Abstract
Traumatic injury induces a local and systemic release of pro-inflammatory cytokines, acute phase proteins, hormones and other inflammatory mediators. The excessive release of these mediators plays an important role in the pathogenesis of shock. In parallel to this pro-inflammatory response, there is a regulatory response characterized by the release of anti-inflammatory mediators, which is thought to represent the host’s attempt to restore immunological equilibrium. Studies in septic patients have suggested the compensatory anti-inflammatory response may result in an “immunodeficient state” that leaves the host susceptible to further infectious insults. A key feature of the anti-inflammatory state in septic patients is a change in the responsiveness of monocytes that has been termed “monocyte deactivation.” This is supported by data that link monocyte deactivation to increased mortality in septic patients. Monocytes with reduced HLA-DR expression have been described in trauma patients.
We collected blood from 25 severely injured patients and evaluated peripheral blood mononuclear cells (PBMC) for HLA-DR expression and TNF-alpha response to LPS stimulation as markers of monocyte deactivation. Levels of intracellular HO-1 were determined in each patient, as HO-1 has been implicated in monocyte deactivation in patients with severe SIRS. HLA-DR expression correlated inversely with Injury Severity Scores and TNF-alpha response to LPS stimulation, but failed to correlate with HO-1 levels in these patients. HLA-DR expression was decreased in normal monocytes stimulated with patient plasma, but this treatment had no effect on HO-1 levels. These results suggest monocyte deactivation in trauma patients is unlikely to be mediated by HO-1.
INTRODUCTION
Severe injury remains one of the leading causes of death and morbidity in patients under 40. Patients who survive the initial trauma and post-traumatic resuscitation have substantial alterations of the immune system. Traumatic injury induces both a local and systemic release of pro-inflammatory cytokines, acute phase proteins, hormones and other inflammatory mediators. The excessive release of these mediators plays an important role in the pathogenesis of shock (1,2,3). In parallel to this pro-inflammatory response, there is a regulatory response characterized by the release of anti-inflammatory cytokines and mediators (3,4), which is thought to represent the host’s attempt to restore immunological equilibrium. Studies in septic patients, however, have suggested the compensatory anti-inflammatory response may result in an “immunodeficient state” that leaves the host susceptible to further infectious insults (5,6). A key feature of the anti-inflammatory state in septic patients is a change in the responsiveness of monocytes that has been termed “monocyte deactivation.” This hypothesis is supported by data that link monocyte deactivation to increased mortality in septic patients (7,8). Monocyte dysfunction in these patients is characterized by 1) a lack of tumor necrosis factor (TNF)-α production upon LPS challenge in vitro, 2) increased production of anti-inflammatory cytokines, such as IL-10 and increased expression of HO-1, and 3) markedly reduced HLA-DR expression resulting in a loss of antigen-presenting capacity. Monocytes with reduced HLA-DR expression have been described in trauma patients, and failure to return to normal levels of HLA-DR expression has been correlated with higher mortality in severely injured trauma patients (9–11).
The mechanisms of monocyte deactivation in trauma patients are complex. While the secretion of pro-inflammatory cytokines is reduced in deactivated monocytes, levels of anti-inflammatory mediators, such as IL-10, remain constant or increase (4,12,13). IL-10 may play a key role in monocyte deactivation since IL-10 expression has been shown to correlate with a reduction of HLA-DR expression and impaired upregulation of LPS-induced HLA-DR can be reversed, at least in part, by the application of an IL-10 neutralizing antibody (14,15).
