Abstract
Background
The hepatic Acute Phase Response(APR) is an organ-specific response to a diverse array of insults and is largely under transcriptional control. Liver-specific transcription factors, Hepatic Nuclear Factors(HNFs)-1α and 4α, play important roles in maintenance of liver phenotype and function and their binding activity changes early after injury. However, their role in modulation of the liver’s response over time is not defined.
Materials and Methods
C57/BL6 mice were anesthetized and exposed to 95°C water for 10sec to create a 15% body surface area full-thickness burn. At specific time points, the mice were sacrificed. An ELISA for IL-6 was performed on serum and hepatic mRNA levels for Fibrinogen-γ and Serum Amyloid A(SAA)-3 were obtained through PCR. Transcriptional factor binding activity was assessed with electrophoretic mobility shift assays.
Results
Serum IL-6 levels peaked at 3h and Fibrinogen-γ and SAA mRNA levels increased more than 6-fold at 12h before returning to control levels at 48h. The binding activity of HNF-4α and HNF-1α rapidly declined after injury(1.5h) but recovered to near control level at 24h and 6h, respectively.
Conclusions
Changes in HNF-4α and HNF-1α binding occurred before changes in acute phase protein mRNA levels and were preceded by the peak in IL-6 levels. The rapid suppression and reconstitution of liver-specific transcription factor binding after injury may represent a mechanism which allows the normal liver phenotype to change and an injury-response phenotype to prevail. The role in the liver’s adaptive response to injury suggests a central role for both HNF-4α and HNF-1α in transcriptional regulation of the hepatic APR.
Keywords: Hepatocyte nuclear factor, DNA-protein binding, cytokine, acute phase protein, burn injury
INTRODUCTION
Injury remains a major problem for both the individual and society due to its physiological, medical, economic and social consequences. It is crucial to understand how the body responds to injury in order to effectively control and manage the damage caused by injury. The acute-phase response (APR) refers to an array of biochemical and metabolic changes that are induced by broad spectrum of stimuli, including infection and inflammation, trauma, burns, ischemic necrosis, and malignant tumors (1, 2) The APR is classically characterized by dramatic changes in the concentration of a group of plasma proteins known as acute phase proteins (APPs) which are synthesized in the liver and function in a wide range of injury-related activities. In response to injury the expression levels of APPs either increase (positive acute-phase proteins, e.g. Fibrinogen-γ and C-reactive protein) or decrease (negative acute-phase (e.g. albumin, transthyretin). The hepatic APR is mediated in part by several cytokines, including interleukin-6 (IL-6). While the APR is considered to be primarily a protective and therefore beneficial mechanism, severe or prolonged exposure to acute-phase conditions can lead to negative clinical consequences (3, 4). A better understanding of the molecular events that are involved in mediating the response to injury will make it possible to develop the therapeutic strategies for preventing the progression of the APR to counterproductive inflammatory states, while preserving its protective effects.
The reaction to injury is a well-orchestrated physiologic response involving the coordinated actions of multiple integrated systems. It initially occurs at the molecular level and involves changes in gene transcription. We hypothesize that the molecular mechanisms regulating the generation of the liver’s acute phase response occur through manipulation of liver-specific transcription factors and their binding activities. Evidence suggests that the liver’s APR is a highly regulated process and this process can be regulated through a small web of liver-specific transcription factors, including Hepatic Nuclear Factor (s) (HNF)-1, 3, 6 and 4, and CCAAT/Enhancer Binding Protein(C/EBP)-α (5). Of particular interest is HNF-4α and HNF-1α, since they are essential factors in the development and maintenance of the normal liver phenotype and are considered the most proximal transcriptional factors in this transcriptional hierarchy (6, 7). However, the role that these liver-specific transcription factors play in mediating the APR in vivo, especially the kinetic correlation of the APR response and HNFs activity has not been thoroughly defined as previous work has examined the APR response to burn only during the first 3 hours following injury (9, 11).
