Abstract
Background
Around 308 million people worldwide are estimated to have impaired glucose tolerance (IGT); 25% to 75% of these will develop diabetes within a decade of initial diagnosis. At diagnosis, half will have tissue-related damage and all have an increased risk for coronary heart disease.
Objectives
The objective of this review was to assess the effects and safety of Chinese herbal medicines for the treatment of people with impaired glucose tolerance or impaired fasting glucose (IFG).
Search strategy
We searched the following databases: The Cochrane Library, PubMed, EMBASE, AMED, a range of Chinese language databases, SIGLE and databases of ongoing trials.
Selection criteria
Randomised clinical trials comparing Chinese herbal medicines with placebo, no treatment, pharmacological or non-pharmacological interventions in people with IGT or IFG were considered.
Data collection and analysis
Two authors independently extracted data. Trials were assessed for risk of bias against key criteria: random sequence generation, allocation concealment, blinding of participants, outcome assessors and intervention providers, incomplete outcome data, selective outcome reporting and other sources of bias.
Main results
This review examined 16 trials lasting four weeks to two years involving 1391 participants receiving 15 different Chinese herbal medicines in eight different comparisons. No trial reported on mortality, morbidity or costs. No serious adverse events like severe hypoglycaemia were observed. Meta-analysis of eight trials showed that those receiving Chinese herbal medicines combined with lifestyle modification were more than twice as likely to have their fasting plasma glucose levels return to normal levels (i.e. fasting plasma glucose <7.8 mmol/L and 2hr blood glucose <11.1 mmol/L) compared to lifestyle modification alone (RR 2.07; 95% confidence intervall (CI) 1.52 to 2.82). Those receiving Chinese herbs were less likely to progress to diabetes over the duration of the trial (RR 0.33; 95% CI 0.19 to 0.58). However, all trials had a considerable risk of bias and none of the specific herbal medicines comparison data was available from more than one study. Moreover, results could have been confounded by rates of natural reversion to normal glucose levels.
Authors’ conclusions
The positive evidence in favour of Chinese herbal medicines for the treatment of IGT or IFG is constrained by the following factors: lack of trials that tested the same herbal medicine, lack of details on co-interventions, unclear methods of randomisation, poor reporting and other risks of bias.
Plain language summary
Chinese herbal medicines for people with impaired glucose tolerance or impaired fasting blood glucose
Around 308 million people worldwide are reported to have ‘impaired glucose tolerance’. These individuals show higher than normal blood sugar (glucose) levels, but do not meet diagnostic criteria for having type 1 or type 2 diabetes. This may provide a window in which to prevent or delay the onset of diabetes and its complications like cardiovascular disease. Within a decade of the initial diagnosis ‘impared glucose tolerance’ 25% to 75% are estimated to progress to diabetes.
This review examined 16 randomised controlled trials of 15 different Chinese herbal medicines. The trials lasted from four weeks to two years (average nine months) and involved altogether 1391 participants. Death from any cause, diabetic complications and economic outcomes were not investigated. No serious adverse events were reported.
The available evidence suggests that Chinese herbal medicines are able to lower and normalise high blood glucose. Due to considerable distortions (bias) in the trials, further high-quality and rigorously evaluated studies are required before any conclusions can confidently be reached about the effects of Chinese herbal medicines for the treatment of impaired glucose tolerance and the delay of diabetes onset.
Background
Description of the condition
In 2007, an estimated 308 million had impaired glucose tolerance (IGT) or about 7.5% of the age group 20 to 79 years. More than 80% live in developing countries (IDF 2008). This is projected to rise to 418 million, or 8.1% by 2025. Around 10% of people with combined impaired fasting glucose (IFG) and IGT will progress to diabetes per year. The progression rate from isolated IGT to diabetes is estimated at 3.7% per year (Engberg 2009). The predicted cumulative 5 to 6 year incidence of development of type 2 diabetes for people with either IGT or IFG is 20% to 34%, those with both IGT and IFG have a progression rate of 38% to 65% (ADA 2003).
IGT and IFG are frequently present for many years until health checks reveal raised plasma glucose levels, or diabetic symptoms develop. By the time a diagnosis of type 2 diabetes is made around half of all people have diabetes related tissue damage. Even after diagnosis, management of type 2 diabetes is not easy. Achieving and maintaining normal glucose levels is difficult, leading to long-term complications. Coronary heart disease has been shown to be more prevalent in people with impaired glucose tolerance compared to those with normal glucose tolerance and is likely to develop before the onset of diabetes or symptoms (Haller 1998).
The concept of IGT was first introduced by the World Health Organisation (WHO) in 1979. Criteria for diagnosing IGT and IFG were revised by the WHO in 1999 (Unwin 2002). People with IGTshow abnormal fasting plasma glucose and abnormal two-hour post-load plasma glucose values. People with IFG only however, demonstrate an abnormal fasting plasma glucose. Currently, the criteria for IGT and IFG are as follows (plasma venous glucose concentrations):
IGT - fasting blood glucose less than 7.0 mmol/L and two-hour post-load blood glucose 7.8 to 11.0 mmol/L (WHO 1999);
IFG - fasting blood glucose 6.1 to 6.9 mmol/L (two-hour post-load blood glucose less than 7.8 mmol/L, if measured) (ADA 1999; WHO 1999). In 2003, the American Diabetes Association (ADA) recommended to change these criteria to 5.6 to 6.9 mmol/L (ADA 2003).
The pathophysiology of the progression to type 2 diabetes is complex and still not fully understood. The progression seems to be governed by two processes: 1) a decline in sensitivity to the action of insulin, and 2) dysfunction and eventual exhaustion of beta-cell function (Polonsky 1996). Beta-cell dysfunction starts some 10 to 12 years prior to the presentation of type 2 diabetes (Davies 2004). This provides a window of opportunity to prevent or delay development of type 2 diabetes, and potentially diminish the risk of cardiovascular and other complications.
‘Lifestyle’ modification is the ideal method of delaying or preventing diabetes as it also reduces cardiovascular risk profile (DPP Research GP 2002). A systematic review concluded that lifestyle education was clearly effective for reducing two-hour plasma glucose and the incidence of type 2 diabetes over one year (Yamaoka 2005). Not all individuals however, will be able to undertake the intensive lifestyle interventions prescribed in these trials. Long-term adherence to the interventions described is a potentially limiting factor (Padwal 2005).
Pharmacological interventions have been employed to delay or prevent the onset of diabetes. Several studies have measured the effects of various interventions in people with IGT on the development of type 2 diabetes mellitus. Therapy with metformin (a biguanide), troglitazone (a thiazolidinedione, see below), or acarbose (an alpha-glucosidase inhibitor) have reduced the progression of IGT to diabetes mellitus by 31%, 49% and 25%, respectively. Metformin is probably the most extensively used pharmacological treatment for people with prediabetes to date. It improves peripheral and liver sensitivity to insulin, reduces basal liver glucose production and increases insulin-stimulated uptake and utilisation of glucose by peripheral tissues (AHFS 1999). The ‘Diabetes Prevention Program’ found that while metformin reduced the incidence of diabetes by 31% compared to placebo, the efficacy of metformin was variable among subgroups (DPP Research GP 2002). Metformin is less effective in those over 60 years of age, those with a body mass index (BMI) less than 35 kg/m2, and those with a fasting plasma glucose (FPG) below 6.1 mmol/L. Gastrointestinal symptoms including diarrhoea, flatulence, nausea and vomiting occurred in 77 of every 100 persons on metformin in the study.
Troglitazone, which was withdrawn from the market due to an increased incidence of drug-induced hepatic damage, markedly reduced the incidence of diabetes during its limited period of use, but this action did not persist (Knowler 2005). Whether other thiazolidinedione drugs used for longer periods can safely prevent or delay diabetes remains to be determined.
The class of drugs called alpha-glucosidase inhibitors are also sometimes used where blood sugar levels are not being stabilized through diet and exercise, or where metformin is not suitable or tolerated. The main alpha-glucosidase inhibitor currently used is acarbose. Acarbose prevents the degradation of complex carbohydrates into glucose, the carbohydrates will remain in the intestine. In the colon, bacteria will digest the complex carbohydrates, thereby causing gastrointestinal side effects such as flatulence (78% of people) and diarrhoea (14% of people). A systematic review of acarbose in people with IGT found that the use of acarbose reduces the incidence of type 2 diabetes, but the effects on glycaemic control are limited (Van de Laar 2006).
Description of the intervention
Chinese herbal medicines include the use of plant, animal and mineral substances in preparations administered as pills, teas and powders. Chinese herbal medicines have long been used for the treatment of IGT and IFG and diabetes in China, Korea and Japan, with strong supportive anecdotal evidence for their efficacy (Liu 2004) and may offer a safe and effective alternative. These herbal medicines tend to be complex formulas combining two or more herbs.
How the intervention might work
Pharmacological studies of the Chinese herbal formulas for the treatment of diabetes indicate that the mechanisms of action of these interventions might be multifactorial. It has been suggested that herbs containing polysaccharides restore the function of the pancreatic tissues causing increased insulin output by the beta-cells. Other herbs have been thought to enhance the microcirculation, increase the availability of insulin and facilitate the metabolism in insulin dependent processes (Jia 2003; Yu 2006). Herbal formulas that exert such a combined effect on insulin and blood glucose control in people with diabetes have relevance to IGT and IFG.
Adverse effects of the intervention
The chronic nature of prediabetes and diabetes means that people are potentially on treatments for a long period of time which increases the likelihood of adverse effects (ADA 2003). Chinese herbal medicines have a long history of being used to treat diabetes and prediabetes in broad and varied population groups. As a consequence there is an accumulated knowledge of the safety in the use for many of the herbal substances. However, all medicinal agents have potentially unexpected effects including toxicity, and herbals are no different. Adverse effects of herbal medications may be intrinsic such as predictable toxicity, overdosage, interaction with other pharmaceutical or idiopathic (allergy, anaphylaxis etc). They may also be extrinsic, relating to misidentification, contamination, lack of standardisation and so on (Bensoussan 1996).
Why it is important to do this review
A Cochrane review of herbal medicines for type 2 diabetes reviewed 66 randomised controlled trials and demonstrated that some herbal medicines with hypoglycaemic effects may be beneficial in type 2 diabetes (Liu 2004). However, no systematic review on the efficacy of Chinese herbal medicines in people with IGT or IFG has been undertaken. In view of the high proportion of people with IGT that go on to develop overt diabetes, and potentially cardiovascular disease, there is considerable interest in exploring therapeutic approaches that will reduce the risk of diabetes in individuals with IGT with minimal or no adverse effects where lifestyle modifications have failed and pharmacological treatment is inappropriate.
Objectives
To assess the effects and safety of Chinese herbal medicines for the treatment of people with impaired glucose tolerance or impaired fasting glucose.
Methods
Criteria for considering studies for this review
Types of studies
Randomised clinical trials were included irrespective of blinding, publication status or language.
Types of participants
People with impaired glucose tolerance (IGT) or impaired fasting glucose (IFG), existing or newly diagnosed.
Types of interventions
Intervention
Chinese herbal medicines involving extracts from herbs, single or mixture herbal preparations regardless of their compositions or forms or Chinese herbal medicines combined with a pharmacological intervention.
Control
placebo;
no treatment;
pharmacological compounds (for example biguanides such as metformin, sulphonylureas);
non-pharmacological interventions (for example diet, exercise)
Co-interventions were allowed as long as all arms of the randomised trial received the same co-intervention(s). Only interventions performed for a minimum duration of four weeks were included.
Types of outcome measures
Primary outcomes
Secondary outcomes
morbidity related to impaired glucose metabolism, the metabolic syndrome or type 2 diabetes: vascular complications (angina pectoris, myocardial infarction, stroke, peripheral vascular disease, amputation), neuropathy, retinopathy, nephropathy, erectile dysfunction, hyperosmolar nonketotic dysregulation);
mortality: mortality related to impaired glucose metabolism, the metabolic syndrome or type 2 diabetes (death from myocardial infarction, stroke, renal disease, or sudden death, death from hyperosmolar nonketotic coma), death from any cause;
insulin: fasting and post-load insulin;
insulin sensitivity;
plasma lipids (triglycerides, total-, high-density lipoprotein (HDL)- and low-density lipoprotein (LDL)-cholesterol);
blood pressure (diastolic and systolic blood pressure);
body weight (or body mass index);
inflammatory markers (for example, C-reactive protein);
quality of life (using a validated instrument);
costs.
Covariates, effect modifiers and confounders
Compliance.
Timing of outcome measurement
Possible influence of treatment duration was addressed in a sensitivity analysis.
Search methods for identification of studies
Electronic searches
We searched the following sources for the identification of trials:
The Cochrane Library
PubMed (contains MEDLINE and a number of additional life science journals);
EMBASE;
Allied and Complementary Medicine Database (AMED);
Chinese Biomedical Literature Database (CBM);
Chinese Medical Current Contents (CMCC);
Traditional Chinese Medical Literature Analysis and Retrieval System (TCMLARS);
Chinese Dissertation Database (CDDB);
Chinese Academic Conference Papers (CACP);
China Medical Academic Conference (CMAC);
The System for Information on Grey Literature in Europe (SIGLE).
The Centralised Information Service For Complementary Medicine (CISCOM) was not searched as the database was not operating when the search was finished (February 2009).
Databases of ongoing trials:
Current Controlled Trials (http://www.controlled-trials.com - with links to other databases of ongoing trials);
UK National Research Register (http://www.update-software.com/National/nrr-frame.html);
USA - CenterWatch Clinical Trials Listing Service (http://www.CenterWatch.com/);
USA - National Institutes of Health (http://clinicalstudies.info.nih.gov/).
We combined three different search strategies as follows:
for IGT and IFG we used the strategy from a previous systematic review of western medicine for IGT and IFG (Van de Laar 2006);
for Chinese herbal medicines we employed strategies used for other Cochrane reviews of Chinese herbal medicines;
for controlled trials we used a sensitive validated search strategy (Robinson 2002).
All the above databases were searched from the available date of inception until the latest issue (Feb 2009). For a detailed MEDLINE search strategy please see under Appendix 1.
Searching other resources
The authors of significant publications or experts in the relevant field were contacted for potential studies. We telephoned authors who had published two or more papers on clinical trials of prediabetes (three authors). When we contacted authors to collect details on included and excluded studies they were asked if they had participated in any other clinical trials of Chinese herbal medicines and IGT. Details of contact are provided in the tables of included and excluded studies. Relevant pharmaceutical companies which produced relevant products, were to be checked and contacted. However none were contacted, the products on the market are aimed at type 2 diabetes not prediabetes.
We searched the reference lists of included trials to identify additional trials. Studies published in any language were included.
Data collection and analysis
Selection of studies
To determine the studies to be assessed further, two authors independently scanned the abstract, title or both sections of every record retrieved. All potentially relevant articles were investigated as full text. Where information was ambiguous or missing in the article the author was contacted where possible. If the author could not be contacted, the decision to include the trial was resolved by consensus. An adapted PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) (Liberati 2009) flow-chart of study selection is attached.
Dealing with duplicate publications
In the case of duplicate publications and companion papers of a primary study, we tried to maximise yield of information by simultaneous evaluation of all available data. In cases of doubt, the original publication (usually the oldest version) obtained priority.
Data extraction and management
For studies that fulfilled the inclusion criteria, two authors abstracted relevant population and intervention characteristics using standard data extraction templates (for details see Characteristics of included studies), with any disagreements resolved by discussion, or if required by a third party. Any relevant missing information on the trial was sought from the original author(s) of the article.
Assessment of risk of bias in included studies
Two authors independently assessed the risk of bias of each of the included studies against key criteria: random sequence generation; allocation concealment; blinding of participants, outcome assessors and intervention providers; incomplete outcome data; selective outcome reporting; and other sources of bias. Studies that did not adequately meet these criteria were considered at high risk of bias. These methods have been updated since the publication of the protocol for this review to reflect guidance from the Cochrane Collaboration (Higgins 2008).
Measures of treatment effect
Dichotomous data
Dichotomous data were expressed as relative risk (RR) ratios rather than odds ratios (OR). This method has been changed since the publication of the protocol to reflect the approach used by other studies in this modality of treatment. It is also a more easily understood statistic in presenting these outcomes.
Continuous data
Weighted mean differences (WMD) and 95% confidence intervals (CI) were calculated for continuous data using a random-effects model. A random-effects model was used in preference to a fixed-effect model due to the expected heterogeneity of the trials. The actual measure of effect of all continuous variables was the differences from baseline to endpoint. The standard deviations (SD) of these differences were essential for the data to be included in the meta-analysis. All SDs of the difference were reported, and it was not necessary to impute SDs.
Time-to-event data
We planned to summarise time-to-event data using use methods of survival analysis and express the treatment effect as ahazard ratio.
Unit of analysis issues
Data were summarised statistically if they were available, sufficiently similar and of sufficient quality. Statistical analysis was performed according to the statistical guidelines referenced in the newest version of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2008).
Dealing with missing data
Relevant missing data were obtained from authors, where possible. Important numerical data such as screened, eligible and randomised participants as well as intention-to-treat (ITT) and per-protocol (PP) population were evaluated. Drop-outs, misses to follow-up and withdrawn study participants were also investigated where possible. Issues of last-observation-carried-forward (LOCF), ITT and PP were critically appraised and compared to specification of primary outcome parameters and power calculation.
Assessment of heterogeneity
In the event of substantial clinical or methodological or statistical heterogeneity, study results were not combined in meta-analysis. Heterogeneity was identified by visual inspection of the forest plots, by using a standard χ2-test and a significance level of α= 0.1. Heterogeneity was also examined with I2 (Higgins 2002), where I2 values of 75% and more indicate a considerable level of heterogeneity (Higgins 2003). When heterogeneity was found, we attempted to determine potential reasons for it by examining individual study characteristics and those of subgroups of the main body of evidence.
Assessment of reporting biases
Funnel plots were used to assess for the potential existence of small study bias. There are a number of explanations for the asymmetry of a funnel plot. Therefore, we planned to carefully interpret results (Lau 2006).
Data synthesis
Data concerning details of study population, intervention and outcomes were extracted independently by two reviewers using a standard data extraction form. The standard data extraction form included at least the following items:
general information: published/unpublished, title, authors, source, contact address, country, urban/rural, language of publication, year of publication, duplicate publications, sponsoring, setting;
trial characteristics: design, duration, randomisation (and method), allocation concealment (and method), blinding (participants, people administering treatment, outcome assessors), check of blinding;
intervention(s): placebo included, intervention(s) (single herb or compound of herbs, dose, route, timing, mode of treatment, expertise of the practitioner), comparison intervention(s) (dose, route, timing), co-medication(s) (dose, route, timing);
participants: sampling (random / convenience), exclusion criteria, total number and number in comparison groups, sex, age, baseline characteristics, diagnostic criteria, duration of diabetes, similarity of groups at baseline (including any co-morbidity), assessment of compliance, withdrawals / losses to follow-up (reasons / description), subgroups;
outcomes: outcomes specified above, any other outcomes assessed, other events, length of follow-up, quality of reporting of outcomes;
results: for outcomes and times of assessment (including a measure of variation), if necessary converted to measures.
Subgroup analysis and investigation of heterogeneity
Subgroup analyses were to be performed if one of the primary outcome parameters demonstrated statistically significant differences between treatment groups. The following subgroup analyses were planned:
glycosylated haemoglobin A1c (HbA1c) level at baseline (subdivided into groups, based on data);
age (subdivided into groups, based on data);
gender;
body mass index (BMI) (subdivided into groups, based on data);
duration of intervention (subdivided into groups, based on data.
Sensitivity analysis
We planned to perform sensitivity analyses in order to explore the influence of the following factors on effect size:
repeating the analysis excluding unpublished studies;
repeating the analysis taking account of risk of bias, as specified above;
repeating the analysis excluding any very long or large studies to establish how much they dominate the results;
repeating the analysis excluding studies using the following filters: diagnostic criteria, language of publication, source of funding (industry versus other), country.
The robustness of the results was also to be tested by repeating the analysis using different measures of effects size (relative risk, odds ratio etc.) and different statistical models (fixed- and random-effects models).
Results
Description of studies
Results of the search
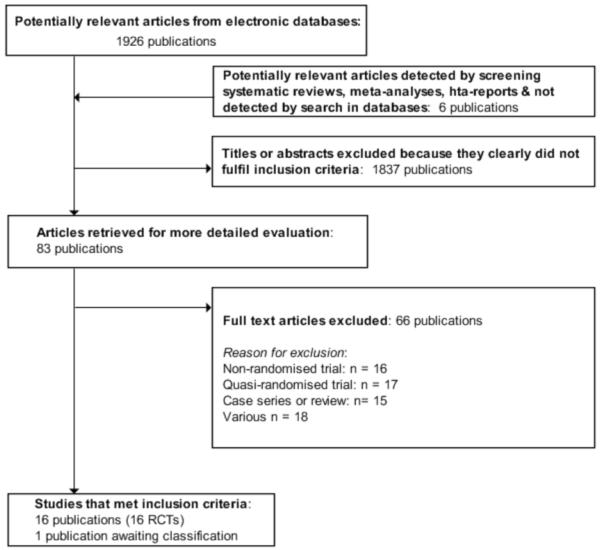
The initial search identified 1926 records, from these 83 full papers were identified for further examination. If there was unclear information in the title or abstract, the full article was retrieved for clarification. The other studies were excluded on the basis of their abstracts because they were not relevant to the question under study, obvious duplicates were removed. After screening the full text of the 83 selected papers, 16 studies finally met the inclusion criteria (Figure 1), and one study is awaiting classification (Liu DQ 2007).
Figure 1.
PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) flow-chart of study selection
Included studies
There were 16 randomised clinical trials included in this review. They reported random allocations of participants with impaired glucose tolerance (IGT) to Chinese herbal medicines versus controls. Nine studies randomised participants to receive Chinese herbal medicines with a co-intervention of lifestyle modification versus a control of lifestyle modification alone (Fan GJ 2004; Hao AZ 2004; Li CP 2004; Tang QZ 2007; Yang B 2004; Yao Z 2001; Wei AS 2001; Zeng YH 2006; Zhou DY 2003). Three trials compared Chinese herbal medicines with placebo (Hioki C 2004; Fang ZH 2007; Wang BQ 2008), two of these trials included a co-intervention of a lifestyle intervention. Two trials compared Chinese herbal medicines with the biguanide metformin (Qu LX 2002; Shi J 2005). There was one three-arm trial comparing Jian pi zhi shen huo xue with lifestyle modification versus the alpha-glucosidase inhibitor, acarbose versus lifestyle modification alone (Tang QZ 2007). One trial compared Jinqi jiangtang and basic IGT education with basic IGT education alone (Wang YX 2005). Finally, there was a single trial that compared Yi qi yang yin huo xue with a co-intervention of an antihypertensive medication (vasodilator) versus the same antihypertensive medication alone (Lu X 2005). The details of the trials are listed under ‘Characteristics of included studies’.
The duration of the trials ranged from four weeks to two years (mean 9.3 months). Of the 16 included trials, eight trials ran for over 12 months (Fan GJ 2004; Li CP 2004; Tang QZ 2007; Wang BQ 2008; Wang YX 2005; Wei AS 2001; Zeng YH 2006; Zhou DY 2003). There were three large trials (more than 100 participants) that ran for over 12 months (Tang QZ 2007; Wang YX 2005; Zeng YH 2006).
All trials had a parallel design. Fourteen trials were two-armed and two were three-armed (Tang QZ 2007; Zeng YH 2006). The third control arm of the Zeng YH 2006 trial was not included in the comparisons or this result section. The control arm had a simple education intervention differing to the co-intervention of the herbal medicine. This comparison did not meet the inclusion criteria set for this review. Data from this arm are not included in the results section.
The trials were all conducted from 2001 onwards. One study is still awaiting classification.
Participants
The sixteen trials included 1391 participants. The average number of participants in the trials was 87, ranging from 42 to 168.
Mean age of the participants was 52, ranging from 44 to 66 years (missing data from Fang ZH 2007). This is consistent with population prevalence which shows that the 40 to 59 years age group currently has the greatest number of persons with impaired glucose tolerance and diabetes (IDF 2008).
There were 719 males and 659 females (no gender data available on 13 withdrawals). One trial (Hioki C 2004) chose to enrol female participants only (n = 81). The aim of this study was primarily to assess insulin resistance and visceral adiposity. One other trial (Hao AZ 2004) had an over-representation of males to females (121 : 47). The overall ratio of the trials is not consistent with the population prevalence of diabetes and prediabetes which shows a female predominance with females ranging 10% higher than males for diabetes, and females ranging 20% higher than males for impaired glucose tolerance (IDF 2008).
Two trials enrolled obese or overweight participants only (Hioki C 2004; Shi J 2005). Three studies (Yang B 2004; Zeng YH 2006; Zhou DY 2003) included participants with a mean body mass index (BMI) in the healthy weight range (ranging from 20.8 to 24.3). Five studies (Tang QZ 2007; Wang BQ 2008; Wang YX 2005; Wei AS 2001; Yao Z 2001) included participants with a mean BMI in the obese range (ranging from 25.3 to 27.2).
One trial enrolled hypertensive participants only (Qu LX 2002).
With regard to ethnicity, all trial participants, with the exception of one trial which involved Japanese women (Hioki C 2004), were Chinese.
All trials included participants with impaired glucose tolerance, only with the exception of one trial (Wang YX 2005) which involved those with impaired fasting tolerance (IFG) in addition to those with IGT.
All trials recruited outpatients from hospitals or clinics.
For an overview of the study populations of the trials, like randomised individuals, intention-to-treat populations and participants finishing the study, please refer to Table 1.
Diagnosis
The diagnostic criteria used in the trials were mainly based on the WHO criteria. Eight trials used WHO 1999 and three trials used WHO 1985 criteria (Yao Z 2001; Zeng YH 2006; Zhou DY 2003) . Four trials used the American Diabetes Association (ADA 1997) criteria (Fang ZH 2007; Lu X 2005; Qu LX 2002; ; Yang B 2004). One trial used a combination of the WHO 1999 and the ADA 1997 criteria (Wang YX 2005). The ADA criteria rely on a fasting plasma glucose level equal or greater than 6.1 mmol/L and less than 7.0 mmol/L. This differs from the WHO 1999 criteria which uses both a fasting plasma glucose less than 7.0 mmol/L AND a 2hr blood glucose after oral glucose tolerance test (oGTT) equal or greater than 7.8 and less than 11.0. The WHO 1985 criteria had a slightly higher range for including people as IGT - fasting plasma glucose less than 7.8 mmol/L. The different diagnostic criteria were subjected to a sensitivity analysis but no significant differences were detected (see Appendix 2).
