Abstract
Rationale
Alcohol dependence is associated with high rates of recidivism. Stress has been shown to increase alcohol craving in alcohol-dependent individuals, but the association between stress-induced craving and alcoholism treatment outcome is not well understood.
Objective
The aim of the present study was to examine the relationship between strength of stress-induced alcohol craving in the human laboratory and subsequent drinking in a cohort of treatment-seeking, alcohol-dependent adults.
Materials and methods
This is a prospective study assessing stress-induced craving in the lab and subsequent treatment outcomes in alcohol-dependent subjects enrolled in a 12-week outpatient study. Stress was induced using a previously developed, individualized, audio recorded stress script and validated with objective (salivary cortisol) and subjective measures of distress. In vivo craving for alcohol was measured pre- and post-challenge using VAS.
Results
Subjects were 28 (16 male, 12 female) alcohol-dependent outpatients. Greater stress-induced craving was associated with a blunted salivary cortisol response, significantly shorter time to alcohol relapse, higher mean drinks per week, fewer percent days abstinent, and lower rates of complete abstinence over the study duration (all p's<0.05). Conversely, no demographic or baseline variables were significant predictors of any outcome variable.
Conclusions
These results suggest that greater stress-related increases in alcohol craving are associated with poorer alcohol treatment outcomes. The findings support the use of stress-induced craving as a predictor of alcohol relapse propensity. Furthermore, treatments that address high stress levels and the associated high levels of alcohol craving are likely to improve treatment outcomes in alcohol dependence.
Keywords: Alcohol, Stress, Relapse, Craving, Treatment, Alcohol dependence, Human laboratory study
Introduction
Alcohol-use disorders, which include both alcohol abuse and dependence, make up one of the most prevalent categories of substance use disorders, affecting more than two billion people worldwide. The Diagnostic and Statistical Manual of Mental Disorders-Fourth Edition (DSM-IV) (American Psychiatric Association 2000) characterizes alcohol dependence as a maladaptive pattern of drinking leading to clinically significant impairment, as manifested by a compulsion to drink, a lack of control over the amount of alcohol consumed, and continued drinking despite realization of the associated problems. Although significant strides have been made in the development of efficacious behavioral and pharmacologic treatments for alcohol dependence, relapse rates remain very high. Relapse, or the return to pathological alcohol use following periods of abstinence, is one of the principal characteristics of dependence on alcohol.
More than 80% of alcohol-dependent individuals relapse during the 6- to 12-month period following treatment (Heinz et al. 2009). A key feature of alcohol dependence and relapse is craving (Childress et al. 1993; Rohsenow and Monti 1999; Waldrop et al. 2007), often elicited by alcohol-related cues, or stress (Breese et al. 2005; Cooney et al. 1997; Mason et al. 2008; Monti et al. 1993; Rohsenow and Monti 1999). The association between stress and alcohol use has been well documented both in the preclinical (Koob 2008) and clinical (Uhart and Wand 2009) literature. In animals, Volpicelli et al. (1990) found that acute stress increased alcohol consumption in rats, and Higley et al. (1993) found that chronic stress (i.e., separation from mother) results in higher alcohol consumption in the adult rhesus monkey. Moreover, studies using the reinstatement model have shown that exposure to stress leads to increased alcohol seeking in abstinent, alcohol-experienced, and dependent laboratory animals (Le et al. 2000, 2001; Liu and Weiss 2002; Shalev et al. 2000; Valdez et al. 2003; Weiss et al. 2001).
Clinical research also demonstrates how psychosocial stress may contribute to both alcohol-seeking behavior and risk of relapse (Brown et al. 1994, 1989; Karlsgodt et al. 2003). A theoretical rationale for the underlying mechanisms responsible for relapse to alcohol use involves negative reinforcement processes whereby the motivational basis of pathological alcohol use is presumed to be the reduction or avoidance of aversive internal states that emerge when alcohol use is discontinued (Baker et al. 2004; Koob 2003). In support of this theoretical approach, prospective studies indicate that individuals with an inability to tolerate affective distress are significantly more likely to drop out of residential substance abuse treatment (Daughters et al. 2005a) and have shorter abstinence durations across addictions (Brandon et al. 2003; Brown et al. 2002; Daughters et al. 2005b). Accordingly, it has been shown that alcoholics confronted with stressful circumstances following treatment are more likely to relapse than those not experiencing such stress (Noone et al. 1999).
Although there is substantial clinical and anecdotal support for stress-induced relapse, empirical support that stress causes relapse or a return to drinking is equivocal. In human laboratory studies, exposure to stress and negative affective cues have been shown to increase alcohol craving and stress-related physiological arousal (Breese et al. 2005; Cooney et al. 1997; Fox et al. 2007; Litt et al. 2000; Marlatt 1990; Mason et al. 2009; Sinha et al. 2009), but whether this increased craving predicts subsequent drinking is unknown. Determining conclusively that stress causes drinking in alcohol-dependent populations poses both methodological and ethical challenges. To date, only four randomized, controlled studies of stress manipulation followed by subsequent drinking in an alcohol-dependent population exist, and the results are mixed. Miller et al. (1974) and Thomas et al. (2011) reported a significant increase in alcohol consumption following acute stress exposure while Higgins and Marlatt (1973) and Pratt and Davidson (2009) found no change. Such contradictory results may be due, in part, to methodological differences and the use of stressors that lack ecological relevance to understanding relapse in alcohol dependence (i.e., anticipation of an electrical shock). Approaches that more closely model common psychosocial stress may provide a more consistent picture. For instance, using personalized guided imagery to induce stress, Sinha et al. (2009) found increased craving for alcohol accompanied by a blunted cortisol response in non-treatment-seeking alcohol-dependent adults. Additionally, Junghanns et al. (2003) reported that blunted cortisol response to a psychosocial stress test is associated with early relapse in recently abstinent alcohol-dependent individuals. These results suggest that stress may mediate return to drinking and that an abnormal physiological stress response in alcohol-dependent individuals may contribute to relapse propensity.
Neurobiological models of addiction have demonstrated that brain stress and reward systems are altered with chronic heavy alcohol use, and these changes may contribute to increased alcohol seeking or “craving” and vulnerability to relapse (Breese et al. 2005; Koob 2008; Le et al. 2001; Weiss et al. 2001). Alterations in the hypothalamo-pituitary-adrenocortical (HPA) axis response have been observed during active drinking (Wand and Dobs 1991), acute withdrawal, and 4 weeks post-withdrawal (Adinoff et al. 1991, 2003; Errico et al. 1993; Lovallo et al. 2000). Moreover, blunted cortisol response has been associated with increased risk of relapse (Junghanns et al. 2003, 2005).
