Abstract
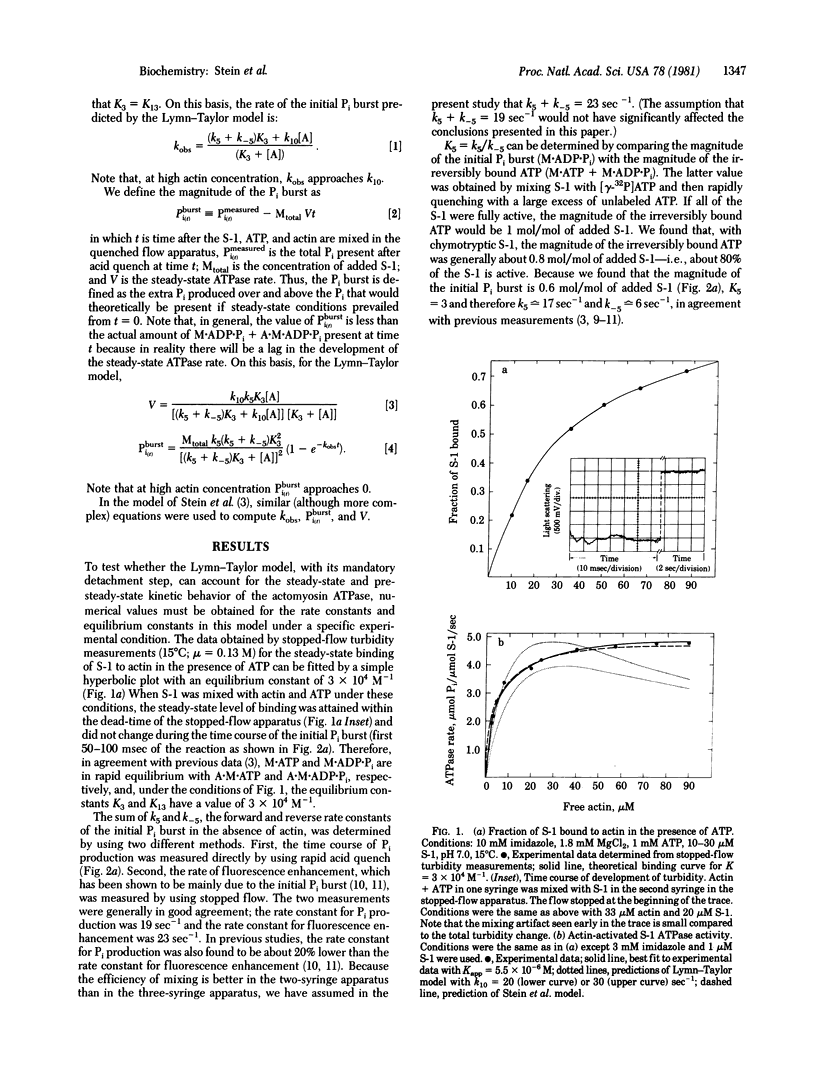
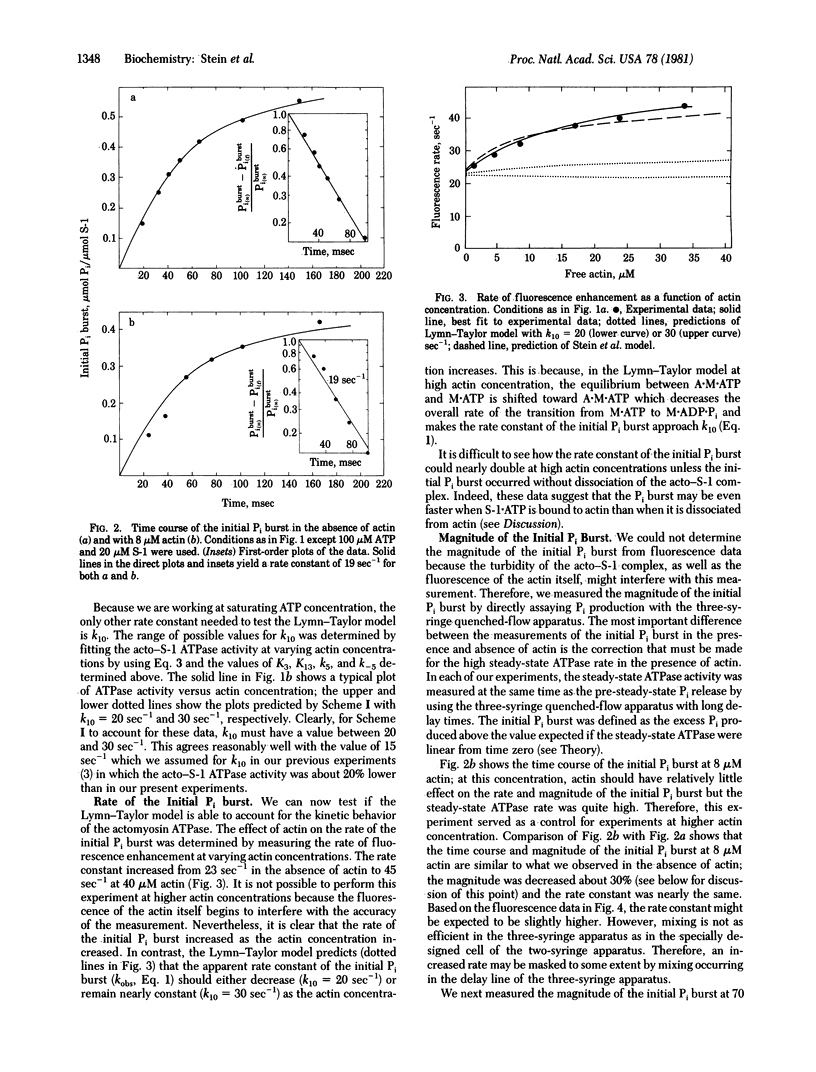
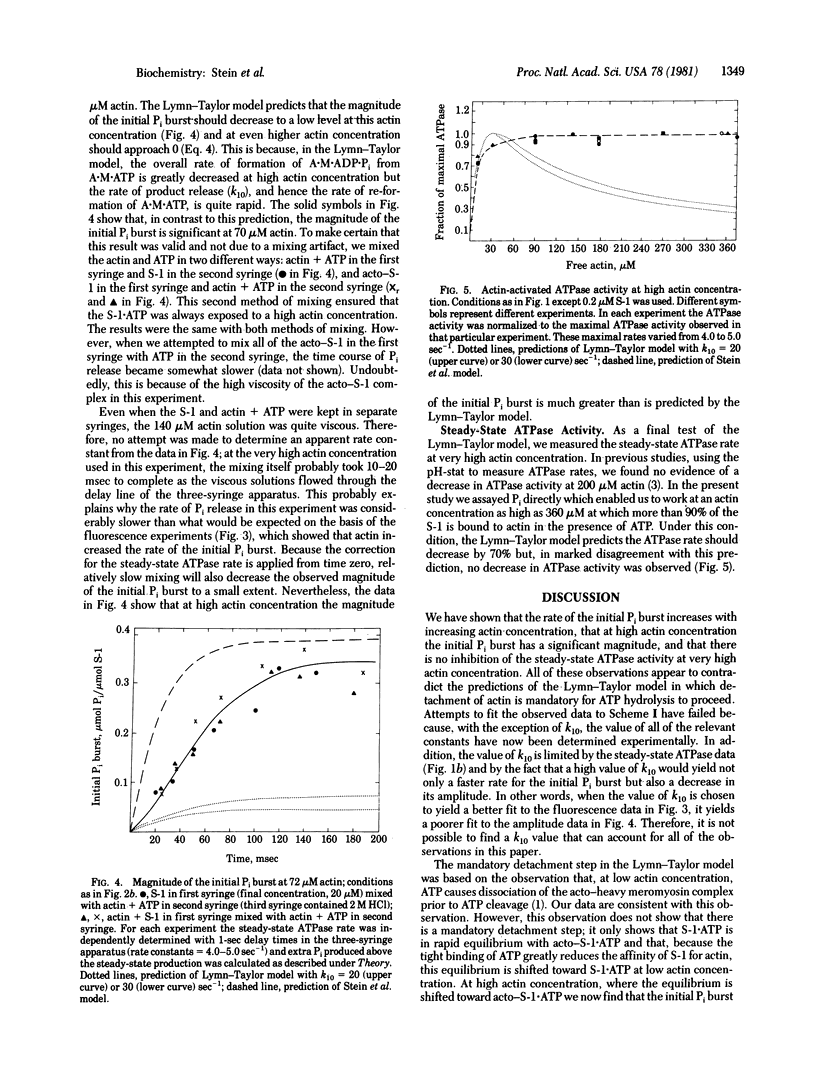
The Lymn-Taylor model for the actomyosin ATPase suggests that during each cycle of ATP hydrolysis the complex of myosin subfragment 1 (S-1) with actin must dissociate into S-1.ATP plus actin before ATP hydrolysis can occur. In the present study we tested whether such a mandatory detachment step occurs by measuring the effect of actin on the rate and magnitude of the ATP hydrolysis step (initial Pi burst) and on the steady-state ATPase rate. We find that the rate of the initial Pi burst markedly increases at high actin concentration although the Lymn-Taylor model predicts the rate should remain nearly constant or decrease. In addition, at high actin concentration, the magnitude of the initial Pi burst is much larger than is predicted by the Lymn-Taylor model. Finally, at 360 microM actin, at which more than 90% of the S-1.ATP is bound to actin, there is no inhibition of the steady-state ATPase activity although the Lymn-Taylor model predicts that 70% inhibition should occur. We conclude that the acto-S-1 complex is not dissociated by ATP during each cycle of ATP hydrolysis; in fact, the rate of the initial Pi burst appears to be even faster when S-1.ATP is bound to actin than when it is dissociated.