Activity | −.324 | .093 | −.249 | .192 | .105 | .587 | .125 | .518 |
| RAIN | −.499 | .007 | −.417 | .024 | −.170 | .379 | −.166 | .388 |
Pearson correlations between formal practice (total minutes) and informal practice (total number of times) and smoking outcomes. N = 27–29. No significant correlations were found in the FFS group.
4. Discussion
This, to our knowledge, is the first randomized clinical trial to evaluate the efficacy of Mindfulness Training as a stand-alone treatment for smoking cessation compared to an active, empirically-supported control condition. Despite comparable treatment retention and completion rates, we found that individuals who received MT demonstrated greater reductions in smoking, which were maintained through the 17-week follow-up interview. These findings are encouraging as behavioral treatments have shown little overall improvement in cessation rates over the past 30 years (Mottillo et al., 2009; Shiffman, 1993). For example, our 17-week point prevalence odds ratio of 6.75, is significantly larger than the average odds ratio of 1.76 found in a recent meta-analysis of previous studies of group counseling (Mottillo et al., 2009), though this may be in part a result of the relatively poor performance of FFS. It was also larger than the odds ratio of behavioral therapies as reported in the current clinical practice guidelines, such as those targeting negative affect (OR = 1.2, abstinence rate = 13.6%), social support (OR = 1.5, abstinence rate = 16.2%), practical counseling (OR = 1.5, abstinence rate = 16.2%), aversive smoking (OR = 1.7, abstinence rate = 17.7%) and medication + counseling (OR = 1.7, abstinence rate = 22.1%) (Fiore et al., 2008).
Though the underpinnings of the actions of MT are just beginning to be understood (Brewer et al., 2010b; Grabovac et al., 2011; Lutz et al., 2008), the results from this study suggest that teaching techniques that in theory, target core components of the addictive process (e.g., craving) may be more effective than teaching the avoidance of cues or fostering positive affective states that have been emphasized in previous treatments. This possibility is particularly relevant in light of evidence suggesting that pharmacotherapies may be more efficacious in targeting background rather than cue-induced craving (Ferguson and Shiffman, 2009). It is plausible that combining MT with a medication may improve both the initial efficacy as well as long-term abstinence rates as 1) the combination may target both background and cue-induced craving concurrently and 2) MT may help to sustain medication effects after discontinuation through the “unlearning” of the addictive process. However, future studies are required to determine whether changes in craving, negative affect or other mechanisms mediate the effects of mindfulness training in smoking cessation. Additionally, as very few subjects in this study opted to use medications, no treatment by medication interactions or differences between groups were noted. Further trials combining MT with medications will help to answer whether there are indeed interactions therein and how these affect the addictive process.
We have previously shown positive relationships between homework completion and substance use outcomes (Carroll et al., 2005). Links between the amount of MT home practice and outcomes have also been studied in various populations, though have yielded mixed results, possibly due to methodological shortcomings (Vettese et al., 2009). Using careful matching for the type and amount of home practice between groups, we found no correlations between the amount of formal or informal homework in the FFS group, but strong correlations in the MT group. These findings help to control for the confounding effects of individuals being motivated to quit (i.e., motivated individuals would presumably do more home practice regardless of group), as well as non-specific effects of doing any type of home practice. Interestingly, many of the positive findings between home practice and outcomes in previous studies may be attributed to self-selection bias, familiarity with mindfulness, and/or treatment expectancies (Vettese et al., 2009). However, even when controlling for these factors (e.g., no mention of the type of treatment until after randomization, no previous mindfulness experience [data not shown] etc.), we still found strong correlations between home practice and outcomes. The correlations between sitting meditation and treatment outcomes are noteworthy as they may suggest that practicing to “sit” through difficult mind-states (including negative affect and craving) may train individuals to do the same when faced with an opportunity to smoke. Alternatively, sitting meditation may be a marker for individuals who are more likely to be able to utilize MT for smoking cessation, thus raising the prospect of individualizing treatment by using this at intake or early in treatment to determine if an individual may benefit from MT, or if other cessation strategies should be emphasized. Overall, these results suggest that MT may be a viable option for smoking cessation treatment for the general population, and given its group format and short treatment period, may prove to be cost effective as well.