In this study we have used a well-established murine burn injury model to systemically induce a robust inflammatory/APR in the liver as evidenced by significant changes in serum IL-6 levels and in mRNA levels of two positive acute phase genes, fibrinogen-γ and serum amyloid alpha (SAA)-3. To investigate the mechanism of transcriptional regulation during the injury response and delineate the possible link of transcriptional events with the APR, we have studied the effect of burn injury on the DNA binding activity of HNF-4α and HNF-1α over an extended period of time. We found that burn injury induced significant changes in HNF-4α and HNF-1α binding activity, which revealed a similar temporal pattern as the induction of serum IL-6, but preceded both the induction and resolution of changes in APR gene expression. Examination of an extended period of time following injury has allowed for a more complete understanding of the kinetics of the APR, including observation of the recovery of binding activity of both HNF-4α and HNF-1α. We propose that the rapid suppression and reconstitution of liver specific transcription factor binding after injury may represent a mechanism for rapidly modulating the APR gene transcription in vivo, and suggest a central role for HNF-4α and HNF-1α in the transcriptional regulation of the liver’s acute phase response.
MATERIALS AND METHODS
Injury model
An injury model for a 15 % body surface area/full thickness burn was performed as previously described (8). Briefly, 18 to 20 gram C57/BL6 male mice (Charles River Laboratories, Boston, MA) were anesthetized via inhalation anesthetic and exposed to water at a temperature of 95°C for a period of 10 sec to create a full thickness burn injury. A plastic template was utilized to ensure that burns were of uniform size. This produced a 15% body surface area burn reproducibly. The control mice were allowed to be recovered from anesthesia and immediately were sacrificed. Sham-burn animals were anesthetized and exposed to room temperature water (20°C) for a period of 10 sec. Mice were observed and given post-injury care according to our institutional animal care standards and protocols. At pre-determined time points 1.5, 3, 6, 12 and 24 h after burn or sham-burn, the mice were sacrificed by cervical dislocation and the liver and serum were immediately harvested. Control animals (time zero) were harvested separately with no experimental treatment. Each experimental group contained four animals per time point.
Enzyme-linked immunosorbent assay (ELISA) for IL-6
Samples of whole blood harvested from animals with burn injury were allowed to clot while on ice and then centrifuged at 10,000 rpm for 10 min at 4° C. The resultant serum was then pooled for each time point and assayed using the IL-6 ELISA assay kit (eBisocience, San Diego, CA) according to the manufacturer’s instructions. The reactions were carried out in triplicate for each pooled sample.
RNA isolation and quantitative PCR
Approximately 150 mg of liver tissue was harvested from the experimental groups detailed above and snap frozen for later RNA isolation and quantitative PCR. Total RNA extraction was performed using the RNeasy Mini Kit (Qiagen). Total RNA was diluted and used in synthesis of cDNA with the Gene-Amp RNA PCR core kit (Applied Biosystems). These procedures were performed according to the manufacturer’s instructions. cDNA was prepared by reverse transcription and amplified with the ABI Prism 7000 Sequence Detection System in TaqMan Universal PCR Master Mix (Applied Biosystems). Probes for fibrinogen-γ, SAA-3 and beta-2-microglobulin (endogenous control) from the TaqMan Gene Expression Assays were utilized for relative quantification (Applied Biosystems). Analysis was performed with the RQ study software for the ABI Prism 7000 Sequence Detection System.
Nuclear extract preparation
The preparation of mouse liver nuclear extracts was carried out as described previously (9). Briefly, nuclear extracts were prepared from fresh liver tissue which was disrupted sharply with a scalpel and then by dounce homogenization in homogenization buffer. Homogenates were then centrifuged at 24,000 rpm for 60 min at 0°C through a 3M sucrose gradient. The pellet was removed and placed in 50 μl to 100 μl of hypertonic lysis buffer. The lysis buffer containing the nuclear pellet was incubated and agitated for 1 h on ice before centrifuging for 10 min at 12,000 rpm at 4°C. Supernatant containing the nuclear proteins were aliquotted and stored at −80°C. Protein concentration was determined using the Bradford method prior to performing experiments.