While stress is considered to be an important factor in the initiation, maintenance, and relapse to alcohol abuse (Brady and Sonne 1999; Marlatt 1979; Pohorecky 1991; Wolffgramm 1991), few studies have directly examined the role of stress on relapse outcomes in treatment-seeking alcohol-dependent individuals. As such, the objective of the present study was to prospectively investigate whether stress-induced alcohol craving and corresponding HPA responses in the laboratory are predictive of alcoholism treatment outcomes in individuals enrolled in a 12-week clinical trial. We hypothesized that stress-induced alcohol craving in the laboratory would predict drinking relapse outcomes in alcohol-dependent individuals seeking treatment, and that blunted HPA axis activity (as measured by salivary cortisol secretion) would be significantly related to drinking outcomes.
Materials and methods
Subjects
Subjects were a cohort of alcohol-dependent, male and female, treatment-seeking individuals enrolled in a 12-week, outpatient alcoholism treatment study investigating the efficacy of two study medications versus placebo. All subjects participated in a stress induction human laboratory session at week 2 of the 12-week trial and subsequently were dichotomized into two stress-induced craving groups (high vs. low) that were equally represented across the three medication groups. All participants satisfied the DSM-IV (American Psychiatric Association 2000) criteria for current alcohol dependence. Subjects were recruited primarily through newspaper ads. This study was conducted at The Scripps Research Institute (TSRI), La Jolla, CA, and approved by the Institutional Review Board as conforming to the ethical standards of the 1964 Declaration of Helsinki.
Inclusion criteria
To be eligible for the study, participants had to meet diagnostic criteria for current alcohol dependence and report drinking an average ≥21 standard drinks per week if male, ≥14 if female, with at least one heavy drinking day (defined as ≥5 drinks per day for males, ≥4 per day for females) per week in the month prior to screening.
Exclusion criteria
Potential subjects were excluded if they had any Axis I disorders including mood or anxiety disorders or if they were dependent on any substance other than alcohol or nicotine. Individuals with clinically significant medical disorders (as determined by a study physician) or those taking medications that could confound study outcomes (e.g., anti-depressants) were also excluded. Other exclusion criteria included an inability to understand study questionnaires and procedures in English, those requiring court-mandated treatment, and/or those in need of alcohol detoxification (or those with a Clinical Institute Withdrawal Assessments (Sullivan et al. 1989) for alcohol total score >8).
Procedures
Initial eligibility was ascertained in a pre-screening telephone interview. Potential subjects then attended a screening interview during which the study was explained, and written informed consent was obtained. Each participant was evaluated for eligibility with a standard clinical interview including the drug, alcohol, and psychiatric modules of the Structured Clinical Interview for DSM-IV (First et al. 1996), and a protocol-specific standardized questionnaire was used to obtain demographics and alcohol and substance use history, including age of first alcohol use and total years of heavy drinking. Baseline measures of drinking were obtained using the Timeline Follow-Back Interview (TLFB) (Sobell and Sobell 2000) to determine quantity and frequency of drinking in the 90 days prior to screening. The alcohol dependence scale (ADS) (Skinner and Horn 1984) was used as a quantitative measure of alcohol dependence severity. The Alcohol Craving Questionnaire (ACQ) (Singleton et al. 1994) was used to assess current alcohol craving, difficulty resisting the urge to drink, and anticipation of positive outcome or relief from negative affective states by drinking. The Beck Depression Inventory II (BDI-II) (Beck et al. 1996) was used to assess baseline mood symptoms. Finally, all eligible participants underwent a clinical laboratory screening of blood and urine and a medical exam by a study physician to rule out clinically significant medical disorders.
Baseline script development session
All subjects participated in a 1-h stress script development session with a trained clinician during the first visit of study participation. During the script development session (modified from Pitman et al. 1987), subjects were asked to identify a recent highly stressful event from their own lives (rated by the subject as ≥ 8 on an 11-point Likert-type scale for stressfulness, adapted from Sinha et al. 2009). A structured stimulus response interview was used to elicit specific details of the stressful event. Subjects were then asked to select from a “menu” those subjective visceral and muscular reactions that he/she remembered as having accompanied the experience (Pitman et al. 1987). Immediately following the script development session, the clinician developed a 1.5-min script that portrayed the stressful experience in the second person, present tense, and incorporated up to five different visceral and muscular reactions or as many as the subject selected, whichever was less. The script was then audio recorded for later use in the laboratory session. Examples of commonly reported stressful situations included breakup with a significant other, a verbal argument with a significant other or family member, or employment-related stress, such as being fired or laid off from work. Stressful situations involving alcohol use or repercussions from alcohol use were not allowed so as not to confound stress-induced craving with alcohol-cue-related craving.
Stress induction laboratory session
The human laboratory session was conducted at week 2 of treatment. Subjects reported to the laboratory at 12:30 p.m. on the day of testing, and the laboratory sessions occurred at approximately 2:00 p.m. to control for effects of circadian rhythm and satiety. Vital signs and a urine specimen were collected, and abstinence from alcohol was confirmed by breath alcohol concentration (BAC). Standardized measures and self-report questionnaires were collected to provide pre-manipulation baseline ratings of drinking, craving, and mood. After completing all baseline assessments, subjects were escorted to a comfortable chair located in a windowless, sound-attenuated testing room adjacent to the control room and separated by a large oneway mirror. Subjects were familiarized with the laboratory procedures during a neutral practice trial. Pre-manipulation salivary cortisol and subjective alcohol craving rating (assessed using four individual 21-point visual analog scale (VAS) craving items) were obtained.
At 2:00 p.m., subjects were provided with headphones and a digital audio recorder and instructed to listen to the audio recording for the entire duration and try to remember and imagine the event as if it were happening at that time. Immediately following the script presentation, salivary cortisol samples and subjective VAS alcohol craving ratings were obtained. Finally, subjects were asked to indicate on an 11-point VAS the degree to which the script evoked a stress response as a validation check of the laboratory-based manipulation of stress exposure. Subjects were then debriefed and guided through a relaxation period. Mood (BDI-II) and craving (ACQ) assessments were repeated to ensure that affect and urge to drink had returned to baseline levels.