4.1. Strengths and Limitations
Strengths of this pilot trial include the random assignment from a diverse community sample, the presence of an active comparison group, and the use of intent-to-treat analysis of our sample using validated outcome measures. This study has several limitations as well. This study was performed at a single site, treatment was provided by only 1–2 therapists per condition, and treatment integrity was not formally assessed. Thus treatment effects may not be generalized beyond the specific therapists in this trial (Crits-Christoph and Mintz, 1991). Also, it was of moderate size, and used an active comparison group, which typically limits the ability to detect differences between contrasts. Notwithstanding, we still found significant between-group differences, though these warrant replication before definitive conclusions may be drawn. Also, MT and FFS are typically delivered over eight weeks as compared to four weeks. Despite significant overall reductions in smoking in both groups, it is possible that participants in both groups may have fared better with extended treatment. Further, the FFS group showed lower abstinence rates than those reported by self-quitters (11%) (Fiore et al., 2008), which may be due to selection inherent in clinical trials, though this bias should be distributed across both arms of this study due to randomization. In addition to the shorter 4-week format, FFS may have also fared unusually poorly as medication treatment was neither emphasized nor de-emphasized, rather than the usual emphasis that has been placed on combination treatment (this was specifically done to isolate the effects of these behavioral therapies, while not withholding proven medication treatment). In fact, the addition of 4–8 sessions of counseling to medication has been shown to produce greater abstinence rates (OR = 1.3, abstinence rate = 26.9%) than medication alone (OR = 1.0, abstinence rate = 21.8%) (Fiore et al., 2008). Given its pilot study nature, our four-month follow-up period is shorter than the six-month period that is standard in the field (Fiore et al., 2008). Finally, a limitation of this study is the exclusion of individuals using psychoactive medications (for example roughly 10% of the US population is currently prescribed selective serotonin reuptake inhibitors (Olfson and Marcus, 2009)). As the establishment of the efficacy of MT in the general population is an important first step, the next step will be to assess its effectiveness in individuals with psychiatric co-morbidities, especially with the high prevalence of smoking in individuals with psychiatric disorders (Ziedonis et al., 1994). Given shared features and possible common mechanisms between addictions and primary Axis I psychiatric disorders (e.g., depression), it is possible that mindfulness training may benefit both concurrently (Brewer et al., 2010b).
In conclusion, results from this study suggest that MT may have promise as a brief, stand-alone treatment for smoking cessation. Larger studies that not only replicate these findings, but evaluate the psychological and neurobiological mechanisms of action of MT, as well as those combining MT with pharmacotherapies are warranted.
Table 3.
Average home practice during treatment and follow-up
| MT | FFS | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Formal home practice | Informal home practice | Formal home practice | Informal home practice | ||||||
| Week(s) | Days/week | Minutes/day | Days/week | Times/day | Days/week | Minutes/day | Days/week | Times/day | |
| 1–4 | 4.6 | 18.0 | 5.1 | 4.8 | 1.5 | (Not calc)* | 3.2 | (Not calc)** | |
| 6 | 4.1 | 20.5 | 3.6 | 5.3 | 2.0 | 14.0 | 2.6 | 4.1 | |
| 12 | 3.3 | 33.3 | 3.6 | 10.4 | 1.3 | 9.6 | 1.6 | 1.3 | |
| 17 | 2.6 | 27.0 | 2.7 | 5.6 | 1.8 | 8.6 | 1.5 | 3.1 | |
Particiants were instructed to listen to a 30 minute CD but were not asked to report the actual number of minutes of listening.
