Abstract
During the last decade, Clostridium difficile infection (CDI) increased markedly inside as well as outside of hospitals. In association with the occurrence of new hypervirulent C. difficile strains, CDI became more important. Until now typing of C. difficile strains has been enabled by PCR-ribotyping. However, this method is restricted to specialized laboratories combined with high maintenance cost. Therefore, we tested MALDI-TOF mass spectrometry for typing of C. difficile to provide a fast method for surveillance of CDI. Using a standard set of 25 different C. difficile PCR ribotypes a database was made by different mass spectra recorded in the SARAMIS™ software (AnagnosTec, Zossen, Germany). The database was validated with 355 C. difficile strains belonging to 29 different PCR ribotypes collected prospectively from all submitted feces samples in 2009. The most frequent PCR ribotypes were type 001 (70%), 027 (4.8%) and 078/126 (4.7%). All three types were recognized by MALDI-TOF MS. We conclude that an extended MALDI-TOF system was capable to recognize specific markers for ribotypes 001, 027 and 078/126 allowing an effective identification of these strains.
Introduction
In recent years the number of Clostridium difficile infections (CDI) increased markedly in hospitals (hospital-acquired CDI) as well as outside hospitals (community-acquired CDI). Since 2003, hypervirulent strains of C. difficile belonging to PCR ribotypes 078 and 027 have been found in North America and in Western Europe. Due to higher toxin production of C. difficile 027, infections result in increased mortality [1].
Until now PCR-ribotyping has been the most effective and widely accepted molecular tool for typing of C. difficile strains. This provides the possibility of monitoring CDI outbreaks and the occurrence of hypervirulent strains.
Today matrix assisted laser desorption/ionization-time of flight mass spectroscopy (MALDI-TOF MS) provides an easy to handle system in order to identify different bacterial pathogens including C. difficile [2, 3]. MALDI-TOF MS is actually utilizable for determination of the bacterial species. There are only a few reports suggesting that MS can be applied for typing of MRSA [4, 5], C. difficile [6] Legionella [7, 8] and Pseudomonas [9].
We performed classical PCR-ribotyping of different C. difficile strains as well as typing via the extended SARAMIS™ MALDI-TOF system and found specific markers for ribotypes 001, 027 and 126/078 allowing clonal identification. Here we report on typing of the frequent C. difficile ribotypes 001, 027, 126 and 078 using MALDI-TOF MS.
Patients and methods
Laboratory and hospital settings
Synlab Medical Care Centre (Weiden, Germany) is located in a rural area in Southern Germany and analyses clinical samples from about 40 hospitals and more than 2,000 physicians serving outpatients in Northern Bavaria [10]. In this region, CDI is known as an increasing nosocomial problem with sporadic severe cases [10, 11, 12].
In the first half of 2009 we cultured approximately 500 C. difficile isolates from stool samples derived from in- and outpatients known with positive C. difficile toxin (Tcd) tests of stools.
Epidemiologic analysis of C. difficile in South Germany
Numbers of Tcd-positive stool samples and numbers of performed Tcd-tests were evaluated by the Hybase system (Cymed AG, Bochum, Germany) linked to the laboratory data system “promed open” (MCS, Eltville, Germany) as described before [10].
C. difficile toxin analysis and culture
Stool samples were collected during 2009 and tested for C. difficile. Tcd examination and culture of C. difficile were performed as previously described [11].
Identification of C. difficile was performed using the identification system for anaerobes “rapid ID 32 A system” (Biomérieux, Nürtingen, Germany) and by MALDI-TOF MS using the Bruker Daltonics microflex LT system (Bruker Daltonik GmbH, Bremen, Germany).