Electrophoretic mobility shift assay (EMSA)
Oligonucleotides corresponding to the HNF-4α binding site in the promoter region of TTR 5′-GATCCCCTAGGCAAGGTTCATA-3′ (5) were annealed and then radiolabeled with [α-32P]-dATP using Klenow fragment of DNA polymerase. An HNF-1α binding site in the albumin promoter (5′-GATCAGTATGGTTAATGATCTACAG-3′) (10) was similarly annealed and radiolabeled. The mobility shift assays were performed in a 15 μl reaction volume utilizing 10 μg of nuclear extract, 2 μg of poly-dIdC, 0.1 μg of bovine serum albumin and 1.5 μl of a 10% glycerol binding buffer. In addition, 1 μl radiolabelled probe was added to each reaction volume with a specific activity of 40,000 cpm. Nuclear extracts from each time point were incubated for 30 min at 20°C in the reaction constituents outlined above and then separated on a 5% polyacrylamide gel at 200V for 90 min. Specificity of the HNF-4α: TTR promoter interactions were confirmed by supershift analysis with anti-HNF-1α or anti-HNF-4α antibody and specific/non-specific competition with non-radiolabeled oligonucleotides. Specific competition assay utilized a ten-fold excess of unlabelled oligonucleotide. Non-specific competition analysis employed a 50-fold excess of a nonspecific oligonucleotide. Gels were transferred to filter paper and dried after separation. Bands were visualized by autoradiography. Quantification of binding activity for each time point was performed using a storage Phosphor screen (Perkin-Elmer, #7001723) and detection by the Cyclone phosphorimager and OptiQuant software (Packard Bioscience). Detection by the phosphorimager was expressed as NetDLU (Light Units)/mm2 and used in further analysis. Changes in binding were normalized to the control level of binding between oligonucleotide and transcription factor of interest. Samples from sham-burn anesthetized animals were similarly assayed and analyzed.
Western-blot analysis
Nuclear extracts was used for immunoblotting by previous described procedures (11). Antibodies against HNF-4α and HNF-1α were purchased from Santa Cruz Biotechnology.
Statistical analysis
Data was compiled using Microsoft Excel™ (Microsoft Corporation. Redmond, WA) and analyzed using SAS 9.1 (SAS Institute, Inc. Cary, NC). Differences between the groups were tested using Analysis of Variance of the absolute or relative values (depending on assay). Values are expressed as the mean ± standard error of the mean (SEM). Statistical significance was set at the p<0.05 level for Tukey’s studentized t-test for ANOVA to minimize Type I statistical error.
RESULTS
Serum IL-6 ELISA
As early as 1.5 h after burn, serum IL-6 increased to more than six fold above control levels (Fig. 1). IL-6 levels further increased to 20 fold control by 3 h and remained there at 6 h after burn. The peak IL-6 activity was 102.2 (±10.1) pg/ml. The IL-6 levels declined forty percent between 6 and 12 h. Despite a precipitous drop by 24 h after injury, the serum IL-6 still remained five-fold greater than control levels. The IL-6 level was elevated at all time points after injury to a statistically significant degree (p<0.05). In the sham-burn group, serum IL-6 levels were undetectable.
FIG. 1. The increase of serum IL-6 level in burn-injury animal model.
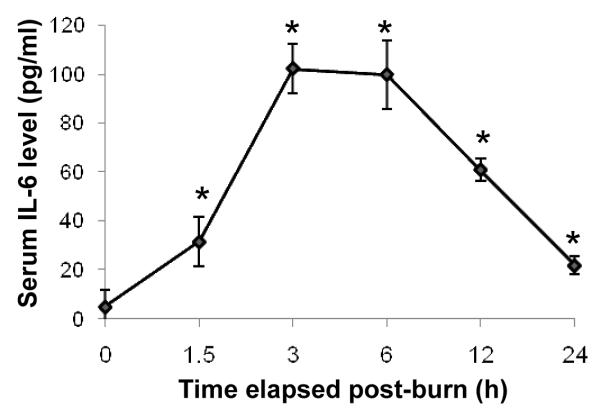
ELISA was used to quantify levels of IL-6 in the serum after burn-injury. Levels of IL-6 remain elevated above control levels at all time points studied. Data shown represent mean ± S.E. from 4 separate animals. *p<0.05 versus control (0 time point).
