Abstract
Objective
The objective was to determine the efficacy of an algorithim containing aspart dosed according to carbohydrate intake and one of 3 initial doses of detemir in stable cardiac surgery patients requiring intravenous (IV) insulin.
Methods
Patients were extubated, off pressors and otherwise stable, requiring at least 1 unit/hr of IV insulin at least 48 hours following surgery. Subjects were randomized to once daily detemir at 50, 65, or 80% of IV basal insulin requirements and received aspart according to carbohydrate intake. The dose of detemir was adjusted daily over 72 hours.
Results
The number of patients with an initial morning glucose 80–130 mg/dl was 36, 63, and 56% of patients at the 50, 65, and 80% doses (p=0.12) (n=82). However, the mean overall glucose at 24 and 72 hours was similar between groups, and 86, 93, and 92% achieved a mean glucose 80–180 mg/dl at 72 hours (p=0.60). Hypoglycemia (<65 mg/dl) only occurred in the 65% (21%) and 80% (12%) groups over the first 72 hours (p=0.02 in the 50% compared to the 65 and 80% groups combined) with one event <40 mg/dl in the 80% group. There was no loss of glycemic control by the end of the once daily dosing interval.
Conclusions
Glycemic targets can be achieved without hypoglycemia by 72 hours in most cardiac surgery patients requiring IV insulin with a regimen containing an initial detemir dose of 50% of basal IV insulin requirements and prandial and supplemental insulin.
BACKGROUND
Glycemic control with intravenous (IV) insulin infusions is known to improve morbidity and mortality post-cardiothoracic surgery (1), and insulin infusions are now considered routine in this setting (2). However, there are little data guiding providers on subsequent care post-infusion, particularly in the post-cardiac surgery patient (2).
One approach is to administer long-acting basal insulin at a dose of 80% of the predicted 24 hour requirements in patients who are receiving minimal exogenous carbohydrate exposure (2–4). However, such an approach may not be applicable to patients whose severity of illness is changing quickly, as is the case in the cardiac surgery population. Furthermore, the approach may not be appropriate for patients without pre-existing evidence of diabetes. In the cardiac surgery population, a large proportion of patients require IV insulin, even where no previous diagnosis of diabetes exists (5). Finally, some experts recommend continuation of the infusion for 3 days postoperatively (1), and many patients are beginning to eat by the first postoperative day. Even the most sophisticated insulin infusion algorithms are perturbed in patients that are eating (6), making it difficult to adequately calculate true basal insulin requirements. One potential solution to this problem is to administer subcutaneous rapid acting insulin, ideally dosed according to carbohydrate intake, in patients who are eating while receiving an insulin infusion. The objective of this study is to determine the efficacy of an algorithm that contains one of three initial subcutaneous doses of detemir, and flexible prandial and supplemental insulin aspart in cardiac surgical patients who are being transitioning off of an IV insulin infusion.
RESEARCH DESIGN AND METHODS
Patients
Patients were eligible if they were requiring at least 1 unit/hour of IV insulin 48 hours following cardiac surgery and were no longer requiring vasopressors or mechanical ventilation. Patients receiving glucocorticoids or enteral or parenteral nutrition were excluded. Other exclusion criteria included pregnancy, end-stage kidney or liver disease, or inability to give consent in English. The protocol was approved by the study institution’s Institutional Review Board and all patients signed informed consent.
Procedures
All patients undergoing cardiac surgery who develop hyperglycemia (two consecutive glucose measurements >150 mg/dl perioperatively) at the study institution are placed on a standardized, hospital-wide, nursing-run insulin infusion algorithm (7) that is continued for a minimum of 48 hours postoperatively. The glucose target range for the infusion algorithm is 100–150 mg/dl.
For the initial dose of detemir, patients were randomly assigned to one of 3 groups and calculated based upon 50%, 65%, or 80% of projected basal IV insulin requirements. The projected total daily basal need was calculated from the average infusion rate in the previous 8 hours (from 9 AM to 5 PM) multiplied by 3. If the insulin infusion was unstable (change greater than 2 unit per hour) in the preceding 6 hours prior to discontinuation, the dose was calculated based upon the 24 hour insulin requirement. Patients received their first dose of detemir at 6 PM, once daily, and the infusion was continued for four additional hours.