Interventions
Fifteen Chinese herbal medicine interventions were examined in the 16 randomised trials (see Characteristics of included studies). Chinese herbal medicine Jinqi jiangtang was tested in two trials (Wang YX 2005; Zhou DY 2003). The trials all tested compounds of complex herbal formulas (see Table 2 ‘Preparation and compositions of the Chinese herbal medicines’). Preparations of herbs were as decoctions, pills, capsules or granules.
The herbal composition of the interventions varied. However, some individual herbs were prevalent in the different formulas. Astragalus membranecus was present in 10 of the 15 interventions for which the ingredients were known. Where Astragalus membranecus was a major part of the formula (either in amounts equal or greater than 20 g or only one of six herbs) it was analysed in a separate analysis. Other commonly used herbs included Shan yao (eight of the 15 interventions) and Ge gen (four of the 15 interventions).
The 16 trials had eight distinct comparisons:
nine trials compared nine Chinese herbal medicines with lifestyle modification as a control and co-intervention (Jiangtang bushen decoction (Fan GJ 2004), Jinqi jiangtang pills (Zhou DY 2003), Liu wei di huang wan pills (Zeng YH 2006), Qimai jiangtang yin decoction (Li CP 2004), Tang kang yin decoction (Yang B 2004), Tang Heng I ( Yao Z 2001), Xiaoke huayu tablets (Hao AZ 2004), Xiaoke yuye decoction (Wei AS 2001) and Jian pi zhi shen huo xue (Tang QZ 2007);
two trials compared Chinese herbal formulas with a placebo with lifestyle modification as a co-intervention: Bofu-tsusho-san (Hioki C 2004) and Dan zhi jiang tang jiao (Fang ZH 2007);
one trial compared Qiwei tangping capsules with a placebo (Wang BQ 2008);
one trial compared Tang ping san with metformin, with a lifestyle modification as co-intervention (Qu LX 2002);
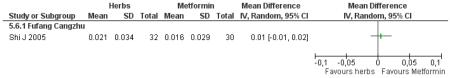
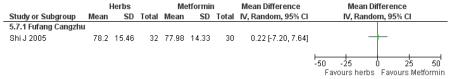
one trial compared Fufang cangzhu decoction with metformin (Shi J 2005);
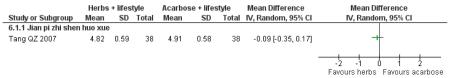
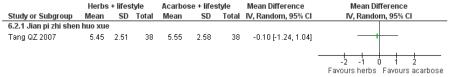
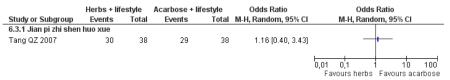
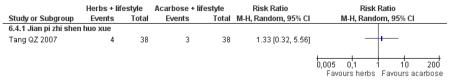
one trial compared Jian pi zhi shen huo xue with acarbose (Tang QZ 2007);
one trial compared Yi qi yang yin huo xue combined with an antihypertensive medication with an antihypertensive medication alone (Lu X 2005);
one trial compared Jinqi jiangtang pills with a basic education on IGT as a co-intervention and as a control (Wang YX 2005).
Tang QZ 2007 and Zeng YH 2006 were three-arm trials. Tang QZ 2007 compared a Jian pi zhi shen huo xue plus lifestyle modification versus acarbose plus lifestyle modification versus lifestyle modification alone. Zeng YH 2006 compared Liu wei di huang tang plus lifestyle modification versus lifestyle modification alone versus a control receiving a diabetes educational pamphlet only.
Lifestyle modification typically involved diet, exercise and education about the disease. In most cases the specific nature of this intervention was poorly documented with the exception of Hioki C 2004 and Zeng YH 2006.
The studies yielded widely differing estimates of effect (a high level of heterogeneity) when results were pooled on most outcomes. This was expected with a review that includes a range of Chinese herbal medicine interventions composed of differing herbs and formulations.
Outcomes
The outcomes reported were mainly metabolic parameters including fasting blood glucose (reported in all studies except Qu LX 2002), 2hr fasting glucose (reported in all studies) and ‘normalisation of fasting blood glucose’ (reported in 10 studies). Normalisation was defined as fasting blood glucose <7.0 mmol/L and 2hr oGTT ≤7.8 mmol/L according to WHO 1999 criteria for all trials except Yao Z 2001, which used the ADA 1997 criteria for normal glucose tolerance of fasting blood glucose less than 6.1 mmol/L. Outcomes on normalisation of blood glucose were recorded as dichotomous data.
Ten studies measured the incidence of diabetes (Fan GJ 2004; Hao AZ 2004; Li CP 2004; Tang QZ 2007; Zhou DY 2003; Wang BQ 2008; Wang YX 2005; Wei AS 2001; Yao Z 2001). Incidence of diabetes refers to the number of participants that have converted to diabetes by the completion of the trial. Eight of the ten trials reporting on this outcome used WHO 1999 criteria to define diabetes (FPG >7.0 mmol/L and 2hr oGTT >11.0 mmol/L).
Four studies measured glycosylated haemoglobin A1c (HbA1c) (Hao AZ 2004; Hioki C 2004; Tang QZ 2007; Wei AS 2001). Zeng YH nominated HbA1c as an outcome but did not report the data.
Eleven studies measured lipids, either total cholesterol, triglycerides or both. Five studies measured HDL-cholesterol. Nine studies measured fasting insulin.
No study investigated mortality, morbidity or cost effectiveness. Lu X 2005 was the only study to measure quality of life. Adverse effects were reported in two of the studies. Other outcomes measured were body mass index (BMI), waist-hip-ratio (WHR) and blood pressure.
Excluded studies
Most of the references identified by the search update were excluded at the first screening step by one reviewer, as they were clearly irrelevant (see Characteristics of excluded studies). The most frequent reasons for exclusion at this level were: article was a review or a commentary; studies of people with diabetes; and clearly non-randomised design.
The full text of 83 studies was retrieved. Sixty-six studies had to be excluded after careful evaluation of the full publication. Seventeen studies were excluded due to inadequate methods of randomisation (odd-even, alternation, and based on clinician’s decision). Sixteen studies were non-randomised trials and 15 were case series. A further 18 studies were excluded as they did not meet the review criteria for the population group, outcomes, duration or intervention. Of these, five studies were excluded due large sampling discrepancies indicating there was no true randomisation. In each case we were unable to contact the authors to resolve the discrepancy. One study was excluded as it was a duplicate.
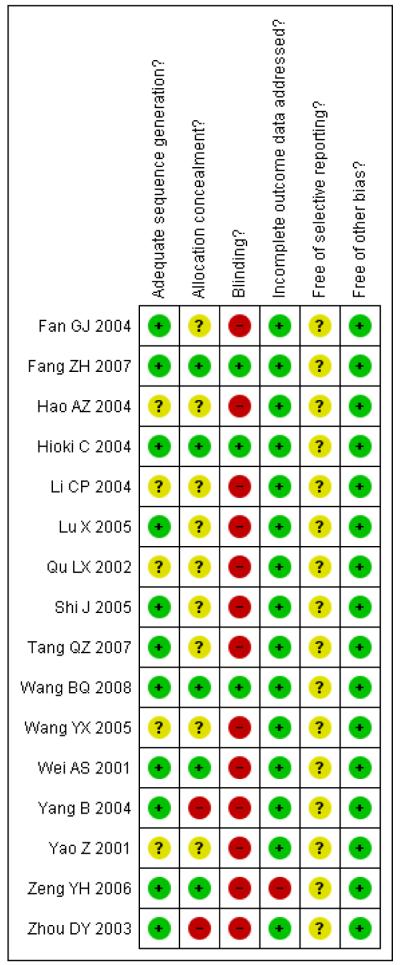
Risk of bias in included studies
Most published reports of trials were lacking in details of trial methodology (see Figure 2). We tried to contact all primary trial authors to clarify randomisation methods. When details were obtained (through phone calls) it was apparent that eleven of the trials had used an adequate sequence generation. The method was not reported for five trials and we were unable to contact the trial authors (Hao AZ 2004; Li CP 2004; Qu LX 2002; Wang YX 2005; Yao Z 2001).
Figure 2.
Risk of bias summary: review authors’ judgements about each risk of bias item for each included study.
Most of the trials provided data on important baseline characteristics of the intervention and control groups to judge the comparability of the two groups.
Three trials (Fang ZH 2007; Hioki C 2004; Wang BQ 2008) could be considered as having a low risk of bias reporting adequate sequence generation, adequate allocation concealment, participants blinded, all participants accounted for and no other apparent bias.
Allocation
Allocation concealment was adequate in only five trials (Fang ZH 2007; Hioki C 2004; Wang BQ 2008; Wei AS 2001; Zeng YH 2006). It was unclear in nine trials and not adequate in two.
Blinding
Participants were blinded in three trials (Fang ZH 2007; Hioki C 2004; Wang BQ 2008). The lack of blinding in the other trials could have resulted in an over- or underestimation of the outcomes as it may have affected the behaviour of the participants.
Incomplete outcome data
Attrition was low or adequately accounted for in most trials.
Selective reporting
In all trials but one (Zeng YH 2006), nominated and expected outcomes were reported. As no trials reviewed had published protocols of their data collection or analysis it is not known if some outcomes were not published.
Other potential sources of bias
Some authors were contacted by phone for further information on methods of randomisation, sequence generation, allocation concealment and, in some cases, clarify data issues. Authors were relying on recall and this may have led to some bias.
Six trials clearly reported the number of drop-outs and withdrawals (Fan GJ 2004; Hioki C 2004; Tang QZ 2007; Wang BQ 2008; Wang YX 2005; Wei AS 2001), although ITT analysis was not implemented; nor was it used in any of the other included trials. Reasons for drop-outs or withdrawals were not always clear.
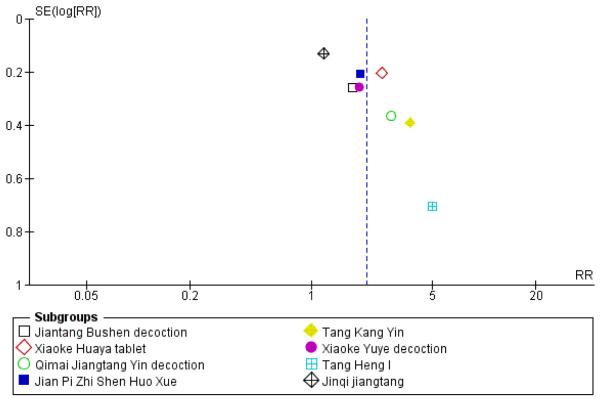
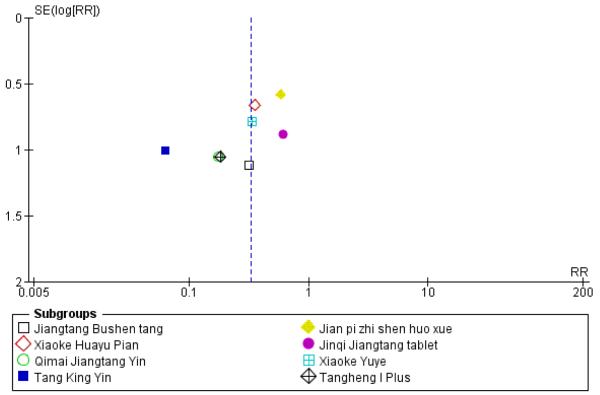
Small study and reporting bias were considered. It is possible that the results are biased as it is possible that studies with negative outcomes have not been published. Outcomes for the first comparison group of studies were explored through funnel plots (see Figure 5 and Figure 6). However, these cannot be considered reliable as there were fewer than 10 studies; in addition funnel plot asymmetry may occur by chance. A sensitivity analysis was conducted to determine if the positive results of the small trial of Tang Heng I (Yao Z 2001) had influenced the meta-analysis of normalisation of fasting blood glucose (RR 1.99; 95% confidence interval (CI) 1.47 to 2.71 versus RR 2.07; 95% CI 1.52 to 2.82).
Figure 5.
Funnel plot of outcome ‘normalisation of fasting blood glucose at trial completion’ (herbal medicines plus lifestyle modification versus lifestyle modification alone)
Figure 6.
Funnel plot of outcome ‘diabetes incidence’ (herbal medicines plus lifestyle modification versus lifestyle modification alone)
Effects of interventions
There were no outcome data in any of the trials on death from any cause, morbidity, diabetes complications, or costs. No serious adverse events or hypoglycaemic episodes were reported.
We were only able to perform meta-analyses on two outcomes in this review and these should be interpreted cautiously. This is mainly due to issues of heterogeneity and because none of the specific herbal medicines comparison data was available from more than one study.
Herbal medicine plus lifestyle modification versus lifestyle modification alone
Nine trials involving 792 participants compared herbal medicines along with lifestyle intervention with lifestyle intervention alone (Fan GJ 2004; Hao AZ 2004; Li CP 2004; Tang QZ 2007; Wei AS 2001; Yang B 2004; Yao Z 2001; Zeng YH 2006;Zhou DY 2003). The average number of trial participants was 50, ranging from 42 to 168 participants. Average trial duration was 8.3 months, ranging from one month to 24 months
Nine different herbal medicines were investigated: Jiangtang Bushen decoction, Xiaoke huaya tablet, Qimai jiangtang yin decoction, Jinqi jiangtang tablets, Xiaoke yuye decoction, Liu wei di huang tang, Tang kang yin decoction, Tang Heng I decoction and Jian pi zhi shen huo xue.
In all trials, with the exception of Zeng YH 2006, the lifestyle intervention was poorly documented.
Normalisation of fasting blood glucose and incidence of diabetes
Normalisation of fasting blood glucose refers to the number of participants who returned to normal blood glucose range at the end of the trial.
Eight trials involving 625 participants reported on the normalisation of fasting blood glucose levels following the intervention.
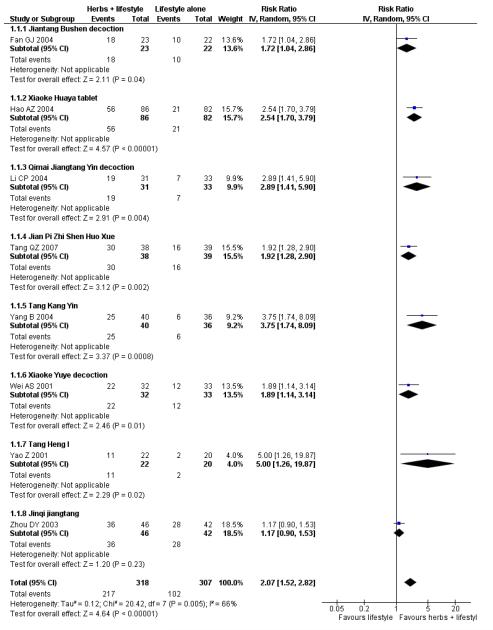
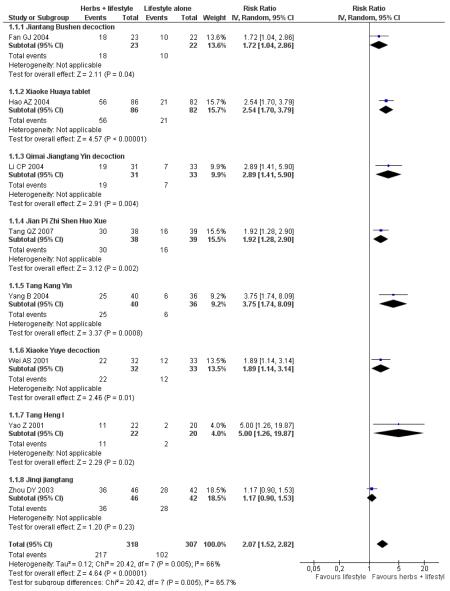
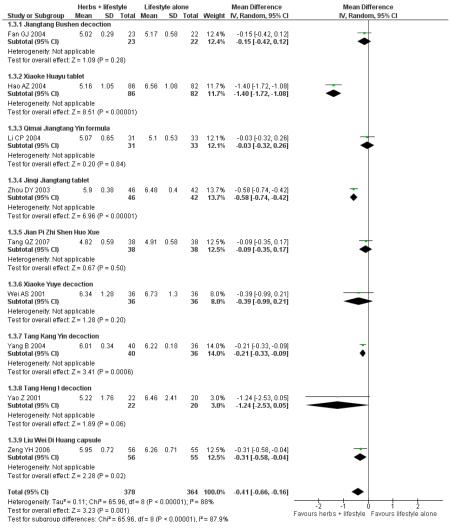
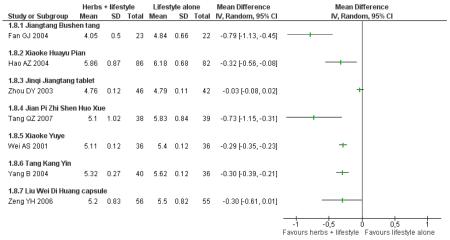
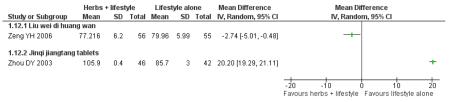
Of the eight trials analysed, those receiving the Chinese herbal intervention were more than twice as likely (RR 2.07; 95% confidence interval (CI) 1.52 to 2.82) to have normalised their fasting blood glucose compared to those receiving lifestyle modification only (Figure 3).
Figure 3.
Forest plot of outcome ‘normalisation of fasting blood glucose at trial completion’ (herbal medicines plus lifestyle modification versus lifestyle modification alone)
The incidence of diabetes refers to the number of participants who had progressed to type 2 diabetes according to WHO or ADA criteria by the end of the trial.
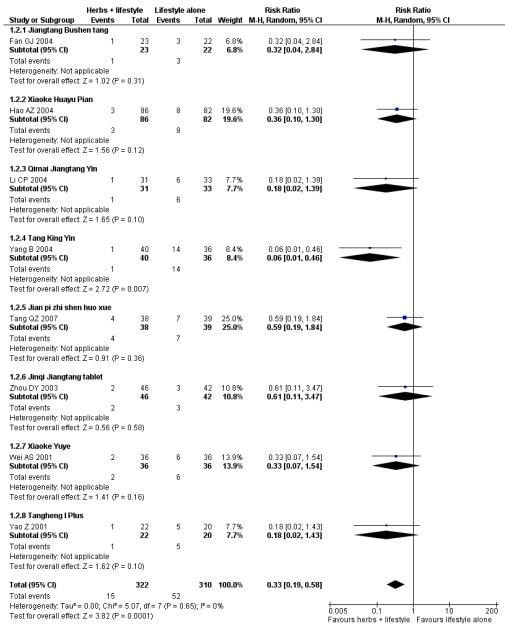
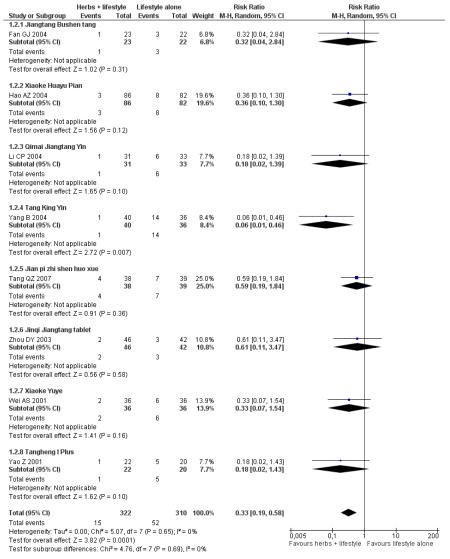
Eight trials reported on the incidence of diabetes in the groups (Figure 4). There was a significant difference in the incidence of diabetes in favour of the Chinese herbal medicines following Tang Keng Yin tablets (Yang B 2004). When the results of the eight trials were pooled there was a significant difference found in favour of the Chinese herbal medicines compared to the lifestyle intervention alone (RR 0.33; 95% CI 0.19 to 0.58). All except three of these trials had a duration of more than 12 months (Yang B 2004 ran for one month, Hao AZ 2004 ran for two months, and Yao Z 2001 for three months).
Figure 4.
Forest plot of outcome ‘diabetes incidence’ (herbal medicines plus lifestyle modification versus lifestyle modification alone)
In the pooling of results on these two measures there was no considerable statistical heterogeneity among the comparisons (normalisation of fasting blood glucose: I2 = 66%; incidence of diabetes: I2 = 0%). It is important to note that there was clinical heterogeneity. The Chinese herbal medicines used in the clinical trials analysed are wide ranging in their ingredients. These ingredients are used for a variety of different clinical purposes. However, they may still be considered as ‘class’ or ‘group’ of oral hypoglycaemic herbal medicines. But any pooled effect size should be interpreted only as crude indicator of the overall direction of the findings. Nevertheless, these findings show that participants receiving Chinese herbal medicines were less likely to develop diabetes and more likely to have normal blood glucose than those in the control group.
Fasting blood glucose and 2hr blood glucose after an oral glucose tolerance test (oGTT)
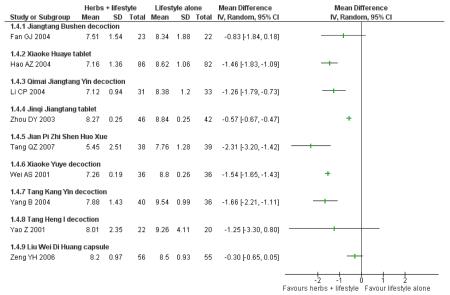
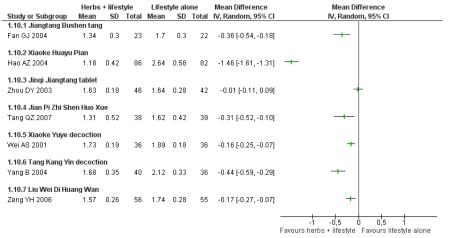
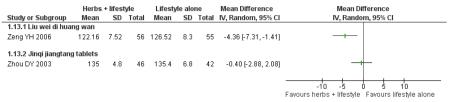
Fasting blood glucose refers to the fasting plasma glucose (FPG) levels (mmol/L) measured in all nine trials in this comparison (continuous data).
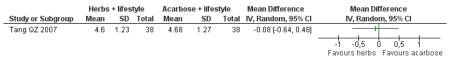
In four of the nine trials, the Chinese herbal medicines combined with lifestyle modification were significantly better at reducing fasting blood glucose levels than lifestyle modification alone. Jinqi jiangtang tablets (Zhou DY 2003) showed a significant reduction (MD −0.58 mmol/L; 95% CI −0.74 to −0.42), as did Hao AZ 2004 (MD −1.40 mmol/L; 95% CI −1.72 to −1.08), Yang B 2004 (MD −0.21 mmol/L; 95% CI −0.33 to −0.09), and Zeng YH 2006 (MD −0.31 mmol/L; 95% CI −0.58 to −0.04). There was no significant difference of fasting blood glucose in the trials of Jiangtang Bushen decoction (Fan GJ 2004), Qimai jiangtang Yin ( Li CP 2004), Jian pi zhi shen huo xue (Tang QZ 2007), Xiaoke yuye ( Wei AS 2001), and Tang heng I ( Yao Z 2001).
Two hour fasting blood glucose refers to blood glucose levels (mmol/L) measured after an oGTT. Six of the nine trials in this comparison reported significantly better results for reducing 2hr fasting blood glucose levels than the lifestyle modification control. There was no significant difference in the trials of Jiangtang bushen decoction (Fan GJ 2004) Tang Heng I decoction (Yao Z 2001) and Liu wei di huang tang (Zeng YH 2006).
When these studies were pooled, considerable heterogeneity was found among the studies (I2 = 90%). This may be due to the type of the intervention, the duration of the intervention or both which prevented a meaningful meta-analysis of these outcomes.
Glycosylated haemoglobin A1c (HbA1c)
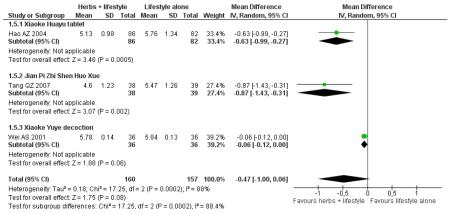
Only three studies reported HbA1c outcomes. Xiaoke huayu tablets (MD −0.6%; 95% CI −1.0 to −0.3) ( Hao AZ 2004), Xiaoke Yuye decoction ( Wei AS 2001) (MD −0.9%; 95% CI −1.4 to −0.3) and Jian pi zhi shen huo xue (MD −0.1%; 95% CI −0.1 to 0.0) ( Tang QZ 2007) combined with lifestyle modification were all statistically significant in reducing HbA1c compared to the control of lifestyle modification alone. No meta-analysis was conducted due to considerable statistical heterogeneity (I2 = 88%).
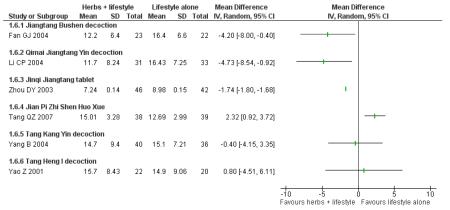
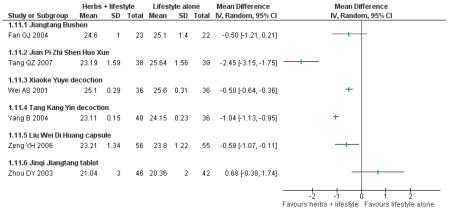
Insulin
In the six trials that measured insulin levels, significantly lower levels were detected in those taking Jiangtang bushen decoction (Fan GJ 2004), Qimai jiangtang decoction (Li CP 2004), and Jinqi jiangtang tablets (Zhou DY 2003). No significant differences in insulin levels were found in those participants taking Tang Kang Yin (Yang B 2004) and Tang Heng I decoction (Yao Z 2001) compared with the lifestyle modification control group. In the trial of Jian pi zhi shen huo xue (Tang QZ 2007) insulin levels of the lifestyle modification control group were significantly lower than those in the Chinese herbal intervention group.
Insulin active index (IAI), a measure of insulin sensitivity, was assessed in one trial of Qimai jiangtang yin ( Li CP 2004). No significant differences were detected.
Lipids
Cholesterol outcomes were measured in seven trials (Fan GJ 2004; Hao AZ 2004; Tang QZ 2007; Wei AS 2001; Yang B 2004; Zeng YH 2006; Zhou DY 2003). Triglycerides were also measured in these seven trials. High density lipoprotein (HDL) cholesterol was measured in two trials (Tang QZ 2007; Zhou DY 2003).