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Chock S. P., Chock P. B., Eisenberg E. Pre-steady-state kinetic evidence for a cyclic interaction of myosin subfragment one with actin during the hydrolysis of adenosine 5'-triphosphate. Biochemistry. 1976 Jul 27;15(15):3244–3253. doi: 10.1021/bi00660a013. [DOI] [PubMed] [Google Scholar]
- Chock S. P., Chock P. B., Eisenberg E. The mechanism of the skeletal muscle myosin ATPase. II. Relationship between the fluorescence enhancement induced by ATP and the initial Pi burst. J Biol Chem. 1979 May 10;254(9):3236–3243. [PubMed] [Google Scholar]
- Chock S. P., Eisenberg E. The mechanism of the skeletal muscle myosin ATPase. I. Identity of the myosin active sites. J Biol Chem. 1979 May 10;254(9):3229–3235. [PubMed] [Google Scholar]
- Eisenberg E., Greene L. E. The relation of muscle biochemistry to muscle physiology. Annu Rev Physiol. 1980;42:293–309. doi: 10.1146/annurev.ph.42.030180.001453. [DOI] [PubMed] [Google Scholar]
- Eisenberg E., Kielley W. W. Troponin-tropomyosin complex. Column chromatographic separation and activity of the three, active troponin components with and without tropomyosin present. J Biol Chem. 1974 Aug 10;249(15):4742–4748. [PubMed] [Google Scholar]
- Eisenberg E., Moos C. The interaction of actin with myosin and heavy meromyosin in solution at low ionic strength. J Biol Chem. 1967 Jun 25;242(12):2945–2951. [PubMed] [Google Scholar]