Glucose was monitored at a minimum of before meals and at bedtime using the Accuchek Inform® glucometer following cessation of the infusion.
Subsequent doses of detemir were titrated upwards or downwards as needed according to a fasting glucose target of 80–130 mg/dL. The detemir was reduced 20% for any glucose <80 mg/dl. On day 1, the dose was increased 5, 10, 15, or 20% for AM glucose greater than 150, 180, 200, or 250 mg/dl respectively. On days 2 and 3, the dose was increased 5, 10, 15, or 20% for a fasting glucose greater than 130, 150, 200, or 250 mg/dl respectively. The patient could be reverted to the IV insulin infusion for persistent hyperglycemia >200 mg/dl.
A simple ordering system for administration of rapid acting insulin analogues according to carbohydrate intake was implemented at the study institution in 2006. All nurses are trained to administer prandial insulin according to carbohydrate intake, using nutrition information that accompanies all meal trays. The carbohydrate counting technique has become the most common method of providing prandial insulin at the study institution. During the study, patients received subcutaneous insulin aspart at 1 unit/10 grams of carbohydrates as soon as a diet was ordered, even while receiving IV insulin therapy. Patients with very large basal insulin requirements (>70 units) received 1 unit/5 grams of carbohydrate intake. Prandial insulin overlap was intended to allow more precise calculation of basal insulin needs. Patients received a supplemental correction factor of 1 unit of aspart per 25 mg/dl increment of glucose above 150 mg/dl. The aspart was continued throughout the first 72 hours of the study without adjustment.
Statistical Analysis
Outcomes of interest included morning glucose and mean glucose, % of patients meeting morning glucose target 80–130 mg/dl or mean glucose target 80–180 mg/dl, and hypoglycemia (defined as any blood glucose <65 mg/dl).
The sample size of 25 in each group was estimated to provide 79% power to detect a difference in means, calculated by one-way analysis of variance, assuming the SD is 15 mg/dL. Patients were analyzed according to respective groups. Differences between groups were determined with the ANOVA or X2 test as appropriate. For hypoglycemia, the 65 and 80% groups were collapsed in order to compare to the 50% group using Fisher’s exact test due to insufficient cell counts. A linear mixed effect model, incorporating repeating measurements for each patient was applied, with treatment group as the main fixed effect and diabetes (defined as known diabetes or HbA1c >6.5%) as the random effect. Values were reported as mean +/− SD unless otherwise stated. P-values less than 0.05 were considered statistically significant. Analyses were performed using JMP8.0 software.
RESULTS
A total of 91 patients consented for the study. Data was available for 82 patients due to withdrawal of consent on one patient, receipt of glargine prior to dose of detemir in two patients, and the remainder no longer required at least 1 unit/hr of insulin following consent (6 patients). Patient characteristics are shown in table 1, and were generally evenly distributed among groups. Coronary artery bypass grafting was the most frequent reason for surgery (66%) and 70% had a diagnosis of type 2 diabetes at admission.
Table 1.