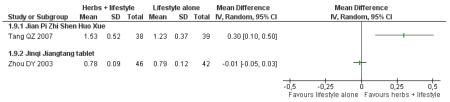
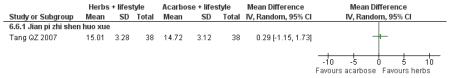
One trial that measured all three outcomes, Jian pi zhi shen huo xue ( Tang QZ 2007) showed a significant improvement in reducing total cholesterol (MD −0.73 mmol/L; 95% CI −1.15 to −0.31), HDL-cholesterol (MD 0.30 mmol/L; 95% CI 0.10 to 0.50) and triglycerides (MD −0.31 mmol/L; 95% CI −0.52 to −0.10) for the combined intervention of Chinese herbal medicines with lifestyle interventions.
Jiangtang bushen tang ( Fan GJ 2004), Tang kang yin ( Yang B 2004), Liu wei di huang tang ( Zeng YH 2006), and Xiaoke huayu pian (Wei AS 2001) also all showed a significant improvement compared to the control in reducing total cholesterol and triglycerides.
Body mass index (BMI)
Five trials comparing herbal medicine with lifestyle modification with lifestyle modification alone measured BMI. Xiaoke yuye decoction (Wei AS 2001), Tang kang yin decoction (Yang B 2004) , and Liu wei di huang capsule (Zeng YH 2006) all demonstrated a significant improvement in BMI. There was no significant improvement in BMI in those taking Jinqi jiangtang pian ( Zhou DY 2003) and Jiangtang bushen tang (Fan GJ 2004).
Blood pressure
Two trials (Zeng YH 2006; Zhou DY 2003) in this comparison group examined blood pressure. Liu wei di huang capsules were statistically significantly more effective than the control group in reducing diastolic (MD −3 mm Hg; 95% CI −4 to −1) and systolic (MD −4 mm Hg; CI 95% −7 to −1) blood pressure. The diastolic blood pressure data for Jinqi Jiangtang pian were unusual in favour of lifestyle intervention alone (MD 20 mm Hg; 95% CI 19 to 21) and we were unable to contact the author to clarify any reporting anomaly. There were no significant differences in the systolic blood pressure (MD −0.4 mm Hg: 95% CI −3 to 2).
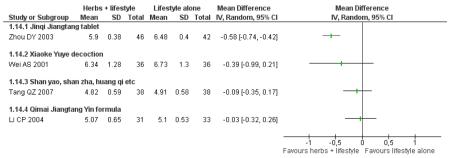
Subgroup analysis: Astragalus membranecus and FBG
Astragalus membranecus was present as a main ingredient in five of the trials and it was analysed in a subgroup analysis. In three of these trials with Astragalus membranecus there was a significant difference of combined herbal medicine with lifestyle interventions in FBG compared to the control (Wang YX 2005; Zhou DY 2003). However, the remaining three trials did not detect a significant difference (Fan GJ 2004; Li CP 2004; Tang QZ 2007).
Herbal medicine plus lifestyle modification versus placebo plus lifestyle modification
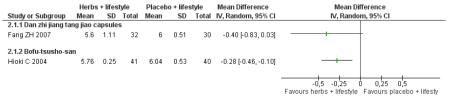
Two trials compared a Chinese herbal medicine with placebo with the co-intervention of lifestyle modification.
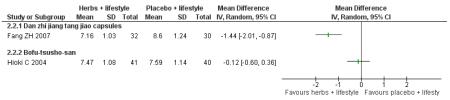
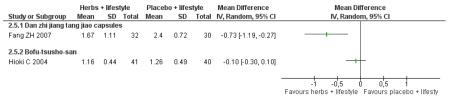
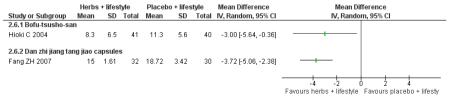
Danzhi jiangtang jiao capsules (Fang ZH 2007) combined with lifestyle modification were significantly better than a placebo and lifestyle modification in improving 2hr-oGTT blood glucose (MD −1.44 mmol/L; 95% CI −2.01 to −0.87). But there was no significant difference between the groups in reducing fasting blood glucose (MD −0.40 mmol/L; 95% CI −0.83 to −0.03). Trigylcerides and insulin levels also showed significant reductions .
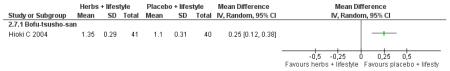
Bofu-Tsusho-San ( Hioki C 2004) significantly improved fasting blood glucose (MD −0.28 mmol/L; 95% CI −46 to −0.10) but not 2hr-oGTT blood glucose (MD −0.12 mmol/L; 95% CI −0.60 to 0.36). There was no significant difference in the HbA1c between those taking Bofu-Tsusho-San and the control group. There was no significant difference in cholesterol levels found in the trial of Bofu-Tsusho-San, and while Bofu-Tsusho-San did not show an improvement in total cholesterol outcomes it demonstrated a significant difference in increasing HDL-cholesterol (MD 0.25 mmol/L; 95% CI 0.12 to 0.38).
Herbal medicine plus lifestyle modification versus metformin plus lifestyle modification
There was no significant difference in the 2-hr glucose tolerance test levels between Tangping san plus lifestyle modification and metformin plus lifestyle modification at the end of the three months intervention. No other outcomes were reported for Tangping San (Qu LX 2002).
Herbal medicine versus placebo
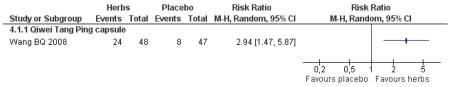
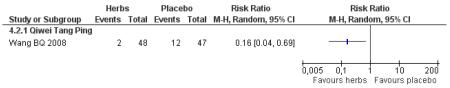
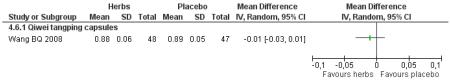
Compared with placebo, those taking Qi wei tang ping capsules (Wang BQ 2008) showed significantly better results for fasting blood glucose and 2hr-oGTT blood glucose. There was a significant higher level of normalisation of fasting blood glucose compared to placebo (RR 2.94, 95% CI 1.47 to 5.87). There was no significant difference in BMI or waist-to-hip ratio in the herbal medicine group compared to those taking placebo.
Herbal medicine versus metformin
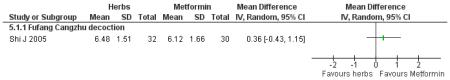
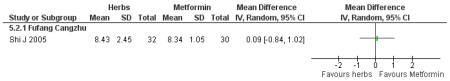
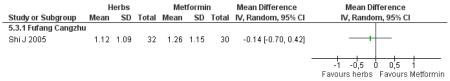
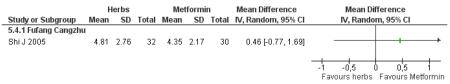
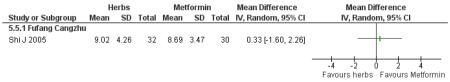
There was no significant difference between Fufang cangzhu decoction and metformin in reducing fasting blood glucose, cholesterol, triglycerides, insulin, weight, or waist-to-hip ratio (Shi J 2005). We were unable to ascertain if this comparison was constructed as a non-inferiority trial.
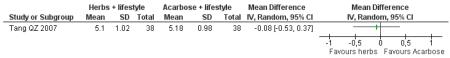
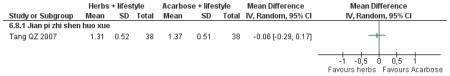
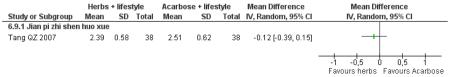
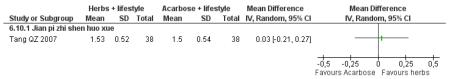
Herbal medicine plus lifestyle modification versus acarbose plus lifestyle modification
There was no significant difference between the Jian pi zhi shen huo xue (Tang QZ 2007) and acarbose in any of the outcome measures (FBG, 2hr-oGTT blood glucose, insulin, lipids or HbA1c). There was also no significant difference between Jian pi zhi shen huo xue and acarbose regarding the normalisation of fasting blood glucose (Tang QZ 2007).
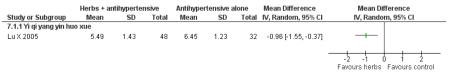
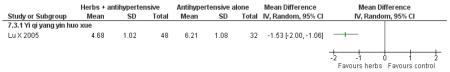
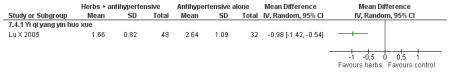
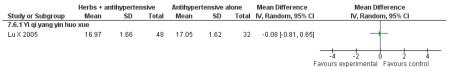
Herbal medicine plus antihypertensive medication versus antihypertensive medication alone
The Chinese herbal medicine, Yi qi yang yin huo xue combined with antihypertensive medication was significantly better than the antihypertensive medication alone in reducing fasting blood glucose (one trial, MD −0.96 mmol/L, 95% CI −1.55 to −0.37). Yi qi yang yin huo xue was also significantly better than the control in reducing cholesterol, triglycerides and increasing HDL-cholesterol. There was no significant difference between Yi qi yang yin huo xue and the control in regard to systolic or diastolic blood pressure. This trial also evaluated quality of life using an instrument comprised of eight scales designed for hypertension based on a study of an American hypertensive population (Testa 1989) and validated for a Chinese population (Du 1994). The trial found that on the five of the eight scales: physical symptoms distress scale, sexual symptoms distress scale, sleep dysfunction scale, positive symptom scale and working performance, there was a significant difference in favour of the herbal medicine group (Lu X 2005). There was no significant difference in the measures of life satisfaction scale, social participation scale and general well-being adjustment scale. However, it was difficult to disentangle the effects of the various compound on quality of life. This study reported that there were no adverse renal or liver findings and in ECG tests.
Herbal medicine plus basic education versus basic education alone
In this comparison one study compared Jinqi jiangtang tablets with a control with a co-intervention of basic education about IGT (Wang YX 2005). This did not involve diet or exercise instruction. Normalisation of blood glucose was significant (RR 10.80; 95% CI 3.47 to 33.66) as was the incidence of diabetes (RR 0.44; 95% 0.22 to 0.87) and reduction of fasting blood glucose (MD −0.88; 95% CI −1.14 to −0.62) and 2-hr oGTT. There was a significant reduction in triglycerides but not total cholesterol in the intervention group compared to the control .
Adverse events
Five of 17 trials reported outcomes for adverse events. In the trial of Bufo-Tsusho-San there were four withdrawals; three from the treatment group for non-compliance because of loose bowels. In Wang BQ there were two instances of abdominal discomfort, one participant from each group (Wang BQ 2008). In the two other studies recording outcomes for adverse events none were reported (Wei AS 2001; Lu X 2005). In the trial of Jinqi Jiangtang (Wang YX 2005) three cases in the intervention group developed mild gastro-intestinal symptoms in the early stage of taking the Chinese herbal medicine. These resolved after one to two weeks.
Discussion
Summary of main results
Sixteen randomised trials were included in this review. There was considerable clinical heterogeneity in the interventions of the included studies. In the 16 studies lasting four weeks to two years there were eight different comparisons, with 15 unique herbal formulations investigated.
In this systematic review we found evidence from eight trials that Chinese herbal medicines combined with lifestyle modification were significantly better at normalising blood glucose levels then lifestyle modification alone (RR 2.07; 95% CI 1.52 to 2.82). In a meta-analysis of eight trials, those receiving Chinese herbs were also more likely to have a reduced incidence of diabetes (RR 0.33; 95% CI 0.19 to 0.58). In the pooling of the results for the meta-analyses of the two measures of normalising blood glucose and incidence of diabetes there was no considerable statistical heterogeneity among the comparisons (I2 = 66% and I2 = 0%, respectively). It is important to note that there is a clinical difference in the herbal composition of these interventions and likely a difference in the active components. But these Chinese herbal medicines are not completely dissimilar. They form part of a ‘group’ of herbal medicines with hypoglycaemic effects designed to normalised elevated blood glucose and prevent diabetes. The population groups according to age, gender and ethnicity were similar. However, any pooled effect size should be interpreted only as a crude indicator of the overall direction of the findings. Further, all of these trials had a high risk of bias. Specifically, none of these trials were blinded and three of the trials reported unclear randomisation procedures. Nevertheless, these findings indicate that participants receiving Chinese herbs were less likely to develop diabetes and more likely to have normal blood glucose than those in the control group. The result therefore provides guidance for future research not for specific clinical practice.
Xiaoke huayu tablets ( Hao AZ 2004), Xiaoke yuye decoction ( Wei AS 2001) and Jian pi zhi shen huo xue ( Tang QZ 2007) combined with lifestyle modification were all statistically significant in reducing glycosylated haemoglobin A1c (HbA1c) compared to the control of lifestyle modification alone.
Compared with placebo and lifestyle modification, Danzhi jiangtang jiao capsules (Fang ZH 2007) with lifestyle modification were significantly better at reducing 2hr blood glucose after oral glucose tolerance testing (oGTT). Bofu-Tsusho-San (Hioki C 2004) combined with lifestyle modification was significantly better at reducing fasting blood glucose (FBG).
Compared with placebo alone, Qiweitang ping (Wang BQ 2008) was significantly better at normalising blood glucose, reducing FBG and 2hr-oGTT blood glucose.
Three trials compared Chinese herbal medicines with a pharmaceutical control. However, these were not clearly specified as non-inferiority or equivalence trials. Compared with metformin, Fufang cangzhu (Shi J 2005) showed no significant difference in reducing FBG. Compared to metformin combined with lifestyle modification, Tangping san (Qu LX 2002) combined with lifestyle modification showed no significant differences in reducing 2hr-oGTT blood glucose.
There was no significant difference between Jian pi zhi shen huo xue (Tang QZ 2007) compared to acarbose, with both groups receiving lifestyle modification, on any of the outcome measures.
In a trial of an antihypertensive pharmaceutical the combination of Yi Qi Yang Yin Huo Xue (Lu X 2005) with the antihypertensive drug was significantly better at reducing FBG, cholesterol, triglycerides and HDL-cholesterol than the antihypertensive drug alone.
Some of the Chinese herbal medicines showed potential for improving cholesterol and triglycerides along with normalising FBG. Jian pi zhi shen huo xue, Jiangtang bushen tang, Tang kang yin, Liu wei di huang tang, and Xiaoke huayu pian all showed a significant improvement compared to the control in reducing total cholesterol and triglycerides.
Overall completeness and applicability of evidence
The age and gender of participants in the included trials was representative of the general global population showing IGT (IDF 2008). Although all but one trial were conducted in a Chinese population this is not thought to impact on the applicability of the interventions to other populations.
Quality of the evidence
Thirteen of the 16 trials included in this review demonstrated a risk of bias in at least two of several key criteria: random sequence generation; allocation concealment; blinding of participants, outcome assessors and intervention providers; incomplete outcome data; selective outcome reporting; and other sources of bias (see Figure 2).
Details of sequence generation and concealment allocation were only reported in one of the published papers. Trial authors were contacted to clarify details. We found that nine trials had used adequate sequence generation methods (Fan GJ 2004; Fang ZH 2007; Hioki C 2004; Lu X 2005; Wang BQ 2008; Wei AS 2001; Yang B 2004; Zeng YH 2006; Zhou DY 2003). Allocation concealment was less frequently understood or adequately performed, with only five of the trial authors providing satisfactory details when questioned (Fang ZH 2007; Hioki C 2004; Wang BQ 2008; Wei AS 2001; Zeng YH 2006). Empirical evidence suggests that failure to meet these criteria, such as adequate allocation concealment, is associated with overestimates of effect.
In clarifying risk of bias with authors it was apparent that the concept of randomisation was not always fully understood. Of the 83 full papers retrieved, 34 claimed to be randomised but after contacting authors only 17 of these were truly randomised (50%). This is lower than the findings of a Cochrane review of Chinese herbal medicines for the treatment of common cold which found more than 95% of the authors misunderstood the concept of randomisation (Wu 2007).
Overall only three of the 16 included trials were well designed and had a fairly low risk of bias (Fang ZH 2007; Hioki C 2004; Wang BQ 2008). The insufficient number of trials prohibited us from performing meaningful sensitivity analyses to clarify robustness of the review results to the exclusion of trials with inadequate methodology.
The double-blind, placebo controlled trial of Dan zhi jiang tang jiao (Fang ZH 2007) reported a significant improvement in 2 hr-oGTT blood glucose, insulin and triglycerides but there was no significant difference in fasting blood glucose. The method of sequence generation and allocation concealment was not reported in the trial but deemed adequate after an interview with one of the authors.
A second double-blind trial compared Bofu-Tsusho-San plus lifestyle modification versus placebo plus lifestyle modification in 81 people over six months. Peope randomised to Bofu-Tsusho-San plus lifestyle modification demonstrated a significantly improved fasting blood glucose but not 2hr-oGTT blood glucose (Hioki C 2004). There was no significant difference in glycosylated haemoglobin A1c (HbA1c) between those taking Bofu-Tsusho-San and the control group.
The third double-blind trial, comparing Qi wei jiangtang yin with placebo, demonstrated a significant improvement in the rate of normalisation of fasting blood glucose, reduction of fasting blood glucose, and 2hr-oGTT (Wang BQ 2008) in those randomised to the placebo group. This trial had a duration of 24 months and a low risk of bias as well as an adequately powered sample. However, as there are no other trials of this herbal medicine in this population group, these results cannot be seen as definitive.
There were few trials (n = 4) that collected HbA1c data. This will be an important outcome measure to collect in future trials. According to the American Diabetes Association Expert Committee on the Diagnosis of Diabetes, the European Association for the Study of Diabetes, and the International Diabetes Federation, the HbA1c will become the preferred diagnostic test for diabetes (ADA 2009).
In many of the trials it is possible that the statistical power may not have been adequate. Several studies have reported rates of natural reversion to normal glucose levels of one third to one half for participants identified with IGT (Forrest 1988; Riccardi 1985). Moreover, rates of reversion to normal glucose levels appeared independent of the duration of follow-up, with a range of two months to 10 years (Rambod 2009). The rate of reversion needs to be built into statistical calculations of the power required to detect a difference in blood glucose and other outcomes.
Twelve of the 16 trials had lifestyle modifications as a co-intervention, all but two trials failed to provide any details on the nature of this intervention. Without thorough details, replication of the trials to build evidence for these interventions is not possible. Further this operates as a potentially confounding factor and calls into question the veracity of the results.
Overall the positive evidence in favour of Chinese herbal medicines for the treatment of impaired glucose tolerance is constrained by the following factors: a lack of trials that tested the same medicine, lack of details on co-interventions, unclear methods of randomisation, poor reporting and other risks of bias.
Potential biases in the review process
We have tried to reduce bias by contacting all trial authors to clarify the methods of randomisation. In this way we were able to eliminate trials that were only quasi-randomised. Nonetheless we were unable to contact the authors of five of the included trials to clarify the methods of randomisation (Hao AZ 2004; Li CP 2004; Qu LX 2002; Wang YX 2005).
Agreements and disagreements with other studies or reviews
As far as we are aware of, no systematic review has been done with a focus on Chinese herbal medicines for people with impaired glucose tolerance or impaired fasting glucose.
Authors’ conclusions
Implications for practice
The available evidence suggests that some Chinese herbal medicines could be considered as a potential treatment in people with impaired glucose tolerance and reduce the incidence of diabetes. Given the sources of potential bias further evidence is required to confirm these trends. A separate systematic review on the efficacy of lifestyle education concluded that lifestyle education was clearly effective for reducing two-hour plasma glucose after an oral glucose tolerance test and the incidence of type 2 diabetes over one year (Yamaoka 2005). Our review adds to this growing body of evidence, in 80% of the included trials lifestyle modification was used as a co-intervention.
Implications for research
Further trials are required before any conclusions can confidently be reached about the effects of Chinese herbal medicines for the treatment of impaired glucose tolerance and the delay of diabetes onset.
Future trials need to be designed in such a way as to address the risk of bias identified in the trials reviewed here. It is essential that such trials have adequate methods of randomisation and allocation concealment and that these methods are clearly reported. Ideally, future trials will involve a control of a pharmacological nature or be placebo-controlled. The Chinese herbal medicines and the control intervention need to be manufactured in such a way that participants and intervention providers can be blinded. If a lifestyle modification is to be used as a co-intervention or control this should be described in detail. The rate of reversion to normal blood glucose needs to be built into statistical calculations of the power of the trial required to detect a difference in blood glucose and other outcomes. Along with fasting blood glucose outcomes, other measurements of efficacy and safety should include glycosylated haemoglobin A1c (HbA1c), health-related quality of life, death from any cause, diabetic complications, economic outcomes and adverse events.
All future Chinese herbal medicines trials should be reported according to the elaborated CONSORT statement for reporting randomised controlled trials of herbal medicines (Gagnier 2006).
Graphs
1 - Herbal medicine plus lifestyle modification versus lifestyle modification alone
1.1 Normalisation of fasting blood glucose at trial completion (n)
1.2 Incidence of diabetes (n)
1.3 Fasting blood glucose (mmol/L)
1.4 2hr fasting blood glucose (mmol/L) [ after oral glucose tolerance test]
1.5 HbA1c (%)
1.6 Insulin (μU/ml)
1.7 IAI (insulin sensitivity)
1.8 Total cholesterol (mmol/L)
1.9 Lipids: HDL (mmol/L)
1.10 Trigylcerides (mmol/L)
1.11 Body Mass Index (kg/m2)
1.12 Diastolic blood pressure (mmHg)
1.13 Systolic Blood Pressure (mmHg)
1.14 Main ingredient Astragalus membranecus (≥30g): Fasting blood glucose (mmol/ml)
2 - Herbal medicine plus lifestyle modification versus placebo plus lifestyle modification
2.1 Reduction in fasting blood glucose (mmol/L)
2.2 Reduction in 2hr fasting blood glucose after oral glucose tolerance test
2.3 Reduction in HbA1c (%)
2.4 Total cholesterol (mmol/L)
2.5 Trigylcerides (mmo/IL)
2.6 Insulin (mu/L)
2.7 Lipids: HDL (mmol/L)
3 - Herbal medicine plus lifestyle modification versus metformin plus lifestyle modification
3.1 Reduction in 2 hr fasting blood glucose after oral glucose tolerance test (mmol/L)
4 - Herbal medicine versus placebo
4.1 Normalisation of fasting blood glucose (n)
4.2 Incidence of diabetes (n)
4.3 Reduction in fasting blood glucose (mmol/L)
4.4 Reduction in 2hr fasting blood glucose after orale glucose tolerance test (mmol/L)
4.5 Body mass index (kg/m2)
4.6 Waist-to-hip ratio (WHR)
5 - Herbal medicine versus metformin
5.1 Reduction in fasting blood glucose (mmol/L)
5.2 Reduction in 2hr fasting blood glucose after orale glucose tolerance test (mmol/L)
5.3 Triglycerides (mmol/L)
5.4 Total cholesterol
5.5 Insulin (mU/L)
5.6 Waist-to-hip ratio (WHR)
5.7 Weight (kg)
6 - Herbal medicine plus lifestyle modification versus acarbose plus lifestyle modification
6.1 Reduction in fasting blood glucose (mmol/L)
6.2 2hr-Glucose tolerance (mmol/L)
6.3 Normalisation of blood glucose (n)
6.4 Incidence of diabetes (n)
6.5 HbA1c (%)
6.6 Insulin (FINS mmol/L)
6.7 Total cholesterol (mmol/L)
6.8 Trigylcerides (mmol/L)
6.9 Lipids: LDL (mmol/L)
6.10 Lipids: HDL (mmol/L)
7 - Herbal medicine plus antihypertensive medication versus antihypertensive medication alone
7.1 Reduction in fasting glucose (mmol/L)
7.2 Reduction in 2hr blood glucose after oral glucose tolerance test (mmol/L)
7.3 Total cholesterol (mmol/L)
7.4 Triglycerides (mmol/L)
7.5 Lipids: HDL (mmol/L)
7.6 Systolic Blood Pressure (Kpa)
7.7 Diastolic blood pressure (kpa)
8 - Herbal medicine versus basic education (diabetes pamphlet)
8.1 Normalisation of fasting blood glucose at trial completion (n)
8.2 Incidence of diabetes (n)
8.3 Fasting blood glucose (mmol/L)
8.4 2hr fasting blood glucose (mmol/L) [ after oral glucose tolerance test]
8.5 Total cholesterol (mmol/L)
8.6 Trigylcerides (mmol/L)
Supplementary Material
Differences between protocol and review.
We chose to alter the way in which the assessment of methodological quality was conducted and focus on a risk of bias assessment as a more useful analysis of the individual trials rather than using the tool originally described in the protocol.
Rather than using fixed effect modelling for continuous data as per the protocol the authors determined that a random-effects model was more appropriate for the relatively heterogeneous nature of herbal medicines. It would not be expected that different herbal formulations would produce the same quantity but that the different interventions would produce a distribution in magnitude of effects on any given outcome.
Dichotomous data were expressed as relative risk (RR) ratios rather than odds ratios (OR). This method has been changed since the publication of the protocol to reflect the approach used by other studies in this modality of treatment. It is also a more easily understood statistic in presenting these outcomes.
A high level of statistical heterogeneity was found in the comparison of Chinese herbal medicines with lifestyle modificationvs lifestyle modification alone on nearly all outcomes. For this reason subgroup analyses were undertaken. Due to the nature of the trials included the subgroup analyses varied from those set out in the trial protocol.
The database CISCOM was not searched as it is no longer active.
Acknowledgements
This work was partially funded by Grant Number R24 AT001293 from the National Center for Complementary and Alternative Medicine (NCCAM) and the Cardiac Health Institute. The contents of this systematic review are solely the responsibility of the authors and do not necessarily represent the official views of the NCCAM or the National Institutes of Health.