Participants were instructed to complete PackTracks® but were not asked to report the number of times/day of doing so.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Addington J, el-Guebaly N, Campbell W, Hodgins DC, Addington D. Smoking cessation treatment for patients with schizophrenia. Am. J. Psychiatry. 1998;155:974–975. doi: 10.1176/ajp.155.7.974. [DOI] [PubMed] [Google Scholar]
- American Lung Association. Freedom From Smoking. [accessed on 7/23/10];2010 http://www.lungusa.org/stop-smoking/how-to-quit/freedom-from-smoking/about.html. [Google Scholar]
- Baker TB, Piper ME, McCarthy DE, Majeskie MR, Fiore MC. Addiction motivation reformulated: an affective processing model of negative reinforcement. Psychol. Rev. 2004;111:33–51. doi: 10.1037/0033-295X.111.1.33. [DOI] [PubMed] [Google Scholar]
- Ball S, Martino S, Nich C, Frankforter T, Van Horn D, Crits-Christoph P, Woody G, Obert J, Farentinos C, Carroll K. Site matters: multisite randomized trial of motivational enhancement therapy in community drug abuse clinics. J. Consult. Clin. Psychol. 2007;75:556–567. doi: 10.1037/0022-006X.75.4.556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bevins RA, Palmatier MI. Extending the role of associative learning processes in nicotine addiction. Behav. Cogn. Neurosci. Rev. 2004;3:143–158. doi: 10.1177/1534582304272005. [DOI] [PubMed] [Google Scholar]
- Bishop SR, Lau M, Shapiro S, Carlson L, Anderson ND, Carmody J, Segal ZV, Abbey S, Speca M, Velting D, Devins G. Mindfulness: a proposed operational definition. Clin. Psychol. 2004;11:230–241. [Google Scholar]
- Bowen S, Chawla N, Collins SE, Witkiewitz K, Hsu S, Grow J, Clifasefi S, Garner M, Douglass A, Larimer ME, Marlatt A. Mindfulness-based relapse prevention for substance use disorders: a pilot efficacy trial. Subst. Abuse. 2009;30:295–305. doi: 10.1080/08897070903250084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowen S, Marlatt A. Surfing the urge: brief mindfulness-based intervention for college student smokers. Psychol. Addict. Behav. 2009;23:666–671. doi: 10.1037/a0017127. [DOI] [PubMed] [Google Scholar]
- Brandon T. Negative affect as motivation to smoke. Curr. Dir. Psychol. Sci. 1994:33–37. [Google Scholar]
- Brandon T, Baker T. The Smoking Consequences Questionnaire: the subjective expected utility of smoking in college students. Psychol. Assess. 1991;3:484–491. [Google Scholar]
- Brewer JA, Bowen S, Smith JT, Marlatt GA, Potenza MN. Mindfulness-based treatments for co-occurring depression and substance use disorders: what can we learn from the brain? Addiction. 2010a;105:1698–1706. doi: 10.1111/j.1360-0443.2009.02890.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brewer JA, Bowen S, Smith JT, Marlatt GA, Potenza MN. Response to commentaries on mindfulness-based treatments for co-occuring depression and substance use disorders: what can we learn from the brain? Addiction. 2010b;105:1709–1710. doi: 10.1111/j.1360-0443.2009.02890.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brewer JA, Sinha R, Chen JA, Michalsen RN, Babuscio TA, Nich C, Grier A, Bergquist KL, Reis DL, Potenza MN, Carroll KM, Rounsaville BJ. Mindfulness training and stress reactivity in substance abuse: results from a randomized, controlled stage I pilot study. Subst. Abuse. 2009;30:306–317. doi: 10.1080/08897070903250241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown RA, Lewinsohn PM, Seeley JR, Wagner EF. Cigarette smoking, major depression, and other psychiatric disorders among adolescents. J. Am. Acad. Child Adolesc. Psychiatry. 1996;35:1602–1610. doi: 10.1097/00004583-199612000-00011. [DOI] [PubMed] [Google Scholar]