Patient Characteristics
| Total N=82 |
50% N=28 |
65% N=29 |
80% N=25 |
P-value | |
|---|---|---|---|---|---|
| African American* | 6 (7.3%) | 1 (3.6%) | 3 (10.3%) | 2 (8.0%) | 0.66 |
| White American | 76 (93%) | 27 (96%) | 26 (90%) | 23 (92%) | |
| Female | 17 (21%) | 5 (18%) | 4 (14%) | 8 (32%) | 0.23 |
| Age (years) | 59 (8.4) | 58 (8.0) | 57 (9.1) | 61 (7.5) | 0.13 |
| BMI (kg/m2) | 33.2 (6.9) | 32.2 (7.9) | 34.1 (7.0) | 33.2 (5.6) | 0.59 |
| Time to extubation (days) | 1.3 (0.78) | 1.3 (0.76) | 1.1 (0.44) | 1.5 (1.1) | 0.30 |
| Days on pressors | 1.1 (0.80) | 0.82 (0.61) | 1.2 (0.77) | 1.2 (0.99) | 0.15 |
| Creatinine (mg/dl) | 0.99 (0.44) | 1.0 (0.41) | 0.90 (0.25) | 1.1 (0.6) | 0.24 |
| Insulin (admission) | 28 (34%) | 10 (36%) | 10 (35%) | 9 (32%) | 0.96 |
| Any glucose-lowering drug (admission) | 56 (68%) | 19 (68%) | 18 (62%) | 19 (76%) | 0.55 |
| Indication for surgery | |||||
| Coronary artery bypass surgery | 54 (66%) | 19 (68%) | 18 (62%) | 17 (68%) | 0.87 |
| Valve | 23 (28%) | 10 (36%) | 9 (31%) | 4 (16%) | 0.25 |
| Aneurysm/dissection | 5 (6.1%) | 1 (3.6%) | 3 (10%) | 1 (4%) | 0.49 |
| Other | 11 (13%) | 4 (14%) | 5 (17%) | 2 (8%) | 0.60 |
| Coronary artery disease | 61 (77%) | 22 (79%) | 20 (71%) | 19 (83%) | 0.62 |
| Diabetes at admission | 57 (70%) | 19 (68%) | 18 (62%) | 20 (80%) | 0.35 |
| Retinopathy | 3 (3.9%) | 3 (11%) | 0 | 0 | 0.05 |
| Nephropathy | 3 (3.8%) | 2 (7.4%) | 0 | 1 (4.2%) | 0.35 |
| Neuropathy | 12 (15%) | 4 (15%) | 4 (14%) | 4 (16%) | 0.98 |
| Hypertension | 70 (86%) | 25 (89%) | 22 (79%) | 23 (92%) | 0.31 |
| Hyperlipidemia | 65 (82%) | 23 (85%) | 20 (74%) | 22 (88%) | 0.37 |
Data are presented as mean (standard deviation) or number (%).
The 65% and 80% groups were collapsed and compared to the 50% group using Fisher’s exact test for the race variable due to insufficient cell counts.
Study outcomes are summarized in table 2. The initial mean dose of detemir was 33, 32, and 41 units (p=0.16). The final mean 6 hour IV insulin infusion requirement, total daily insulin dose at 24 hours, and overall basal insulin as a % of total insulin was similar. Three patients (2 patients in the 50% group and 1 patient in the 80% group) reverted back to the insulin drip due to persistent hyperglycemia.
Table 2.
Study Data
| Total N=82 |
50% N=28 |
65% N=29 |
80% N=25 |
P-value | |
|---|---|---|---|---|---|
| Final 6 hour IV insulin dose (units) | 14.2 (11.5) | 17.0 (17) | 12.7 (7.4) | 12.8 (7.6) | 0.28 |
| Initial Detemir dose (units) | 35 (18) | 33 (19) | 32 (13) | 41 (21) | 0.17 |
| 24 hour total insulin (units) | 66 (27) | 66 (34) | 61 (18) | 71 (27) | 0.42 |
| 24 hour carbohydrate intake (grams) | 128 (77) | 119 (78) | 122 (70) | 144 (84) | 0.48 |
| Reversion to insulin infusion (%) | 3 (4%) | 2 (7%) | 0 | 1 (4%) | NA |
| % basal insulin overall | 0.68 (0.15) | 0.67 (0.16) | 0.68 (0.15) | 0.70 (0.15) | 0.76 |
| Morning glucose | |||||
| Day 1 Mean (mg/dl) | 139 (47) | 144 (48) | 141 (51) | 130 (31) | 0.49 |
| % at target (80–130 mg/dl) | 42 (51%) | 10 (36%) | 18 (63%) | 14 (56%) | 0.12 |
| 3-Day Mean (mg/dl) | 110 (20) | 105 (30) | 112 (24) | 0.72 | |
| % at target (80–130 mg/dl) | 53 (65%) | 18 (64%) | 20 (69%) | 15 (60%) | 0.79 |
| Total glucose | |||||
| Day 1 Mean (mg/dl) | 154 (44) | 154 (49) | 156 (45) | 150 (40) | 0.89 |
| % at target (80–180 mg/dl) | 60 (73%) | 21 (75%) | 20 (69%) | 19 (76%) | 0.70 |
| 3-day Mean (mg/dl) | 138 (31) | 142 (36) | 135 (29) | 138 (29) | 0.70 |
| % at target (80–180 mg/dl) | 74 (90%) | 24 (86%) | 27 (93%) | 23 (92%) | 0.60 |
| Hypoglycemia* | |||||
| Day 1 | 2 (2.4%) | 0 | 1 (3.5%) | 2 (8%) | NA |
| Overall | 9 (11%) | 0 | 6 (21%) | 3 (12%) | 0.02 |
| <40 mg/dl Overall | 1 (1.2%) | 0 | 0 | 1 (4%) | NA |
Data are presented as mean (standard deviation) or number (%).