Laboratory assessments
Stress-induced craving was assessed using four individual 21-point VAS items adapted from the ACQ (Singleton et al. 1994). Each VAS item endpoint was anchored with a 0 on the left indicating no craving and a 20 on the right indicating severe craving. The items represented strength of craving (“How strong is your craving to drink alcohol”), impulse (“It would be hard to turn down a drink right now”), control (“If I could drink alcohol right now, I would drink it”), and relief drinking (“Having a drink would make things just perfect”). Total stress-induced craving was calculated by summing the four individual items (range=0–80).
Subjective stress induction was assessed using an 11-point VAS in which subjects were asked to indicate the degree to which the story induced stress (0=“not at all,” 10=“extremely high”).
Salivary cortisol
Saliva samples were collected pre- and post-stress manipulation as a marker of HPA axis response. Participants were instructed to place a cotton roll between their tongue and cheek for approximately 1 min or until the swab was completely saturated. The saliva swab was then collected in plastic tubes that were placed directly on ice and stored at −20°C. Saliva samples were assayed in duplicate at the Parsons Laboratory at TSRI to obtain levels of free cortisol following standard radioimmuno-assay kits with no modifications.
Procedures and assessments over the 10-week treatment period
All participants received manualized, weekly, individual, abstinence-oriented counseling (www.alcoholfree.info) with a trained clinician. At each weekly study visit, a urine specimen was collected to screen for drugs of abuse, and BAC level was assessed with a breathalyzer to confirm abstinence. The Timeline Follow-Back Interview (Sobell and Sobell 2000) was used to determine quantity and frequency of drinking between weekly visits with a standard drink equivalent to 12 oz of beer (4–6% abv), 5 oz of wine, or 1½oz of 80-proof liquor. All subjects received weekly drinking diaries for recording alcohol consumption. Drinking diaries were collected weekly, and corroborated drinking information was obtained in the TLFB interview. Weekly mood and craving ratings were obtained using the BDI-II (Beck et al. 1996) and ACQ (Singleton et al. 1994), respectively.
Data analysis
Associations between stress-induced craving in the laboratory and prospective treatment outcomes were assessed for the remaining 10 weeks of treatment. The following alcohol use measures were computed from the TLFB and used for outcome analyses covering the interval from the lab session (week 2) to end of treatment (week 12): (1) latency to first drink, the interval to the first day of alcohol use; (2) alcohol use, the mean number of drinks per week while on study; (3) percentage of abstinent days, the total number of abstinent days during study; and (4) complete abstinence duration, the number of individuals achieving complete abstinence for the 10-week treatment interval following the lab session.
High and low stress-induced craving groups were defined on the basis of a mean split of the VAS total craving difference score (post-stress VAS total craving minus pre-stress total VAS craving). Those with stress-induced VAS difference scores < the mean VAS craving difference score were assigned to the low stress-induced craving group; those with scores ≥ mean VAS craving difference score were assigned to the high stress-induced craving group. Demographic and baseline characteristics were first examined for group differences and association with treatment outcome measures. If any demographic or baseline measure was significantly associated with any treatment outcome, it would be considered as a potential covariate in examining the association between stress-induced craving and the specific treatment outcome. Differences in demographic variables between subjects with low versus high stress-induced craving responses to stress script were assessed by t test or X2 for continuous and categorical variables, respectively.
Complete abstinence in the study was defined as abstinence from study entry to study completion. Differences between low and high stress-induced craving groups in the proportion of subjects with complete abstinence were assessed by chi-square test. Latency to first drink was defined as the interval between the stress induction laboratory session and the first drink. Kaplan–Meier curves were constructed, and Breslow-Wilcoxon analysis was performed to make univariate comparisons of differences in drink latency between craving groups. Multivariate analysis of drink latency was performed using the Cox proportional hazards regression method. Mean number of drinks was defined as the number of drinks consumed divided by the number of weeks of study participation. Percentage of abstinent days was defined as the number of days abstinent divided by the number of days of study participation. In addition to stress-induced VAS craving score in the lab, cortisol response and continuous baseline variables were evaluated as predictors of treatment response including age, age at onset of alcohol dependence, baseline ADS score, baseline BDI-II total score, baseline total ACQ score, and baseline (past 90 days) drinks per week. Dichotomous variables included in models were family history of alcohol abuse, medication treatment group, and gender. Backward stepwise linear regression was used to characterize the relationship between baseline variables, stress-induced VAS total craving scores, and drinking outcomes, using the same covariates listed above. Covariates were eliminated in each step at p<0.2 to arrive at parsimonious models including only independently significant covariates. Regression assumptions were confirmed with collinearity and outlier diagnostics. Tolerance values for predictors were >0.9, and no Mahalanobis value exceeded the critical value of 13.82. All tests were 2-tailed, and a p value <0.05 was considered statistically significant. All analyses were performed using PASW 17.0 (IBM Corp., Somers, NY)
Results
Participants were 28 treatment-seeking, alcohol-dependent, males (n=16) and females (n=12) enrolled in a 12-week outpatient study for alcoholism treatment. The average age was approximately 40 years, ranging from 21 to 60 years. Table 1 shows demographic and baseline characteristics of the sample. The sample was dichotomized into two groups based on a mean split (M=6) of the change scores obtained from subtracting the stress-induced VAS total craving score obtained in the lab session by subtracting pre-stress VAS total craving scores from post-stress VAS total craving score. The high stress-induced craving group (n=15) was defined as those individuals with a stress-induced VAS total craving change score ≥6. The low stress-induced craving group (n=13) was defined as those individuals with a stress-induced VAS total craving score change <6. Pre-stress VAS total craving scores were comparable between the high (M=26±17.98) and low stress-induced craving groups (M=26.7±12.48). However, following stress induction, an independent samples t test revealed a significant difference (p<0.001) in VAS total craving scores between the high (M=57.8±26.03) and low stress-induced craving groups (M=24.08±24.64). Importantly, there were no significant group differences in the manipulation check of stress induction ratings such that both groups indicated their personalized script provocation as stressful (M=9.03±1.72 for high craving; M=9.15±1.45 for low craving; maximum stress rating possible=10). Moreover, there were no significant differences between the high and low stress-induced craving groups on any demographic or baseline variable.
Table 1.