- Brown RA, Palm KM, Strong DR, Lejuez CW, Kahler CW, Zvolensky MJ, Hayes SC, Wilson KG, Gifford EV. Distress tolerance treatment for early-lapse smokers: rationale, program description, and preliminary findings. Behav. Modif. 2008;32:302–332. doi: 10.1177/0145445507309024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carmody J, Baer RA. Relationships between mindfulness practice and levels of mindfulness, medical and psychological symptoms and well-being in a mindfulness-based stress reduction program. J. Behav. Med. 2008;31:23–33. doi: 10.1007/s10865-007-9130-7. [DOI] [PubMed] [Google Scholar]
- Carmody T, Vieten C, Astin J. Negative affect, emotional acceptance, and smoking cessation. J. Psychoactive Drugs. 2007;39:499–508. doi: 10.1080/02791072.2007.10399889. [DOI] [PubMed] [Google Scholar]
- Carroll K. Recent advances in the psychotherapy of addictive disorders. Curr. Psychiatry Rep. 2005;7:329–336. doi: 10.1007/s11920-005-0032-5. [DOI] [PubMed] [Google Scholar]
- Carroll KM, Ball SA, Martino S, Nich C, Babuscio TA, Nuro KF, Gordon MA, Portnoy GA, Rounsaville BJ. Computer-assisted delivery of cognitive-behavioral therapy for addiction: a randomized trial of CBT4CBT. Am. J. Psychiatry. 2008;165:881–888. doi: 10.1176/appi.ajp.2008.07111835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll KM, Nich C, Ball SA. Practice makes progress? Homework assignments and outcome in treatment of cocaine dependence. J. Consult. Clin. Psychol. 2005;73:749–755. doi: 10.1037/0022-006X.73.4.749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter BL, Lam CY, Robinson JD, Paris MM, Waters AJ, Wetter DW, Cinciripini PM. Real-time craving and mood assessments before and after smoking. Nicotine Tob. Res. 2008;10:1165–1169. doi: 10.1080/14622200802163084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter BL, Tiffany ST. Meta-analysis of cue-reactivity in addiction research. Addiction. 1999;94:327–340. [PubMed] [Google Scholar]
- Carter BL, Tiffany ST. The cue-availability paradigm: the effects of cigarette availability on cue reactivity in smokers. Exp. Clin. Psychopharmacol. 2001;9:183–190. doi: 10.1037//1064-1297.9.2.183. [DOI] [PubMed] [Google Scholar]
- Center for Disease Control. Smoking and Tobacco Use. 2007 http://www.cdc.gov/tobacco/basic_information/FastFacts.htm#toll.
- Cook JW, Spring B, McChargue D, Hedeker D. Hedonic capacity, cigarette craving, and diminished positive mood. Nicotine Tob. Res. 2004;6:39–47. doi: 10.1080/14622200310001656849. [DOI] [PubMed] [Google Scholar]
- Cox LS, Tiffany ST, Christen AG. Evaluation of the brief questionnaire of smoking urges (QSU-brief) in laboratory and clinical settings. Nicotine Tob. Res. 2001;3:7–16. doi: 10.1080/14622200020032051. [DOI] [PubMed] [Google Scholar]
- Crits-Christoph P, Mintz J. Implications of therapist effects for the design and analysis of comparative studies of psychotherapies. J. Consult. Clin. Psychol. 1991;59:20–26. doi: 10.1037//0022-006x.59.1.20. [DOI] [PubMed] [Google Scholar]
- Curtin J, McCarthy D, Piper M, Baker T. Implicit and explicit drug motivational processes: a model of boundary conditions. In: Wiers RW, Stacy AW, editors. Handbook of Implicit Cognition and Addiction. Thousand Oaks: Sage Publications, Inc.; 2006. pp. 233–250. [Google Scholar]
- Davis JM, Fleming MF, Bonus KA, Baker TB. A pilot study on mindfulness based stress reduction for smokers. BMC Complement. Altern. Med. 2007;7:2. doi: 10.1186/1472-6882-7-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson SG, Shiffman S. The relevance and treatment of cue-induced cravings in tobacco dependence. J. Subst. Abuse Treat. 2009;36:235–243. doi: 10.1016/j.jsat.2008.06.005. [DOI] [PubMed] [Google Scholar]