Heme oxygenase (HO)-1 is a stress-inducible protein shown to be associated with protection from cellular injury and oxidative stress. HO-1 and its three metabolites, carbon monoxide, biliverdin and free iron are known to possess anti-inflammatory properties (16). Several studies have suggested that HO-1 may limit tissue damage in response to different pro-inflammatory stimuli (17,18), and recent studies have shown that HO-1 mediates at least some of the anti-inflammatory effects of IL-10 (19,20). HO-1 can be induced under a variety of circumstances, including free radical stress, bacterial LPS exposure and hyperoxia, underscoring its importance as an immune mediator (16). Inhibition of HO-1 activity in mice reverses the inhibitory effect of IL-10 on LPS-induced production of TNF-α (20). Intracellular HO-1 expression was increased in patients with severe SIRS, and exposure of normal monocytes to serum from these patients increased HO-1 expression to the level seen in the monocytes of the patients (21). Taken together, these findings suggest a role for HO-1 in monocyte deactivation or regulation of responses of deactivated monocytes.
PATIENTS AND METHODS
Patients
We collected blood from 25 severely injured trauma patients, admitted to the Trauma Service at the University of New Mexico Hospital. All samples were obtained in accordance with guidelines and under protocols approved by the Human Research Review Committee at the University of New Mexico Health Sciences Center. Enrollment criteria included ICU admission or Injury Severity Score of greater than 16 as a marker of severe injury, age greater than 18 years, and negative pregnancy test. Of the patients, 18 were men and 7 were women, with a mean average age of 40.3 ± 17.8 years (range 19–73). The mean Injury Severity Score was 31.4 ± 13.9 (range 9–55). No patient was treated with any immunosuppressant or steroids. No patients were included that were admitted to the ICU for merely observation status. Of the patients included, two patients had single system injury from a penetrating injury to the extremity, the remaining patients had multi-system trauma.
Blood Samples
We collected blood samples from the patients at enrollment (within 24 hours of admission) and at 48 and 72 hours from enrollment. Monocyte intracellular HO-1 and HLA-DR surface marker expression were determined at 48 hours. Plasma cytokine concentrations were determined from the 24, 48 and 72 hour blood samples. The TNF-alpha response of PBMC to LPS stimulation was determined at 72 hours.
Plasma Cytokine Concentrations
Blood was collected from patients at 24 hours in heparinized tubes. Samples were centrifuged at 1000 rpm for 10 minutes and then plasma was collected and stored at −80ºC for IL-10 and TGF-beta concentrations. IL-10 concentrations were then determined with Milliplex Human Cytokine Immunoassay according to manufacturer’s instructions (Millipore). TGF-beta concentrations were determined with enzyme-linked immunosorbent assay (ELISA) according to manufacturer’s instructions (BD Bioscience).
Determination of Intracellular HO-1
Intracellular HO-1 in monocytes was measured by flow cytometry. Whole blood samples were collected at 48 hours in EDTA-treated tubes, and 200 μl was used for measurements. Twenty microliters of phycoerythrin-conjugated anti-CD14 (BD Biosciences) was added and the samples were incubated for 10 minutes at room temperature. Samples were kept in the dark from this step. Two milliliters of RBC lysis solution (eBioscience) was added, and the samples were incubated at room temperature for 10 minutes. Cells were washed with 4 ml of phosphate buffered saline (PBS, Invitrogen) and centrifuged at 1600 rpm for 5 minutes at 20ºC. Three milliliters of PAB (0.1% BSA/0.05% sodium azide in PBS, Sigma) staining buffer was added to each sample and centrifuged at 1600 rpm for 5 minutes for 2 cycles. One milliliter of 3% paraformaldehyde (Sigma) was used to fix the cells and the samples were incubated at room temperature for 10 minutes. After centrifugation, samples were washed with PBS, and then treated with 1 ml of 0.2% saponin/0.1% BSA/0.05% azide in PBS for a 30 minute incubation to permeabilize the cells. Samples were washed with wash buffer (0.2% saponin/0.1% BSA/0.05% azide). After centrifugation, 2 μl of rabbit anti-human HO-1 antibody (Stressgen) was added to each sample, and the samples were incubated at room temperature for 30 minutes. Samples were washed with wash buffer and 1 μl of FITC goat anti-rabbit IgG (Sigma) was added as a secondary antibody. Samples were incubated at room temp for 30 minutes, washed with wash buffer and then resuspended in 600 μl 2% paraformaldehyde (Sigma). The fluorescence of each sample was analyzed with a flow cytometer and expressed as the geometric mean fluorescence intensity (GMFI) after subtracting the GMFI for cells stained with the secondary antibody alone.