Induction of changes in post-burn transcription of acute phase protein targets
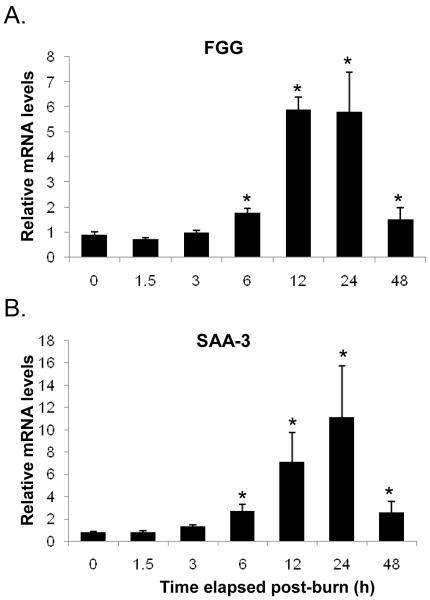
At the early time points post-injury, fibrinogen-γ message (mRNA) remained near control levels. There was a nearly two fold induction of Fibrinogen-γ transcription by 6 h post burn. This eventually peaked at 12 and 24 h after injury in mRNA levels approximately six fold higher than control (Fig. 2A). Similarly, SAA-3 mRNA levels exhibited marked induction after injury rising significantly at 12 and 24 h to seven and eleven fold, respectively compared to control (Fig. 2B).
FIG. 2. The expression of hepatic APP genes in response to burn injury.
The mRNA levels of Fibrinogen-γ (FGG) (A) and SAA-3 (B) were shown following injury. Data shown represent mean ± S.E. from 4 separate animals. *p<0.05 versus control (0 time point).
Changes in binding of HNF-4α and HNF-1α post-Injury
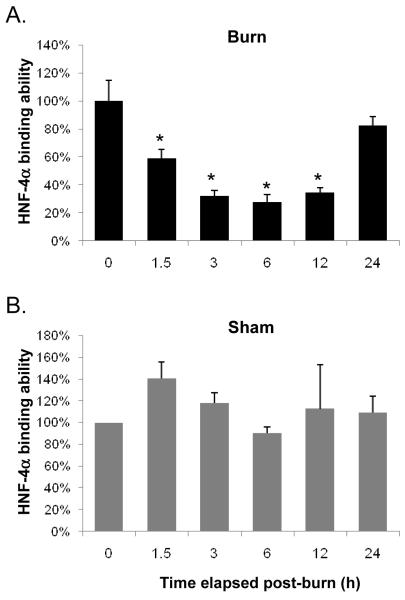
HNF-4α binding declined to 59% of control by 1.5 h post-burn and continued to do so until reaching a nadir at 28% control levels at 6 h. The binding efficacy slightly increased at the 12 h time point, but not to a statistically significant level compared with at 3 h and 6 h time points. HNF-4α binding recovered to 83% of control levels by 24 h post-injury (Fig. 3A). These changes in binding were statistically different from control up to 12 h (p<0.05) but not at 24 h (p>0.05) post-burn. In sham-burn group the binding ability of HNF-4α was not statistically different from control at any time point measured (p>0.05) (Fig. 3B).
FIG. 3. A rapid decrease in HNF-4α binding ability after burn-injury.
Binding efficacy of HNF-4α assayed by EMSA in burn injury (A) and sham-burn (B) animals are shown. Data shown represent mean ± S.E. from 3 separate experiments. *p<0.05 versus control (zero time point).