- Fiore M, JaÈn C, Baker T, Bailey W, Benowitz N, Curry S, Dorfman S, Froelicher E, Goldstein M, Healton C. US Department of Health and Human Services. Rockville, MD: Public Health Service; 2008. Treating Tobacco Use and Dependence: 2008 Update. Clinical Practice Guideline; pp. 101–103. [Google Scholar]
- Fiore MC, Bailey WC, Cohen SJ, Dorfman SF, Goldstein MG, Gritz ER, Heyman RB, Jaén CR, Kottke TE, Lando HA. US Department of Health and Human Services. Washington, DC: Public Health Service; 2000. Treating tobacco Use and Dependence: Clinical Practice Guideline; p. 196. [Google Scholar]
- Gifford EV, Kohlenberg BS, Hayes SC, Antonuccio DO, Piasecki MM, Rasmussen-Hall ML, Palm KM. Acceptance-based treatment for smoking cessation. Behav. Ther. 2004;35:689–705. [Google Scholar]
- Grabovac AD, Lau MA, Willett BR. Mechanisms of mindfulness: a Buddhist psychological model. Mindfulness. 2011 [Google Scholar]
- Gunaratana H. Mindfulness in Plain English. Somerville, MA: Wisdom Publications; 2002. [Google Scholar]
- Hall SM, Munoz RF, Reus VI, Sees KL. Nicotine, negative affect, and depression. J. Consult. Clin. Psychol. 1993;61:761–767. doi: 10.1037//0022-006x.61.5.761. [DOI] [PubMed] [Google Scholar]
- Hedeker D, Mermelstein RJ, Demirtas H. Analysis of binary outcomes with missing data: missing = smoking, last observation carried forward, and a little multiple imputation. Addiction. 2007;102:1564–1573. doi: 10.1111/j.1360-0443.2007.01946.x. [DOI] [PubMed] [Google Scholar]
- Hernandez-Lopez M, Luciano MC, Bricker JB, Roales-Nieto JG, Montesinos F. Acceptance and commitment therapy for smoking cessation: a preliminary study of its effectiveness in comparison with cognitive behavioral therapy. Psychol. Addict. Behav. 2009;23:723–730. doi: 10.1037/a0017632. [DOI] [PubMed] [Google Scholar]
- Hofmann SG, Sawyer AT, Witt AA, Oh D. The effect of mindfulness-based therapy on anxiety and depression: a meta-analytic review. J. Consult. Clin. Psychol. 2010;78:169–183. doi: 10.1037/a0018555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollis S, Campbell F. What is meant by intention to treat analysis? Survey of published randomized controlled trials. BMJ. 1999;319:670–674. doi: 10.1136/bmj.319.7211.670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huston-Lyons D, Kornetsky C. Effects of nicotine on the threshold for rewarding brain stimulation in rats. Pharmacol. Biochem. Behav. 1992;41:755–759. doi: 10.1016/0091-3057(92)90223-3. [DOI] [PubMed] [Google Scholar]
- Hyman SE. The neurobiology of addiction: implications for voluntary control of behavior. Am. J. Bioeth. 2007;7:8–11. doi: 10.1080/15265160601063969. [DOI] [PubMed] [Google Scholar]
- Imperato A, Mulas A, Di Chiara G. Nicotine preferentially stimulates dopamine release in the limbic system of freely moving rats. Eur. J. Pharmacol. 1986;132:337–338. doi: 10.1016/0014-2999(86)90629-1. [DOI] [PubMed] [Google Scholar]
- Javors MA, Hatch JP, Lamb RJ. Cut-off levels for breath carbon monoxide as a marker for cigarette smoking. Addiction. 2005;100:159–167. doi: 10.1111/j.1360-0443.2004.00957.x. [DOI] [PubMed] [Google Scholar]
- Jha P, Chaloupka FJ, Corrao M, Jacob B. Reducing the burden of smoking world-wide: effectiveness of interventions and their coverage. Drug Alcohol Rev. 2006;25:597–609. doi: 10.1080/09595230600944511. [DOI] [PubMed] [Google Scholar]
- Kabat-Zinn J. An outpatient program in behavioral medicine for chronic pain patients based on the practice of mindfulness meditation: theoretical considerations and preliminary results. Gen. Hosp. Psychiatry. 1982;4:33–47. doi: 10.1016/0163-8343(82)90026-3. [DOI] [PubMed] [Google Scholar]