Demographic and baseline characteristics
| Low stress-induced craving (n = 13) | High stress-induced craving (n = 15) | |
|---|---|---|
| Demographic characteristics | ||
| Age, years | 41.85 (13.25) | 37.60 (8.98) |
| Sex, male | 62% | 54% |
| Race, white | 62% | 53% |
| Years of education | 12.69 (2.14) | 14.07 (1.98) |
| Drinking characteristics | ||
| Drinking days per weeka | 4.66 (2.10) | 4.77 (1.65) |
| Drinks per drinking daya | 11.41 (9.61) | 13.35 (7.68) |
| Years heavy drinking | 15.00 (11.31) | 10.57 (8.10) |
| Drinks per weeka | 57.85 (31.17) | 61.47 (28.15) |
| Number of DSM-IV criteria met | 6.0 (1.0) | 6.60 (.74) |
| ADS | 16.08 (6.82) | 20.73 (5.95) |
| Clinical characteristics | ||
| BDI-II | 9.0 (4.81) | 9.40 (5.91) |
| ACQ total craving score | 42.08 (17.38) | 41.87 (20.31) |
There were no significant differences between groups. Values given are means (SD) or percents
During the 90 days before screening
Stress-induced cortisol measures in the laboratory session
There was a significant directional difference in stress-induced cortisol response for the high vs. low stress-induced craving groups. A 2×2 (group × cortisol time) repeated measures ANOVA revealed a significantly blunted cortisol response following stress induction in the high stress-induced craving group (M=0.09±.05; pre-post difference score= −0.5) in comparison to the increased cortisol response of the low stress-induced craving group (M=0.14±.07; pre-post difference score=+0.03) following stress induction (F(1,22)=3.73, p<0.05).
Stress-induced craving and cortisol as predictors of treatment outcomes
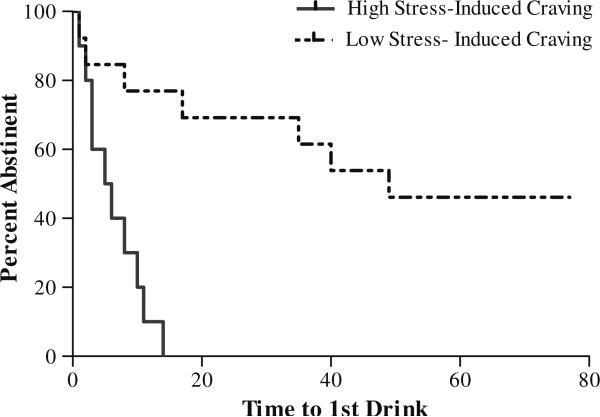
Higher stress-induced VAS total craving for alcohol in the human laboratory session was significantly associated with a shorter time to relapse (p<0.001) than lower stress-induced VAS total craving; see Fig. 1. This was confirmed via Cox regression analysis (χ2=12.69; p<0.001; hazard ratio, 9.106; 95% confidence interval, 2.70–30.70). The median latency to first drink was 5.5 vs. 49 days for high vs. low stress-induced craving groups. In addition, the high stress-induced craving group drank significantly more drinks per week (M=24.11±16.99 vs. M=4.02±8.19; p<0.05), had significantly fewer percent days abstinent (M=4.75±18 vs. M=71.8± 30.1; p<0.01), and were significantly less likely to achieve complete abstinence for the study duration (0% vs. 46%) than the low stress-induced craving group.
Fig. 1.
Kaplan–Meier curves showing latency to first drink in high and low stress-induced craving groups (χ2=12.69; p<0.001; hazard ratio, 9.11; 95% confidence interval, 2.70–30.70). The percent remaining abstinent is shown on the y-axis, and the time during the remaining 10 weeks of study when relapse occurred is shown on the x-axis. The high stress-induced craving group (black line) had a significantly shorter latency to relapse (5.5 days) than did the low stress-induced craving group (dotted line; 49 days) (p<0.001)
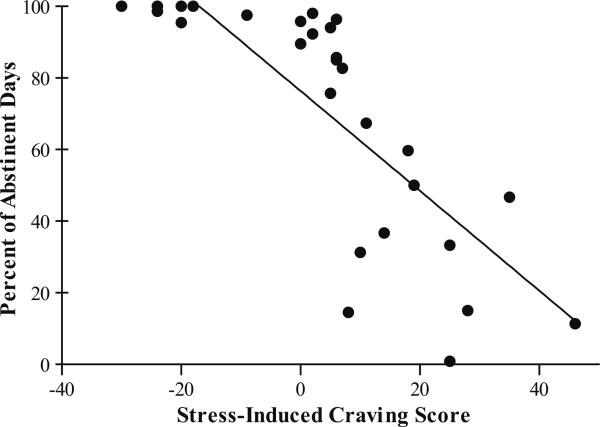
A linear regression analysis indicated that lower stress-induced VAS total craving significantly predicted greater percent days abstinent (β=−2.35, p=0.03, R2=0.22; Fig. 2). The low stress-induced craving group had an average of 72% of days abstinent versus an average of 5% of days abstinent on study observed in the high stress-induced craving group. Moreover, the low stress-induced craving group was significantly more likely to achieve complete abstinence for the study duration than the high stress-induced craving group (χ2=7.02, p<0.001).
Fig. 2.
Scatterplot of relationship between stress-induced VAS total craving score and percent abstinent days during the study. The low stress-induced craving group had significantly more days abstinent (M=72%) than did the high stress-induced craving group (M=5%; β=−2.35, p=0.03, R2=0.22)
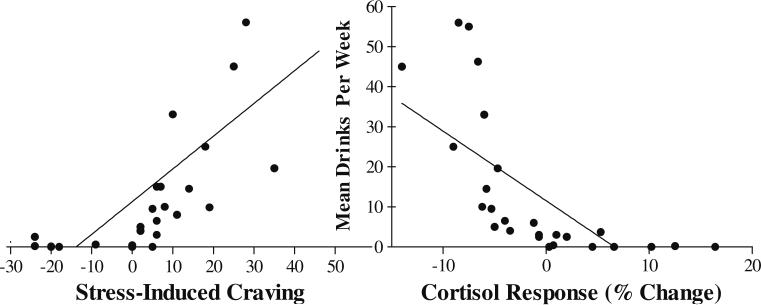
A linear regression model containing stress-induced craving and cortisol response as predictors of mean drinks per week revealed these variables as independent significant predictors of mean drinks per week and accounted for more than a third of the variance in the amount of alcohol consumed per week (F(2, 24)=6.69, p=.005, R2=0.38). In this model, higher stress-induced craving (β=0.41, p=0.02) and blunted stress-induced cortisol response (β=0.−45, p=0.01) were associated with increased drinks per week; see Fig. 3.