Surface Expression of HLA-DR
Expression of HLA-DR on monocytes was determined by flow cytometry. Whole blood samples were collected in EDTA-treated tubes at 48 hours, and 200 μl was used for measurements. Ten microliters of FITC-conjugated anti-CD14 (Miltenyi Biotec) and 20 μl of PerCp-Cy5.5-conjugated anti-HLA-DR (BD Bioscience) was added to the samples and incubated for 10 minutes in the dark. Two milliliters of RBC lysis solution was added to each sample and incubated at room temperature for 10 minutes. Cells were washed with 4 ml of PBS and centrifuged at 1600 rpm for 5 minutes at 4ºC. Cells were washed twice with 3 ml of PAB staining buffer. Cells were then fixed with 2% paraformaldehyde and the fluorescence of each sample was analyzed with a flow cytometer and expressed as the GMFI after subtracting the GMFI for the corresponding isotype control.
Analysis of Normal Monocyte Response to Plasma from Trauma Patients
Blood from normal volunteers was drawn into heparinized tubes. PBMC were obtained from peripheral blood of healthy volunteers by gradient separation using Ficoll Paque Plus (GE Healthcare). Briefly, 5 ml of whole blood was diluted 1:1 with PBS and carefully layered over 3 ml of Ficoll Paque Plus. Samples were then centrifuged at 1400 rpm for 30 minutes. PBMC were washed two times in PBS and resuspended at a concentration of 12 × 106/ml in RPMI-1640 medium (containing 10% heat-inactivated FBS-HyClone, Sigma). A volume of 1 ml of cells in RPMI-1640 medium was then added to a polypropylene tube and incubated with 1/3 volume of patient plasma for 22 hours at 37ºC in a CO2 incubator. Patient plasma samples with HLA-DR percentage of <30% were used in the patient plasma incubation experiments, ensuring monocyte deactivation. A limited number of samples of patient plasma with HLA-DR expression > 30% were used in the incubation experiments, and we found a similar decrease in HLA-DR expression, but not to the extent seen with plasma with <30% expression (data not shown). We chose to use plasma from patients with <30% expression of HLA-DR for a more exaggerated response so that we could more clearly examine the role of HO-1. A second tube was treated with normal volunteer plasma in a similar fashion. After the incubation period, samples were washed with PAB and HLA-DR expression and intracellular HO-1 levels in the treated monocytes were determined as described above by flow cytometry. HLA-DR and HO-1 levels were analyzed by two color fluorescence on CD14+ monocytes as described above and were expressed as the GMFI.
In a separate set of experiments, HLA-DR expression was evaluated on monocytes from healthy volunteers after incubation with the HO-1 inhibitor zinc protoporphyrin (ZnPP). PBMC were obtained as described above and incubated for 30 minutes with 5 μM of the HO-1 inhibitor ZnPP, or the non-inhibitory control compound, copper protoporphyrin (CuPP), before addition of patient or normal volunteer plasma as described above. After incubation, HLA-DR expression was determined by flow cytometry and expressed as the GMFI.
Analysis of TNF-alpha production
TNF-alpha production from monocytes of trauma patients in response to lipopolysaccharide (LPS) (E. coli 055:B5, Sigma) stimulation was measured by ELISA. Blood was collected from patients at 72 hours in heparinized tubes. PBMC were obtained by gradient separation using Ficoll Paque Plus as described above. PBMC were cultured in 96-well plates at a concentration of 0.5 × 106 cells per well in 200 μl of complete RPMI-1640 medium and incubated with LPS (10ng/ml) at 37ºC for 4 hours. After 4 h, supernatants were collected, centrifuged to remove any cells, and stored at −80ºC for TNF-alpha determinations. TNF-alpha concentrations were determined by ELISA kit (BD Biosciences) according to the manufacturer’s instructions.