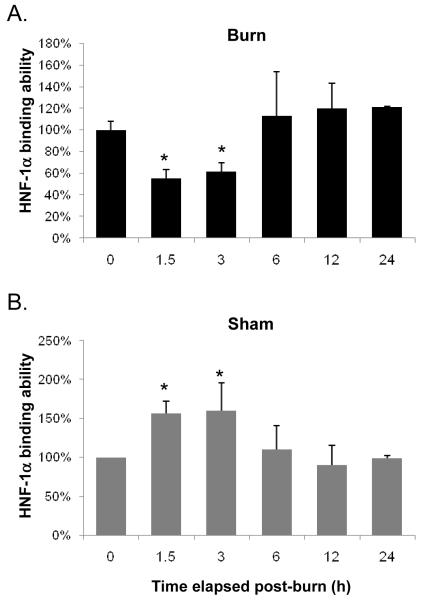
HNF-1α exhibited similar changes in binding to those seen in HNF-4α. Specifically, HNF-1α binding abruptly declined to 56% control levels by 1.5 h after injury. The lowest level in HNF-1α binding was reached at 1.5 h after which it increased slightly to 63% of control at 3 h. Binding then increased to levels statistically equivalent to control at all later time points (Fig. 4A). The declines in HNF-1α binding at the early time points (1.5 h and 3 h post-injury) were different from control levels but not different from one another (p>0.05). Unlike the statistical similarity of the sham-burn groups in HNF-4α EMSA, binding of HNF-1α actually increases above control levels to a significant degree at the early time points and falls back to control levels at later time points (Fig. 4B). Western Blot analysis of nuclear extracts showed no change in protein concentrations of HNF-4α or HNF-1α at anytime measured after injury. These data indicate that burn injury leads to a rapid and significant decrease in HNF-4 and HNF-1 binding activity with reconstitution of binding activity within 24 and 6 hours, respectively. This change is not due to a decrease in HNF protein concentration (Fig. 5).
FIG. 4. A rapid decrease in HNF-1α binding ability after burn-injury.
Binding efficacy of HNF-1α assayed by EMSA in burn injury (A) and sham-burn (B) animals are shown. Data shown represent mean ± S.E. from 3 separate experiments. *p<0.05 versus control (zero time point).
FIG. 5. No significant change in HNF-1α and HNF-4α protein concentrations after burn injury.
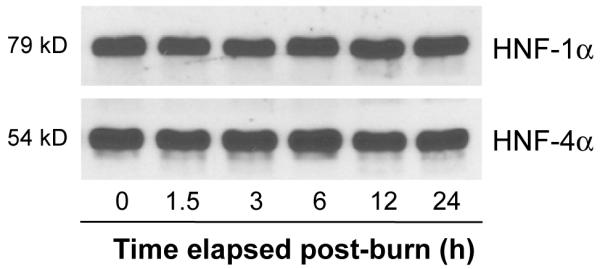
Protein expression was examined by Western blot using the same nuclear extracts as those used for running EMSA. No significant difference was found over the time period post- injury we examined.
DISCUSSION
In the present study, we demonstrated the time-limited nature and reversibility of the acute phase response and that changes in the APR are mirrored by changes in the activities of two important liver specific transcription factors: HNF-1α and HNF-4α. We demonstrated that the acute phase cytokine IL-6 is induced quite markedly as early as 1.5 h after burn injury. IL-6 is generally considered a major inducer of the acute phase response (12, 13). IL-6 also may directly lead to the reduction of HNF’s binding ability. This idea is supported by Andrejko’s work (14) showing that HNF-1α DNA binding activity decreased in the IL-6 +/+ but not IL-6 −/− mice with cecal ligation and puncture (CLP), a sepsis animal model. IL-6 may be one of the more significant proximal signaling molecules leading to the induction of an acute phase response and the transcriptional manipulation of APP genes in the liver. IL-6 also makes an ideal marker to confirm that we are causing an appropriate inflammatory stimulus. It is unlikely to be the sole proximal signaling molecule in such a complex and highly regulated process and other cytokines and signaling pathways likely play a role in the induction, maintenance and regression of the APR. However, IL-6 peaked at 3 and 6 h at twenty fold greater concentration than control (102.2±10.1 pg/ml) and it fell back towards pre-injury levels represent a significant change in the systems homeostasis and can be expected to result in changes in the transcriptional output of the liver. This allows us to look for changes in transcriptional regulatory mechanisms and correlate them with the kinetics of the systemic injury response.