Published notes
Characteristics of studies
Characteristics of included studies
| Methods | Parallel randomised controlled trial |
| Participants | SETTING: Outpatients at Guangxi TCM College Affiliated No.1 Hospital, China |
| WHO PARTICIPATED: 45 (M/F 21/24; 23 in the treatment group, age 54.6 yrs; 22 in the control, age 57.45 yrs) | |
| INCLUSION CRITERIA: IGT diagnosed by WHO criteria (WHO 1999) | |
| EXCLUSION CRITERIA: <35 yrs, BMI <19 kg/m, serious liver or kidney disorders, hypertension, IGT induced by other organic diseases, drugs or stress. | |
| CO-MORBIDITIES: none reported | |
| CO-MEDICATIONS: none reported | |
| Interventions | INTERVENTION: Jiangtang bushen tang (gou qi 10g, chuan duan xu 10g, nu zhen zi 15g, han lian cao 15g, di gu pi 15g, sheng huang qi 15g, sheng di huang 15g, ge gen 12g, huang lian 5g, sang bai pi 10g, zhi mu 6g) plus diet and exercise; Dosage: 1 decoction every two days |
| CONTROL: lifestyle modification | |
| Outcomes | FBG (mmol/L), 2hr-GTT (mmol/L), Triglycerides (mmol/L), total cholesterol (mmol/L), BMI (kg/m2), fasting insulin (mmol/L), TCM symptoms; |
| Outcomes assessed at baseline, 3 months, 6 months and 12 months. | |
| Study details | DURATION OF INTERVENTION: 12 months |
| DURATION OF FOLLOW-UP: 12 months | |
| RUN-IN PERIOD: none | |
| Publication details | LANGUAGE OF PUBLICATION: Chinese |
| COMMERCIAL FUNDING: No | |
| NON-COMMERCIAL FUNDING: Not reported | |
| PUBLICATION STATUS (PEER REVIEW JOURNAL): Yes | |
| PUBLICATION STATUS (JOURNAL SUPPLEMENT): No | |
| PUBLICATION STATUS (ABSTRACT): Yes | |
| Stated aim of study | “To evaluate the intervention effect of diet, exercise and Jiangtang Bushen Recipe (JBR, a Chinese herbal recipe) in preventing the progress of patients with impaired glucose tolerance (IGT) to diabetes mellitus (DM) type 2.” |
| Notes | |
Risk of bias table
| Item | Authors’ judgement |
Support for judgement |
|---|---|---|
| Adequate sequence generation? | Yes | Quote from phone call: “numbers randomisation from random table” |
| Allocation concealment? | Unclear | Phone call: Participants did not know the group to which they were to be allocated. |
| No information provided about whether researchers knew the allocation. | ||
| Blinding? | No | No blinding of participants, intervention provider or outcomes assessor. |
| Incomplete outcome data addressed? |
Yes | All participants are reported. Six withdrawals are explained. One participant left the intervention as they did not want to take the decoction, the other withdrawal did not give a reason. There were four withdrawals from the control where contact was lost. |
| Free of selective reporting? | Unclear | No protocol provided but all nominated and expected outcomes are reported. |
| Free of other bias? | Yes | None identified. |
| Methods | Parallel placebo controlled randomised trial |
| Participants | SETTING: Outpatient and inpatients, No 1 Affiliated Hospital of An Hui University of Chinese Medicine, China |
| WHO PARTCIPATED: 62 (in treatment group M/F 18/14; age 40-67 yrs; in the control group M/F 17/13, age 39-65 yrs) | |
| INCLUSION CRITERIA: IGT diagnosed by ADA criteria (ADA 1997) and traditional Chinese medicine (TCM) diagnosis of qi and yin deficiency or blood stagnation | |
| EXCLUSION CRITERIA: None reported | |
| CO-MORBIDITIES: none reported | |
| CO-MEDICATIONS: none report | |
| Interventions | INTERVENTION: Dan zhi jiang tang jiao capsules: mu dan pi, shui zhi, tu si zi, ze xie, huang jing, tai zi shen plus liu wei di huang tang oral, 5 capsules (0.35g per capsule) 3 time per day after meals plus lifestyle modification (diet & lifestyle advice) |
| CONTROL: placebo plus lifestyle modification (diet & lifestyle advice) | |
| Outcomes | FBG (mmol/L), 2hr-GTT (mmol/L), insulin (mu/L), triglycerides (mmol/L), traditional Chinese medicine patterns and symptoms. |
| Outcomes were assessed at baseline and trial completion (12 wks). | |
| Study details | DURATION OF INTERVENTION: 12 weeks |
| DURATION OF FOLLOW-UP: 12 weeks | |
| RUN-IN PERIOD: none | |
| Publication details | LANGUAGE OF PUBLICATION: Chinese |
| COMMERCIAL FUNDING: no | |
| NON-COMMERCIAL FUNDING: no | |
| PUBLICATION STATUS (PEER REVIEW JOURNAL): yes | |
| PUBLICATION STATUS (JOURNAL SUPPLEMENT): no | |
| PUBLICATION STATUS (ABSTRACT): yes | |
| Stated aim of study | To observe the intervention effects of Dan zhi jiang tang jiao on IGT. |
| Notes | |
Risk of bias table
| Item | Authors’ judgement |
Support for judgement |
|---|---|---|
| Adequate sequence generation? | Yes | Quote (from the report): “the randomly divided into two groups”. |
| Quote (from phone interview): numbers generated through a random table | ||
| Allocation concealment? | Yes | Concealed envelopes with random numbers were used. |
| Blinding? | Yes | From phone interview: participants were blinded with the use of a placebo and provided with the same diet & lifestyle advice as the treatment group; clinicians were blinded also; not known if the assessors were blinded. |
| Incomplete outcome data addressed? |
Yes | No missing participants or withdrawals. |
| Free of selective reporting? | Unclear | No protocol provided but all nominated outcomes reported, reported TCM patterns and symptoms also. |
| Free of other bias? | Yes | None identified. |
| Methods | Parallel placebo-controlled randomised trial |
| Participants | SETTING: Outpatients at the General Hospital of the People’s Liberation Army, China |
| WHO PARTCIPATED: n=168 (in treatment group M/F 62/24; mean age 55.6 yrs, duration of disease 1mth -2yrs; in the control group M/F 59/23, mean age 53.8, duration of disease 1mth-2yrs). | |
| INCLUSION CRITERIA: IGT (WHO 1999) and hypertension plus high total cholesterol or high triglycerides or low HDL. | |
| EXCLUSION CRITERIA: IGT due to endocrinological disorders, liver disease, drugs, stress. | |
| CO-MORBIDITIES: hypertension, hypercholestemia or high triglycerides or low high density lipoprotein (HDL). | |
| CO-MEDICATIONS: none reported | |
| Interventions | INTERVENTION: Xiaoke huaya tablet (Zhi My, Gui JIan Yu etc), dosage: 0.5g three times per day plus lifestyle modification (diet & exercise) |
| CONTROL: lifestyle modification (diet & exercise alone) | |
| Outcomes | FBG (mmol/L), 2hr-GTT (mmol/L), HbA1c (%), triglycerides (mmol/L), total cholesterol (mmol/L), normalisation of FBG (n), incidence of diabetes (n) |
| Outcomes were measured at baseline and trial completion (8 wks). | |
| Study details | DURATION OF INTERVENTION: 8 weeks |
| DURATION OF FOLLOW-UP: 8 weeks | |
| RUN-IN PERIOD: none | |
| Publication details | LANGUAGE OF PUBLICATION: Chinese |
| COMMERCIAL FUNDING: No | |
| NON-COMMERCIAL FUNDING: Yes (Translational Funding Project) | |
| PUBLICATION STATUS (PEER REVIEW JOURNAL): Yes | |
| PUBLICATION STATUS (JOURNAL SUPPLEMENT): No | |
| PUBLICATION STATUS (ABSTRACT): Yes | |
| Stated aim of study | Quote “to validate the therapeutic effects of Xiaoke Huaya tablet in the impaired glucose tolerance population ” |
| Notes | |
Risk of bias table
| Item | Authors’ judgement |
Support for judgement |
|---|---|---|
| Adequate sequence generation? | Unclear | Randomisation method not mentioned in paper and unable to contact author. |
| Allocation concealment? | Unclear | Allocation concealment not mentioned in paper and unable to contact author. |
| Blinding? | No | Participants were not blinded. It is not clear if the outcome assessor or intervention providers were blinded. Unable to contact author. |
| Incomplete outcome data addressed? |
Yes | All participants reported. No withdrawals. |
| Free of selective reporting? | Unclear | No protocol but all nominated and expected outcomes reported. |
| Free of other bias? | Yes | None identified. |
| Methods | Parallel placebo-controlled randomised trial |
| Participants | SETTING: Outpatients at the Obesity Clinic of Kyoto Prefectual University, Japan |
| WHO PARTCIPATED: n= 81 (44 in treatment group mean age 52.6 yrs, mean weight 90.8 kg, mean BMI 36.7; in the 41 in control group mean age 54.8, mean weight 90.3, mean BMI 36.1) | |
| INCLUSION CRITERIA: IGT (WHO 1999) and obese | |
| EXCLUSION CRITERIA: People with kidney, heart and/or liver disease, any metabolic or endocrine disease, psychiatric disorders and cancer. | |
| CO-MORBIDITIES: obesity | |
| CO-MEDICATIONS: none reported | |
| Interventions | INTERVENTION: Bofu-tsusho-san (Scutellariae Radix, Glycyrrhizae Radix, Platycodi Radix, Gypsum Fibrosum, Atractylodis Rhizoma, Rhei Rhizoma, Schizonepetae Spica, Gardeniae Fructus, Paeoniae Radix, Cnidium Rhizoma, Angelicae Radix, Menthae Herba, Ledebouriellae Radix, Ephedrae Herba, Forsythiae Fructus, Zingiberis Rhizoma, Talcum, Natrium Sulphuricum), dry extract, three times a day (t.i.d) 30 mins before meals, for 24 wks plus lifestyle modification (diet & exercise). |
| CONTROL: placebo three times a day (t.i.d) 30 mins before meals, for 24wks plus lifestyle modification (diet & exercise) | |
| Lifestyle modification for all participants involved a diet of 1200 kcal/day, analysed based on food ingestion records, and exercise (5000 steps/day) determined by pedometer recordings. | |
| Outcomes | FBG (mg/dL), 2hr-GTT (mg/dL), HbA1c (%), triglycerides (mg/dL), HDL (mg/dL), LDL (mg/dL), total cholesterol (mg/dL), fasting insulin (μU/mL), 2hr insulin (μU/mL), insulin AUC, HOMA-IR. |
| Outcomes for all measures were assessed at baseline, 12 weeks, and 24 weeks. | |
| Note: For FBG mg/dL & 2hr-GTT conversion to mmol/L: mg/dl of glucose to mmol/l, divided by 18. | |
| For total cholesterol, HDL, LDL mg/dL conversion to mmol/L: convert mg/dl of HDL or LDL cholesterol to mmol/l, divided by 38.67. | |
| For triglycerides mg/dL conversion to mmol/L: mg/dl of triglycerides to mmol/l, divide by 89. | |
| Study details | DURATION OF INTERVENTION: 24 weeks |
| DURATION OF FOLLOW-UP: 24 weeks | |
| RUN-IN PERIOD: after 2 months of lifestyle modification (diet and exercise therapy as described above), the active drug or placebo was introduced. | |
| Publication details | LANGUAGE OF PUBLICATION: English and Japanese |
| COMMERCIAL FUNDING: no | |
| NON-COMMERCIAL FUNDING: This study was supported, in part, by a Grant-in-Aid (No.14571106; to TY) for Scientific Research from the Ministry of Education, Culture, Sports, Science and Technology of Japan. | |
| PUBLICATION STATUS (PEER REVIEW JOURNAL): yes | |
| PUBLICATION STATUS (JOURNAL SUPPLEMENT): no | |
| PUBLICATION STATUS (ABSTRACT): yes | |
| Stated aim of study | The aim of the study was to determine whether BF was effective in decreasing visceral adiposity and insulin resistance. |
| Notes | |
Risk of bias table
| Item | Authors’ judgement |
Support for judgement |
|---|---|---|
| Adequate sequence generation? | Yes | Randomisation by random number table |
| Allocation concealment? | Yes | As a placebo controlled study it is likely that allocation was concealed. |
| Blinding? | Yes | Participants were blinded (placebo controlled), outcome assessors blinded. |
| Incomplete outcome data addressed? |
Yes | All withdrawals are explained and all participant data included. Four withdrawals; 3 from treatment group for non-compliance because of loose bowels; 1 withdrew from control for non-compliance. The data of these subjects was excluded from the analysis. The baseline data of all 85 subjects was not significantly different from those of the 81 women. |
| Free of selective reporting? | Unclear | No protocol provided but all nominated and expected outcomes are reported. |
| Free of other bias? | Yes | None identified. |
| Methods | Parallel randomised controlled trial |
| Participants | SETTING: inpatients and outpatients at Nanning TCM hospital, China |
| WHO PARTCIPATED: n= 64; (28/36 (M/F); mean age 50.9yrs; 31 in treatment group; 33 in control group) | |
| INCLUSION CRITERIA: IGT (WHO 1999) | |
| EXCLUSION CRITERIA: People <40yrs, BMI <19kg/m, hypertension, IGT induced by other organic diseases, drugs or stress. | |
| CO-MORBIDITIES: none reported | |
| CO-MEDICATIONS: none reported | |
| Interventions | INTERVENTION: Qimai jiangtang yin (huang qi 20g, ge gen 20g, mai dong 10g, nu zhen zi 10g, san qi 10g, yu jin 10g, sheng di huang 15g) decoction taken 1 dose (100ml) every two days in 1st and 2nd months plus lifestyle modification (diet & lifestyle advice) |
| CONTROL: Lifestyle modification (diet & lifestyle advice) | |
| Outcomes | FBG (mmol/L), 2hr GTT (mmol/L), fasting insulin, IAI, normalisation rate of IGT (n), incidence of diabetes |
| Outcomes were measured at baseline and at trial completion (12 months). | |
| Study details | DURATION OF INTERVENTION: 12 months |
| DURATION OF FOLLOW-UP: 12 months | |
| RUN-IN PERIOD: none | |
| Publication details | LANGUAGE OF PUBLICATION: Chinese |
| COMMERCIAL FUNDING: no | |
| NON-COMMERCIAL FUNDING: no | |
| PUBLICATION STATUS (PEER REVIEW JOURNAL): yes | |
| PUBLICATION STATUS (JOURNAL SUPPLEMENT): no | |
| PUBLICATION STATUS (ABSTRACT): yes | |
| Stated aim of study | To observe the effect of Qimai Jingtang yin on impaired glucose tolerance. |
| Notes | |
Risk of bias table
| Item | Authors’ judgement |
Support for judgement |
|---|---|---|
| Adequate sequence generation? | Unclear | Method not reported in published article and unable to contact authors to get further information. |
| Allocation concealment? | Unclear | Unable to contact authors to get further information. |
| Blinding? | No | Probably not as the control group were not taking a placebo. |
| Incomplete outcome data addressed? |
Yes | All participant data reported. |
| Free of selective reporting? | Unclear | No protocol provided, but all nominated and expected outcomes reported. |
| Free of other bias? | Yes | None identified. |
| Methods | Parallel randomised controlled trial (block randomisation, 3 participants in the intervention group : 2 in the control group) |
| Participants | SETTING: Outpatients presenting at Guigang City TCM Hospital, China |
| WHO PARTCIPATED: 80 (48 in treatment group, M/F 29/19, mean age 48.08 yrs; 32 in control group, M/F 19/13, mean age 47.62 yrs) | |
| INCLUSION CRITERIA: IGT (ADA 1997): FBG ≥ and less than 7.0 AND 2-hr TT (75g) ≥7.8<11.1 PLUS either hypertension systolic ≥18.7kpa and/or diastolic ≥12.0; OR total cholesterol ≥5.7mmol/L OR triglycerides ≥2.26 mmol/L OR HDL ≤1.04 mmol/L. | |
| EXCLUSION CRITERIA: none reported | |
| CO-MORBIDITIES: primary hypertension | |
| CO-MEDICATIONS: none reported | |
| Interventions | INTERVENTION: Yi qi yang yin huo xue (Huang qi, Dang gui, Shan yao, Sang bai pi, Sang ye, Sang zhi) 100 ml per dose, 3 times per day, plus Beijing Jiang Ya No. 0 tablets (Blood pressure lowering medication: Pterofen 12.5 mg, Dihydralazine Sulfate 12.5 mg, reserpine 0.1 mg) |
| CONTROL: Beijing Jiang Ya No. 0 tablets (Blood pressure lowering medication: Pterofen 12.5 mg, Dihydralazine Sulfate 12.5 mg, reserpine 0.1 mg) | |
| Outcomes | Blood pressure, fasting blood glucose (mmol/L), 2hr-GTT (mmol/L), total cholestrol (mmol/L), triglycerides (mmol/L), HDL (mmol/L), and quality of life assessment (Chinese scale, Du 1994). |
| Outcomes were measured at baseline and trial completion (28d) | |
| This study reported that there were no adverse findings in renal, liver, and ECG tests. | |
| Study details | DURATION OF INTERVENTION: 28 days |
| DURATION OF FOLLOW-UP: 28 days | |
| RUN-IN PERIOD: none | |
| Publication details | LANGUAGE OF PUBLICATION: Chinese |
| COMMERCIAL FUNDING: No | |
| NON-COMMERCIAL FUNDING: No | |
| PUBLICATION STATUS (PEER REVIEW JOURNAL): yes | |
| PUBLICATION STATUS (JOURNAL SUPPLEMENT): no | |
| PUBLICATION STATUS (ABSTRACT): yes | |
| Stated aim of study | “To observe the influences of combination of Chinese and western therapies on the life quality and blood-fat [lipids] of primary hypertension patients with declined glucose tolerance.” |
| Notes | |
Risk of bias table
| Item | Authors’ judgement |
Support for judgement |
|---|---|---|
| Adequate sequence generation? | Yes | Phone call: random table used randomisation sequence |
| Allocation concealment? | Unclear | No information in the report or from phone call. |
| Blinding? | No | Noticeably different interventions provided, blinding is unlikely and not mentioned. |
| Incomplete outcome data addressed? |
Yes | No withdrawals or exclusions, all participant data reported. |
| Free of selective reporting? | Unclear | Nominated and expected outcomes were reported but no protocol was available. |
| Free of other bias? | Yes | None identified. |
| Methods | Parallel randomised controlled trial |
| Participants | SETTING: inpatients and outpatients at Huaian County Hospital |
| WHO PARTCIPATED: n=60 (30 in treatment group, M/F 21/9, mean age 48yrs and 30 in control group, M/F 17/13, mean age 49yrs) | |
| INCLUSION CRITERIA: IGT (ADA 1997) | |
| EXCLUSION CRITERIA: none reported | |
| CO-MORBIDITIES: none reported | |
| CO-MEDICATIONS: none reported | |
| Interventions | INTERVENTION: Tang ping san (Huang Qi 30g, Shan Yao 10g, Sheng Di Huang 10g, Shu Di Huang 10g, Gou Qi Zi 10g, He Shou Wu 10g, Xian Ling Pi 10g, Dan Shen 30g, Ze Xie 10g, Sang Ye 10g), oral, decoction, once per day (b.i.d), plus lifestyle modification (diet & lifestyle advice) |
| CONTROL: Metformin, oral, 0.25g per dose, three times a day (t.i.d), plus lifestyle modification (diet & lifestyle advice) | |
| Outcomes | 2hr-GTT (mmol/L) measured at baseline, at trial completion (3 months) and at follow- up (6 months) |
| Study details | DURATION OF INTERVENTION: 12 weeks |
| DURATION OF FOLLOW-UP: 3 months | |
| RUN-IN PERIOD: none | |
| Publication details | LANGUAGE OF PUBLICATION: Chinese |
| COMMERCIAL FUNDING: No | |
| NON-COMMERCIAL FUNDING: Yes (Scientific research funding by Municipal Government). | |
| PUBLICATION STATUS (PEER REVIEW JOURNAL): Yes | |
| PUBLICATION STATUS (JOURNAL SUPPLEMENT): no | |
| PUBLICATION STATUS (ABSTRACT): no | |
| Stated aim of study | Quote “to observe the treatment of impaired glucose with Tangping san”. |
| Notes | |
Risk of bias table
| Item | Authors’ judgement |
Support for judgement |
|---|---|---|
| Adequate sequence generation? | Unclear | The method of randomisation was not stated. We were unable to contact the author about the method used. |
| Allocation concealment? | Unclear | Comment: unable to contact the author about the method used. |
| Blinding? | No | Comment: both received an intervention but of a different nature (powder vs pill). It’s likely that participants and intervention providers knew the intervention they were receiving. |
| Incomplete outcome data addressed? |
Yes | All participants data reported. |
| Free of selective reporting? | Unclear | No protocol but all expected and nominated outcomes reported. |
| Free of other bias? | Yes | None reported. |
| Methods | Parallel randomised controlled trial |
| Participants | SETTING: Inpatients and outpatients at Liyuan Hospital, China |
| WHO PARTCIPATED: 62 people (32 in intervention group, M/F 17/15, mean age 65.3 yrs, mean disease duration 3.1yrs, mean weight 82.3kg; 30 in control group, M/F 15/15, mean age 66.1 yrs, mean disease duration 3.6yrs, mean weight 82.1kg) | |
| INCLUSION CRITERIA: IGT according to WHO,1998 | |
| EXCLUSION CRITERIA: none reported | |
| CO-MORBIDITIES: none reported | |
| CO-MEDICATIONS: none reported | |
| Interventions | INTERVENTION: Fufang cangzhu decoction (cang zhu 15g, yi yi ren 24g, sang shen 20g, shan yao 30g, huang bai 10g, li zhi hue 20g, di long 10g) oral, decoction, 150ml, bid |
| CONTROL: metformin 0.25g, oral, tablet, tid | |
| Outcomes | FBG (mmol/L), 1hr-GTT (mmol/L), 2hr-GTT (mmol/L), weight (kg), waist-hip ratio (WHR), triglycerides (mmol/L), total cholesterol (mmol/L), fasting insulin (mU/L) |
| All outcomes measured at baseline and at trial completion (8 weeks). | |
| Study details | DURATION OF INTERVENTION: 8 weeks |
| DURATION OF FOLLOW-UP: 8 weeks | |
| RUN-IN PERIOD: none | |
| Publication details | LANGUAGE OF PUBLICATION: Chinese |
| COMMERCIAL FUNDING: no | |
| NON-COMMERCIAL FUNDING: no | |
| PUBLICATION STATUS (PEER REVIEW JOURNAL): yes | |
| PUBLICATION STATUS (JOURNAL SUPPLEMENT): no | |
| PUBLICATION STATUS (ABSTRACT): yes | |
| Stated aim of study | Quote “To observe therapeutic effect of Fufang Cangzhu Decoction on senile obesity or overweight with impaired glucose tolerance (IGT)”. |
| Notes | |
Risk of bias table
| Item | Authors’ judgement |
Support for judgement |
|---|---|---|
| Adequate sequence generation? | Yes | The authors were interviewed by phone and stated that the randomisation was done by use of a random table. |
| Allocation concealment? | Unclear | No details provided on allocation concealment. |
| Blinding? | No | Assessors blinded; participants could not be blinded as one group was taking capsule and the other a decoction. Intervention provider not blinded. |
| Incomplete outcome data addressed? |
Yes | All participants reported. No withdrawals. |
| Free of selective reporting? | Unclear | All expected and nominated outcomes reported. |
| Free of other bias? | Yes | None reported. |
| Methods | Parallel randomised controlled trial, three arms: Chinese herbal medicines plus diet & lifestyle vs acabose plus diet & lifestyle vs diet & lifestyle |
| Participants | SETTING: outpatients at the Intergrated Medicine Hospital of Guangdong Province, China |
| WHO PARTCIPATED: 120 participants (40 in intervention I, M/F 24/16, mean age 53.5yrs; 40 in intervention II, M/F 20/20, mean age 54.8yrs; 40 in the control, M/F 22/18, mean age 50.6yrs) | |
| INCLUSION CRITERIA: IGT WHO 1999 | |
| EXCLUSION CRITERIA: <35yrs, BMI <19, hypertension level 3, severe liver or kidney diseases, other endocrine diseases, any IGT caused by medication or high levels of stress. | |
| CO-MORBIDITIES: none stated | |
| CO-MEDICATIONS: none stated | |
| Interventions | INTERVENTION I: Jian Pi Zhi Shen Huo Xue (shan yao 30g, shan zha 30g, huang qi 20g, fu ling 20g, shan zhu yu 15g, tao ren 10g) plus diet & lifestyle once per day. |