PCR ribotyping
After extraction of bacterial DNA, amplification reactions were performed with specific primers according to Bidet et al. [13]. In variation to this protocol, amplification reactions were performed in a 26.5 μl volume containing 15 μl H2Obidest., 2.6 μl 10× FastStart Taq Polymerase PCR-buffer + MgCl2 (Roche), 1.25 μl of each primer, 0.25 μl dNTP (20 mM, Pharmacia), 0.15 μl FastStart Taq DNA polymerase (5 U/μl, Roche) and 6 μl template DNA. PCR was performed in a thermal cycler, for 1 cycle of 360 s at 95°C for initial denaturation and 40 cycles of denaturation at 95°C for 30 s, annealing at 55°C for 30 s and extension at 72°C for 45 s. Final extension was carried out for 180 s at 72°C.
Detection of PCR products
Amplification products were separated using 8% polyacrylamide gels (for 2.5 h at 80 V, 21 mA in 1× TBE buffer). DNA banding patterns were visualized on a UV-transilluminator (Sigma) after staining for 15 min in ethidium bromide (20 μg/ml) and destaining for 5 min in H2Obidest. A molecular mass standard (DNA molecular weight marker VI, Roche) was included for normalization of the gel patterns. PCR ribotype profiles were compared to band patterns of 25 C. difficile reference strains (Cardiff-ECDC collection). C. difficile isolates that did not match one of the reference strains were sent for further typing to the German Reference Laboratory for Gastrointestinal Infections (University of Freiburg, Freiburg, Germany) or to Leiden University Medical Center (LUMC, Leiden, The Netherlands).
AXIMA@SARAMIS™ MALDI-TOF MS
MALDI-TOF MS for typing of C. difficile was performed in the laboratory of AnagnosTec Association for analytical biochemistry and diagnostics mbH (Zossen, Germany) according to the internal standard operating procedure.
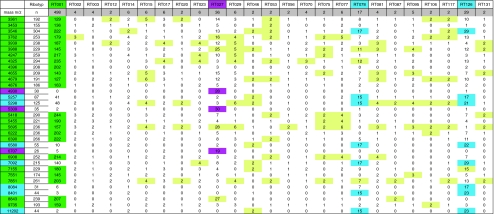
After anaerobic cultivation of C. difficile isolates and examination of the culture morphology and purity, respective cells were transferred from blood-agar plates after 24 h growth at 37°C to a free spot on the FlexiMass-DS target using the direct smear method. Then, 1.0 μl alpha-cyano-4-hydroxy cinamic acid (CHCA) matrix solution was added to the samples within less than two minutes after transferring of cells to the target. The matrix solution (saturated CHCA in 33% ethanol, 33% acetonitrile, 3% triflouro acetic acid and 31% water) extracted the proteins. Afterwards, the matrix was dried at room temperature on the bench (not under air flow). Finally, the target was inserted into the mass spectrometer and spectra were generated by double-measurement with adequate quality control criteria. Mass analyses were performed in the linear mode with delayed, positive ion delayed extraction (acceleration voltage: 20 kV) on an AXIMA Confidence (Shimadzu Europe, Duisburg, Germany) mass spectrometer equipped with a nitrogen laser (λ = 337 nm). Spectra were accumulated from 500 automatically acquired laser pulse cycles. All spectra were processed by the BioTech Launchpad software (Shimadzu Europe, Duisburg, Germany) with baseline correction, peak filtering and smoothing. The resulting peak lists were exported to the SARAMIS™ software package. The spectra were compared to the superspectra for species identification. All spectra were transferred into the database for cluster analysis and biomarker analysis (Table 1).
Table 1.
Number of tested C. difficile isolates (double measurement) and distribution of the detected masses
green specific masses of PCR-ribotype 001, magenta specific masses of PCR-ribotype 027, blue specific masses of PCR-ribotypes 078 and 126, yellow highly frequent masses among sporadic strains, RT ribotype, m/z mass-to-charge-relation
Results and discussion
Development of a MALDI-TOF database using ECDC-Brazier collection
Using the ECDC-Brazier collection encompassing 25 different PCR ribotypes we developed an initial reference database. After recording the mass spectra of each isolate by AXIMA@SARAMIS™ MALDI-TOF MS the data were stored along with the information of PCR ribotypes in a SARAMIS™ database (Spectral Archive and Microbial Identification System). An internal calculation algorithm (Superspectra™ function for selection of specific biomarkers) was applied to recognize if certain peaks or combinations of certain peaks might be associated with specific PCR ribotypes. After identification of frequently occurring masses indicative for a certain subtype, it was examined how many “indicative masses” have to be determined in combination with the system to automatically recognize the respective ribotype. Masses of ribosomal protein fragments observed by MALDI-TOF MS analyses applied to the 25 reference PCR ribotypes are summarized in Table 1. Using this database, four individual PCR ribotypes could be recognized (Table 1). Types other than 001, 027 and 078/126 could not be separated due to the lack of specific masses.