Statistical Analysis
Graphical and statistical analyses were performed using Prism software version 5.0 (GraphPad). Linear regression and correlation analyses were used to analyze patient data. Student’s t test was used to compare mean values of HO-1 and HLA-DR for monocytes incubated with plasma from patients and healthy volunteers. Statistical significance was determined at p < 0.05. For n = 25 only relatively strong correlations greater than 0.53 will have 80% statistical power (two-sided α= 0.05).
RESULTS
Monocyte Deactivation in Trauma Patients and Intracellular HO-1
The expression of HLA-DR on trauma patient monocytes was determined by two color flow cytometric analysis using CD14 as a monocyte marker (Figure 1). Six of the 25 patients had <30% of monocytes with HLA-DR expression greater than the isotype control, consistent with deactivated monocytes. Overall in 25 severely injured trauma patients, we found an inverse correlation between HLA-DR levels (GMFI) as well as the % of monocytes positive for HLA-DR and their Injury Severity Scores (p < 0.001) (Figure 2). TNF-alpha production from PBMC of trauma patients in response to LPS stimulation correlated with HLA-DR expression (p < 0.001), consistent with the description of deactivated monocytes. In addition, TNF-alpha production inversely correlated with Injury Severity Scores in the patients (p < 0.005). Intracellular HO-1 levels in CD14+ monocytes were increased in trauma patients compared to normal volunteers (patients, 545.9 ± 40.3 GMFI versus controls, 242.4 ± 24 GMFI, p < 0.05), but had no correlation with either Injury Severity Score (r = 0.0047, p = 0.98) or HLA-DR levels (r = 0.067, p = 0.77) in the patient samples (Figure 2). We found no correlation between HO-1 levels and TNF-alpha production after LPS stimulation. We looked at the anti-inflammatory cytokines IL-10 and TGF-beta as possible mediators of monocyte deactivation after trauma. Both cytokines were elevated in trauma patients compared to healthy volunteers (IL-10, 202.9 ± 53.9 pg/ml in patients vs. 6.05 ± 1.7 pg/ml in controls; and TGF-β 4.13 ± 0.3 pg/ml in patients vs. 2.5 ± 1.8 pg/ml in controls). However, neither IL-10 nor TGF-beta levels correlated with Injury Severity Score or markers of monocyte deactivation in our patient population. We looked at blood transfusion as a possible surrogate for ischemia and found that HLA-DR levels inversely correlated with ISS in both patients receiving blood transfusions (p=0.03) and in those that did not (p=0.02).
Figure 1.
Representative flow cytometry results for HLA-DR on CD14+ monocytes in patient samples. A. demonstrates a patient with greater than 95% HLA-DR expression on CD14+ monocytes, while B. demonstrates a patient with <30% HLA-DR expression on CD14+ monocytes. Quadrants were established using appropriate isotype control antibodies.
Figure 2.
Linear regression analyses demonstrating inverse correlation of HLA-DR expression and TNF-alpha production after LPS stimulation with Injury Severity Score. TNF-alpha production after LPS stimulation positively correlated with HLA-DR expression as markers of monocyte deactivation. Intracellular HO-1 failed to correlate with HLA-DR expression on CD14+ monocytes.
Normal Monocyte Response to Plasma from Trauma Patients
HLA-DR expression in normal monocytes treated with plasma from trauma patients with deactivated monocytes showed a significant decrease in HLA-DR surface expression when compared to monocytes treated with plasma from healthy volunteers (Figure 3). The fluorescence intensity of intracellular HO-1 in normal monocytes stimulated with plasma from trauma patients was not significantly increased compared to HO-1 levels in normal monocytes stimulated with plasma from healthy volunteers, although there was a trend toward increased HO-1 in the CD14+ monocytes treated with patient plasma. There was no difference in total HO-1 levels in PBMC between the 2 groups (Figure 4).