| INTERVENTION II: Acarbose 50mg per dose, three times a day plus diet & lifestyle | |
| CONTROL: diet & lifestyle alone | |
| Outcomes | BMI (kg/m2), FBG (mmol/L), 2hr-GTT (mmol/L), HbA1c (%), trigylcerides (mmol/L), total cholesterol (mmol/L), HDL (mmol/L0, LDL (mmol/L), FINS (mmol/L), ISI, normalisation of blood glucose (n), incidence of diabetes (n) |
| All outcomes assessed at baseline, 6 months and at trial completion (12 months). | |
| Study details | DURATION OF INTERVENTION: 12 months. |
| After 6 months a 2hr-GTT to check if participants had progressed to diabetes which was the endpoint, if normal GT or still IGT one or more treatment courses given. | |
| DURATION OF FOLLOW-UP: 12 months | |
| RUN-IN PERIOD: none | |
| Publication details | LANGUAGE OF PUBLICATION: Chinese |
| COMMERCIAL FUNDING: no | |
| NON-COMMERCIAL FUNDING: no | |
| PUBLICATION STATUS (PEER REVIEW JOURNAL): yes | |
| PUBLICATION STATUS (JOURNAL SUPPLEMENT): no | |
| PUBLICATION STATUS (ABSTRACT): yes | |
| Stated aim of study | Quote “to study the method of strengthen the spleen, nourish the kidney and activate the blood method for the treatment of impaired glucose tolerance.” |
| Notes | |
Risk of bias table
| Item | Authors’ judgement |
Support for judgement |
|---|---|---|
| Adequate sequence generation? | Yes | A random table was used to divide the sample into 3 groups (40 in each group). |
| Allocation concealment? | Unclear | No information provided in the report on allocation concealment. |
| Blinding? | No | Not reported, unlikely given the design of the trial consisting of a chinese herbal powder, western pharmaceutical tablet and a group taking no medication. |
| Incomplete outcome data addressed? |
Yes | No missing data. 5 withdrawals: 2 from intervention I; reason given: could not continue with medication; 2 from intervention II reason given: could not continue with medication; 1 from the control; reason given: participant left the area. |
| Free of selective reporting? | Unclear | No protocol provided and all nominated outcomes reported. |
| Free of other bias? | Yes | None reported. |
| Methods | Parallel randomised controlled trial |
| Participants | SETTING: China |
| WHO PARTCIPATED: 95 participants (48 in intervention, M/F 32/16, mean age 53.5yrs; 47 in control, M/F 29/18, mean age 53.5yrs) | |
| INCLUSION CRITERIA: IGT (WHO 1999); >30yrs<60yrs; not taking any medication that influences glucose levels for at lease one month; willingness to comply with the trial and examination; | |
| EXCLUSION CRITERIA: Level 3 hypertension; serious heart, liver or kidney dysfunction; mental diseases; allergic condition; pregnancy or lactation. | |
| DIAGNOSTIC CRITERIA: IGT WHO 1999 | |
| CO-MORBIDITIES: none stated | |
| CO-MEDICATIONS: none stated | |
| Interventions | INTERVENTION: Qiweitangping capsule (huang qi, huang qin, zi su zi, dang shen, da huang, da zao, shu di huang, chai hu, dan shen, yu jin, yin chen, tian hua fen, shi gao, che qian zi, shan yao, we wei zi, shan zhu yu, zhi mu, gou qi zi, ge gen, bai he, gua lou, wu yao, di huang, hua jiao, wang bu liu xing, gan cao) 3 capsule twice daily |
| CONTROL: placebo 3 capsules twice daily oral | |
| Outcomes | FBG (mmol/L), 2hr-GTT (mmol/L), BMI (kg/m2), WHR |
| All outcomes were measured at baseline and trial completion (24 months). | |
| Adverse effects: one participant from treatment and one from the control developed abdominal discomfort. Both were resolved without any treatment | |
| Study details | DURATION OF INTERVENTION: 24 months |
| DURATION OF FOLLOW-UP: 24 months | |
| RUN-IN PERIOD: none | |
| Publication details | LANGUAGE OF PUBLICATION: Chinese |
| COMMERCIAL FUNDING: no | |
| NON-COMMERCIAL FUNDING: no | |
| PUBLICATION STATUS (PEER REVIEW JOURNAL): yes | |
| PUBLICATION STATUS (JOURNAL SUPPLEMENT): no | |
| PUBLICATION STATUS (ABSTRACT): yes | |
| Stated aim of study | Quote “To observe the intervention effects of Qiweitangping capsule on impaired glucose tolerance (IGT) and on the morbidity of diabetes mellitus (DM)and the effect of conversing IFF to normal glucose tolerance (NGT) ” |
| Notes | |
Risk of bias table
| Item | Authors’ judgement |
Support for judgement |
|---|---|---|
| Adequate sequence generation? | Yes | SAS was used to generate the allocation sequence. |
| Allocation concealment? | Yes | Both the participant and the intervention provider did not know the allocation groups. |
| Blinding? | Yes | Participants, doctor and outcomes assessor all blinded. |
| Incomplete outcome data addressed? |
Yes | All participant data reported. 5 withdrawals. |
| Free of selective reporting? | Unclear | No protocol reported but all expected and nominated outcome measures were reported. |
| Free of other bias? | Yes | None identified. |
| Methods | Parallel randomised controlled trial |
| Participants | SETTING: Tianjin No. 1 Zhong Xin Hospital outpatients, China |
| WHO PARTCIPATED: n=159 (81 in the intervention group, M/F 40/41; 78 in the control group, M/F 37/41) | |
| INCLUSION CRITERIA: IGT (WHO 1999) and/or IFG (ADA 1997) | |
| EXCLUSION CRITERIA: None reported | |
| CO-MORBIDITIES: None reported | |
| CO-MEDICATIONS: None reported | |
| Interventions | INTERVENTION: Jinqi Jiangtang 4-7 tablets each dose, three times a day plus basic education (no diet or exercise) |
| CONTROL (ROUTE, TOTAL DOSE/DAY, FREQUENCY): basic education alone (no diet or exercise) | |
| Outcomes | Fasting blood glucose (mmol/L), 2hr-GTT (mmol/L), normalisation of glucose tolerance (n), total cholesterol (mmol/L), triglycerides (mmol/L), systolic blood pressure (mm/Hg), diastolic blood pressure (mm/Hg) |
| Adverse effects: 3 cases in the intervention group developed mild GIT symptoms in the early stage of taking the Chinese herbal medicine. These resolved after one to two weeks. No other adverse effects were observed. | |
| Study details | DURATION OF INTERVENTION: 2 years |
| DURATION OF FOLLOW-UP: 2 years | |
| RUN-IN PERIOD: none | |
| Publication details | LANGUAGE OF PUBLICATION: Chinese |
| COMMERCIAL FUNDING: no | |
| NON-COMMERCIAL FUNDING: no | |
| PUBLICATION STATUS (PEER REVIEW JOURNAL): yes | |
| PUBLICATION STATUS (JOURNAL SUPPLEMENT): no | |
| PUBLICATION STATUS (ABSTRACT): no | |
| Stated aim of study | Quote “To observe the effect of Jinqi Jiangtang tablet on preventing patients with impaired glucose becoming diabetes” |
| Notes | |
Risk of bias table
| Item | Authors’ judgement |
Support for judgement |
|---|---|---|
| Adequate sequence generation? | Unclear | Stated as randomised but unable to contact the authors. |
| Allocation concealment? | Unclear | No information available in report. |
| Blinding? | No | Unlikely due to the nature of the medication intervention (tablets vs no medication). |
| Incomplete outcome data addressed? |
Yes | Three withdrawals reported in the control group. All data for the other participants reported. |
| Free of selective reporting? | Unclear | No protocol provided but all expected and nominated outcomes reported. |
| Free of other bias? | Yes | None identified. |
| Methods | Parallel randomised controlled trial |
| Participants | SETTING: Outpatients at the Foshan TCM Hospital, Guangdong Province, China |
| WHO PARTCIPATED: n=72 (36 in the intervention group, M/F 19/13, mean age 46.3yrs; 36 in the control group, M/F 20/13, mean age 47.1yrs) | |
| INCLUSION CRITERIA: IGT (WHO 1999) | |
| EXCLUSION CRITERIA: none reported | |
| CO-MORBIDITIES: none reported | |
| CO-MEDICATIONS none reported | |
| Interventions | INTERVENTION (ROUTE, TOTAL DOSE/DAY, FREQUENCY): Xiaoke Yuye (Huang qi, huang jing, he shou wu, zhi mu) 10ml three times per day, plus lifestyle modification (diet and exercise) |
| CONTROL: lifestyle modification (diet and exercise) | |
| Outcomes | Fasting blood glucose (mmol/L), 2hr-GTT (mmol/L), HbA1c (%), total cholesterol (mmol/L), BMI, normalisation of fasting blood glucose (n), incidence of diabetes (n) |
| All outcomes were measured at baseline and at trial completion (24 months) | |
| No adverse effects in the treatment group. | |
| Study details | DURATION OF INTERVENTION: 2 years |
| DURATION OF FOLLOW-UP: 2 years | |
| RUN-IN PERIOD: none | |
| Publication details | LANGUAGE OF PUBLICATION: Chinese |
| COMMERCIAL FUNDING: no | |
| NON-COMMERCIAL FUNDING: no | |
| PUBLICATION STATUS (PEER REVIEW JOURNAL): yes | |
| PUBLICATION STATUS (JOURNAL SUPPLEMENT): no | |
| PUBLICATION STATUS (ABSTRACT): yes | |
| Stated aim of study | Quote “To observe the effects of Xiaoke yuye on patients with impaired glucose tolerance.” |
| Notes | |
Risk of bias table
| Item | Authors’ judgement |
Support for judgement |
|---|---|---|
| Adequate sequence generation? | Yes | Authors were interviewed by phone and stated that a random table was used. |
| Allocation concealment? | Yes | Allocation concealment not mentioned in the report. Information obtained from phone interview that numbers drawn from a sealed box designated the random number allocation. |
| Blinding? | No | No placebo so probably not blinded. |
| Incomplete outcome data addressed? |
Yes | 7 withdrawals reported, no reason given. Four in the intervention group and 3 in the control group. Baseline data reported included participants only. No difference at baseline. |
| Free of selective reporting? | Unclear | No protocol provided but all nominated outcomes reported. |
| Free of other bias? | Yes | None identified. |
| Methods | Parallel randomised controlled trial |
| Participants | SETTING: Hospital outpatients and patients recruited from general company check-up, Huabei Petroleum Two Hospital, Hebei, China |
| WHO PARTCIPATED: n=76 (40 in the intervention group M/F 31/19, mean age 44.2yrs; 36 in the control M/F 23/13, mean age 43.9yrs) | |
| INCLUSION CRITERIA: IGT (ADA 1997): FBG <7.0 and 2hr-GTT (75g) ≥7.8 <11.1 | |
| EXCLUSION CRITERIA: <20yrs <65yrs; IGT induced by other disorders, drugs or stress, pregnancy, serious liver, kidney or heart disorders. | |
| CO-MORBIDITIES: none reported | |
| CO-MEDICATIONS: none reported | |
| Interventions | INTERVENTION: Tang Kang Yin decoction (ren shen 6g, huang lian 10g, nu zhen zi 15g, xia ku cao 30g, fan shi liu ye 30g) oral, 200ml, 2x/day, plus lifestyle modification (diet & exercise) |
| CONTROL: lifestyle modification (diet & lifestyle) | |
| Outcomes | Fasting blood glucose (mmol/L), 2hr-GTT (mmol/L), insulin (ng/ml), triglycerides (mmol/L), total cholesterol (mmol/L), BMI, normalisation of fasting bloood glucose (n), incidence of diabetes (n) |
| All outcomes were assessed at baseline and trial completion (30 days). | |
| Study details | DURATION OF INTERVENTION: 30 days |
| DURATION OF FOLLOW-UP: 30 days | |
| RUN-IN PERIOD: none | |
| Publication details | LANGUAGE OF PUBLICATION: Chinese |
| COMMERCIAL FUNDING: no | |
| NON-COMMERCIAL FUNDING: no | |
| PUBLICATION STATUS (PEER REVIEW JOURNAL): no | |
| PUBLICATION STATUS (JOURNAL SUPPLEMENT): no | |
| PUBLICATION STATUS (ABSTRACT): yes | |
| Stated aim of study | Quote “To observe the clinical effect of intervention of Tangganyin decoction on patients with Impaired glucose tolerance ( IGT) .” |
| Notes | |
Risk of bias table
| Item | Authors’ judgement |
Support for judgement |
|---|---|---|
| Adequate sequence generation? | Yes | The authors were interviewed by phone and stated that randomisation was conducted by using a random table from the internet. |
| Allocation concealment? | No | The intervention provider knew the which group each participant was to be allocated to. |
| Blinding? | No | Not stated in the report. From phone interview “participants and intervention provider were not blinded”. |
| Incomplete outcome data addressed? |
Yes | All participants reported. No withdrawals. |
| Free of selective reporting? | Unclear | No protocol provided but all expected and nominated outcomes reported. |
| Free of other bias? | Yes | None identified. |
| Methods | Parallel randomised controlled trial |
| Participants | SETTING: Hospital outpatients and patients recruited from general company check-up; Shugang Hospital, Shanghai, China |
| WHO PARTCIPATED: 42 (22 in the intervention group, M/F 8/14; and 20 in the control group M/F 8/12) | |
| INCLUSION CRITERIA: IGT (WHO 1985) | |
| EXCLUSION CRITERIA: disorders that interfere with glucose metabolism | |
| CO-MORBIDITIES: none reported | |
| CO-MEDICATIONS: none reported | |
| Interventions | INTERVENTION: Tangheng I, 2 bags twice a day for 3 mth splus lifestyle modification (diet & exercise) |
| CONTROL: lifestyle modification (diet and exercise) for 3 months | |
| Outcomes | FBG (mmol/L), 2hr-GTT (mmol/L), insulin (ng/mL), BMI, systolic blood pressure (mmHg), diastolic blood pressure (mmHg) |
| All outcomes were assessed at baseline and trial completion (3 months) | |
| Study details | DURATION OF INTERVENTION: 3 months |
| DURATION OF FOLLOW-UP: 3 months | |
| RUN-IN PERIOD: none | |
| Publication details | LANGUAGE OF PUBLICATION: Chinese |
| COMMERCIAL FUNDING: no | |
| NON-COMMERCIAL FUNDING: no | |
| PUBLICATION STATUS (PEER REVIEW JOURNAL): yes | |
| PUBLICATION STATUS (JOURNAL SUPPLEMENT): no | |
| PUBLICATION STATUS (ABSTRACT): yes | |
| Stated aim of study | Quote “To observe the intervention on 42 cases of impaired glucose with Tangheng I.” |
| Notes | |
| Item | Authors’ judgement |
Support for judgement |
|---|---|---|
| Adequate sequence generation? | Unclear | Method not reported and unable to contact authors. |
| Allocation concealment? | Unclear | Method not reported and unable to contact authors. |
| Blinding? | No | No placebo so probably not blinded. |
| Incomplete outcome data addressed? |
Yes | No missing data. No withdrawals. |
| Free of selective reporting? | Unclear | No protocol provided. All expected and nominated outcomes appropriately reported. |
| Free of other bias? | Yes | None identified. |
| Methods | Parallel randomised controlled trial |
| Participants | SETTING: Hospital outpatients, Guangdong Province People’s Hospital, China |
| WHO PARTCIPATED: n=166 (I: n=56, 29/27 m/f, mean age 53.12yrs; II: n=55 m/f 29/26, mean age 52.68yrs; Control: n=55, m/f 30/25, mean age 52.45yrs) | |
| INCLUSION CRITERIA: IGT (WHO 1985) | |
| EXCLUSION CRITERIA: none reported | |
| CO-MORBIDITIES: none reported | |
| CO-MEDICATIONS:none reported | |
| Interventions | INTERVENTION 1: Liu wei di huang capsule, oral, 2 capsules bid, plus lifestyle modification involving a strict diet prescription, frequent diabetes lectures, moderate exercise. |
| INTERVENTION 2: lifestyle modification as per intervention 1. | |
| CONTROL: pamphlet on diabetes only (no diet or exercise). | |
| Outcomes | FBG (mmol/L), 2-hr GTT (mmol/L), HbA1c (%), total cholesterol (mmol/L), triglycerides (mmol/L), BMI (kg/m2), blood pressure |
| Study details | DURATION OF INTERVENTION: 18 months |
| DURATION OF FOLLOW-UP: 18 months | |
| RUN-IN PERIOD: none | |
| Publication details | LANGUAGE OF PUBLICATION: Chinese |
| COMMERCIAL FUNDING: no | |
| NON-COMMERCIAL FUNDING: no | |
| PUBLICATION STATUS (PEER REVIEW JOURNAL): yes | |
| PUBLICATION STATUS (JOURNAL SUPPLEMENT): no | |
| PUBLICATION STATUS (ABSTRACT): yes | |
| Stated aim of study | Quote “To study the effect of Liu wei di huang pill for the treatment of IGT to reduce the risk of CVD”. |
| Notes | Two articles about this study, one reports only two groups (2000) and the other three groups (2006). |
Risk of bias table
| Item | Authors’ judgement |
Support for judgement |
|---|---|---|
| Adequate sequence generation? | Yes | The authors were interviewed by phone and advised that randomisation by a random table. |
| Allocation concealment? | Yes | Allocation unknown to groups and intervention provider until after allocation. |
| Blinding? | No | No placebo so not blinded. |
| Incomplete outcome data addressed? |
No | HbA1c was nominated as a collected outcome but not reported. No withdrawals. |
| Free of selective reporting? | Unclear | A study protocol is not available but the published data includes all nominated and expected outcomes. |
| Free of other bias? | Yes | None identified. |
| Methods | Parallel randomised controlled trial |
| Participants | SETTING: Outpatients, Zhejiang Province, Hangzhou Red Cross Hospital, China |
| WHO PARTCIPATED: n=88 (46 in the intervention group M/F 18/28, mean age 55.6 yrs; 42 in the control M/F 17/25, mean age 54.0 yrs) | |
| INCLUSION CRITERIA: IGT (WHO 1985) | |
| EXCLUSION CRITERIA: none reported | |
| CO-MORBIDITIES: none reported | |
| CO-MEDICATIONS:none reported | |
| Interventions | INTERVENTION: Jinqi Jiangtang tablet, oral, 7 tablets, 0.42g per tablet, three times per day, 30mins before meals plus lifestyle modification (diet and exercise) |
| CONTROL: lifestyle modification (diet and exercise) | |
| Outcomes | Fasting blood glucose (mmol/L), 2hr-GTT (mmol/L), fasting insulin, 2hr-insulin, triglycerides, total cholesterol, HDL, systolic blood pressure (kpa), diastolic blood pressure (kpa), normalisation of fasting blood glucose (n), incidence of diabetes (n) |
| Blood pressure measurement converted as follows: 1 mmHg = 0.133 kPa | |
| All outcomes reported at baseline, 3 months, 6 months and trial completion (12 months) | |
| Study details | DURATION OF INTERVENTION: 12 months |
| DURATION OF FOLLOW-UP: 12 months | |
| RUN-IN PERIOD: none | |
| Publication details | LANGUAGE OF PUBLICATION: Chinese |
| COMMERCIAL FUNDING: no | |
| NON-COMMERCIAL FUNDING: no | |
| PUBLICATION STATUS (PEER REVIEW JOURNAL): yes | |
| PUBLICATION STATUS (JOURNAL SUPPLEMENT): no | |
| PUBLICATION STATUS (ABSTRACT): no | |
| Stated aim of study | To observe the effect of Jingqi Jiangtang tablet on non-overweight people with impaired glucose tolerance. |
| Notes | |
Risk of bias table
| Item | Authors’ judgement |
Support for judgement |
|---|---|---|
| Adequate sequence generation? | Yes | The authors were interviewed by phone and advised that randomisation by a random table |
| Allocation concealment? | No | Not mentioned in the report. From the phone interview: not adequate allocation concealment |
| Blinding? | No | No placebo so probably not blinded. |
| Incomplete outcome data addressed? |
Yes | All participant data reported, no withdrawals. |
| Free of selective reporting? | Unclear | No protocol provided. All expected and nominated outcomes appropriately reported. |
| Free of other bias? | Yes | None identified. |
ADA: American Diabetes Association; AUC: area under the curve; BMI: body-mass index; FBG: fasting blood glucose; GTT: (oral) glucose tolerance test; HbA1c: glycosylated haemoglobin A1c; HDL: high-density cholesterol; IGT: impaired glucose tolerance; M/F: male / female; LDL: low-density cholesterol; TCM: Traditional Chinese Medicine; t.i.d.: three times daily
Characteristics of excluded studies
| Reason for exclusion | Quasi-randomised study comparing the effects of a Chinese herbal formula (ren shen 10g, huang qi 30g, sheng di huang 15g, shan yao 15, bai zhu 15g, ban xia 10g, fu ling 10g, cang zhu 10g, dang shen 12g, chi shao 10g, chuan xiong 10g, shan zha 10g, ge gen 10g, tian hua fen 15g) plus diet and lifestyle advice versus diet and lifestyle advice alone in 80 participants with impaired glucose tolerance over 4 months. The report stated that the study was randomised. Information obtained from phone interview indicated that allocation was based on clinician’s decision. |
| Reason for exclusion | Quasi-randomised study comparing the effects of a Chinese herbal medicine formula (Shu di 20g, shan zhu yu 10g, shan yao 10g, ze xie 10g, mu dan pi 10g, fu ling 10g, ban xia 10g, chen pi 10g, dan shen 10g, gou qi zi 10g, shan zha 10g) versus metformin in 90 participants with impaired glucose tolerance over 4 months. The report stated that the study was randomised. Information obtained from phone interview indicated that allocation was based on clinician’s decision. |
| Reason for exclusion | Non-randomised study of Ke Tang Ling in people with IGT. |
| Reason for exclusion | Non-randomised study of Ke Tang Ling in people with IGT. Possible duplicate of Cai X 2001. |
| Reason for exclusion | Trial comparing two herbal medicines in people with IGT. This study did not meet the review criteria as it was an ineligible comparison. |
| Reason for exclusion | Case series of 48 people with IGT taking Jianpi nichan tang. |
| Reason for exclusion | Randomised study comparing the effects of Yuye Tang (Shanyao 30g, Shenghuangqi (raw Huangqi) 30g, Zhimu 10g, Jineijin 6g, Gegen 10g, Wuweizi 6g, Tianhuafen 30g, Huanqin 10g) versus dimethyldiguanide tablets in people with impaired glucose tolerance over 8 wks. The study reported that it randomly divided the sample into two groups. There is a large sampling discrepancy between the control (n = 43) and intervention group (n = 72) implying this was not random. Method of randomisation not reported. Unable to contact the authors. |
| Reason for exclusion | Non-randomised study of Jinqi jiangtang tablets (n = 42) versus metformin (n = 38) in 80 participants over 3 months. |
| Reason for exclusion | A three-arm study comparing the effects of Ketangling granules (sheng di 10g, shu di 15g, huang jing 10g, huang lian 5g, huang bai 6g, tian hua fen 10g, ze xie 15g, dan shen 10g, chuan xiong 10g, zhi da huang 6g) plus diet & lifestyle modification (n = 32) compared to diet & lifestyle alone (n = 14) and compared to no intervention (n = 16) in people with impaired glucose tolerance. The discrepancy in sampling numbers indicates that it is unlikely that the study was truly randomised. |
| Reason for exclusion | Quasi-randomised three-arm study comparing the effects of a Chinese herbal formula (huang qi, huang jing, ge gen, zhe xie, chai hui, gui jian yu etc) in 66 people with impaired glucose tolerance over 3 months. The report stated that the study was randomised. Information obtained from phone interview indicated that allocation was based on alternation. No blinding. |
| Reason for exclusion | Case series of a chinese herbal medicine in people with IGT. |