- Kandel D, Davies M. Adult sequelae of adolescent depressive symptoms. Arch. Gen. Psychiatry. 1986;43:255–262. doi: 10.1001/archpsyc.1986.01800030073007. [DOI] [PubMed] [Google Scholar]
- Kassel JD, Stroud LR, Paronis CA. Smoking, stress, and negative affect: correlation, causation, and context across stages of smoking. Psychol. Bull. 2003;129:270–304. doi: 10.1037/0033-2909.129.2.270. [DOI] [PubMed] [Google Scholar]
- Lando HA, McGovern PG, Barrios FX, Etringer BD. Comparative evaluation of American Cancer Society and American Lung Association smoking cessation clinics. Am. J. Public Health. 1990;80:554–559. doi: 10.2105/ajph.80.5.554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Law M, Tang J. An analysis of the effectiveness of interventions intended to help people stop smoking. Arch. Intern. Med. 1995;155:1933–1941. [PubMed] [Google Scholar]
- Leknes S, Tracey I. A common neurobiology for pain and pleasure. Nat. Rev. Neurosci. 2008;9:314–320. doi: 10.1038/nrn2333. [DOI] [PubMed] [Google Scholar]
- Lutz A, Slagter HA, Dunne JD, Davidson RJ. Attention regulation and monitoring in meditation. Trends Cog. Sci. 2008;12:163–169. doi: 10.1016/j.tics.2008.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marlatt GA, Donovan DM. Relapse Prevention: Maintenance Strategies in the Treatment of Addictive Behaviors. New York, NY: Guilford Press; 2005. p. 416. [Google Scholar]
- Mottillo S, Filion K, BÈlisle P, Joseph L, Gervais A, O'Loughlin J, Paradis G, Pihl R, Pilote L, Rinfret S. Behavioural interventions for smoking cessation: a meta-analysis of randomized controlled trials. Eur. Heart J. 2009;30:718–730. doi: 10.1093/eurheartj/ehn552. [DOI] [PubMed] [Google Scholar]
- Muraven M, Baumeister RF. Self-regulation and depletion of limited resources: does self-control resemble a muscle? Psychol. Bull. 2000;126:247–259. doi: 10.1037/0033-2909.126.2.247. [DOI] [PubMed] [Google Scholar]
- Niaura R, Abrams D. Smoking cessation: progress, priorities, and prospectus. J. Consult. Clin. Psychol. 2002;70:494–509. doi: 10.1037//0022-006x.70.3.494. [DOI] [PubMed] [Google Scholar]
- Olfson M, Marcus SC. National patterns in antidepressant medication treatment. Arch. Gen. Psychiatry. 2009;66:848–856. doi: 10.1001/archgenpsychiatry.2009.81. [DOI] [PubMed] [Google Scholar]
- Perkins K, Karelitz J, Conklin C, Sayette M, Giedgowd G. Acute Negative Affect Relief from Smoking Depends on the Affect Situation and Measure but Not on Nicotine. Biol. Psychiatry. 2010;15:707–714. doi: 10.1016/j.biopsych.2009.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perkins KA. Does smoking cue-induced craving tell us anything important about nicotine dependence? Addiction. 2009;104:1610–1616. doi: 10.1111/j.1360-0443.2009.02550.x. [DOI] [PubMed] [Google Scholar]
- Piasecki T, Kenford S, Smith S, Fiore M, Baker T. Listening to nicotine: negative affect and the smoking withdrawal conundrum. Psychol. Sci. 1997;8:184. [Google Scholar]
- Rose J, Levin E. Inter-relationships between conditioned and primary reinforcement in the maintenance of cigarette smoking. Br. J. Addict. 1991;86:605–609. doi: 10.1111/j.1360-0443.1991.tb01816.x. [DOI] [PubMed] [Google Scholar]
- Segal Z, Williams JMG, Teasdale JD. Mindfulness Based Cognitive Therapy for Depression: A New Approach to Preventing Relapse. New York, NY: The Guilford Press; 2002. [Google Scholar]
- Shiffman S. Smoking cessation treatment: any progress? J. Consult. Clin. Psychol. 1993;61:718–718. doi: 10.1037//0022-006x.61.5.718. [DOI] [PubMed] [Google Scholar]