Figure 3.
Surface HLA-DR expression on CD14+ monocytes after incubation with healthy volunteer plasma (control) or trauma patient plasma with monocyte deactivation for 22 hours. * P < 0.01 vs. control plasma (n=4).
Figure 4.
Intracellular HO-1 levels after incubation of normal PBMCs with healthy volunteer plasma (control) and trauma patient plasma with monocyte deactivation for 22 hours. Neither HO-1 levels in CD14+ monocytes nor total HO-1 levels were significantly changed following incubation with patient plasma compared to healthy volunteer plasma (n=6).
Effect of HO-1 Inhibition on the Monocyte Response to Plasma from Trauma Patients
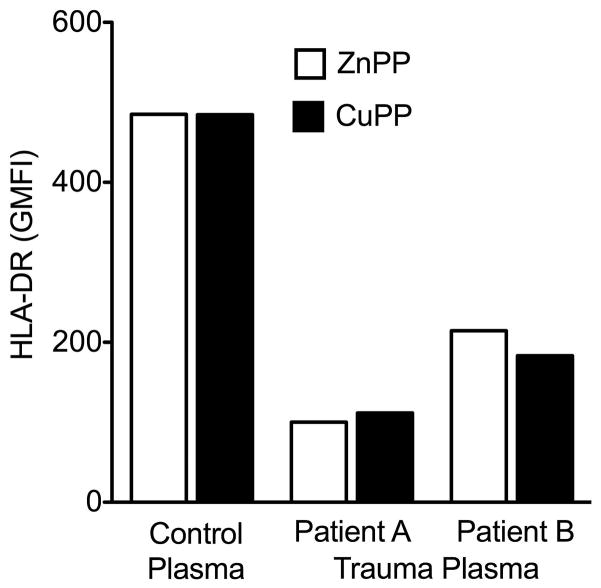
To determine directly whether HO-1 was playing a role in decreased expression of HLA-DR, we exposed normal monocytes to plasma from healthy volunteers or trauma patients in the presence of an HO-1 inhibitor (Zn-protoporphyrin) or an inactive control compound (Cu-protoporphyrin) (Figure 5). The results show that monocytes incubated with trauma plasma had equally decreased HLA-DR expression regardless of the presence of HO-1 inhibition.
Figure 5.
Surface HLA-DR expression on CD14+ monocytes after incubation with the HO-1 inhibitor ZnPP, or CuPP as control, for 30 minutes followed by healthy volunteer plasma (control) or trauma patient plasma with monocyte deactivation for 22 hours. The experiment was repeated using a different PBMC donor and different plasma with similar results.
DISCUSSION
Trauma patients who survive the initial injury and posttraumatic resuscitation period are often confronted with substantial derangements in the immune system. Approximately 20% of deaths after trauma are considered late deaths and occur as a result of multiple organ dysfunction syndrome (MODS). MODS is a syndrome in which organs and tissues fail or become dysfunctional but are not involved in the original injury. Essentially all patients have a septic-like picture, but infection is rarely found as the precipitating cause, suggesting host-derived non-bacterial factors are involved in the pathogenesis. The stimulus for deterioration of the immune response after severe injury remains unknown. A marked suppression in cell-mediated immunity following an excessive inflammatory response appears to be responsible for the increased susceptibility to subsequent sepsis.
A decreased HLA-DR expression and attenuated ex-vivo TNF-alpha response to LPS stimulation has been observed in septic patients (22). The decrease in HLA-DR expression and reduced ex vivo TNF-alpha production during sepsis correlated with prognosis and outcome (7,8). In trauma patients, HLA-DR expression is diminished within 48 hours of injury. In patients with an uneventful course, the HLA-DR recovery was within 1 week, while patients with sustained depression of HLA-DR expression developed infectious complications or MODS (11,23). A complete failure of recovery of HLA-DR expression was associated with death (9,11).