Fibrinogen-γ has been used by many investigators as a marker of the acute phase response (2, 15). Fibrinogen-γ and SAA-3 are generally considered positive acute phase proteins, as their production is increased as part of the APR and in this respect can be used as markers for the kinetics of the APR in the liver. After burn injury, we observed an increase in transcription of both fibrinogen-γ and SAA-3. Increases are seen at 12 and 24 h after injury and lag the humoral response by IL-6. This is consistent with the idea that the liver’s transcriptional response is proximately mediated in part by changes in inflammatory cytokine signaling. Cytokine signaling networks influence a number of global responses to injury and stress, including the organ specific acute phase response (1, 12).
Having defined the time dependent and reversible nature of the liver’s APR and a proximal link to IL-6 signaling, a connection to transcriptional regulatory mechanism would require that changes in these mechanisms conform to the overall kinetic pattern of the response. We observed that HNF-4α binding declined very early at 1.5 h and reached a nadir at 3~6 h post-burn well before changes are seen in APP mRNAs. Fibrinogen-γ reached its peak at 12 and 24 h post-injury, at 24 h the HNF-4α binding was returning to near normal levels, although still decreased relative to control. Similarly, SAA-3 mRNA reached peak levels at 12 and 24 h after injury, also lagging the peak changes in HNF-4α binding. HNF-1α binding changes also precede the changes we see in APP mRNA transcription. The decline in HNF-1α binding at 1.5 and 3 h while not as large as the changes seen in HNF-4α occurs despite an elevation in HNF-1α binding seen in sham-burn animals. This suggests that a more profound suppression of HNF-1α binding may occur in the absence of anesthesia.
The present study expands on earlier data where we have demonstrated that binding of both HNF-4α and HNF-1α decrease early after injury 1.5 h and 3 h after burn injury. These changes in binding continued at the 6 and 12 h time points for both transcription factors and do not return to control levels until APP mRNAs are no longer changing. Although prior studies demonstrated a clear decline in binding of liver specific transcription factors early after injury, the question remained whether or not these transcriptional regulatory changes are also reversed acutely in a non-lethal traumatic injury model. This would appear to be the case for HNF-4α and HNF-1α, whose binding activities return to non-injured levels in a pattern that precedes the return to a non-injured liver phenotype. The acute phase changes in transcription factor binding, specifically HNF-4α and HNF-1α, suggest that suppression of binding in these factors manifest a ‘switching off’ of the normal liver phenotype during the acute phase response. This rapid modulation of binding efficacy may represent a key mechanism by which the hepatic acute phase response is modulated.
Studies of transcription factor binding require the presence of an intact protein capable of combining with target sequences. We have shown no change in the amount of HNF-4α and HNF-1α protein at various time points after injury by Western Blot. The changes in HNF-1α binding appear to be due to a significant change in the dissociation constant (Kd) for HNF-1α early after injury (9). Early changes in HNF-4α binding after injury appear to be due to a post-translational modification by phosphorylation (11) which markedly affects its Kd. Data from the present study suggest that changes in binding are not due to simple changes in HNF-4α and HNF-1α concentration but are due to posttranslational changes that are reversible over time. Prior studies demonstrated decreases in binding at time points early after injury in a burn model but did not follow these changes longitudinally in order to demonstrate their reversal (9). Our findings of a time limited decline in binding efficacy is an important extension of our previous work.