Fig. 3.
Scatterplot showing the inverse relationship of stress-induced change in alcohol craving (left) and cortisol response (right) from baseline as independent significant predictors of mean drinks per week (R2=0.38). In this model, higher stress-induced craving and corresponding blunted stress-induced cortisol response were associated with increased drinks per week (p<0.05)
Stress-induced cortisol responses were not independent predictors of latency to relapse, percent days abstinent, or complete abstinence. Moreover, demographic measures of age, gender, age of onset of alcohol dependence, family history, and baseline amount of alcohol use in the 90 days prior to study, ADS, ACQ, and BDI-II were not significant independent predictors of any treatment outcome.
Discussion
This study prospectively examined the relationship between stress-induced craving in a human laboratory session and treatment outcomes in alcohol-dependent individuals. The potential role of stress in increasing risk of alcohol relapse was initially supported by work showing that alcohol-dependent individuals, but not social drinkers, were more susceptible to stress-induced alcohol consumption in laboratory situations (Miller et al. 1974) and that negative emotion or stress induction resulted in increased craving for alcohol (Breese et al. 2005; Cooney et al. 1997; Fox et al. 2007; Nesic and Duka 2006; Sinha et al. 2009). To our knowledge, however, this is the first study to provide direct evidence that stress-induced alcohol craving in the lab predicts subsequent treatment response in alcohol-dependent individuals. Importantly, there was no difference in the stressfulness of the stress induction ratings between the two groups with an average stress severity rating greater than 9 (maximum stress rating possible=10). The stress severity rating of the script alone did not predict or correlate with cortisol or craving response. This is especially interesting given the equivocal nature of the stress and relapse relationship and significantly contributes to the mixed body of literature. It was not simply the exposure to stress, or even the subjective quantification of stress, but the overall behavior response of increased craving following stress exposure that predicted treatment outcomes. Stress-induced craving in the laboratory session (but not age, gender, age of onset of alcohol dependence, parental alcohol abuse, baseline amount of alcohol use, ACQ ratings, or BDIII scores) predicted all four drinking outcomes—(1) latency to 1st drink, (2) quantity of alcohol use, (3) percentage of abstinent days, and (4) complete abstinence duration—suggesting the increased specificity of stress-induced craving relative to other predictors of treatment response. Greater stress-induced craving predicted a shorter time to initial alcohol lapse, increased drinks per week, fewer percent days abstinent, and reduced likelihood of maintaining complete abstinence for the study duration.
Blunted HPA response to stress was observed in the high stress-induced craving group, but not the low stress-induced craving group, and was predictive of subsequent alcohol use. These data support previous findings that chronic alcohol consumption is associated with dysregulation of the HPA axis. Specifically that some alcohol-dependent individuals show a blunted HPA response to acute intervening stressors (Berman et al. 1990; Bernardy et al. 1996; Ehrenreich et al. 1997; Errico et al. 1993; Lovallo et al. 2000; Pratt and Davidson 2009; Sinha et al. 2009; Wand and Dobs 1991) and these attenuated HPA responses are predictive of early relapse to alcohol (Adinoff et al. 2005; Junghanns et al. 2003). Our current findings are consistent with these data and extend them by documenting a significant inverse correlation between high stress-induced alcohol craving and attenuated cortisol responses. Elevated basal cortisol levels are consistent with an overactive HPA system associated with chronic alcohol abuse and acute and protracted withdrawal (Breese et al. 2005; Funk et al. 2006; Koob and Kreek 2007; Koob et al. 1998). The inability to mount an adequate stress response in the high stress-induced craving group may reflect altered HPA axis function, which may suggest weakened ability to effectively cope with stress during protracted abstinence (Adinoff et al. 2005) and thus contribute to the increased relapse susceptibility observed in this study.
The current findings have significant clinical implications. First, stress-induced craving and associated blunted HPA responses may be used as prognostic markers to assess relapse propensity. Such assessments could inform clinicians of the need to tailor interventions for stress regulation and reduction of stress-induced craving. Second, while assessing stress-induced craving and hormonal responses can be informative in identifying individuals who are highly susceptible to relapse, the findings also underscore the importance of developing treatments that address management of stress-induced alcohol craving and regulation of stress-related HPA axis responses in alcohol relapse prevention. There are currently no empirically validated treatments that address stress-induced alcohol craving and related HPA response. Based on these findings, treatments that target stress regulation and attenuation of stress-related alcohol craving may be important for reducing relapse susceptibility. Finally, laboratory models of stress-induced craving could be effective in screening pharmacologic agents or testing cognitive and/or behavioral strategies to reduce stress-induced alcohol craving and HPA responsivity and improve treatment outcomes.
The current study has limitations that need to be acknowledged. Although the sample size is comparable to that of prior investigations, replication with a larger sample will be important to ascertain generalizability. Moreover, individuals were excluded from participating if they had any comorbid psychiatric disorders and/or other drug use or dependence. Given the high rates of comorbidity in alcoholics (Kessler et al. 2005; Zilberman et al. 2003), results may not generalize to broader clinical populations. Additionally, the rather non-specific nature of salivary cortisol to assess HPA response warrants future studies that more comprehensively assess stress-induced alterations in HPA function. Despite these caveats, the current study provides previously undocumented evidence that stress-related increases in alcohol craving in the laboratory are associated with treatment outcomes in treatment-seeking alcohol-dependent individuals. The findings suggest that stress-induced alcohol craving and attenuated hormonal responses could be used diagnostically to evaluate relapse propensity. They also support the need to address the effects of stress-induced craving and HPA response on alcohol relapse susceptibility via pharmacologic and cognitive and/or behavioral treatment development efforts.
In summary, the findings provide direct evidence of the ability of a laboratory paradigm of stress-induced craving to predict treatment outcomes in treatment seekers with alcohol dependence. The clinical importance of stress-induced craving is highlighted in this study by the observed associations between stress-induced craving in the lab setting, blunted cortisol response, and subsequent treatment outcomes. Further investigations are needed to replicate the current findings and to identify behavioral and pharmacologic interventions that may serve to alter stress-induced craving and ultimately treatment outcomes.