We demonstrated a decreased HLA-DR surface expression on monocytes of severely injured trauma patients at 48 hours, as previously described (24). In addition, we showed a correlation with monocyte suppression, as defined by HLA-DR expression and ex vivo TNF-alpha production, and injury as measured by Injury Severity Score.
Previous studies have shown an increase in intracellular HO-1 levels in patients with a systemic inflammatory response (21). We also demonstrated an increase in intracellular HO-1 levels in monocytes after trauma, but these values failed to correlate with either the Injury Severity Score (r = 0.0047) or markers of monocyte suppression (r = 0.067), suggesting HO-1 is unlikely to mediate monocyte deactivation after trauma.
The anti-inflammatory cytokines IL-10 and TGF-beta have been proposed to play a role in the mechanism of monocyte deactivation in patients with sepsis (25,26). However, we found no correlation between plasma IL-10 levels or TGF-beta levels in patients with either HLA-DR expression or TNF-alpha production after LPS stimulation, suggesting these cytokines likely do not mediate monocyte deactivation after severe injury.
We found that intracellular monocyte HO-1 levels in normal PBMC incubated with plasma from patients with deactivated monocytes was slightly increased, but not significantly, when compared to PBMC treated with plasma from healthy volunteers. These findings are contrary to those described by Mohri, et al (21). In that study, a heterogeneous population of patients with systemic inflammatory response syndrome was evaluated. In addition, serum from patients meeting SIRS criteria, but not confirmed monocyte suppression, was used to stimulate normal monocytes in the Mohri study. In our study, only patients with demonstrated monocyte HLA-DR expression <30% were used in the normal monocyte exposure experiments. When we looked at intracellular HO-1 expression in normal volunteer monocytes after treatment with plasma from patients with greater than 75% HLA-DR expression, we found a similar rise (data not shown).
To further evaluate the role of HO-1 in monocyte deactivation, we then looked at the effects of the HO-1 inhibitor, ZnPP, on HLA-DR levels on normal monocytes after exposure to trauma patient plasma. Incubation with the HO-1 inhibitor ZnPP had no effect on the decrease of HLA-DR surface expression on treated monocytes. Taken together, these findings confirm that HO-1 likely does not play a role in monocyte deactivation after trauma.
Although we demonstrate that intracellular HO-1 expression increases in monocytes from patients after severe injury compared to monocytes from healthy volunteers, we failed to show any correlation with HO-1 and monocyte deactivation in our population. We did demonstrate that significantly injured patients are more likely to have deactivated monocytes, confirming this group of patients is at risk for further infectious complications and increased mortality. There was a trend toward more infectious complications in the six patients with monocyte deactivation, but there was not enough power to analyze this outcome (p = 0.056). Early identification of this group of patients after trauma may be useful for tailoring approaches that minimize further insults or disruption of a compromised immune system.
Acknowledgments
Supported By: CTSA KL2 Mentored Career Development Scholars Program
The authors gratefully acknowledge Carol Morris for her assistance and technical expertise in performing the experiments described. Orrin Myers provided statistical assistance. This work was partially supported by DHHS/NIH/NCRR grant #1UL1RR031977-01, The University of New Mexico Clinical and Translational Science Center.