The liver plays a central role in homeostatic synthesis and metabolism. Prolonged phenotype switching might be harmful as the body’s reserves of hepatic function are exhausted if this broader interpretation of APR transcriptional changes is indeed the case. Thus, the return of HNF-4α and HNF-1α binding might well be as adaptive as its deficiency when appropriately timed. Over 70 years ago, Cuthbertson postulated the existence of an ‘ebb’ and ‘flow’ phase to injury response (16). Although one may criticize the aforementioned temporal division for imprecision, the changes observed by Cuthbertson all require hepatic involvement. In some ways, the time limited nature of binding changes in HNF-4α and HNF-1α may reflect hepatic adherence to a broader agenda of metabolic injury response. Further studies will no doubt shed light on the interplay between these temporally synchronous but as yet unlinked phenomena.
In conclusion, the hepatic acute phase response is a time-limited organ specific response to remote injury. These changes are reflected as increased IL-6, a major inducer of the hepatic acute phase response. Changes in the transcription of the classic acute phase proteins fibrinogen-γ and SSA-3 follow the increases in IL-6. Based on this observation, binding of transcription factors should exhibit a decrease preceding the changes seen in relative quantity of transcript. Indeed, we observed a decrease in binding of HNF-4α and HNF-1α by 1.5 h after injury to a statistically significant degree. These observations are indicative of a regulatory role for HNF-4α and HNF-1α in modifying APR gene expression. However, a direct linear relationship between a given APP production and an individual transcription factor would be misleading because transcriptional regulation is complex and involves the interactions of many different individual transcriptional factors and many other components of the cell’s transcriptional apparatus. We believe that proximal to changes in acute phase serum protein and mRNA levels injury causes significant changes in the activities of transcription factors such as HNF-1α and 4α to allow the change in liver phenotype seen after injury. The early changes in the transcriptional environment are required to allow the liver phenotype change seen after injury and that is what we are seeing in the changes in HNF-4α and HNF-1α activity. Further work will be needed to find a direct relationship between individual transcription factors and individual acute phase protein genes by utilizing knockout animals or other molecular level strategies. Figure 6 illustrates our hypothesis. Our findings support this hypothesis that the molecular mechanisms regulating the generation of the acute phase response likely involve the manipulation of liver-specific transcription factors binding activity and HNF-4α and HNF-1α may play a central role in the adaptive response to injury by the liver. The characterization and understanding of the transcriptional kinetics taking place during an injury response may equip clinicians with the ability to foreshadow their patient’s molecular and ultimately physiological response to serious, perhaps life-threatening maladies and represents the first step in the potential manipulation of these responses.
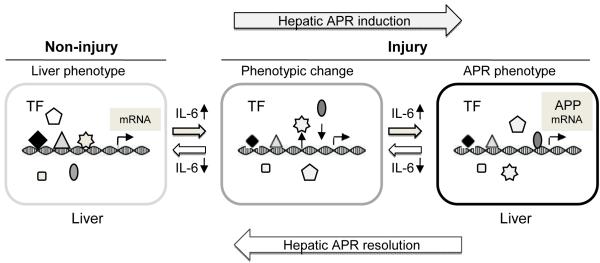
FIG. 6. A proposal mechanism of the role of transcription factors in the hepatic APR.
After burn-injury, IL-6 levels rapidly increase in the serum. For the liver respond to APP production, a rapid change in the phenotype is required. These phenotype changes involve in part the modification of liver-specific transcription factor activates, and allowing the liver synthetic programming to change and the induction of acute phase response by regulating mRNA expression of acute phase proteins. As the injury signaling resolves, IL-6 levels fall and the liver’s phenotype shifts from the APR to a non-injury status. TF: transcription factor, ◆and △ denote HNF-4α and HNF-1α, and other symbols indicate additional transcription factors and co-regulators; APR: acute phase response; APP: acute phase protein.
Acknowledgments
This study was supported by NIH grant (R01DK064945).