Results of the analysis of 355 German clinical isolates
Ribotyping was performed on 355 C. difficile isolates, collected in the first half of 2009, revealing 29 different ribotypes. At least 330 isolates were clinical isolates from patients. Our C. difficile collection from patients showed the following constitution:
Ribotype (RT) 001 (n = 248; 70.0%) was the most prevalent strain, followed by RT 027 (n = 17; 4.8%), RT 078, 015 (n = 7; 2.0%), RT 126 (n = 6; 2.7%), RT 095 (n = 5; 1.4%), RT 77 (n = 4; 1.1%), RT 003, 014, 017, 023, (n = 3; 0.9%), RT 002, 029, 045, 046, 070, 081, 149 (n = 2; 0.6%), and RT 005, 012, 020, 053, 056, 075, 087, 106, 117, 131 (n = 1; 0.3%).
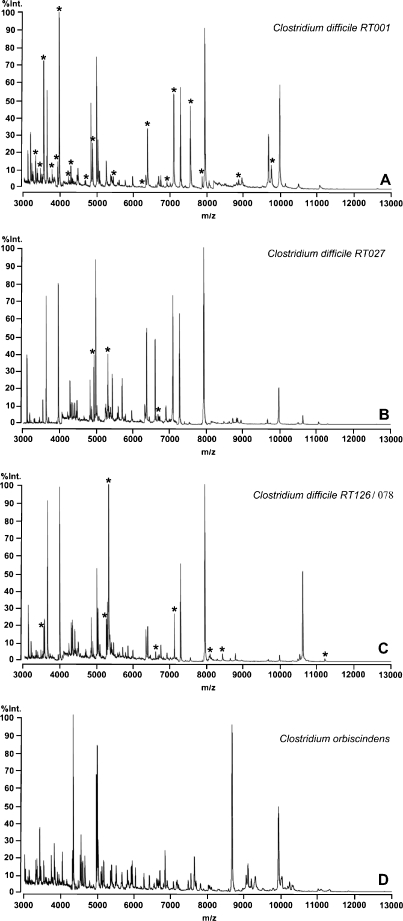
Figure 1 shows spectra representative for the most common strains 001, 027 and 126/078. Stars show indicative masses for the respective ribotype.
Fig. 1.
Overview of the mass spectra of C. difficile PCR-ribotypes 001 (A), 027 (B) and 126/078 (C) in comparison to the mass spectrum of Clostridium orbiscindens (D). Each peak represents the mass of an intact protein detected in the analyses while the height of the peak depends on the amount of proteins measured. Specific masses are indicated by stars. RT ribotype, Int. intensity, m/z mass-to-charge-relation
Validation of the MALDI-TOF MS database with 355 German isolates
On the basis of the specific masses found for ribotypes 001, 027 and 078/126 (see Table 1) we verified our database on the collected German isolates. While masses in yellow fields were not indicative for the given strains, masses in green fields were indicative for C. difficile 001 (n = 248), masses in magenta fields for C. difficile 027 (n = 17) and masses in blue fields for C. difficile 126/078 (n = 6/7).
All 001 isolates (n = 248) were recognized by the presence of 9–18 of the “green masses”. The definition of less than nine masses resulted such that strains other than 001 were erroneously matched to C. difficile 001 while determination of more than 18 masses resulted in disregarding 001 isolates.