References
- 1.Martin C, Boisson C, Haccoun M, Thomachot L, Mege JL. Patterns of cytokine evolution (tumor necrosis factor-alpha and interleukin-6) after septic shock, hemorrhagic shock and severe trauma. Crit Care Med. 1997;25:1813–1819. doi: 10.1097/00003246-199711000-00018. [DOI] [PubMed] [Google Scholar]
- 2.Roumen RM, Hendriks T, van der Ven-Jongekrijg J, Nieuwenhuijzen GA, Sauerwein RW, van der Meer JW, Goris RJ. Cytokine patterns in patients after major vascular surgery, hemorrhagic shock, and severe blunt trauma. Relation with subsequent adult respiratory distress syndrome and multiple organ failure. Ann Surg. 1993;218:769–776. doi: 10.1097/00000658-199312000-00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Keel M, Trentz O. Pathophysiology of polytrauma. Injury. 2005;36:691–709. doi: 10.1016/j.injury.2004.12.037. [DOI] [PubMed] [Google Scholar]
- 4.Mokart D, Capo C, Blache JL, Delpero JR, Houvenaeghel G, Martin C, Mege JL. Early post-operative compensatory anti-inflammatory response syndrome is associated with septic complications after major surgical trauma in patients with cancer. Br J Surg. 2002;89:1450–1456. doi: 10.1046/j.1365-2168.2002.02218.x. [DOI] [PubMed] [Google Scholar]
- 5.Bone RC. Sir Isaac Newton, sepsis and CARS. Crit Care Med. 1996;24:1125–1128. doi: 10.1097/00003246-199607000-00010. [DOI] [PubMed] [Google Scholar]
- 6.Cohen J. The immunopathogenesis of sepsis. Nature. 2002;420:885–891. doi: 10.1038/nature01326. [DOI] [PubMed] [Google Scholar]
- 7.Schinkel C, Sendtner R, Zimmer S, Faist E. Functional analysis of monocyte subsets in surgical sepsis. J Trauma. 1998;44:743–748. doi: 10.1097/00005373-199805000-00001. [DOI] [PubMed] [Google Scholar]
- 8.Volk HD, Reinke P, Krausch D, Zuckermann H, Asadullah K, Muller JM, Docke WD, Kox WJ. Monocyte deactivation – rationale for a new therapeutic strategy in sepsis. Intensive Care Med. 1996;22:S474–S481. doi: 10.1007/BF01743727. [DOI] [PubMed] [Google Scholar]
- 9.Hershman MJ, Cheadle WG, Wellhausen SR, Davidson PF, Polk HC., Jr Monocyte HLA-DR antigen expression characterizes clinical outcome in the trauma patient. Br J Surg. 1990;77:204–207. doi: 10.1002/bjs.1800770225. [DOI] [PubMed] [Google Scholar]
- 10.Polk HC, Jr, George CD, Wellhausen SR, Cost K, Davidson PR, Regan MP, Borzotta AP. A systematic study of host defense processes in badly injured patients. Ann Surg. 1986;204:282–299. [PMC free article] [PubMed] [Google Scholar]
- 11.Livingston DH, Appel SH, Wellhausen SR, Sonnenfeld G, Polk HC., Jr Depressed interferon gamma production and monocyte HLA-DR expression after severe injury. Arch Surg. 1988;123:1309–1312. doi: 10.1001/archsurg.1988.01400350023002. [DOI] [PubMed] [Google Scholar]
- 12.Ogata M, Okamoto K, Kohriyama K, Kawasaki T, Itoh H, Shigematsu A. Role of interleukin-10 on hyporesponsiveness of endotoxin during surgery. Crit Care Med. 2000;28:3166–3170. doi: 10.1097/00003246-200009000-00007. [DOI] [PubMed] [Google Scholar]
- 13.Gordon S. Alternative activation of macrophages. Nat Rev Immunol. 2002;3:23–35. doi: 10.1038/nri978. [DOI] [PubMed] [Google Scholar]
- 14.Strassmann G, Patil-Koota V, Finkelman F, Fong M, Kambayashi T. Evidence for the involvement of interleukin-10 in the differential deactivation of murine peritoneal macrophages by prostaglandin E2. J Exp Med. 1994;180:2365–2370. doi: 10.1084/jem.180.6.2365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Klava A, Windsor AC, Farmery SM, Woodhouse LF, Reynolds JV, Ramsden CW, Boylston AW, Guillou PJ. Interleukin-10. A role in the development of postoperative immunosuppression. Arch Surg. 1997;132:425–429. doi: 10.1001/archsurg.1997.01430280099016. [DOI] [PubMed] [Google Scholar]