- Johnson K. A., Taylor E. W. Intermediate states of subfragment 1 and actosubfragment 1 ATPase: reevaluation of the mechanism. Biochemistry. 1978 Aug 22;17(17):3432–3442. doi: 10.1021/bi00610a002. [DOI] [PubMed] [Google Scholar]
- KIELLEY W. W., HARRINGTON W. F. A model for the myosin molecule. Biochim Biophys Acta. 1960 Jul 15;41:401–421. doi: 10.1016/0006-3002(60)90037-8. [DOI] [PubMed] [Google Scholar]
- Lymn R. W., Taylor E. W. Mechanism of adenosine triphosphate hydrolysis by actomyosin. Biochemistry. 1971 Dec 7;10(25):4617–4624. doi: 10.1021/bi00801a004. [DOI] [PubMed] [Google Scholar]
- Sleep J. A., Hutton R. L. Actin mediated release of ATP from a myosin-ATP complex. Biochemistry. 1978 Dec 12;17(25):5423–5430. doi: 10.1021/bi00618a016. [DOI] [PubMed] [Google Scholar]
- Stein L. A., Schwarz R. P., Jr, Chock P. B., Eisenberg E. Mechanism of actomyosin adenosine triphosphatase. Evidence that adenosine 5'-triphosphate hydrolysis can occur without dissociation of the actomyosin complex. Biochemistry. 1979 Sep 4;18(18):3895–3909. doi: 10.1021/bi00585a009. [DOI] [PubMed] [Google Scholar]
- Weeds A. G., Taylor R. S. Separation of subfragment-1 isoenzymes from rabbit skeletal muscle myosin. Nature. 1975 Sep 4;257(5521):54–56. doi: 10.1038/257054a0. [DOI] [PubMed] [Google Scholar]