To identify 027 isolates, definition of the three “magenta masses” was sufficient using ten strains of ribotypes 027. Using this setting C. difficile 027 provided from German Robert-Koch-Institute (n = 7) were also correctly identified.
Because of the high molecular similarity of ribotype 126 and the hypervirulent strain 078, only PCR-ribotyping was able to discriminate between these two strains. Generated mass spectra of ribotypes 126 and 078 showed that both strains share seven specific “blue masses”. Thus, typing of ribotypes 126 and 078 was sufficient for verification of the group of ribotypes 126/078.
Recently it was noticed that C. difficile strains 001, 017 and 027 were associated with fatal outcome in Germany [14]. As demonstrated here, strains 001 and 027 might be identified simultaneously to C. difficile identification when using MALDI-TOF MS. However, due to the low number of 017 isolates it was not possible to identify diagnostic peaks for this strain. Although this limitation counts for other sporadic strains found in our region, 98.9% of the isolates (268 of 271) belonging to types 001, 027 and 078/126 were identified correctly by MALDI-TOF MS.
However, the finding that most strains were isolated only sporadically (<10 isolates per strain per 6 months) implies that it was not possible to determine specific masses for these strains. On the other hand, in a population exhibiting a different composition of C. difficile strains, strains other than 001, 027 and 126 might be isolated in high numbers probably allowing strain identification by MALDI-TOF MS. Consequently, and besides technical conditions, identification of C. difficile strains by MALDI-TOFMS also depends on the prevalence of certain strains within a region.
However, one observation limits usage of our settings for application in other regions. Although the 027 isolates from the Robert-Koch-Institute were correctly identified when using 027 identification criteria (e.g. the three magenta masses), they also shared masses of ribotype 001. The 027 isolates (n = 7) from the Robert-Koch Institute derived from regions other than Northern Bavaria in Germany indicating regional specificity of C. difficile MALDI-TOF MS spectra. Hence, determination of regional settings for C. difficile strain identification by MALDI-TOF MS is essential. Furthermore, it also seems necessary to monitor strain identification by both MALDI-TOF MS and ribotyping over time to notice variation of spectra of C. difficile strains.
Analysing C. difficile by the Bruker Daltonics microflex LT system (Bruker Daltonik GmbH, Bremen, Germany) resulted in correct species identification. However, in our laboratory it was not possible to identify different ribotype strains. Recently, preliminary results of a study have been presented in which the Bruker system correctly recognized C. difficile PCR ribotypes 027 and 078 after composition of a new database encompassing at least ten spectra of each type [6].
We were able to type C. difficile isolates using an extended MALDI-TOF MS system and we identified specific markers for the most frequent C. difficile ribotype 001 in Southern Germany including the highly virulent strain NAP1/027. However, typing of other, more sporadic strains was not possible due to the lack of sufficient numbers of C. difficile isolates. In order to use this effective system for typing of more sporadic strains as well, future work will have to analyze more isolates of the respective ribotypes to generate a broad database of reference mass spectra. In the end, MALDI-typing will provide a suitable tool for C. difficile strains and surveillance of CDI.