- Shiffman S, Engberg JB, Paty JA, Perz WG, Gnys M, Kassel JD, Hickcox M. A day at a time: predicting smoking lapse from daily urge. J. Abnorm. Psychol. 1997;106:104–116. doi: 10.1037//0021-843x.106.1.104. [DOI] [PubMed] [Google Scholar]
- Shiffman S, Waters AJ. Negative affect and smoking lapses: a prospective analysis. J. Consult. Clin. Psychol. 2004;72:192–201. doi: 10.1037/0022-006X.72.2.192. [DOI] [PubMed] [Google Scholar]
- Singer J, Willett J. Applied Longitudinal Data Analysis: Modeling Change and Event Occurrence. New York: Oxford University Press; 2003. [Google Scholar]
- Sobell L, Sobell M. Timeline follow-back. In: Allen JP, editor. Measuring Alcohol Consumption: Psychosocial and Biochemical Methods. Totwa, NJ: Humana Press; 1992. pp. 41–72. [Google Scholar]
- Strong DR, Kahler CW, Leventhal AM, Abrantes AM, Lloyd-Richardson E, Niaura R, Brown RA. Impact of bupropion and cognitive behavioral treatment for depression on positive affect, negative affect, and urges to smoke during cessation treatment. Nicotine Tob. Res. 2009;11:1142–1153. doi: 10.1093/ntr/ntp111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teasdale JD, Moore RG, Hayhurst H, Pope M, Williams S, Segal ZV. Metacognitive awareness and prevention of relapse in depression: empirical evidence. J. Consult. Clin. Psychol. 2002;70:275–287. doi: 10.1037//0022-006x.70.2.275. [DOI] [PubMed] [Google Scholar]
- Tiffany ST. A cognitive model of drug urges and drug-use behavior: role of automatic and nonautomatic processes. Psychol. Rev. 1990;97:147–168. doi: 10.1037/0033-295x.97.2.147. [DOI] [PubMed] [Google Scholar]
- Tiffany ST, Carter BL. Is craving the source of compulsive drug use? J. Psychopharmacol. 1998;12:23–30. doi: 10.1177/026988119801200104. [DOI] [PubMed] [Google Scholar]
- Tiffany ST, Conklin CA. A cognitive processing model of alcohol craving and compulsive alcohol use. Addiction. 2000;95:145–153. doi: 10.1080/09652140050111717. [DOI] [PubMed] [Google Scholar]
- Vettese LC, Toneatto T, Stea JN, Nguyen L, Wang JJ. Do mindfulness meditation participants do their homework? And does it make a difference? A review of the empirical evidence. J. Cogn. Psychotherapy. 2009;23:198–225. [Google Scholar]
- Warburton DM, Mancuso G. Evaluation of the information processing and mood effects of a transdermal nicotine patch. Psychopharmacology (Berl.) 1998;135:305–310. doi: 10.1007/s002130050514. [DOI] [PubMed] [Google Scholar]
- Wu TC, Tashkin DP, Djahed B, Rose JE. Pulmonary hazards of smoking marijuana as compared with tobacco. N. Engl. J. Med. 1988;318:347–351. doi: 10.1056/NEJM198802113180603. [DOI] [PubMed] [Google Scholar]
- Zgierska A, Rabago D, Chawla N, Kushner K, Koehler R, Marlatt A. Mindfulness meditation for substance use disorders: a systematic review. Subst. Abuse. 2009;30:266–294. doi: 10.1080/08897070903250019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zgierska A, Rabago D, Zuelsdorff M, Coe C, Miller M, Fleming M. Mindfulness meditation for alcohol relapse prevention: a feasibility pilot study. J. Addict. Med. 2008;2:165–173. doi: 10.1097/ADM.0b013e31816f8546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziedonis DM, Kosten TR, Glazer WM, Frances RJ. Nicotine Dependence and Schizophrenia. Washington, DC: American Psychiatric Association; 1994. [DOI] [PubMed] [Google Scholar]
- Zinser M, Baker T, Sherman J, Cannon D. Relation between self-reported affect and drug urges and cravings in continuing and withdrawing smokers. J. Abnorm. Psychol. 1992;92:617–629. doi: 10.1037//0021-843x.101.4.617. [DOI] [PubMed] [Google Scholar]