| Reason for exclusion | Case series of the effects of a chinese herbal medicine (ren shen 15g, ge gen 20g, bai zhu 10g, fu ling 30g, shan yao 30g, huang qi 30g, shan zhu yu 15g, shui zhi 10g, cang zhu 10, xuan shen 10g, tian hua fen 15g, huang lian 12g For obesity add ji nei jin 15g, shan zha 15g, yi mi 15g; For yin deficient heat add zhi mu 10g, sheng di huang 10g, and delete ren shen, huang qi) in 26 participants with impaired glucose tolerance. |
| Reason for exclusion | Case series of Huanyan kuguasu pills in people with IGT. |
| Reason for exclusion | Case series. |
| Reason for exclusion | Quasi-randomised study comparing the effects of Danshen injection plus xuezhi kang versus xuezhi kang (extract of cholestin, red yeast Chinese rice) in 62 participants with IGT over 4 weeks. The report stated that the study was randomised. Information obtained from phone interview indicated that allocation was based on the judgement of the clinician. |
| Reason for exclusion | Case series of chinese herbal medicine in 60 people with IGT. |
| Reason for exclusion | Quasi-randomised study comparing the effects of a Chinese herbal medicine (huang qi 20g, shan yao 20g, dan shen 20g, sheng di huang 20g, shu di huang 20g, tian hua fen 40g, fu ling 15g, chi shao 15g, chai hu 12g, shu da huang 10g, zhi shi 10g, huang qin 10g, qu mai 10g) versus metformin in 64 people over 12 months. The method of allocation to groups was inadequate. |
| From a phone call to the author it was ascertained that participants were allocated to the treatment according to the judgement of the clinician. |
| Reason for exclusion | Quasi-randomised study comparing the effects of Xiao yang tang (tao ren 15g, mu dan pi 15g, dan shen 15g, xuan shen 15g, da huang 10g, yu jin 10g, chuan bei mu 10g, lai fu zi 10g) versus metformin in 90 people with impaired glucose tolerance. The method of randomisation was alternation. |
| From a phone call it was determined that the participants were randomised on the basis of alternation. |
| Reason for exclusion | Randomised study comparing yangxing tongmai tables versus metformin in 67 people with insulin resistance. The criteria for inclusion in the study included people with IGT, diabetes or abnormal ISI. These criteria do not match the required ones for this review. |
| Reason for exclusion | Non-randomised study of Qiweibaizhu san and Gan cao shaoyao tang in 31 people with IGT. |
| Reason for exclusion | Non-randomised study of a Chinese herbal extract (bereberine) in people with IGT and hyperlipedemia. |
| Reason for exclusion | Case series. |
| Reason for exclusion | Quasi-randomised study comparing the effects of Hu Ben Hui Ni (shu di 20g, shan zhu yu 10g, shan yao 10g, ze xie 10g, mu dan pi 10g, fu ling 10g, ban xia 10g, chen pi 10g, dan shen 10g, gou qi zi 10g, shan zha 10g) in 90 people with impaired glucose tolerance. |
| Report: “randomly divided into control group of 40 and treatment group of 42 cases. Phone interview: allocation was according to the preference of the participant. |
| Reason for exclusion | Duplicate of LI HB 2003 |
| Reason for exclusion | Quasi-randomised study comparing the effect of Yiqi jianpi fang (dang shen 20g, ge gen 20g, huang qi 15g, fu ling 15g, bai zhu 15g, cang zhu 15g, tian hua fen 15g, shen qu 15g) plus captopril vs captopril alone in 64 participants with impaired glucose tolerance. The study duration was 21 days (<4wks). |
| The report stated that participants were randomly divided into treatment group A and control group B. From phone contact with the authors the subjects were divided into groups according to ages and other characteristics by the clinician. | |
| Same study with more detailed information as Li HB 2002 |
| Reason for exclusion | Case series. |
| Reason for exclusion | Case series of Jianpi sanjing tang in 31 people with IGT. |
| Reason for exclusion | This trial compared different herbal medicines. |
| Reason for exclusion | Case series. |
| Reason for exclusion | Randomised study comparing Jiangtang bushen fang plus diet & lifestyle advice vs diet & lifestyle advice alone in 51 participants. Only TCM symptom outcomes were reported. These reported outcomes did not fall into our designated categories. |
| Reason for exclusion | Randomised study comparing Chinese herbal formula in 156 people with impaired glucose tolerance. Outcomes (FBG, 2hr-GTT, HbA1c and clinical signs and symptoms) were reported as grouped data only: absolutely effective, effective, not effective. |
| Reason for exclusion | Quasi-randomised study comparing Jinqi Jiangtang plus diet & exercise vs diet and exercise in 62 participants over 3 months. The report stated that the trial was “randomised”. Contact with the author revealed that participants were “casually grouped”. |
| Reason for exclusion | Randomised study comparing the effect of a chinese herbal decoction plus diet, exercise and education versus diet, exercise and education alone. The trial did not state if it was randomised. There is a sampling discrepancy, with 34 allocated to the treatment group and 22 to the control. Unable to contact the authors. |
| Reason for exclusion | Non-randomised study comparing Xuexi II capsules plus diet & exercise versus diet & exercise alone in 50 people with IGT. |
| Reason for exclusion | Randomised study observing Yi ming decoction plus insulin vs insulin alone in 131 in people with type 2 diabetes. This population group did not meet the criteria of this review. |
| Reason for exclusion | Case series. |
| Reason for exclusion | The study used a mixed intervention of Chinese herbs and western medicine and was quasi randomised. |
| Reason for exclusion | Case series of 40 people with IGT. |
| Reason for exclusion | This study included subjects with both IGT and diabetes. |
| Reason for exclusion | Review of traditional methods of treating people with IGT. |
| Reason for exclusion | Participants were all diabetes type 2. This population group did not meet the criteria of this review. |
| Reason for exclusion | Non-randomised study comparing Liu wei di huang wan plus diet & exercise versus diet & exercise alone in 64 participants. |
| Reason for exclusion | Case series. |
| Reason for exclusion | Randomised study comparing Yiqi ziyin granules (Huang qi 10g, sang shen 10g, xuan shen 10g, tai zi shen 15g) plus diet & lifestyle advice versus diet & lifestyle advice alone in 61 participants. There is a discrepancy in the sampling, 41 in the treatment group versus 20 in the control group. |
| Reason for exclusion | Randomised study comparing the effect of Fufang Yin Yang huo chongji versus rosiglitazone in 90 or 60 participants. Participant numbers reported in the tables were 45 in each group and in the text 30 in each group is reported. Authors refused to provide any further information about the study. |
| Reason for exclusion | Quasi-randomised study of Shenqi di huang tang plus diet & exercise versus metformin plus diet & exercise in 80 participants over two and a half years. |
| Reason for exclusion | Quasi-randomised, parallel trial of Tang No. 1 granules plus education versus education alone in 140 people for 6 months. Randomisation was based on the visiting sequence of the participants. |
| Reason for exclusion | Review of traditional methods of treating people with IGT. |
| Reason for exclusion | Review of traditional methods of treating people with IGT. |
| Reason for exclusion | A review of traditional methods of treating people with IGT, not a clinical trial. |
| Reason for exclusion | Randomised controlled trial of Jianpi bushen pill plus diet and exercise compared to multivitamin supplement and diet and exercise in 136 participants. The control intervention did not fall into the category of the inclusion criteria. |
| Reason for exclusion | Non-randomised parallel study of Chinese herbal medicine in combinations with vitamin C or pioglitazone in 150 participants (30 participants in 5 groups). |
| Reason for exclusion | Quasi-randomised, parallel study comparing Jianpi Jiangtang Yin (fu ling 20g, huang qi 30g, cang zhu 15g, yi yi ren 25g, ge gen 15g, mai dong 15g, xuan shen 15g, san qi 15g, dang gui 15g, huang lian 10g. Additions: 1) 5 palm sweat, excessive thirst & drinking: zhi mu 20g, tian hua fen 15g; 2) vexation and irritability, insomnia: bai zi ren 20g, ye jiao teng 15g, huang lian 10g; 3) dizziness and swollen eyes, reddish complexion & ears: mu dan pi 15g, gou teng 15g vs diet and exercise in 160 participants. Method of randomisation was odd-even. |
| Reason for exclusion | Study of different methods of treating people with IGT. |
| Reason for exclusion | Non-randomised. |
| Reason for exclusion | Review of traditional methods of treating people with IGT. |
| Reason for exclusion | Quasi-randomised study comparing Jinqi jiangtang tablets plus diet & lifestyle advice vs diet & lifestyle advice in 72 participants. (Randomisation based on age). |
| Reason for exclusion | A study of Shengmai injection into elderly people. There were no blood glucose outcomes reported. |
| Reason for exclusion | Non-randomised trial of Jinqi jiangtang pills in people with IGT. |
| Reason for exclusion | Quasi-randomised (odd-even method) study comparing Chinese herbal medicine (Zhu ru 10g, zhi shi 12g, chen pi 12g, fa xia 12g, fu ling 10g, sheng jiang 5g, yu zhu 15g, dan shen 15g, ze xie 12g, gan cao 3g) plus vigorous exercise, diet and education vs vigorous exercise, diet and education alone in 72 participants. |
| Reason for exclusion | Review of traditional methods of treating people with IGT. |
| Reason for exclusion | Randomised study comparing the effect of Jinqi jiangtang tablet vs placebo in 57 people. The trial objective was to assess efficacy of the herbal medicine on the excretion rate of microalbuminuria. No blood glucose outcomes were reported. |
| Reason for exclusion | Non-randomised case series of a chinese herbal formula. |
| Reason for exclusion | Randomised study comparing Shen qi jiang tang ke li vs placebo in 60 participants with impaired glucose tolerance. The study duration was 14 days (<4 wks). |
| Reason for exclusion | Quasi-randomised study comparing Huaqi Jiangtang vs diet and exercise in 72 participants with impaired glucose tolerance. Althought the report stated the participants were randomised, from the phone interview the author advised that the allocation to treatment group was based on the preference of the participants. |
| Reason for exclusion | Quasi-randomised study comparing the effects of Jinqi jiangtang tablet in 46 participants with impaired glucose tolerance. The method of randomisation was alternation. From phone interview it was revealed that the allocation to treatment group was based on the judgement of the clinician. |
IGT: impaired glucose tolerance
Characteristics of studies awaiting classification
| Methods | Parallel, randomised controlled trial |
| Participants | SETTING: Outpatients, Hospital, China |
| WHO PARTCIPATED: 160 (80 in treatment group, M/F 50/30, mean age 44.3 yrs; 80 in control group, M/F 48/32, mean age 44.1yrs) | |
| INCLUSION CRITERIA: IGT (WHO 1999). | |
| EXCLUSION CRITERIA: Excluded hyperthyroid, depression, cancer. | |
| CO-MORBIDITIES: none reported | |
| CO-MEDICATIONS: none reported | |
| Interventions | INTERVENTION: Jie yu huo xue decoction (dang gui 15g, chai hu 15g, Yu Zhu 10g, huang qi 30g, chuan xiong 10g, chi shao 15g, san qi 3g, ge gen 15g, huang jing 15g, yu jin 15g, ren shen 25g, xia ku cao 10g) , oral, 100ml decoction per dose, 3 times per day; plus llifestyle modification (diet and lifestyle advice). This prescription was added to if the following symptoms were present: |
| If heat in palms, thirst, increased drinking: + zhi mu 20g, wu wei zi 10g. | |
| if restlessness, tendency to anger, insomnia: + bai zi ren 20g, ye jiao teng 15g, huang lian 10g. | |
| if dizziness, stuffiness in eyes, reddish face & ears with heat: +mu dan pi, gou teng 15g. | |
| CONTROL: Lifestyle modification (diet and lifestyle advice). | |
| Outcomes | FBG (mmol/L), 2hr-GTT (mmol/L), normalisation of glucose levels (n). |
| Outcomes were measured at baseline and at trial completion (12 months). | |
| Notes | We are trying to contact the authors to clarify a discrepancy in the publication. The number randomised to the intervention group is reported as 80. In Table 1 of the published report, the incidence of diabetes gives different numbers for the intervention group with totals of 88 (42 normalised, 40 IGT, 6 diabetes mellitus). |
IGT: impaired glucose tolerance; FBG: fasting blood glucose; M/F: male/female
Characteristics of ongoing studies
Footnotes
Summary of findings tables
Additional tables
1 Overview of study populations
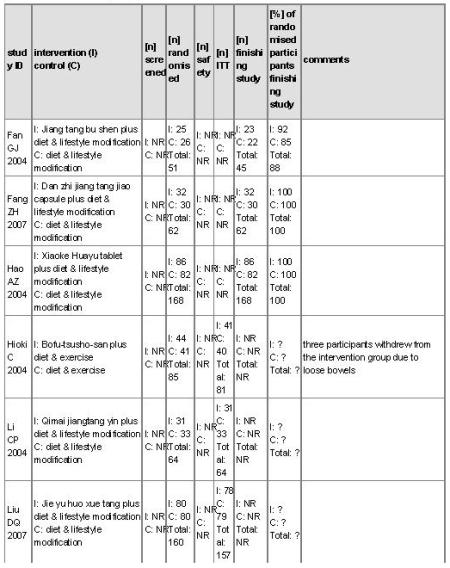 |
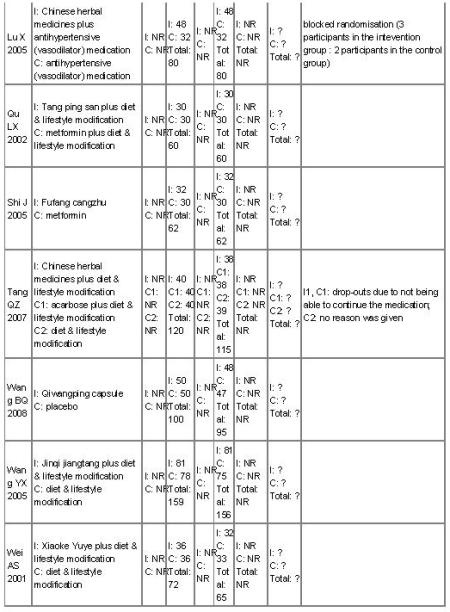 |
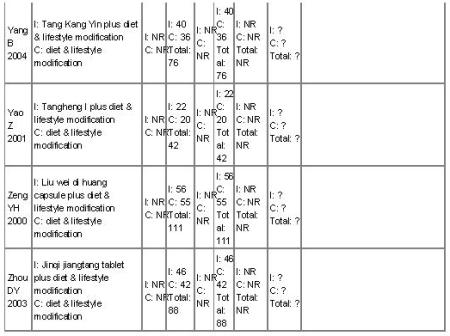 |
?: unclear; ITT = intention-to-treat; NR: not reported
2 Preparation and composition of Chinese herbal medicines in the included trials
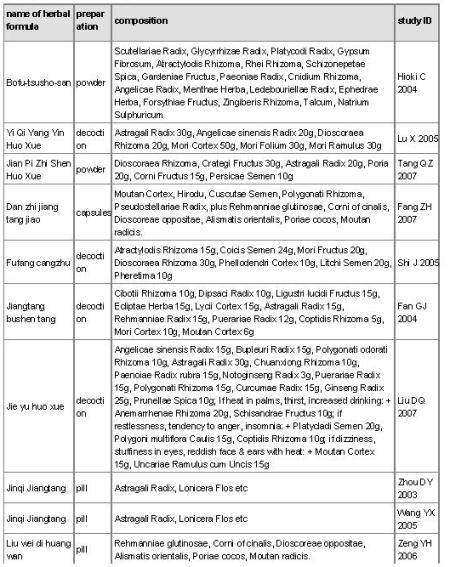 |
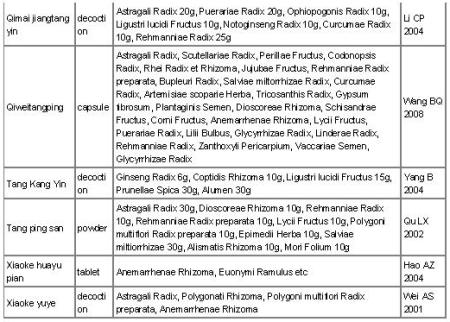 |
Data and analyses
1 Herbal medicine plus lifestyle modification versus lifestyle modification alone
| Outcome or Subgroup | Studies | Participants | Statistical Method | Effect Estimate |
|---|---|---|---|---|
| 1.1 Normalisation of fasting blood glucose at trial completion (n) |
8 | 625 | Risk Ratio ( IV , Random , 95% CI ) | 2.07 [1.52, 2.82] |
| 1.1.1 Jiantang Bushen decoction | 1 | 45 | Risk Ratio ( IV , Random , 95% CI ) | 1.72 [1.04, 2.86] |
| 1.1.2 Xiaoke Huaya tablet | 1 | 168 | Risk Ratio ( IV , Random , 95% CI ) | 2.54 [1.70, 3.79] |
| 1.1.3 Qimai Jiangtang Yin decoction |
1 | 64 | Risk Ratio ( IV , Random , 95% CI ) | 2.89 [1.41, 5.90] |
| 1.1.4 Jian Pi Zhi Shen Huo Xue | 1 | 77 | Risk Ratio ( IV , Random , 95% CI ) | 1.92 [1.28, 2.90] |
| 1.1.5 Tang Kang Yin | 1 | 76 | Risk Ratio ( IV , Random , 95% CI ) | 3.75 [1.74, 8.09] |
| 1.1.6 Xiaoke Yuye decoction | 1 | 65 | Risk Ratio ( IV , Random , 95% CI ) | 1.89 [1.14, 3.14] |
| 1.1.7 Tang Heng I | 1 | 42 | Risk Ratio ( IV , Random , 95% CI ) | 5.00 [1.26, 19.87] |
| 1.1.8 Jinqi jiangtang | 1 | 88 | Risk Ratio ( IV , Random , 95% CI ) | 1.17 [0.90, 1.53] |
| 1.2 Incidence of diabetes (n) | 8 | 632 | Risk Ratio ( M-H , Random , 95% CI ) |
0.33 [0.19, 0.58] |
| 1.2.1 Jiangtang Bushen tang | 1 | 45 | Risk Ratio ( M-H , Random , 95% CI ) |
0.32 [0.04, 2.84] |
| 1.2.2 Xiaoke Huayu Pian | 1 | 168 | Risk Ratio ( M-H , Random , 95% CI ) |
.36 [0.10, 1.30] |
| 1.2.3 Qimai Jiangtang Yin | 1 | 64 | Risk Ratio ( M-H , Random , 95% CI ) |
0.18 [0.02, 1.39] |
| 1.2.4 Tang King Yin | 1 | 76 | Risk Ratio ( M-H , Random , 95% CI ) |
0.06 [0.01, 0.46] |
| 1.2.5 Jian pi zhi shen huo xue | 1 | 77 | Risk Ratio ( M-H , Random , 95% CI ) |
0.59 [0.19, 1.84] |
| 1.2.6 Jinqi Jiangtang tablet | 1 | 88 | Risk Ratio ( M-H , Random , 95% CI ) |
0.61 [0.11, 3.47] |
| 1.2.7 Xiaoke Yuye | 1 | 72 | Risk Ratio ( M-H , Random , 95% CI ) |
0.33 [0.07, 1.54] |
| 1.2.8 Tangheng I Plus | 1 | 42 | Risk Ratio ( M-H , Random , 95% CI ) |
0.18 [0.02, 1.43] |
| 1.3 Fasting blood glucose (mmol/L) | 9 | 742 | Mean Difference ( IV , Random , 95% CI ) |
−0.41 [−0.66, −0.16] |
| 1.3.1 Jiangtang Bushen decoction | 1 | 45 | Mean Difference ( IV , Random , 95% CI ) |
0.15 [ 0.42, 0.12] |
| 1.3.2 Xiaoke Huayu tablet | 1 | 168 | Mean Difference ( IV , Random , 95% CI ) |
−1.40 [−1.72, −1.08] |
| 1.3.3 Qimai Jiangtang Yin formula | 1 | 64 | Mean Difference ( IV , Random , 95% CI ) |
−0.03 [−0.32, 0.26] |
| 1.3.4 Jinqi Jiangtang tablet | 1 | 88 | Mean Difference ( IV , Random , 95% CI ) |
−0.58 [−0.74, −0.42] |
| 1.3.5 Jian Pi Zhi Shen Huo Xue | 1 | 76 | Mean Difference ( IV , Random , 95% CI ) |
−0.09 [−0.35, 0.17] |
| 1.3.6 Xiaoke Yuye decoction | 1 | 72 | Mean Difference ( IV , Random , 95% CI ) |
−0.39 [−0.99, 0.21] |
| 1.3.7 Tang Kang Yin decoction | 1 | 76 | Mean Difference ( IV , Random , 95% CI ) |
−0.21 [−0.33, −0.09] |
| 1.3.8 Tang Heng I decoction | 1 | 42 | Mean Difference ( IV , Random , 95% CI ) |
−1.24 [−2.53, 0.05] |
| 1.3.9 Liu Wei Di Huang capsule | 1 | 111 | Mean Difference ( IV , Random , 95% CI ) |
−0.31 [−0.58, −0.04] |
| 1.4 2hr fasting blood glucose (mmol/L) [ after oral glucose tolerance test] |
9 | Mean Difference ( IV , Random , 95% CI ) |
No totals | |
| 1.4.1 Jiangtang Bushen decoction | 1 | Mean Difference ( IV , Random , 95% CI ) |
No totals | |
| 1.4.2 Xiaoke Huaye tablet | 1 | Mean Difference ( IV , Random , 95% CI ) |
No totals | |
| 1.4.3 Qimai Jiangtang Yin decoction |
1 | Mean Difference ( IV , Random , 95% CI ) |
No totals | |
| 1.4.4 Jinqi Jiangtang tablet | 1 | Mean Difference ( IV , Random , 95% CI ) |
No totals | |
| 1.4.5 Jian Pi Zhi Shen Huo Xue | 1 | Mean Difference ( IV , Random , 95% CI ) |
No totals | |
| 1.4.6 Xiaoke Yuye decoction | 1 | Mean Difference ( IV , Random , 95% CI ) |
No totals | |
| 1.4.7 Tang Kang Yin decoction | 1 | Mean Difference ( IV , Random , 95% CI ) |
No totals | |
| 1.4.8 Tang Heng I decoction | 1 | Mean Difference ( IV , Random , 95% CI ) |
No totals | |
| 1.4.9 Liu Wei Di Huang capsule | 1 | Mean Difference ( IV , Random , 95% CI ) |
No totals | |
| 1.5 HbA1c (%) | 3 | 317 | Mean Difference ( IV , Random , 95% CI ) |
−0.47 [−1.00, 0.06] |
| 1.5.1 Xiaoke Huayu tablet | 1 | 168 | Mean Difference ( IV , Random , 95% CI ) |
−0.63 [−0.99, −0.27] |
| 1.5.2 Jian Pi Zhi Shen Huo Xue | 1 | 77 | Mean Difference ( IV , Random , 95% CI ) |
−0.87 [−1.43, −0.31] |
| 1.5.3 Xiaoke Yuye decoction | 1 | 72 | Mean Difference ( IV , Random , 95% CI ) |
−0.06 [−0.12, 0.00] |
| 1.6 Insulin (μU/ml) | 6 | Mean Difference ( IV , Random , 95% CI ) |
No totals | |
| 1.6.1 Jiangtang Bushen decoction | 1 | Mean Difference ( IV , Random , 95% CI ) |
No totals | |
| 1.6.2 Qimai Jiangtang Yin decoction |
1 | Mean Difference ( IV , Random , 95% CI ) |
No totals | |
| 1.6.3 Jinqi Jiangtang tablet | 1 | Mean Difference ( IV , Random , 95% CI ) |
No totals | |
| 1.6.4 Jian Pi Zhi Shen Huo Xue | 1 | Mean Difference ( IV , Random , 95% CI ) |
No totals | |
| 1.6.5 Tang Kang Yin decoction | 1 | Mean Difference ( IV , Random , 95% CI ) |
No totals | |
| 1.6.6 Tang Heng I decoction | 1 | Mean Difference ( IV , Random , 95% CI ) |
No totals | |
| 1.7 IAI (insulin sensitivity) | 1 | Mean Difference ( IV , Random , 95% CI ) |
No totals | |
| 1.7.1 Qimai Jiangtang Yin decoction |
1 | Mean Difference ( IV , Random , 95% CI ) |
No totals | |
| 1.8 Total cholesterol (mmol/L) | 7 | Mean Difference ( IV , Random , 95% CI ) |
No totals | |
| 1.8.1 Jiangtang Bushen tang | 1 | Mean Difference ( IV , Random , 95% CI ) |
No totals | |
| 1.8.2 Xiaoke Huayu Pian | 1 | Mean Difference ( IV , Random , 95% CI ) |
No totals | |
| 1.8.3 Jinqi Jiangtang tablet | 1 | Mean Difference ( IV , Random , 95% CI ) |
No totals | |
| 1.8.4 Jian Pi Zhi Shen Huo Xue | 1 | Mean Difference ( IV , Random , 95% CI ) |
No totals | |
| 1.8.5 Xiaoke Yuye | 1 | Mean Difference ( IV , Random , 95% CI ) |
No totals | |
| 1.8.6 Tang Kang Yin | 1 | Mean Difference ( IV , Random , 95% CI ) |
No totals | |
| 1.8.7 Liu Wei Di Huang capsule | 1 | Mean Difference ( IV , Random , 95% CI ) |
No totals | |
| 1.9 Lipids: HDL (mmol/L) | 2 | Mean Difference ( IV , Random , 95% CI ) |
No totals | |
| 1.9.1 Jian Pi Zhi Shen Huo Xue | 1 | Mean Difference ( IV , Random , 95% CI ) |
No totals | |
| 1.9.2 Jinqi Jiangtang tablet | 1 | Mean Difference ( IV , Random , 95% CI ) |
No totals | |
| 1.10 Trigylcerides (mmol/L) | 7 | Mean Difference ( IV , Random , 95% CI ) |
No totals | |
| 1.10.1 Jiangtang Bushen tang | 1 | Mean Difference ( IV , Random , 95% CI ) |
No totals | |
| 1.10.2 Xiaoke Huayu Pian | 1 | Mean Difference ( IV , Random , 95% CI ) |
No totals | |
| 1.10.3 Jinqi Jiangtang tablet | 1 | Mean Difference ( IV , Random , 95% CI ) |
No totals | |
| 1.10.4 Jian Pi Zhi Shen Huo Xue | 1 | Mean Difference ( IV , Random , 95% CI ) |
No totals | |
| 1.10.5 Xiaoke Yuye decoction | 1 | Mean Difference ( IV , Random , 95% CI ) |
No totals | |
| 1.10.6 Tang Kang Yin decoction | 1 | Mean Difference ( IV , Random , 95% CI ) |
No totals | |
| 1.10.7 Liu Wei Di Huang Wan | 1 | Mean Difference ( IV , Random , 95% CI ) |
No totals | |
| 1.11 Body Mass Index (kg/m2) | 6 | Mean Difference ( IV , Random , 95% CI ) |
No totals | |
| 1.11.1 Jiangtang Bushen | 1 | Mean Difference ( IV , Random , 95% CI ) |
No totals | |
| 1.11.2 Jian Pi Zhi Shen Huo Xue | 1 | Mean Difference ( IV , Random , 95% CI ) |
No totals | |
| 1.11.3 Xiaoke Yuye decoction | 1 | Mean Difference ( IV , Random , 95% CI ) |
No totals | |
| 1.11.4 Tang Kang Yin decoction | 1 | Mean Difference ( IV , Random , 95% CI ) |
No totals | |
| 1.11.5 Liu Wei Di Huang capsule | 1 | Mean Difference ( IV , Random , 95% CI ) |
No totals | |
| 1.11.6 Jinqi Jiangtang tablet | 1 | Mean Difference ( IV , Random , 95% CI ) |
No totals | |
| 1.12 Diastolic blood pressure (mmHg) |
2 | Mean Difference ( IV , Random , 95% CI ) |
No totals | |
| 1.12.1 Liu wei di huang wan | 1 | Mean Difference ( IV , Random , 95% CI ) |
No totals | |
| 1.12.2 Jinqi jiangtang tablets | 1 | Mean Difference ( IV , Random , 95% CI ) |
No totals | |
| 1.13 Systolic Blood Pressure (mmHg) |
2 | Mean Difference ( IV , Random , 95% CI ) |
No totals | |
| 1.13.1 Liu wei di huang wan | 1 | Mean Difference ( IV , Random , 95% CI ) |
No totals | |
| 1.13.2 Jinqi jiangtang tablets | 1 | Mean Difference ( IV , Random , 95% CI ) |
No totals | |
| 1.14 Main ingredient Astragalus membranecus (≥30g): Fasting blood glucose (mmol/ml) |
4 | Mean Difference ( IV , Random , 95% CI ) |
No totals | |
| 1.14.1 Jinqi Jiangtang tablet | 1 | Mean Difference ( IV , Random , 95% CI ) |
No totals | |
| 1.14.2 Xiaoke Yuye decoction | 1 | Mean Difference ( IV , Random , 95% CI ) |
No totals | |
| 1.14.3 Shan yao, shan zha, huang qi etc |
1 | Mean Difference ( IV , Random , 95% CI ) |
No totals | |
| 1.14.4 Qimai Jiangtang Yin formula |
1 | Mean Difference ( IV , Random , 95% CI ) |
No totals | |