Acknowledgments
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
References
- 1.McDonald LC, Killgore GE, Thompson A, Owens RC, Jr, Kazakova SV, Sambol SP, Johnson S, Gerding DN. An epidemic, toxin gene-variant strain of Clostridium difficile. N Engl J Med. 2005;353:2433–2441. doi: 10.1056/NEJMoa051590. [DOI] [PubMed] [Google Scholar]
- 2.van Veen SQ, Claas EC, Kuijper EJ. High-throughput identification of bacteria and yeast by matrix-assisted laser desorption ionization-time of flight mass spectrometry in conventional medical microbiology laboratories. J Clin Microbiol. 2010;48:900–907. doi: 10.1128/JCM.02071-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Veloo ACM, Knoester M, Degener JE, Kuijper EJ (2011) Comparison of two matrix-assisted laser desorption ionisation-time of flight mass spectrometry methods for the identification of clinically relevant anaerobic bacteria. Clin Microbiol Infect. doi:10.1111/j.1469-0691.2011.03467.x [DOI] [PubMed]
- 4.Jackson KA, Edwards-Jones V, Sutton CW, Fox AJ. Optimisation of intact cell MALDI method for fingerprinting of methicillin-resistant Staphylococcus aureus. J Microbiol Methods. 2005;62:273–284. doi: 10.1016/j.mimet.2005.04.015. [DOI] [PubMed] [Google Scholar]
- 5.Hall TA, Sampath R, Blyn LB, Ranken R, Ivy C, Melton R, Matthews H, White N, Li F, Harpin V, Ecker DJ, McDougal LK, Limbago B, Ross T, Wolk DM, Wysocki V, Carroll KC. Rapid molecular genotyping and clonal complex assignment of Staphylococcus aureus isolates by PCR coupled to electrospray ionization-mass spectrometry. J Clin Microbiol. 2009;47:1733–1741. doi: 10.1128/JCM.02175-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Knetsch CW, Corver J, Kuijper EJ (2010) Rapid typing of Clostridium difficile by Maldi-Tof MS. Abstract P15 of 3rd International Clostridium difficile symposium, September 22–24th, 2010, Bled, Slovenia
- 7.Fujinami Y, Kikkawa HS, Kurosaki Y, Sakurada K, Yoshino M, Yasuda J. Rapid discrimination of Legionella by matrix-assisted laser desorption ionization time-of-flight mass spectrometry. Microbiol Res. 2011;166:77–86. doi: 10.1016/j.micres.2010.02.005. [DOI] [PubMed] [Google Scholar]
- 8.Gaia V, Casati S, Tonolla M. Rapid identification of Legionella spp. By MALDI-TOF MS based protein mass fingerprinting. Syst Appl Microbiol. 2011;34:40–44. doi: 10.1016/j.syapm.2010.11.007. [DOI] [PubMed] [Google Scholar]
- 9.Hotta Y, Teramoto K, Sato H, Yoshikawa H, Hosoda A, Tamura H. Classification of genus Pseudomonas by MALDI-TOF MS based ribosomal protein coding in S10-spc-alpha operon at strain level. J Proteome Res. 2010;9:6722–6728. doi: 10.1021/pr100868d. [DOI] [PubMed] [Google Scholar]
- 10.Borgmann S, Jakobiak T, Gruber H, Schröder H, Sagel U. Prescription of broad-spectrum antibiotics to outpatients do not match increased prevalence and antibiotic resistance of respiratory pathogens in Bavaria. Pol J Microbiol. 2009;58:105–110. [PubMed] [Google Scholar]
- 11.Borgmann S, Kist M, Jakobiak T, Reil M, Scholz E, von Eichel-Streiber C, Gruber H, Brazier JS, Schulte B (2008) Increased number of Clostridium difficile infections and prevalence of Clostridium difficile PCR ribotype 001 in southern Germany. Euro Surveill 13(49):pii:19057 [PubMed]
- 12.Borgmann S, Jakobiak T, Gruber H, Reil M, Schroder H, Kist M, Sagel U (2010) Association of ciprofloxacin prescriptions to outpatients to Clostridium difficile infections. Euro Surveill 15(5):pii:19479 [PubMed]
- 13.Bidet P, Barbut F, Lalande V, Burghoffer B, Petit JC. Development of a new PCR-ribotyping method for Clostridium difficile based on ribosomal RNA gene sequencing. FEMS Microbiol Lett. 1999;175:261–266. doi: 10.1111/j.1574-6968.1999.tb13629.x. [DOI] [PubMed] [Google Scholar]
- 14.Arvand M, Hauri AM, Zaiss NH, Witte W, Bettge-Weller G (2009) Clostridium difficile ribotypes 001, 017, and 027 are associated with lethal C. difficile infection in Hesse, Germany. Euro Surveill 14(45):pii:19403 [DOI] [PubMed]