REFERENCES
- 1.Gabay C, Kushner I. Acute-phase proteins and other systemic responses to inflammation. N Engl J Med. 1999;340:448–454. doi: 10.1056/NEJM199902113400607. [DOI] [PubMed] [Google Scholar]
- 2.Baumann H, Gauldie J. The acute phase response. Immunol Today. 1994;15:74–80. doi: 10.1016/0167-5699(94)90137-6. [DOI] [PubMed] [Google Scholar]
- 3.Cerra FB. The multiple organ failure syndrome. Hosp Pract (Off Ed) 1990;25:169–176. doi: 10.1080/21548331.1990.11703990. [DOI] [PubMed] [Google Scholar]
- 4.Beal AL, Cerra FB. Multiple organ failure syndrome in the 1990s. Systemic inflammatory response and organ dysfunction. Jama. 1994;271:226–233. [PubMed] [Google Scholar]
- 5.Sladek FM, Zhong WM, Lai E, Darnell JE. Liver-enriched transcription factor HNF-4 is a novel member of the steroid hormone receptor superfamily. Genes Dev. 1990;4:2353–2365. doi: 10.1101/gad.4.12b.2353. [DOI] [PubMed] [Google Scholar]
- 6.Sladek FM. Orphan receptor HNF-4 and liver-specific gene expression. Receptor. 1994;4:64. [PubMed] [Google Scholar]
- 7.Kuo CJ, Conley PB, Chen L, Sladek FM, Darnell JE, Crabtree GR. A transcriptional hierarchy involved in mammalian cell-type specification. Nature. 1992;355:457–461. doi: 10.1038/355457a0. [DOI] [PubMed] [Google Scholar]
- 8.Burke PA, Drotar M, Luo M, Yaffe M, Forse RA. Rapid modulation of liver-specific transcription factors after injury. Surgery. 1994;116:285–292. discussion 292-283. [PubMed] [Google Scholar]
- 9.Burke PA, Luo M, Zhu J, Yaffe MB, Forse RA. Injury induces rapid changes in hepatocyte nuclear factor-1: DNA binding. Surgery. 1996;120:374–380. doi: 10.1016/s0039-6060(96)80312-6. discussion 380-371. [DOI] [PubMed] [Google Scholar]
- 10.Mendel DB, Crabtree GR. HNF-1, a member of a novel class of dimerizing homeodomain proteins. J Biol Chem. 1991;266:677–680. [PubMed] [Google Scholar]
- 11.Li X, Salisbury-Rowswell J, Murdock AD, Forse RA, Burke PA. Hepatocyte nuclear factor 4 response to injury involves a rapid decrease in DNA binding and transactivation via a JAK2 signal transduction pathway. Biochem J. 2002;368:203–211. doi: 10.1042/BJ20020233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Castell JV, Gomez-Lechon MJ, David M, Fabra R, Trullenque R, Heinrich PC. Acute-phase response of human hepatocytes: regulation of acute-phase protein synthesis by interleukin-6. Hepatology. 1990;12:1179–1186. doi: 10.1002/hep.1840120517. [DOI] [PubMed] [Google Scholar]
- 13.Heinrich PC, Castell JV, Andus T. Interleukin-6 and the acute phase response. Biochem J. 1990;265:621–636. doi: 10.1042/bj2650621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Andrejko KM, Raj NR, Kim PK, Cereda M, Deutschman CS. IL-6 modulates sepsis-induced decreases in transcription of hepatic organic anion and bile acid transporters. Shock. 2008;29:490–496. doi: 10.1097/shk.0b013e318150762b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kushner I. The phenomenon of the acute phase response. Ann N Y Acad Sci. 1982;389:39–48. doi: 10.1111/j.1749-6632.1982.tb22124.x. [DOI] [PubMed] [Google Scholar]
- 16.Cuthbertson DP. The disturbance of metabolism produced by bony and non-bony injury, with notes on certain abnormal conditions of bone. Biochem J. 1930;24:1244–1263. doi: 10.1042/bj0241244. [DOI] [PMC free article] [PubMed] [Google Scholar]