- 16.Burt TD, Seu L, Mold JE, Kappas A, McCune JM. Naïve T cells are activated and proliferate in response to Heme oxygenase-1 inhibitor tin mesoporphyrin. J Immuno. 2010;185:5279–5288. doi: 10.4049/jimmunol.0903127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Howard MS, Scalea T, Paskamik A, Yang B. Superoxide dismutase prevents hyptotension after hemorrhagic shock and aortic cross clamping. Am J Med Sci. 1996;312:155–159. doi: 10.1097/00000441-199610000-00002. [DOI] [PubMed] [Google Scholar]
- 18.Kato H, Amersi F, Buelow R, Melinek J, Coito AJ, Ke B, Busuttil RW, Kupiec-Weglinksi JW. Heme-oxygenase-1 overexpression protects rat livers from ischemia/reperfusion injury with extended cold preservation. Am J Transplant. 2001;1:121–128. [PubMed] [Google Scholar]
- 19.Tamion F, Richard V, Renet S, Thuillez C. Protective effects of heme-oxygenase expression against endotoxic shock: Inhibition of tumor necrosis factor-α and augmentation of interleukin-10. J Trauma. 2006;61:1078–1084. doi: 10.1097/01.ta.0000239359.41464.ef. [DOI] [PubMed] [Google Scholar]
- 20.Lee TS, Chau LY. Heme oxygenase-1 mediates the anti-inflammatory effect of interleukin-10 in mice. Nat Med. 2002;8:240–246. doi: 10.1038/nm0302-240. [DOI] [PubMed] [Google Scholar]
- 21.Mohri T, Ogura H, Koh T, Fujita K, Sumi Y, Yoshiya K, Matsushima A, Hosotsubo H, Kuwagata Y, Tanaka H, Shimazu T, Sugimoto H. Enhanced expression of intracellular heme oxygenase-1 in deactivated monocytes from patients with severe systemic inflammatory response syndrome. J Trauma. 2006;61:616–623. doi: 10.1097/01.ta.0000238228.67894.d7. discussion 623. [DOI] [PubMed] [Google Scholar]
- 22.Meisel C, Schefold JC, Pschowski R, Baumann T, Hetzger K, Gregor J, Weber-Carstens S, Hasper D, Keh D, Zuckermann H, Reinke P, Volk HD. Granulocyte-macrophage colony-stimulating factor to reverse sepsis-associated immunosuppression: a double-blind, randomized, placebo-controlled multicenter trial. Am J Respir Crit Care Med. 2009;180:640–648. doi: 10.1164/rccm.200903-0363OC. [DOI] [PubMed] [Google Scholar]
- 23.Lendemans S, Kreuzfelder E, Waydhas C, Nast-Kolb D, Flohe S. Clinical course and prognostic significance of immunological and functional parameters after severe trauma. Unfallchirug. 2004;107:203–210. doi: 10.1007/s00113-004-0729-7. [DOI] [PubMed] [Google Scholar]
- 24.Flohe S, Flohe B, Schade FU, Waydhas C. Immune response of severely injured patients – the influence of surgical intervention and therapeutic impact. Langenbacks Arch Surg. 2007;392:639–648. doi: 10.1007/s00423-007-0203-4. [DOI] [PubMed] [Google Scholar]
- 25.Fumeaux T, Pugin J. Role of interleukin-10 in the intracellular sequestration of human leukocyte antigen-DR in monocytes during septic shock. Am J Respir Crit Med. 2002;166:1475–1482. doi: 10.1164/rccm.200203-217OC. [DOI] [PubMed] [Google Scholar]
- 26.Randow F, Syrbe U, Meisel C, Krausch D, Zuckermann H, Platzer C, Volk HD. Mechanism of endotoxin densitization: involvement of interleukin-10 and transforming growth factor-beta. J Exp Med. 181:1887–1892. doi: 10.1084/jem.181.5.1887. [DOI] [PMC free article] [PubMed] [Google Scholar]