The 65 and 80% groups are collapsed and compared to the 50% group using Fisher’s exact test for the race variable due to insufficient cell counts.
The mean morning glucose following cessation of the infusion was similar at 24 and 72 hours. After exclusion of 3 protocol violations, differences were not statistically significant. The percentage of patients reaching the morning glucose target 80–130 mg/dl was 36, 63, and 65% on day 1 (p=0.12) and 64, 69, and 60% over the first three days (p=0.34). Fasting hyperglycemia on day 1 was a significant predictor of hyperglycemia on day 2 (p=0.02) but not on day 3 (p=0.53). Specifically, 38% and 15% of patients who had fasting hyperglycemia on day 1 also had fasting hyperglycemia on day 2 and 3 respectively.
The overall (morning, lunch, dinner, and bed time) mean glucose was similar (p=0.89) at 24 hours and 72 hours (p=0.70) across groups. The percentage of patients at target 80–180 mg/dl over the 72 hour period was 86, 93, and 92% (p=0.60). Overall, there did not appear to be a loss of glycemic control throughout the once daily dosing period (figure 1).
Fig. 1. Duration of Detemir Effect.
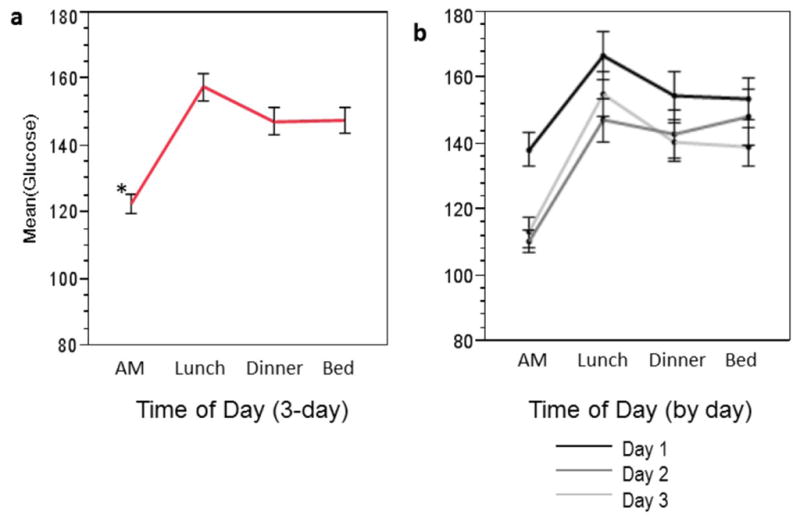
Each error bar is constructed using 1 standard error from the mean. The mean glucose pre-breakfast, lunch, dinner (pre-dose), and bedtime were 122 +/− 41, 158 +/− 58, 147 +/− 55, and 148 +/− 52 mg/dl. *p<0.0001 versus the pre-dinner time point, using Bonferroni’s correction.
Hypoglycemia only occurred in groups 2 (6 patients) and 3 (3 patients) over the first 3 days post-cessation of the drip (p=0.02 in the 50% group compared to the 65 and 80% groups combined). One patient in the 80% group with a history of diabetes had a severe low glucose (<40 mg/dl). It was determined that the etiology was related to inadequate carbohydrate coverage during the infusion, which resulted in an overestimation of basal insulin requirements. Hypoglycemia was most likely to occur in the morning compared to other times of the day (2.8 vs. 0.71% of all readings, p=0.03). Of the 12 patients who had a glucose <80 mg/dl in the 24 hours after drip cessation, 2 patients had hypoglycemia on the subsequent day.
The mixed model demonstrated that glucose decreased over time (p=0.0015), and patients without diabetes had significantly lower glucose levels than patients with diabetes (p<0.0001), but there was no effect of treatment group (p=0.32 for group 1 and p=0.69 for group 2, using group 3 as the referent).