Acknowledgments
Funding for this project was provided by NIAAA grant numbers RO1AA012602 and R37AA014028 to BJM and the Pearson Center for Alcoholism and Addiction Research.
References
- Adinoff B, Risher-Flowers D, De Jong J, Ravitz B, Bone GH, Nutt DJ, Roehrich L, Martin PR, Linnoila M. Disturbances of hypothalamic-pituitary-adrenal axis functioning during ethanol withdrawal in six men. AJ Psychiatry. 1991;148:1023–1025. doi: 10.1176/ajp.148.8.1023. [DOI] [PubMed] [Google Scholar]
- Adinoff B, Ruether K, Krebaum S, Iranmanesh A, Williams MJ. Increased salivary cortisol concentrations during chronic alcohol intoxication in a naturalistic clinical sample of men. Alcohol Clin Exp Res. 2003;27:1420–1427. doi: 10.1097/01.ALC.0000087581.13912.64. [DOI] [PubMed] [Google Scholar]
- Adinoff B, Junghanns K, Kiefer F, Krishnan-Sarin S. Suppression of the HPA axis stress-response: implications for relapse. Alcohol Clin Exp Res. 2005;29:1351–1355. doi: 10.1097/01.ALC.0000176356.97620.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- American Psychiatric Association . In: Diagnostic and statistical manual of mental disorders, fourth edition, text revision. First M, editor. American Psychiatric Association; Washington, DC: 2000. [Google Scholar]
- Baker TB, Piper ME, McCarthy DE, Majeskie MR, Fiore MC. Addiction motivation reformulated: an affective processing model of negative reinforcement. Psychol Rev. 2004;111:33–51. doi: 10.1037/0033-295X.111.1.33. [DOI] [PubMed] [Google Scholar]
- Beck AT, Steer RA, Brown GK. Beck depression inventory-II. Psychological Corporation; San Antonio: 1996. [Google Scholar]
- Berman JD, Cook DM, Buchman M, Keith LD. Diminished adrenocorticotropin response to insulin-induced hypoglycemia in nondepressed, actively drinking male alcoholics. J Clin Endocrinol Metab. 1990;71:712–717. doi: 10.1210/jcem-71-3-712. [DOI] [PubMed] [Google Scholar]
- Bernardy NC, King AC, Parsons OA, Lovallo WR. Altered cortisol response in sober alcoholics: an examination of contributing factors. Alcohol. 1996;13:493–498. doi: 10.1016/0741-8329(96)00043-2. [DOI] [PubMed] [Google Scholar]
- Brady KT, Sonne SC. The role of stress in alcohol use, alcoholism treatment, and relapse. Alcohol Res Health. 1999;23:263–271. [PMC free article] [PubMed] [Google Scholar]
- Brandon TH, Herzog TA, Webb MS. It ain't over till it's over: the case for offering relapse-prevention interventions to former smokers. Am J Med Sci. 2003;326:197–200. doi: 10.1097/00000441-200310000-00009. [DOI] [PubMed] [Google Scholar]
- Breese GR, Chu K, Dayas CV, Funk D, Knapp DJ, Koob GF, Le DA, O'Dell LE, Overstreet DH, Roberts AJ, Sinha R, Valdez GR, Weiss F. Stress enhancement of craving during sobriety: a risk for relapse. Alcohol Clin Exp Res. 2005;29:185–195. doi: 10.1097/01.alc.0000153544.83656.3c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown SA, Vik PW, Creamer VA. Characteristics of relapse following adolescent substance abuse treatment. Addict Behav. 1989;14:291–300. doi: 10.1016/0306-4603(89)90060-9. [DOI] [PubMed] [Google Scholar]
- Brown SA, Myers MG, Mott MA, Vik PW. Correlates of success following treatment for adolescent substance abuse. Appl Prev Psychol. 1994;3:61–73. [Google Scholar]
- Brown RA, Lejuez CW, Kahler CW, Strong DR. Distress tolerance and duration of past smoking cessation attempts. J Abnorm Psychol. 2002;111:180–185. [PubMed] [Google Scholar]
- Childress AR, Hole AV, Ehrman RN, Robbins SJ, McLellan AT, O'Brien CP. Cue reactivity and cue reactivity interventions in drug dependence. NIDA Res Monogr. 1993;137:73–95. [PubMed] [Google Scholar]
- Cooney NL, Litt MD, Morse PA, Bauer LO, Gaupp L. Alcohol cue reactivity, negative-mood reactivity, and relapse in treated alcoholic men. J Abnorm Psychol. 1997;106:243–250. doi: 10.1037//0021-843x.106.2.243. [DOI] [PubMed] [Google Scholar]
- Daughters SB, Lejuez CW, Bornovalova MA, Kahler CW, Strong DR, Brown RA. Distress tolerance as a predictor of early treatment dropout in a residential substance abuse treatment facility. J Abnorm Psychol. 2005a;114:729–734. doi: 10.1037/0021-843X.114.4.729. [DOI] [PubMed] [Google Scholar]
- Daughters SB, Lejuez CW, Kahler CW, Strong DR, Brown RA. Psychological distress tolerance and duration of most recent abstinence attempt among residential treatment-seeking substance abusers. Psychol Addict Behav. 2005b;19:208–211. doi: 10.1037/0893-164X.19.2.208. [DOI] [PubMed] [Google Scholar]
- Ehrenreich H, Schuck J, Stender N, Pilz J, Gefeller O, Schilling L, Poser W, Kaw S. Endocrine and hemodynamic effects of stress versus systemic CRF in alcoholics during early and medium term abstinence. Alcohol Clin Exp Res. 1997;21:1285–1293. [PubMed] [Google Scholar]
- Errico AL, Parsons OA, King AC, Lovallo WR. Attenuated cortisol response to biobehavioral stressors in sober alcoholics. J Stud Alcohol. 1993;54:393–398. doi: 10.15288/jsa.1993.54.393. [DOI] [PubMed] [Google Scholar]
- First M, Spitzer R, Gibbon M, Williams J. Structured clinical interview for DSM-IV Axis I disorders, patient edition. Biometrics Research Department, New York State Psychiatric Institute; New York: 1996. [Google Scholar]
- Fox HC, Bergquist KL, Hong KI, Sinha R. Stress-induced and alcohol cue-induced craving in recently abstinent alcohol-dependent individuals. Alcohol Clin Exp Res. 2007;31:395–403. doi: 10.1111/j.1530-0277.2006.00320.x. [DOI] [PubMed] [Google Scholar]