| 1.15 Hypoglycaemic effects of herbal medicines with lifestyle modification compared lifestyle modification alone |
9 | Other data | No numeric data |
2 Herbal medicine plus lifestyle modification versus placebo plus lifestyle modification
| Outcome or Subgroup | Studies | Participants | Statistical Method | Effect Estimate |
|---|---|---|---|---|
| 2.1 Reduction in fasting blood glucose (mmol/L) |
2 | Mean Difference ( IV , Random , 95% CI ) |
No totals | |
| 2.1.1 Dan zhi jiang tang jiao capsules |
1 | Mean Difference ( IV , Random , 95% CI ) |
No totals | |
| 2.1.2 Bofu-tsusho-san | 1 | Mean Difference ( IV , Random , 95% CI ) |
No totals | |
| 2.2 Reduction in 2hr fasting blood glucose after oral glucose tolerance test |
2 | Mean Difference ( IV , Random , 95% CI ) |
No totals | |
| 2.2.1 Dan zhi jiang tang jiao capsules |
1 | Mean Difference ( IV , Random , 95% CI ) |
No totals | |
| 2.2.2 Bofu-tsusho-san | 1 | Mean Difference ( IV , Random , 95% CI ) |
No totals | |
| 2.3 Reduction in HbA1c (%) | 1 | Mean Difference ( IV , Random , 95% CI ) |
No totals | |
| 2.3.1 Bofu-tsusho-san | 1 | Mean Difference ( IV , Random , 95% CI ) |
No totals | |
| 2.4 Total cholesterol (mmol/L) | 1 | Mean Difference ( IV , Random , 95% CI ) |
No totals | |
| 2.4.1 Bofu-tsusho-san | 1 | Mean Difference ( IV , Random , 95% CI ) |
No totals | |
| 2.5 Trigylcerides (mmo/lL) | 2 | Mean Difference ( IV , Random , 95% CI ) |
No totals | |
| 2.5.1 Dan zhi jiang tang jiao capsules |
1 | Mean Difference ( IV , Random , 95% CI ) |
No totals | |
| 2.5.2 Bofu-tsusho-san | 1 | Mean Difference ( IV , Random , 95% CI ) |
No totals | |
| 2.6 Insulin (mu/L) | 2 | Mean Difference ( IV , Random , 95% CI ) |
No totals | |
| 2.6.1 Bofu-tsusho-san | 1 | Mean Difference ( IV , Random , 95% CI ) |
No totals | |
| 2.6.2 Dan zhi jiang tang jiao capsules |
1 | Mean Difference ( IV , Random , 95% CI ) |
No totals | |
| 2.7 Lipids: HDL (mmol/L) | 1 | Mean Difference ( IV , Random , 95% CI ) |
No totals | |
| 2.7.1 Bofu-tsusho-san | 1 | Mean Difference ( IV , Random , 95% CI ) |
No totals |
3 Herbal medicine plus lifestyle modification versus metformin plus lifestyle modification
| Outcome or Subgroup | Studies | Participants | Statistical Method | Effect Estimate |
|---|---|---|---|---|
| 3.1 Reduction in 2 hr fasting blood glucose after oral glucose tolerance test (mmol/L) |
1 | Mean Difference ( IV , Random , 95% CI ) |
No totals | |
| 3.1.1 Tangping San | 1 | Mean Difference ( IV , Random , 95% CI ) |
No totals |
4 Herbal medicine versus placebo
| Outcome or Subgroup | Studies | Participants | Statistical Method | Effect Estimate |
|---|---|---|---|---|
| 4.1 Normalisation of fasting blood glucose (n) |
1 | Risk Ratio ( M-H , Random , 95% CI ) |
No totals | |
| 4.1.1 Qiwei Tang Ping capsule | 1 | Risk Ratio ( M-H , Random , 95% CI ) |
No totals | |
| 4.2 Incidence of diabetes (n) | 1 | Risk Ratio ( M-H , Random , 95% CI ) |
No totals | |
| 4.2.1 Qiwei Tang Ping | 1 | Risk Ratio ( M-H , Random , 95% CI ) |
No totals | |
| 4.3 Reduction in fasting blood glucose (mmol/L) |
1 | Mean Difference ( IV , Random , 95% CI ) |
No totals | |
| 4.3.1 Qiwei tangping capsules | 1 | Mean Difference ( IV , Random , 95% CI ) |
No totals | |
| 4.4 Reduction in 2hr fasting blood glucose after orale glucose tolerance test (mmol/L) |
1 | Mean Difference ( IV , Random , 95% CI ) |
No totals | |
| 4.4.1 Qiwei tangping capsules | 1 | Mean Difference ( IV , Random , 95% CI ) |
No totals | |
| 4.5 Body mass index (kg/m2) | 1 | Mean Difference ( IV , Random , 95% CI ) |
No totals | |
| 4.5.1 Qiwei tangping capsules | 1 | Mean Difference ( IV , Random , 95% CI ) |
No totals | |
| 4.6 Waist-to-hip ratio (WHR) | 1 | Mean Difference ( IV , Random , 95% CI ) |
No totals | |
| 4.6.1 Qiwei tangping capsules | 1 | Mean Difference ( IV , Random , 95% CI ) |
No totals |
5 Herbal medicine versus metformin
| Outcome or Subgroup | Studies | Participants | Statistical Method | Effect Estimate |
|---|---|---|---|---|
| 5.1 Reduction in fasting blood glucose (mmol/L) |
1 | Mean Difference ( IV , Random , 95% CI ) |
No totals | |
| 5.1.1 Fufang Cangzhu decoction | 1 | Mean Difference ( IV , Random , 95% CI ) |
No totals | |
| 5.2 Reduction in 2hr fasting blood glucose after orale glucose tolerance test (mmol/L) |
1 | Mean Difference ( IV , Random , 95% CI ) |
No totals | |
| 5.2.1 Fufang Cangzhu | 1 | Mean Difference ( IV , Random , 95% CI ) |
No totals | |
| 5.3 Triglycerides (mmol/L) | 1 | Mean Difference ( IV , Random , 95% CI ) |
No totals | |
| 5.3.1 Fufang Cangzhu | 1 | Mean Difference ( IV , Random , 95% CI ) |
No totals | |
| 5.4 Total cholesterol | 1 | Mean Difference ( IV , Random , 95% CI ) |
No totals | |
| 5.4.1 Fufang Cangzhu | 1 | Mean Difference ( IV , Random , 95% CI ) |
No totals | |
| 5.5 Insulin (mU/L) | 1 | Mean Difference ( IV , Random , 95% CI ) |
No totals | |
| 5.5.1 Fufang Cangzhu | 1 | Mean Difference ( IV , Random , 95% CI ) |
No totals | |
| 5.6 Waist-to-hip ratio (WHR) | 1 | Mean Difference ( IV , Random , 95% CI ) |
No totals | |
| 5.6.1 Fufang Cangzhu | 1 | Mean Difference ( IV , Random , 95% CI ) |
No totals | |
| 5.7 Weight (kg) | 1 | Mean Difference ( IV , Random , 95% CI ) |
No totals | |
| 5.7.1 Fufang Cangzhu | 1 | Mean Difference ( IV , Random , 95% CI ) |
No totals |
6 Herbal medicine plus lifestyle modification versus acarbose plus lifestyle modification
| Outcome or Subgroup | Studies | Participants | Statistical Method | Effect Estimate |
|---|---|---|---|---|
| 6.1 Reduction in fasting blood glucose (mmol/L) |
1 | Mean Difference ( IV , Random , 95% CI ) |
No totals | |
| 6.1.1 Jian pi zhi shen huo xue | 1 | Mean Difference ( IV , Random , 95% CI ) |
No totals | |
| 6.2 2hr-Glucose tolerance (mmol/L) | 1 | Mean Difference ( IV , Random , 95% CI ) |
No totals | |
| 6.2.1 Jian pi zhi shen huo xue | 1 | Mean Difference ( IV , Random , 95% CI ) |
No totals | |
| 6.3 Normalisation of blood glucose (n) |
1 | Odds Ratio ( M-H , Random , 95% CI ) |
No totals | |
| 6.3.1 Jian pi zhi shen huo xue | 1 | Odds Ratio ( M-H , Random , 95% CI ) |
No totals | |
| 6.4 Incidence of diabetes (n) | 1 | Risk Ratio ( M-H , Random , 95% CI ) |
No totals | |
| 6.4.1 Jian pi zhi shen huo xue | 1 | Risk Ratio ( M-H , Random , 95% CI ) |
No totals | |
| 6.5 HbA1c (%) | 1 | Mean Difference ( IV , Random , 95% CI ) |
No totals | |
| 6.6 Insulin (FINS mmol/L) | 1 | Mean Difference ( IV , Random , 95% CI ) |
No totals | |
| 6.6.1 Jian pi zhi shen huo xue | 1 | Mean Difference ( IV , Random , 95% CI ) |
No totals | |
| 6.7 Total cholesterol (mmol/L) | 1 | Mean Difference ( IV , Random , 95% CI ) |
No totals | |
| 6.8 Trigylcerides (mmol/L) | 1 | Mean Difference ( IV , Random , 95% CI ) |
No totals | |
| 6.8.1 Jian pi zhi shen huo xue | 1 | Mean Difference ( IV , Random , 95% CI ) |
No totals | |
| 6.9 Lipids: LDL (mmol/L) | 1 | Mean Difference ( IV , Random , 95% CI ) |
No totals | |
| 6.9.1 Jian pi zhi shen huo xue | 1 | Mean Difference ( IV , Random , 95% CI ) |
No totals | |
| 6.10 Lipids: HDL (mmol/L) | 1 | Mean Difference ( IV , Random , 95% CI ) |
No totals | |
| 6.10.1 Jian pi zhi shen huo xue | 1 | Mean Difference ( IV , Random , 95% CI ) |
No totals |
7 Herbal medicine plus antihypertensive medication versus antihypertensive medication alone
| Outcome or Subgroup | Studies | Participants | Statistical Method | Effect Estimate |
|---|---|---|---|---|
| 7.1 Reduction in fasting glucose (mmol/L) |
1 | Mean Difference ( IV , Random , 95% CI ) |
No totals | |
| 7.1.1 Yi qi yang yin huo xue | 1 | Mean Difference ( IV , Random , 95% CI ) |
No totals | |
| 7.2 Reduction in 2hr blood glucose after oral glucose tolerance test (mmol/L) |
1 | Mean Difference ( IV , Random , 95% CI ) |
No totals | |
| 7.2.1 Yi qi yang yin huo xue | 1 | Mean Difference ( IV , Random , 95% CI ) |
No totals | |
| 7.3 Total cholesterol (mmol/L) | 1 | Mean Difference ( IV , Random , 95% CI ) |
No totals | |
| 7.3.1 Yi qi yang yin huo xue | 1 | Mean Difference ( IV , Random , 95% CI ) |
No totals | |
| 7.4 Triglycerides (mmol/L) | 1 | Mean Difference ( IV , Random , 95% CI ) |
No totals | |
| 7.4.1 Yi qi yang yin huo xue | 1 | Mean Difference ( IV , Random , 95% CI ) |
No totals | |
| 7.5 Lipids: HDL (mmol/L) | 1 | Mean Difference ( IV , Random , 95% CI ) |
No totals | |
| 7.5.1 Yi qi yang yin huo xue | 1 | Mean Difference ( IV , Random , 95% CI ) |
No totals | |
| 7.6 Systolic Blood Pressure (kpa) | 1 | Mean Difference ( IV , Random , 95% CI ) |
No totals | |
| 7.6.1 Yi qi yang yin huo xue | 1 | Mean Difference ( IV , Random , 95% CI ) |
No totals | |
| 7.7 Diastolic blood pressure (kpa) | 1 | Mean Difference ( IV , Random , 95% CI ) |
No totals | |
| 7.7.1 Yi qi yang yin huo xue | 1 | Mean Difference ( IV , Random , 95% CI ) |
No totals |
8 Herbal medicine versus basic education (diabetes pamphlet)
| Outcome or Subgroup | Studies | Participants | Statistical Method | Effect Estimate |
|---|---|---|---|---|
| 8.1 Normalisation of fasting blood glucose at trial completion (n) |
1 | Risk Ratio ( IV , Random , 95% CI ) | No totals | |
| 8.1.1 Jinqi Jiangtang tablet | 1 | Risk Ratio ( IV , Random , 95% CI ) | No totals | |
| 8.2 Incidence of diabetes (n) | 1 | Risk Ratio ( M-H , Random , 95% CI ) |
No totals | |
| 8.2.1 Jinqi Jiangtang tablet | 1 | Risk Ratio ( M-H , Random , 95% CI ) |
No totals | |
| 8.3 Fasting blood glucose (mmol/L) | 1 | Mean Difference ( IV , Random , 95% CI ) |
No totals | |
| 8.3.1 06 Jinqi Jiangtang tablet | 1 | Mean Difference ( IV , Random , 95% CI ) |
No totals | |
| 8.4 2hr fasting blood glucose (mmol/L) [ after oral glucose tolerance test] |
1 | Mean Difference ( IV , Random , 95% CI ) |
No totals | |
| 8.4.1 Jinqi Jiangtang tablet | 1 | Mean Difference ( IV , Random , 95% CI ) |
No totals | |
| 8.5 Total cholesterol (mmol/L) | 1 | Mean Difference ( IV , Random , 95% CI ) |
No totals | |
| 8.5.1 Jinqi Jiangtang tablet | 1 | Mean Difference ( IV , Random , 95% CI ) |
No totals | |
| 8.6 Trigylcerides (mmol/L) | 1 | Mean Difference ( IV , Random , 95% CI ) |
No totals | |
| 8.6.1 Jinqi Jiangtang tablet | 1 | Mean Difference ( IV , Random , 95% CI ) |
No totals |
Other data tables
1 Herbal medicine plus lifestyle modification versus lifestyle modification alone
1.15 Hypoglycaemic effects of herbal medicines with lifestyle modification compared lifestyle modification alone
| Study ID | Intervention | FBG levels (mmol/L) |
2hr GTT FBG (mmol/L) |
Normalisation of FBG: RR [95% CI] |
HbA1c (%) |
|---|---|---|---|---|---|
| Fan GJ 2004 | Jiantang bushen Tang | −0.15 [0.42, 0.12] | −0.83 [−1.84, 0.18] | 1.72 [1.04, 2.86] | - |
| Hao AZ 2004 | Xiaoke Huaya tablet | −1.40 [−1.72, − 1.08] |
−1.46 [−1.83, −1.09] | 2.54 [1.70, 3.79] | −0.63 [−0.99, − 0.27] |
| Li CP 2004 | Qimai Jiangtang Yin formula | −0.03 [−0.32, 0.26] |
2.89 [1.41, 5.90] | - | |
| Tang QZ 2007 | Shan yao, Shan zha, Huang qi etc |
−0.09 [−0.35, 0.17] |
−2.31 [−3.20, −1.42] | 1.92 [1.28, 2.90] | −0.87 [−1.43, − 0.31] |
| Wei AS 2001 | Xiaoke Yuye decoction | −0.39 [0.99, 021] | −1.54 [−1.65, −1.43] | 1.89 [1.14, 3.14] | −0.06 [−0.12, 0.00] |
| Yang B 2004 | Tang Kang Yin decoction | −0.21 [−0.33, − 0.09] |
−1.66 [−2.21, −1.11] | - | - |
| Yao Z 2001 | Tang Heng I | −1.24 [−2.53, 0.05] |
−1.25 [−3.30, 0.80] | 5.00 [1.26, 19.87] | - |
| Zeng YH 2006 | Liu Wei Di Huang capsule | −1.19 [−1.46, − 0.92] |
−2.77 [−3.17, −2.37] | - | - |
| Zhou DY 2003 | Jinqi Jiangtang tablet | −0.58 [−0.74, − 0.42] |
−0.57 [−0.67, −0.47] | - | - |
Feedback
Appendices
1 MEDLINE search strategy
| Electronic searches |
|---|
| Unless otherwise stated, search terms were free text terms; exp = exploded MeSH: Medical Subject Heading (Medline medical index term); the dollar sign ($) stands for any character(s); the question mark (?) = substitute for one or no characters; ab = abstract; ti = titel; ot = original titel; pt = publication type; sh = MeSH: Medical subject heading (MEDLINE medical index term); adj = adjacency. |
| I. Impaired glucose tolerance / impaired fasting blood glucose: |
| 1.exp Glucose Intolerance/ |
| 2.exp Diabetes Mellitus/pc [Prevention & Control] |
| 3.exp Glucose Tolerance Test/ |
| 4.exp Insulin Resistance/ |
| 5.exp Metabolic Syndrome X/ |
| 6.IGT.ab,ti,ot. |
| 7.glucose intoleranc$.ab,ti,ot. |
| 8.(glucose adj tolerance test$).ab,ti,ot. |
| 9.OGTT.ab,ti,ot. |
| 10.insulin$ resistanc$.ab,ti,ot. |
| 11.(impaired fasting and (blood or glucos$ or gly?emia$)).ab,ti,ot. |
| 12.(impaired glucose and (toleranc$ or stat$ or respons$ or control$)).ab,ti,ot. |
| 13.(impaired glucose and (regul$ or metab$ or homeost$)).ab,ti,ot. |
| 14.(reduced glucose and (metabol$ or toleranc$)).ab,ti,ot. |
| 15.glucose intolerant$.ab,ti,ot. |
| 16.pr?ediabet$.ab,ti,ot. |
| 17.pr?e diabet$.ab,ti,ot. |
| 18.metabolic syndr$.ab,ti,ot. |
| 19.syndrom$ X.ab,ti,ot. |
| 20.((borderline or mild or chemical) adj diabet$).ab,ti,ot. |
| 21.((impaired insulin$ or reduced insulin$) adj secret$).ab,ti,ot. |
| 22.or/1-21 |
| II. Chinese herbal medicines: |
| 23.exp Medicine, Oriental Traditional/ |
| 24.exp Drugs, Chinese Herbal/ |
| 25.exp Plants, Medicinal/ |
| 26.exp Plant Extracts/ |
| 27.exp Phytotherapy/ |
| 28.exp Medicine, Ayurvedic/ |
| 29.integrative medicin$.ab,ti,ot. |
| 30.(medicin$ adj5 (chines$ or oriental$ or tibetan$)).ab,ti,ot. |
| 31.(herbal medicin$ or medicin$ herbal$).ab,ti,ot. |
| 32.(plant$ medicin$ or medicin$ plant$).ab,ti,ot. |
| 33.plant$ extract$.ab,ti,ot. |
| 34.phytotherap$.ab,ti,ot. |
| 35.medicine ayurvedic.ab,ti,ot. |
| 36.(chinese adj (herb$ or drug$ or formul$ or plant$ or presri$ or remed$ or materia medica)).ab,ti,ot. |
| 37.((herb$ or drug$ or formul$ or plant$ or presri$ or remed$ or materia medica) adj chinese).ab,ti,ot. |
| 38.((chines$ or oriental$ or tibetan$) adj medicine$).ab,ti,ot. |
| 39.or/23-38 |
| III. Impaired glucose tolerance/Impaired fasting blood glucose and Chinese herbal medicine: |
| 40.22 and 39 |
| IV. RCT / CCT (sensitive search): |
| Part 1: |
| 41.randomized controlled trial.pt. |
| 42.controlled clinical trial.pt. |
| 43.randomized controlled trials.sh. |
| 44.random allocation.sh. |
| 45.double-blind method.sh. |
| 46.single-blind method.sh. |
| 47.or/41-46 |
| Part 2: |
| 48.clinical trial.pt. |
| 49.exp clinical trials/ |
| 50.(clinic$ adj25 trial$).ab,ti,ot. |
| 51.((singl$ or doubl$ or trebl$ or tripl$) adj (mask$ or blind$)).ab,ti,ot. |
| 52.placebos.sh. |
| 53.placebo$.ab,ti,ot. |
| 54.random$.ab,ti,ot. |
| 55.research design.sh. |
| 56.(latin adj square).ab,ti,ot. |
| 57.or/48-56 |
| Part 3: |
| 58.comparative study.pt. |
| 59.exp evaluation studies/ |
| 60.follow-up studies.sh. |
| 61.prospective studies.sh. |
| 62.(control$ or prospectiv$ or volunteer$).ab,ti,ot. |
| 63.cross-over studies.sh. |
| 64.or/58-63 |
| 65.47 or 57 or 64 |
| V. Meta-analyses, systematic reviews, health technology assessments: |
| 66.exp meta-analysis/ |
| 67.exp Review Literature/ |
| 68.meta-analysis.pt. |
| 69.review.pt. |
| 70.or/66-69 |
| 71.letter.pt. |
| 72.comment.pt. |
| 73.editorial.pt. |
| 74.historical-article.pt. |
| 75.or/71-74 |
| 76.70 not 75 |
| 77.((systematic$ or quantitativ$ or methodologic$) adj (review$ or overview$)).ab,ti,ot. |
| 78meta?anal$.ab,ti,ot. |
| 79.(integrativ$ research review$ or research integration$).ab,ti,ot. |
| 80.quantitativ$ synthes$.ab,ti,ot. |
| 81.(pooling$ or pooled analys$ or mantel$ haenszel$).ab,ti,ot. |
| 82.(peto$ or der?simonian$ or fixed effect$ or random effect$).ab,ti,ot. |
| 83.or/77-82 |
| 84.76 or 83 |
| VI. Impaired glucose tolerance / impaired fasting blood glucose AND Chinese herbal medicine AND RCTs / CCTs: |
| 85.40 and 65 |
| VII. Impaired glucose tolerance / impaired fasting blood glucose AND Chinese herbal medicine AND systematic reviews: |
| 86.40 and 85 |
| VIII. VI OR VII: |
| 87.85 or 86 |
| 88.limit 87 to animal |
| 89.limit 87 to human |
| 90.88 not 89 |
| 91.87 not 90 |
References:
Robinson KA, Dickersin K. Development of a highly sensitive search strategy for the retrieval of reports of controlled trials using PubMed. International Journal of Epidemiology 2002;31(1):150-3.
Van de Laar FA, Lucassen PLBJ, Akkermans RP, Van de Lisdonk EH, De Grauw WJC. Alpha-glucosidase inhibitors for people with impaired glucose tolerance or impaired fasting blood glucose. In: Cochrane Database of Systematic Reviews, Issue 4, 2004.
2 Sensitivity analysis on diagnostic criteria
| outcome | sensitivity analysis on diagnostic criteria | result |
|---|---|---|
| 1.1 Normalisation of fasting blood glucose |
Exclude WHO 1985 (Yao Z 2001) | RR 2.35 (95% CI 1.71 to 3.23) I2 = 51% |
| 1.2 Incidence of diabetes | Exclude WHO 1985 (Yao Z 2001; Zhou DY 2003) | RR 0.29 (95% CI 0.15 to 0.56] I2 = 41% |
| 1.3 Fasting blood glucose | Exclude ADA 1997 (Yang B 2004) | RR −0.64 (95% CI −0.94 to − 0.35] I2 = 90% |
| Exclude WHO 1985 (Yao Z 2001; Zeng YH 2006; Zhou DY 2003) | RR −0.50 (95% CI −0.81 to − 0.18) I2 = 92% |
|
| Exclude both WHO 1985 and ADA 1997 | RR −0.54 (95% CI −0.93 to − 0.16) I2 = 92% |
Footnotes
Sources of support
- No sources of support provided
- University of Western Sydney, Australia
- Cardiac Health Institute, Australia
Contributions of authors
Conceiving the review: SG
Designing the review: SG, JL, NK, DC, AB, HK
Coordinating the review: SG
Undertaking manual searches: SG, JL, XL
Screening search results: SG, JL, XL
Organising retrieval of papers: SG, JL, XL
Screening retrieved papers against inclusion criteria: SG, JL
Appraising quality of papers: SG, JL
Abstracting data from papers: SG, JL, XL
Writing to authors of papers for additional information: SG, JL, XL
Obtaining and screening data on unpublished studies: SG, JL, XL
Data management for the review: SG
Entering data into RevMan: SG
Analysis of data: SG
Interpretation of data: SG, JL
Writing the review: SG, JL, XL, NK, DC, AB, HK
Securing funding for the review: SG, DC, AB, HK
Guarantor for the review (one author): Suzanne Grant
Declarations of interest Suzanne Grant is currently undertaking a randomised controlled trial of Chinese herbal medicines to treat impaired glucose tolerance or impaired fasting blood glucose. There are no other known potential conflicts of interest.
References to studies
Included studies
- Fan GJ 2004.Published and unpublished data Fan GJ, Luo GB, Qin ML, Li SL, Tang AH, Tang JY. Effect of jiangtang bushen recipe in intervention treatment of patients with impaired glucose tolerance [Jiang tang bushen fang dui tang nai liang di jian huan zhe gan yu zhi liao de ying xiang] Chinese Journal of Intergrated Traditional and Western Medicine [Zhong Guo Zhong Xi Yi Jie He Za Zhi] 2004;24(4):317–20.
- Fang ZH 2007.Published and unpublished data Fang ZH. Observation of the effectiveness of Dan zhi jiang tang capsules for IGT [Dan Zhi Jiang Tang Nang Gan Yu Pu Tao Tang Nai Liang Di Jian Liao Xiao Guan Cha] Journal of Emergency Traditional Chinese Medicine [Zhong Guo Zhong Yi Ji Zheng] 2007;16:402–04.
- Hao AZ 2004.Hao AZ, Hu J, Liu ZF, Cui Y. Therapeutic effect of Xiaoke Huayu tablet on impaired glucose tolerance [Xiao ke hua yu pian gan yu zhi liao tang nai liang jian di de lin chuang guan cha] Medical Journal of Chinese People’s Liberation Army [Jie Fang Jun Yi Xue Za Zhi] 2004;29(11):993–94. [Google Scholar]
- Hioki C 2004.Hioki C, Yoshimoto K, Yoshida T. Efficacy of Bofu-Tsusho-san, an oriental herbal medicine, in obese Japanese women with impaired glucose tolerance. Clinical and Experimental Pharmacology and Physiology. 2004;31:614–19. doi: 10.1111/j.1440-1681.2004.04056.x. [DOI] [PubMed] [Google Scholar]
- Li CP 2004.Li CP, Xie B, Huang J, Li HB, Li QM. Clinical observation of the effect of Qimai jingtang yin on impaired glucose tolerance [Qi mai jiang tang yin dui tang nai liang di jian gan yu lin chuang liao xiao guan cha] Journal of Sichuan Traditional Chinese Medicine [Si Chuan Zhong Yi] 2004;22(10):32–3. [Google Scholar]
- Lu X 2005.Published and unpublished data Lu X. Combination of Chinese and Western Therapies: Its influences on Life Quality, Lipids and Primary Hypertension in patients with impaired glucose tolerance [Zhong xi yi jie he liao fa dui yuan fa xing gao ya bing tang nai liang di jian zhe sheng huo zhi liang he xue zhi de ying xiang] Journal of Shaanxi College of Traditional Chinese Medicine. 2005;28(4):11–13.
- Qu LX 2002.Published data only (unpublished sought but not used)Qu LX. Clinical observation on the treatment of impaired glucose with Tangping san [Tang ping san zhi liao tang nai liang yi chang de lin chuang guan cha] Zhejiang Journal of Integrated Traditional Chinese and Western Medicine [Zhe Jiang Zhong Xi Yi Jie He Za Zhi] 2002;12(1):32.
- Shi J 2005.Published and unpublished data Shi J, Hu YY, Wang QH. Clinical observation of 32 cases of senile obesity or overweight with abnormal glucose tolerance treated with Fufang Cangzhu tang [Fu fang can zhu tang zhi liao lao nian fei pang huo chao zhong he bing tang nai liang yi chang 32 li lin chuang guan cha] Journal of Traditional Chinese Medicine [Zhong Yi Za Zhi] 2005;46(1):24–5.
- Tang QZ 2007.Tang QZ, Liao ZS, Feng MJ, Yao XL, Zhu ZZ, Liu M. The clinical study of Jianpi zishen huo xue (stengthen the spleen, nourish the kidney and activate the blood) method for IGT in 38 cases [Jian pi zi shen hup xue fa gan yu tang nai liang di jian 38 li lin chuang yan jiu] Journal of New Chinese Medicine [Xin Zhong Yi] 2007;39(9):88–90. [Google Scholar]
- Wang BQ 2008.Published and unpublished data Wang B, Jiao X, Chen X. Clinical study on the intervention of Qiweitangping capsule on impaired glucose tolerance [Qi wei tang ping jiao nang yu tang nai liang di jian de lin chuang yan jiu] Chinese Journal of Difficult and Complicated Cases [Yi Nan Bing Za Zhi] 2008;7(3):155–7.
- Wang YX 2005.Published and unpublished data Wang YX. The effect of Jianqi Jiangtang tablet on preventing patients with impaired glucose tolerance (IGT) & impaired fasting glucose (IFG) becoming diabetes [Jin qi jiang tang pian dui tang tiao jie shou sun zhe yu fang tang niao bing de zuo yong] Tianjin Medical Journal [Tian Jin Yi Yao] 2005;33(12):793–4.