There were no differences in morbidity, (including infection, length of hospital stay, transfusion, arrhythmia, acute renal failure, and inotrope use) or mortality among groups. There was one death due to arrhythmia in a patient in the 80% group that was not related to hypoglycemia.
CONCLUSIONS
In this study, cardiac surgery patients requiring significant amounts of IV insulin at 48 hours post-operatively had similar overall mean glucose levels by 24 and 72 hours using an algorithm that contains an initial dose of detemir at 50, 65, or 80% of basal IV insulin requirements, and flexible mealtime and supplemental insulin aspart. The study is limited by small sample size. However, important lessons can still be learned. Firstly, there was a difference in hypoglycemia between the 50% group and the other 2 groups, suggesting that the 50% dose is safer for most patients. An observational study of cardiac surgery patients also reported a low incidence of hypoglycemia (1%) over an unspecified time period using the 50% conversion factor (8). Secondly, the small, albeit nonsignificant difference in the initial dose of detemir and initial AM glucose is completely lost by day 2, suggesting that the initial dose will have little impact on overall glycemia. Thirdly, most patients reached the target glucose range regardless of the initial dose, highlighting the greater relative importance of titration and bolus insulin compared to the initial basal insulin dose on overall glycemia.
The reason for lack of difference between dosing groups could be related to supplemental and prandial insulin, but this was not clearly established in the analysis. Another study in an unselected hospitalized population also reported no difference in primary glycemic outcomes with the use of insulin glargine, administered at 80%, 60% or 40% of the total insulin drip requirements (9). The study included more heterogeneous disease states, and prandial insulin was not standardized. The investigators anticipated that controlling for these factors would improve precision. However, the results suggest that other factors, such as diabetes status and oral intake added more variability than expected. Other studies report difficulty maintaining glycemic control in patients who are eating while receiving an insulin infusion (6,10). This may add further complexity to the calculation of basal insulin requirements from insulin infusions. Finally, there may not be a practical difference between doses differing by only 15% among patients that are likely to have significant insulin resistance following cardiac surgery.
The comparatively high basal insulin doses and the observation that hypoglycemia was most likely to occur in the morning suggest that the prandial insulin dose could have been higher, particularly at breakfast. However, the prandial insulin dosing strategy was felt to be an acceptable starting point that would be easy to implement in order sets throughout a large institution with varying knowledge of diabetes. We ensured that this prandial insulin dose was not excessive by pre-specifying a minimum basal infusion requirement of 1 unit/hour. Post-breakfast hyperglycemia may be due to greater insulin resistance in the morning, meal-specific factors, or system factors (such as late insulin coverage at shift change). Post-breakfast hyperglycemia was also reported in both arms of a randomized study comparing NPH/regular and Detemir/Aspart among hospitalized patients (11). However, since the focus of the study was a simplified algorithm that emphasized basal insulin dosing, adjustments in prandial insulin were deferred until after the third dose of detemir. To our knowledge, this is the first study to report the implementation of prandial insulin dosed according to carbohydrate intake in hospitalized patients. Future studies will require more precise prandial insulin dosing and a higher proportion of prandial coverage.
Although patients were initially hospitalized in an intensive care setting, the glucose targets for the study are based upon guidelines for non-critically ill patients because patients were no longer critically ill (2). Daily dose titration was based upon previously published inpatient protocols (11,12). More aggressive upward dose titration may be beneficial in patients with hyperglycemia on the first day following cessation of the drip, since these patients were likely to be persistently hyperglycemic. In contrast, most cases of hypoglycemia were not predictable based upon glucose measurements from the previous day. Lastly, there was no evidence for loss of glycemic control by the end of each 24 hour dosing interval, providing support for once daily dosing, although the study was not specifically designed to address this question.
In conclusion, effective overall glycemic control can be achieved using an algorithmic approach in the majority of cardiac surgery patients with persistent IV insulin requirements, regardless of the initial basal insulin conversion factor. The 50% conversion factor is preferred in most patients due to less hypoglycemia, although patients with diabetes or residual IV insulin requirements may require a higher conversion factor or more aggressive titration.