- Funk CK, O'Dell LE, Crawford EF, Koob GF. Corticotropin-releasing factor within the central nucleus of the amygdala mediates enhanced ethanol self-administration in withdrawn, ethanol-dependent rats. J Neurosci. 2006;26:11324–11332. doi: 10.1523/JNEUROSCI.3096-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinz A, Beck A, Grusser SM, Grace AA, Wrase J. Identifying the neural circuitry of alcohol craving and relapse vulnerability. Addict Biol. 2009;14:108–118. doi: 10.1111/j.1369-1600.2008.00136.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins RL, Marlatt GA. Effects of anxiety arousal on the consumption of alcohol by alcoholics and social drinkers. J Consult Clin Psychol. 1973;41:426–433. doi: 10.1037/h0035366. [DOI] [PubMed] [Google Scholar]
- Higley JD, Thompson WW, Champoux M, Goldman D, Hasert MF, Kraemer GW, Scanlan JM, Suomi SJ, Linnoila M. Paternal and maternal genetic and environmental contributions to cerebrospinal fluid monoamine metabolites in rhesus monkeys (Macaca mulatta). Arch Gen Psychiatry. 1993;50:615–623. doi: 10.1001/archpsyc.1993.01820200025003. [DOI] [PubMed] [Google Scholar]
- Junghanns K, Backhaus J, Tietz U, Lange W, Bernzen J, Wetterling T, Rink L, Driessen M. Impaired serum cortisol stress response is a predictor of early relapse. Alcohol Alcohol. 2003;38:189–193. doi: 10.1093/alcalc/agg052. [DOI] [PubMed] [Google Scholar]
- Junghanns K, Tietz U, Dibbelt L, Kuether M, Jurth R, Ehrenthal D, Blank S, Backhaus J. Attenuated salivary cortisol secretion under cue exposure is associated with early relapse. Alcohol Alcohol. 2005;40:80–85. doi: 10.1093/alcalc/agh107. [DOI] [PubMed] [Google Scholar]
- Karlsgodt KH, Lukas SE, Elman I. Psychosocial stress and the duration of cocaine use in non-treatment seeking individuals with cocaine dependence. Am J Drug Alcohol Abuse. 2003;29:539–551. doi: 10.1081/ada-120023457. [DOI] [PubMed] [Google Scholar]
- Kessler RC, Chiu WT, Demler O, Merikangas KR, Walters EE. Prevalence, severity, and comorbidity of 12-month DSM-IV disorders in the National Comorbidity Survey Replication. Arch Gen Psychiatry. 2005;62:617–627. doi: 10.1001/archpsyc.62.6.617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF. Alcoholism: allostasis and beyond. Alcohol Clin Exp Res. 2003;27:232–243. doi: 10.1097/01.ALC.0000057122.36127.C2. [DOI] [PubMed] [Google Scholar]
- Koob GF. A role for brain stress systems in addiction. Neuron. 2008;59:11–34. doi: 10.1016/j.neuron.2008.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob G, Kreek MJ. Stress, dysregulation of drug reward pathways, and the transition to drug dependence. AJ Psychiatry. 2007;164:1149–1159. doi: 10.1176/appi.ajp.2007.05030503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF, Roberts AJ, Schulteis G, Parsons LH, Heyser CJ, Hyytia P, Merlo-Pich E, Weiss F. Neurocircuitry targets in ethanol reward and dependence. Alcohol Clin Exp Res. 1998;22:3–9. [PubMed] [Google Scholar]
- Le AD, Harding S, Juzytsch W, Watchus J, Shalev U, Shaham Y. The role of corticotrophin-releasing factor in stress-induced relapse to alcohol-seeking behavior in rats. Psychopharmacol Berl. 2000;150:317–324. doi: 10.1007/s002130000411. [DOI] [PubMed] [Google Scholar]
- Le AD, Kiianmaa K, Cunningham CL, Engel JA, Ericson M, Soderpalm B, Koob GF, Roberts AJ, Weiss F, Hyytia P, Janhunen S, Mikkola J, Backstrom P, Ponomarev I, Crabbe JC. Neurobiological processes in alcohol addiction. Alcohol Clin Exp Res. 2001;25:144S–151S. doi: 10.1111/j.1530-0277.2001.tb02389.x. [DOI] [PubMed] [Google Scholar]
- Litt MD, Cooney NL, Morse P. Reactivity to alcohol-related stimuli in the laboratory and in the field: predictors of craving in treated alcoholics. Addiction. 2000;95:889–900. doi: 10.1046/j.1360-0443.2000.9568896.x. [DOI] [PubMed] [Google Scholar]
- Liu X, Weiss F. Additive effect of stress and drug cues on reinstatement of ethanol seeking: exacerbation by history of dependence and role of concurrent activation of corticotropin-releasing factor and opioid mechanisms. J Neurosci. 2002;22:7856–7861. doi: 10.1523/JNEUROSCI.22-18-07856.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovallo WR, Dickensheets SL, Myers DA, Thomas TL, Nixon SJ. Blunted stress cortisol response in abstinent alcoholic and polysubstance-abusing men. Alcohol Clin Exp Res. 2000;24:651–658. [PubMed] [Google Scholar]
- Marlatt GA. A cognitive-behavioral model of the relapse process. NIDA Res Monogr. 1979:191–200. doi: 10.1037/e497382006-015. [DOI] [PubMed] [Google Scholar]
- Marlatt GA. Cue exposure and relapse prevention in the treatment of addictive behaviors. Addict Behav. 1990;15:395–399. doi: 10.1016/0306-4603(90)90048-3. [DOI] [PubMed] [Google Scholar]
- Mason BJ, Light JM, Escher T, Drobes DJ. Effect of positive and negative affective stimuli and beverage cues on measures of craving in non treatment-seeking alcoholics. Psychopharmacology (Berl) 2008;200(1):141–50. doi: 10.1007/s00213-008-1192-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason BJ, Light JM, Williams LD, Drobes DJ. Proof-of-concept human laboratory study for protracted abstinence in alcohol dependence: effects of gabapentin. Addict Biol. 2009;14:73–83. doi: 10.1111/j.1369-1600.2008.00133.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller PM, Hersen M, Eisler RM, Hilsman G. Effects of social stress on operant drinking of alcoholics and social drinkers. Behav Res Ther. 1974;12:67–72. doi: 10.1016/0005-7967(74)90094-1. [DOI] [PubMed] [Google Scholar]
- Monti PM, Rohsenow DJ, Rubonis AV, Niaura RS, Sirota AD, Colby SM, Abrams DB. Alcohol cue reactivity: effects of detoxification and extended exposure. J Stud Alcohol. 1993;54:235–245. doi: 10.15288/jsa.1993.54.235. [DOI] [PubMed] [Google Scholar]
- Nesic J, Duka T. Gender specific effects of a mild stressor on alcohol cue reactivity in heavy social drinkers. Pharmacol Biochem Behav. 2006;83:239–248. doi: 10.1016/j.pbb.2006.02.006. [DOI] [PubMed] [Google Scholar]
- Noone M, Dua J, Markham R. Stress, cognitive factors, and coping resources as predictors of relapse in alcoholics. Addict Behav. 1999;24:687–693. doi: 10.1016/s0306-4603(98)00087-2. [DOI] [PubMed] [Google Scholar]
- Pitman RK, Orr SP, Forgue DF, de Jong JB, Claiborn JM. Psychophysiologic assessment of posttraumatic stress disorder imagery in Vietnam combat veterans. Arch Gen Psychiatry. 1987;44:970–975. doi: 10.1001/archpsyc.1987.01800230050009. [DOI] [PubMed] [Google Scholar]
- Pohorecky LA. Stress and alcohol interaction: an update of human research. Alcohol Clin Exp Res. 1991;15:438–459. doi: 10.1111/j.1530-0277.1991.tb00543.x. [DOI] [PubMed] [Google Scholar]
- Pratt WM, Davidson D. Role of the HPA axis and the A118G polymorphism of the mu-opioid receptor in stress-induced drinking behavior. Alcohol Alcohol. 2009;44:358–365. doi: 10.1093/alcalc/agp007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohsenow DJ, Monti PM. Does urge to drink predict relapse after treatment? Alcohol Res Health. 1999;23:225–232. [PMC free article] [PubMed] [Google Scholar]
- Shalev U, Highfield D, Yap J, Shaham Y. Stress and relapse to drug seeking in rats: studies on the generality of the effect. Psychopharmacol Berl. 2000;150:337–346. doi: 10.1007/s002130000441. [DOI] [PubMed] [Google Scholar]
- Singleton E, Tiffany S, Henningfield J. Development and validation of a new questionnaire to assess craving for alcohol: problems of drug dependence.. Proceeding of the 56th Annual Meeting, The College on Problems of Drug Dependence.; National Institute on Drug Abuse, Rockville, MD. 1994. p. 289. [Google Scholar]
- Sinha R, Fox HC, Hong KA, Bergquist K, Bhagwagar Z, Siedlarz KM. Enhanced negative emotion and alcohol craving, and altered physiological responses following stress and cue exposure in alcohol dependent individuals. Neuropsychopharmacology. 2009;34:1198–1208. doi: 10.1038/npp.2008.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skinner H, Horn J. Alcohol dependence scale: users guide. Addiction Research Foundation Toronto; Canada: 1984. [Google Scholar]
- Sobell L, Sobell M. Alcohol timeline followback (TFLB). In: AP Association, editor. Handbook of psychicatric measures. American Psychiatric Association; Washington: 2000. pp. 477–479. [Google Scholar]
- Sullivan JT, Sykora K, Schneiderman J, Naranjo CA, Sellers EM. Assessment of alcohol withdrawal: the revised clinical institute withdrawal assessment for alcohol scale (CIWA-Ar). Br J Addict. 1989;84:1353–1357. doi: 10.1111/j.1360-0443.1989.tb00737.x. [DOI] [PubMed] [Google Scholar]
- Thomas SE, Bacon AK, Randall PK, Brady KT, See RE. An acute psychosocial stressor increases drinking in non-treatment-seeking alcoholics. Psychopharmacology (Berl) 2011 doi: 10.1007/s00213-010-2163-6. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uhart M, Wand GS. Stress, alcohol and drug interaction: an update of human research. Addict Biol. 2009;14:43–64. doi: 10.1111/j.1369-1600.2008.00131.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valdez GR, Zorrilla EP, Roberts AJ, Koob GF. Antagonism of corticotropin-releasing factor attenuates the enhanced responsiveness to stress observed during protracted ethanol abstinence. Alcohol. 2003;29:55–60. doi: 10.1016/s0741-8329(03)00020-x. [DOI] [PubMed] [Google Scholar]
- Volpicelli JR, Ulm RR, Hopson N. The bidirectional effects of shock on alcohol preference in rats. Alcohol Clin Exp Res. 1990;14:913–916. doi: 10.1111/j.1530-0277.1990.tb01837.x. [DOI] [PubMed] [Google Scholar]
- Waldrop AE, Ana EJ, Saladin ME, McRae AL, Brady KT. Differences in early onset alcohol use and heavy drinking among persons with childhood and adulthood trauma. Am J Addict. 2007;16:439–442. doi: 10.1080/10550490701643484. [DOI] [PubMed] [Google Scholar]
- Wand GS, Dobs AS. Alterations in the hypothalamic-pituitary-adrenal axis in actively drinking alcoholics. J Clin Endocrinol Metab. 1991;72:1290–1295. doi: 10.1210/jcem-72-6-1290. [DOI] [PubMed] [Google Scholar]
- Weiss F, Ciccocioppo R, Parsons LH, Katner S, Liu X, Zorrilla EP, Valdez GR, Ben-Shahar O, Angeletti S, Richter RR. Compulsive drug-seeking behavior and relapse. Neuroadaptation, stress, and conditioning factors. Ann NY Acad Sci. 2001;937:1–26. doi: 10.1111/j.1749-6632.2001.tb03556.x. [DOI] [PubMed] [Google Scholar]
- Wolffgramm J. An ethopharmacological approach to the development of drug addiction. Neurosci Biobehav Rev. 1991;15:515–519. doi: 10.1016/s0149-7634(05)80142-3. [DOI] [PubMed] [Google Scholar]
- Zilberman ML, Tavares H, Blume SB, el-Guebaly N. Substance use disorders: sex differences and psychiatric comorbidities. Can J Psychiatry. 2003;48:5–13. doi: 10.1177/070674370304800103. [DOI] [PubMed] [Google Scholar]