Acknowledgments
This study was supported by an investigator-initiated grant from Novo Nordisk and by NIH grant number 1K23DK080891-02. The authors wish to thank the Ohio State University Clinical Research Center (supported by Award Number UL1RR025755 from the National Center for Research Resources) for assistance with data collection and analysis, and Rita Burris, study coordinator.
References
- 1.Furnary AP, Wu Y, Bookin SO. Effect of hyperglycemia and continuous intravenous insulin infusions on outcomes of cardiac surgical procedures: the Portland Diabetic Project. Endocr Pract. 2004;10(suppl 2):21–33. doi: 10.4158/EP.10.S2.21. [DOI] [PubMed] [Google Scholar]
- 2.Moghissi ES, Korytkowski MT, DiNardo M, et al. American Association of Clinical Endocrinologists and American Diabetes Association Consensus Statement on Inpatient Glycemic Control. Endocr Pract. 2009;15:353–369. doi: 10.4158/EP09102.RA. [DOI] [PubMed] [Google Scholar]
- 3.Bode BW, Braithwaite SS, Steed RD, Davidson PC. Intravenous insulin infusion therapy: indications, methods, and transition to subcutaneous insulin therapy. Endocr Pract. 2004;10 (suppl 2):71–80. doi: 10.4158/EP.10.S2.71. [DOI] [PubMed] [Google Scholar]
- 4.Furnary AP, Braithwaite SS. Effects of outcome on in-hospital transition from intravenous insulin infusion to subcutaneous therapy. Am J Cardiol. 2006;98:557–564. doi: 10.1016/j.amjcard.2006.02.065. [DOI] [PubMed] [Google Scholar]
- 5.Knapik P, Nadziakiewicz P, Urbanska E, Saucha W, Herdynska M, Zembala M. Cardiopulmonary bypass increases postoperative glycemia and insulin consumption after coronary surgery. Ann Thorac Surg. 2009;87:1859–1865. doi: 10.1016/j.athoracsur.2009.02.066. [DOI] [PubMed] [Google Scholar]
- 6.Davidson PC, Steed RD, Bode BW. Glucommander: a computer-directed intravenous insulin system shown to be safe, simple, and effective in 120,618 h of operation. Diabetes Care. 2005;28:2418–2423. doi: 10.2337/diacare.28.10.2418. [DOI] [PubMed] [Google Scholar]
- 7.Goldberg PA. Memoirs of a root canal salesman: the successful implementation of a hospital-wide intravenous insulin infusion protocol. Endocr Pract. 2006;12(suppl 3):79–85. doi: 10.4158/EP.12.S3.79. [DOI] [PubMed] [Google Scholar]
- 8.Olansky L, Sam S, Lober C, Yared JP, Hoogwerf B. Cleveland Clinic Cardiovascular Intensive Care Unit Insulin Conversion Protocol. J Diabetes Sci Technol. 2009;3:478–486. doi: 10.1177/193229680900300311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schmeltz LR, DeSantis AJ, Schmidt K, et al. Conversion of intravenous insulin infusions to subcutaneously administered insulin glargine in patients with hyperglycemia. Endocr Pract. 2006;12:641–650. doi: 10.4158/EP.12.6.641. [DOI] [PubMed] [Google Scholar]
- 10.Smiley D, Rhee M, Peng L, Roediger L, Mulligan P, Satterwhite L, Bowen P, Umpierrez GE. Safety and efficacy of continuous insulin infusion in noncritical care settings. J Hosp Med. 2010;5:212–217. doi: 10.1002/jhm.646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Umpierrez GE, Hor T, Smiley D, et al. Comparison of Inpatient Insulin Regimens with Detemir plus Aspart Versus Neutral Protamine Hagedorn plus Regular in Medical Patients with Type 2 Diabetes. J Clin Endocrinol Metab. 2009;94:564–569. doi: 10.1210/jc.2008-1441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Umpierrez GE, Smiley D, Zisman A, et al. Randomized Study of Basal-Bolus Insulin Therapy in the Inpatient Management of Patients With Type 2 Diabetes (RABBIT 2 Trial) Diabetes Care. 2007;30:2181–2186. doi: 10.2337/dc07-0295. [DOI] [PubMed] [Google Scholar]