- Wei As 2001.Published and unpublished data Wei AS, Sun FL, Lang JM. Effects of Xiaoke Yuye on patients with impaired glucose tolerance [Xiaoke yuye zhi liao tang nai liang jian tui lin chuang guan cha] Journal of Shandong University of TCM [Shang Dong Zhong Yi Yao Da Xue Xue Bao] 2001;25(4):259–60.
- Yang B 2004.Published and unpublished data Yang B, Wang WX, Huo W, Min HQ, Liu DX, He Z. Clinical observation of intervention of tangkangyin decoction on patients with impaired glucose tolerance [Tang kang yin gan yu zhi liao tang nai liang di jian lin chuang guan cha] Hebei Journal of Traditional Chinese Medicine [He Bei Zhong Yi] 2004;26(12):893–5.
- Yao Z 2001.Yao Z, Yu FH, Zhang M, Xu PY. Clinical observation on the intervention of 42 cases of impaired glucose with Tangheng 1. Gansu Journal of Traditional Chinese Medicine [Gan Su Zhong Yi] 2001;14(3):30–31. [Google Scholar]
- Zeng YH 2006.Published and unpublished data Zeng YH, Zhu HX, Chen P, Huang XH, Chen YL, Lan LJ, Rong YL, Wang YS, Yang HZ. The study on Liu wei di huang pill for the treatment of IGT to reduce the risk of CVD [Liu Wei Di Huang Wan Zhi Liao IGT Jiang Di Xin Xue Guan Ji Bing Wei Xian Yin Su De Yan Jiu] China Journal of Cardiovascular Rehabilitation Medicine (Xin Xue Guan Kang Fu Yi Xue Za Zhi) 2006;15(6):606–7.
- Zhou DY 2003.Published and unpublished dataZhou DY, Chen HP. Observation on the effect of Jinqi Jiangtang tablet on non-overweight impaired glucose tolerance [Jin qi jiang tang pian dui fei fei pang xing tang nai liang jian tui de liao liao guan cha] Chinese Traditional and Herbal Drugs [Zhong Cao Yao] 2003;34(5):449–50. Excluded studies
- An SH 2007a.Published and unpublished data An SH. The clinical observation of the nourishing kidney and expelling phlegm method for the treatment of IGT [Bu shen qu tan fa zhi liao tang nai liang di jian de lin chuang guan cha] Journal of Sichuan Traditional Chinese Medicine [Si Chuan Zhong Yi] 2007;25(5):44–5.
- An SH 2007b.Published and unpublished data An SH. Chinese herbal medicine for IGT in 40 cases [Zhong yao zhi liao tang nai liang di jian 40 li] Journal of Liaoning University of TCM [Liao Ning Zhong Yi Yao Da Xue Xue Bao] 2007;9(3):115.
- Cai X 2001.Cai X, Dai FF, Wang WL, Ji Y. The change after treatment by Ke Tang Ling in IGT patients [Tang nai liang di jian bing ren tang hua xue hong dan bai ji ke tang ling zhi liao hou de bian hua] Chinese Medical Journal of Communications [Jiao Tong Yi Xue] 2001;15(1):47–8. [Google Scholar]
- Cai X 2002.Cai X, Dai FF, Wang WL, Ji Y. Exploration of change after treatment by Ke Tang Ling in IGT patients [Tang nai liang di jian bing ren tang hua xue hong dan bai ji jing ke tang liang zhi liao hou de bian hua jian ce] Journal of Mathematical Medicine [Shu Li Yi Yao Xue Za Zhi] 2002;15(2):136–7. [Google Scholar]
- Chen C 2005.Chen C. Intervention action of Shenqi jiangtang capsule on blood sugar and blood lipid in patients with impaired glucose tolerance [Shenqi Jiangtang Jiaonang dui tang nai liang di jian huan zhe xue tang ji xue zhi shui ping de gan yu zuo yong] Modern Journal of Integrated Traditional Chinese and Western Medicine [Xian Dai Zhong Xi Yi Jie He Za Zhi] 2005;14(13):1681–1682. [Google Scholar]
- Chen G 2001.Chen G. Jianpi Nichan Tang for IGT in 48 cases [Jianpi Nichan Tang zhi liao tang nai liang dijian 48 li] Journal of Sichuan of Traditional Chinese Medicine [Sichuan Zhong Yi] 2001;19(11):43. [Google Scholar]
- Chen JM 2006.Chen JM, Pan XQ. Observation of 72 cases of low glucose tolerance treated with Yuye decoction [Yu Ye Tang Jia Jian zhi liao tang nai liang di jian de lin chuang guan cha] Journal of Hubei College of TCM [Hubei Zhong Yi Xue Yuna Xue Bao] 2006;8(2):55. [Google Scholar]
- Chen Y 2005.Published and unpublished data Chen Y, Liu GH, Yang Y, Qi BQ. Therapeutic observation on the comparison of Jinqi jiangtang tablet and metformin in treating impaired glucose tolerance [Jin qi jiang tang pian yu er jia shuang gua bi jiao zhi liao tang nai liang jian di de liao xiao guan cha] Tianjin Medical Journal [Tian Jin Yi Yao] 2005;33(11):726–7.
- Dai FF 2005.Published and unpublished data Dai FF. Clinical observation on the intervention of 32 cases of impaired glucose with Ketangling granule [Ke tang ling ke li gan yu zhi liao tang nai liang jian di 32 li lin chuang guan cha] Jiangsu Journal of Traditional Chinese Medicine [Jiang Su Zhong Yu Yao] 2005;26(11):24. 2005.
- Ding P 2007.Published and unpublished data Ding P, Luo PYY, Xu JH. Clinical effectiveness observation on strengthening the spleen soothing liver method for IGT [Jian pi shu gan fa zhi liao pu tao tang nai liang di jian de lin chuang liao xiao guan cha] Henan Traditional Chinese Medicine. 2007;27(6):30–1.
- Fan GJ 2003.Fan GJ, Liu CH. TCM for IGT [Zhong yi yao zhi liao tang nai liang di jian] Chinese Journal of Diabetes [Tang Niao Bing Zhi You] 2003;2(2):54–5. [Google Scholar]
- Fan JB 2000.Fan JB, Wang C, Zhang ZS. Clinical observation on herbal treatment of impaired glucose [Zhong yao zhi liao tang nai liang yi chang de lin chuang guan cha] Journal of Henyang Medical College [Heng Yang Yi Xue Yuan Xue Bao] 2000;28(5):493. [Google Scholar]
- Gao S 2007.Gao SR. Effectiveness analysis of Huanyan Kugansu Pill for IGt [Huanyuan Kuguasu Hanpian zhi liao tang nai liang di jian liao xiao fen xi] Proceedings of Clinical Medicine Journal [Lin Chuang Yi Yao Shi Jian Za Zhi] 2007;16:2. [Google Scholar]
- Gu HX 2005.Gu HX, Wang YJ. Effectiveness observation on interventions for IGT in 67 cases [Gan yu zhi liao tang nai liang di jian 67 li lin chuang liao xiao guan cha] Jilin Medical Journal [Ji Lin Yi Xue] 2005;26(2):200. [Google Scholar]
- He Y 2000.He Y. The effect of danshen injection on middle and elderly people with aged IGT [Danshen zhu she ye dui zhong lao nian tang nai liang jian di se ying xiang] Modern Rehabilitation [Xian Dai Kang Fu] 2000;4(5):176. [Google Scholar]
- Hu XX 2001.Hu XX. Clinical observation on self-modified presciption for IGT in 60 cases [Zi ni fang zhi liao tang nai liang di jian 60 li lin chuang guan cha] Chinese General Practise [Zhong Guo Quan Ke Sheng] 2001;4(5):385. [Google Scholar]
- Huang JX 2003.Published and unpublished data Huang JX, Ye XC. Herbal treatment of 33 cases of impaired glucose tolerance [Zhong yao zhi liao tang nai liang di jian 33 li] Journal of Practical Traditional Chinese Medicine. 2003;19(8):411.
- Huang SL 2005.Published and unpublished data Huang SL, Mai M. Clinical research on revcersing impaired glucose by xiaoyang tang [Xiaoyang tang ni zhuan tang nai liang di jian de lin chuang yan jiu] Chinese Journal of Traditional Medical Science and Technology [Zhong Guo Zhong Yi Yao Ke Ji] 2005;12(2):73–4.
- Huang XP 2003.Huang XP. Effects of yangxing tongmai tablets on erythrocyte insulin receptor of insulin resistance syndrome patients [Yangxin tongmai pian dui yi dao su di kang zong eh zheng bing ren hong xi bao yi dao su shou ti de ying xiang] Chinese Journal of Integrative Medicine on Cardio/Cerebrovascular Disease [Zhong Xi Yi Jie He Xin Nao Xue Guan Bing Za Zhi] 2003;1(7):373–5. [Google Scholar]
- Ji HM 2002.Ji HM. Therapeutic efect of qiwei baizhu san and gancao shaoyao decoction in treating 31 patients with impaired glucose tolerance [Qiwei Baizhu San Jia Gancao Shaoyao Tang zhi liao pu tao tang nai liang di jian huan zhe 31 li] Central Plains Medical Journal [Zhong Yuan Yi Kan] 2002;34(5):24–5. [Google Scholar]
- Ju S 2007.Ju SB, Tan LR, Su W, Rong KW. Intervention effect of berberine hydrochloride liposomes for IGT complicated with hyperlipedemia [Yan suan xiao chai jian zhi zhi ti dui tang nai liang di jian ban gao zhi xue zheng de gan yu zuo yong] Journsl of Practical Traditional Chinese Medicine [Shi Yong Zhong Yi Yao Za Zhi] 2007;23(8):490–2. [Google Scholar]
- Kuang KA 2001.Kuang KA. Understanding of Wumei Shaoyao Tang for IGT in 20 cases [Wumei Shaoyao Tang zhi liao pu tao tang nai liang di jian 20 li ti hui] Acta Chinese Medicine and Pharmacology [Zhong Yi Yao Xue Bao] 2001;29(5):11. [Google Scholar]
- Li CL 2007.Published and unpublished data Li CL, Zhang MZ. The effect of Hu Ben Hui Ni prescription on ICR [Hu Ben Hui Ni Fang Dui Tang Tiao Jie Zhang Ai Gn Yu Zhi Liao Ying Xiang] Li Shi Zhen Medicine and Materia Medica Research [Shi Zhen GUo Yi GUo Yao] 2007;18(8):2001–3.
- Li HB 2002.Li HB, Xu ZX, Ling LF, Hu ZH. The interference of glucose tolerance reduction by qi-benefiting [Yi qi jian pi fang dui yuan fa xing gao xue ya] New Journal of Traditional Chinese Medicine [Xin Zhong Yi] 2002;34(11):35–36. [Google Scholar]
- Li HB 2003.Li HB. The effect of yiqi jianpi fang on reduced glucose tolerance in primary hypertension [Yi qi jian pi zhong yao dui yuan fa xing gao xue ya] Journal of Guangxi Traditional Chinese Medical University [Guang Xi Zhong Yi Xue Yuan Xue Bao] 2003;34(11):35–36. [Google Scholar]
- Lian JE 2004.Lian JE. Syndrome differentiateion and treatment for IGT in 60 cases [Bian zheng fen xing zhi liao pu tao tang nai liang di jian 60 li] Zhejiang Journal of Traditional Chinese Medicine [Zhe Jiang Zhong Yi Za Zhi] 2004;12:521. [Google Scholar]
- Liu DH 2001.Liu DH. Jianpi Sanjing Tang for IGT in 31 cases [Jianpi Sanjing Tang shi liao pu tao tang nai liang dijian 31 cases] Journal of New Chinese Medicine [Xin Zhong Yi] 2001;33(2):59–60. [Google Scholar]
- Liu PY 2008.Liu PY, Liu JY, Peng Ji, Xiong JF, Xiao J, Li Y, et al. The Comparative Study of Intervention Effect by Different Methods on the Population with Impaired Glucose Tolerance [Bu tong fang fa dui tang nai liang jian di gan yu xiao guo de bi jiao yan jiu] Shenzhen Journal of Integrated Traditional Chinese and Western Medicine [Shen Zhen Zhong Xi Yi Jie He Za Zhi] 2008;18(3):145–7. [Google Scholar]
- Lu YZ 2003.Lu YZ, Wu Y. Clinical Study of Supplementing Qi and Nourishing Yin Decoction on 20 Caese of Impaired Glucose Tolerance (IGT) with Anti-After Ovariotomy [Yiqi Yangyin Fang zhi liao luan chao qie chu hou tang nai liang di jian 20 li lin chuang guan cha] Shanxi Journal of TCM [Shanxi Zhong Yi] 2003;19(3):47. [Google Scholar]
- Luo GB 2005.Luo GB, Fan GJ, Li SL, Tang AH, Jia ML. Clinical observation on the intervention of impaired glucose tolerance with the qi and yin deficiency [Du qi yin liang xu xing tang nai liang di jian zhe gan yu zhi liao de lin chuang guan cha] Chinese Journal of Basic Medicine in Tradtional Chinese Medicine [Zhong Guo Zhong Yu Ji Chu Yi Xue Za Zhi] 2005;11(11):845–6. [Google Scholar]
- Luo HX 2008.Luo HX. Clinical observation of liver soothing, spleen strengthening and expelling phlegm method for early diabetes [Shugan Jianpi Huatan fa Zhi liao tang niao bing qian qi de lin chuang guan cha] Inner Mongol Journal of Traditional Chinese Medicine [Nei Meng Gu Zhong Yi Yao] 2008;27(7):18. [Google Scholar]
- Mao LH 2003.Published and unpublished data Mao LH. Clinical observation of impaired glucose tolerance by jinqi melbine in old people [Jin qi jiang tang pian gan yu zhi liao lao nian ren tang nai liang di jian lin chuan guan cha] Journal of Henan University of Science and Technology [Henan Ke Ji Da sxue Xue Bao] 2003;21(3):200–2.
- Meng FX 2000.Meng FX. Impact of Jiangtang xiaozhi yin on glucose and fat metabolism in 34 patients with IGT [Jiangtang Xiaozhi yin dui 34 li IGT zhe tang zhi dai xie de ying xiang] Chinese Journal of Information on Traditional Chinese Medicine [Zhongguo Zhong Yi Yao Xin Xi Za Zhi] 2000;7(8):31–2. [Google Scholar]
- Niu ZY 2003.Published and unpublished data Niu ZY. Treatment of 50 cases of impaired glucose tolerance and early stage of diabetes with Xuexi II capsules [Xue xi er hao jiao nang zhi liao tang nai liang shou sun ji zao qi tang niao bing 50 li] Henan Traditional Chinese Medicine [Henan Zhong Yi] 2003;23(9):30–31.
- Ouyang AJ 2003.Ouyang AJ. Clinical observation on Yi ming decoction [Yi ming tang zhi liao tan shi yong sheng xing er xing tang niao bing yi dao su di kang de lin chuan 32 guan cha] Journal of TCM University of Hunan. 2003;23(5):52–3. [Google Scholar]
- Shi Y 2000.Shi Y. Changing observation on treatment of IGT [Pu tao tang nai liang di jian gan yu zhi liao de bian hua guan cha] Chinese Journal of Prevention and Control of Chronic Non-communicable Diseases [Zhong guo man xing bing yu fang yu kong zhi] 2000;8(6):1. [Google Scholar]
- Sun BR 2005.Sun BR. Medical intervention for IGT in 104 cases [Tang nai liang di jian huan zhe yao wu gan yu zhi liao 104 li lin chuang guan cha] Journal of Guangdong College of Pharmacy [Guangdong Yao Xue Yuan Xue Bao] 2005;21(3):371–2. [Google Scholar]
- Sun Y 2005.Sun Y. Tonifying-qi nurishing-yin strengthening-kidney method for IGT in 40 cases [Yiqi yangyin bushen fa zhi liao tang nai liang di jian 40 li] Journal of Practical Traditional Chinese Internal Medicine [Shi Yong Zhong Yi Nei Ke Za Zhi] 2005;19(6):567. [Google Scholar]
- Sun YW 2002.Sun YW, Yuan L. Treatment of sixty cases of hidden diabetes with ziyin jiantang capsule [Zi yin jiang tang jiao nang zhi liao yin ni xing tang niao ning 60 li liao xiao guan cha] Research of Traditional Chinese Medicine [Zhong Yi Za Zhi] 2002;18(4):9. [Google Scholar]
- Wang BH 2004.Wang BH. Study progress of TCM in IGT [Tang nai liang di jian (IGT) de zhong yi yan jiu jin zhan] Beijing Journal of Traditional Chinese Medicine [Beijing Zhong Yi] 2004;23(5):299–300. [Google Scholar]
- Wang D 1999.Wang DH, Liu WF, Xi MH, Zhang H. Analysis of the effectiveness of soothing-liver clearing-heat and promoting-blood circulation expelling-stagnation method for II DM in 120 cases [Shugan qingre huoxue huoyu fa zhi liao II xing tang niao bing 120 li liao xiao fen xi] Tianjin Journal of Traditional Chinese Medicine [Tianjin Zhongyi] 1999;16(2):15–7. [Google Scholar]
- Wang H 2002.Published data only (unpublished sought but not used) Wang H, Liang XP, Yu XM, Zuo Y. Observation on intervention of liu wei di huang pill on IGT [Liu wei di huang wan dui IGT de gan yu] Liaoning Journal of Traditional Chinese Medicine [Liao Ning Zhong Yi Za Zhi] 2002;29(12):758–9.
- Wang H 2003.Wang H. Strengthening qi and nourishing yin methods for treating IGT [Yiqi Ziyin Fa gan yu zhi liao pu tao tang nai liang di jian liao xiao guan cha] Guangdong Medicial Journal [Guangdong Yi Xue] 2003;24(7):1012. [Google Scholar]
- Wang H 2004.Published data only (unpublished sought but not used) Wang H. Observation on the effect of Yiqi Ziyin granule in IGT [Yi qi zi yin zhong yao pei fang ke li dui tang nai liang di jian gan yu de guan cha] Shanxi Journal of Traditional Medicine. 2004;20(3):10–1.
- Wang J 2005.Published data only (unpublished sought but not used) Wang J, Chen YY. The effect of Fufang yinyanghuo chongji on indicators of reduced glucose tolerance among aged metabolic syndrome [Fu fang yin yang huo chong ji dui lao nian dai xie zong he zheng tang nai liang di jian xiang guan zhi biao de ying xiang] Chinese Journal of Traditional Medical Science and Technology. 2005;12(3):186–7.
- Wang YF 2008.Wang YF. Observation of Shenqi Dihuang Tang intervention for blood and lipids in patients with IGT [Shenqi Dihuang Tang Dui tang nai liang jian di huan zhe xue tang, xue zhi de gan yu guan cha] Hebei Journal of Traditional Chinese Medicine. 2008;30(5):473–474. [Google Scholar]
- Wei Y 2008.Wei Y, Hong YZ, Ye X. Effect of Tang No. 1 Granule in Treating Patients with Impaired Glucose Tolerance. Chinese Journal of Integrated Medicine. 2008;14(4):298–302. doi: 10.1007/s11655-008-0298-7. [DOI] [PubMed] [Google Scholar]
- Wu G 2007.Wu GT. Effectiveness and importance of interventions for IGT from Evidence Based Medicine [Cong xun zheng yi xue kan tang nai liang di jian huan zhe gan yu de you xiao xing he zhong yao xing] Journal of Tongji University (Medical Science) [Tong Ji Da Xue Xue Bao (Yi Xue Ban)] 2007;28(3):6–10. [Google Scholar]
- Wu S 2004.Wu ST. Failure of spleen to disperse the essence and IGT [Pi bu san jing yu tang nai liang di jian] China Journal of Traditional Chinese Medicine and Pharmacy [Zhong Guo Yi Yao Xue Bao] 2004;19(8):463–5. [Google Scholar]
- Wu ZX 2006.Wu ZX. The treatment of IGT [Tang nai liang di jian de gan yu zhi liao] Chinese Practical Journal of Rural Doctor [Zhong Guo Shi Yong Xiang Cun Yi Sheng Za Zhi] 2006;13(12):54–5. [Google Scholar]
- Xin XX 2007.Xin XX. Clinical observation of Jianpi bushen pill for IGT in 68 cases [Jian pi bu shen wan gan yu zhi liao tang nai liang di jian 68 li lin chuang guan cha] Hebei Journal of Traditional Chinese Medicine [Hebei Zhong Yi] 2007;29(4):302–3. [Google Scholar]
- Xu YJ 2008.Published and unpublished data Xu YJ. A clinical investigation of damp and phlegm - transforming heat-clearing toxins-resolving principle for impaired glucose tolerance (IGT) [Zao Shi Hua Tan Qing Re Jie Du Zhong Yao Zhi Liao Tang Nai Liang Di Jian De Lin Chuang Yan Jiu] Journal of Chinese Modern Traditional Chinese Medicine. 2008;4(1):1–3.
- Xue LH 2008.Xue LH. Clinical observation of Jianpi Jiangtang Yin for IGT in 80 cases [Jian Pi Jiang Tang Yin Gan Yu Tang Nai Liang Shou Sun 80 Li Lin Chuang Guan Cha] Journal of Practical Traditional Chinese Internal Medicine. 2008;22(8):35–6. [Google Scholar]
- Yang E 2007.Yang E, Yu XY, Mo ZE, Li XF. Impact of different methods for IGT [Bu tong fang shi de gan yu cuo shi dui tang nai liang shou sun huan zhe de ying xiang] Journal of Practical Diagnosis and Therapy [Shi Yong Zhen Duan yu Zhi Liao Za Zhi] 2007;21(4):269–70. [Google Scholar]
- Yang SJ 2001.Yang SJ, Chen JX, Yang FQ. Clinical observation on interventions for IGT [Tang nai liang di jian gan yu zhi liao de lin chuang guan cha] Chinese Journal of Rural Medicine and Pharmacy [Zhong Guo Xiang Cun Yi Yao Za Zhi] 2001;8(6):18. [Google Scholar]
- Yang WX 2007.Yang WX, Gao TS. Study progress of metabolism syndromes [Dai xie zong he zheng de zhong yi yan jiu jin zhan] Journal of Liaoning University of TCM [Liaoning Zhong Yi Yao Da Xue Xue Bao] 2007;9(4):55–7. [Google Scholar]
- Yin B 2007.Published and unpublished data Yin B. The clinical observation of jin qi jiang tang for IGT in 41 cases [Jin qi jiang tang pian gan yu zhi liao IGT 41 li lin chuang guan cha] Journal of Practical Diabetology [Shi Yong Tang Niao Bing Za Zhi] 2007;2(3):35.
- Yin B 2007.Published and unpublished data Yin B. The clinical observation of jin qi jiang tang for IGT in 41 cases [Jin qi jiang tang pian gan yu zhi liao IGT 41 li lin chuang guan cha] Journal of Practical Diabetology [Shi Yong Tang Niao Bing Za Zhi] 2007;2(3):35.
- Yin L 2004.Yin L, Wan CC. The impact of Shengmai injection for elderly patients with IGT [Shengmai Zhushe Ye dui lao nian tang nai liang di jian zhe zao qi niao wei liang bai dan bai de ying xiang] Chinese Journal of Integrated Traditional and Western Nephrology [Zhong Guo Zhong Xi Yi Jie He Shen Bing Za Zhi] 2004;5(12):728. [Google Scholar]
- Yu FH 2005.Yu FH, Zhang LQ. Clinical observation on Jinqi Jiangtang Pill for IGT [Jinqi Jiangtang Pian gan yu zhi liao tang nai liang shou sun de lin chuang guan cha] Tianjin Medicine Journal [Tianjin Yi Yao] 2005;33(5):300. [Google Scholar]
- Yuan WL 2008.Published and unpublished data Yuan WL, Hu JH, Tang B. Observation of the Therapeutic Effect of Phlegm Treatment for IGT [Cong tan lun zhi tang nai liang di jian liao xiao quan cha] Journal of Sichuan Traditional Chinese Medicine. 2008;26(2):60–1.
- Zhang L 2006.Zhang LQ. Progress of prevention and treatment of IGT [Tang nai liang shou sun de fang zhi xin jin zhan] Nursing and Rehabilitation Journal [Hu Li yu Kang Fu] 2006;5(4):254–5. [Google Scholar]
- Zhang RR 2005.Zhang RR, Liu Y. The effect of Jinqi Jiangtang tablet on the excretion rate of microalbuminuria in patients with impaired glucose tolerance [Jin qi jiang tang pian dui IGT huan zhe niao wei liang bai dan bai pai xie lu de ying xiang] Tianjin Medical Journal. 2005;33(5):301–2. [Google Scholar]
- Zhang X 2003.Zhang X, Guo YN. Clinical observation on the treatment of IGT by nourishing blood, dispelling phlegm and regulating fu [Huo xue que tan tong fu fa zhi lioa tang nai liang jian di de lin chuang guan cha] Liaoning Journal of Traditional Chinese Medicine [Liaoning Zhong Yi Za Zhi] 2003;30(6):472. [Google Scholar]
- Zhou XL 2006.Zhou XL, Sun YY. Intervention of impaired glucose with Shenqi jiangtang keli and comment on the function of the B cell [Shen qi jiang tang ke li dui tang mai liang jian di zhe gan yu zhi liao ji yi dao B xi bao gong neng ping jia] Journal of Emergency in Traditional Chinese Medicine [Zhong Guo Zhong Yi Ji Zheng] 2006;15(4):369–70. [Google Scholar]
- Zhou ZN 2001.Published and unpublished data Zhou ZN. Observation of curative effect of self-prepared Huaqi jiangtang decoction on impaired glucose tolerance [Zi yi hua qi jiang tang fang yu tang nai liang yi chang liao xiao guan cha] Guangxi Journal of Traditional Chinese Medicine [Guang Xi Zhong Yi Yao] 2001;24(6):13–5.
- Zhou ZN 2002.Published and unpublished data Zhou Zn. Research on the effect of Jinqi Jiangtang tablet on impaired glucose tolerance [Jin qi jiang tang pian dui tang nai liang di jian de liao xiao tan suo] Journal of Practical Traditional Chinese Medicine [Shi Yong Zhong Yi Yao Za Zhi] 2002;18(11):3–4. 2002. Studies awaiting classification
- Liu DQ 2007.Liu DQ, Wang KF, Kan LZ, Zheng XQ. The clinical observation of Jie Yu Huo Xue decoction for IGT [JIeyu Huoxue Tang gan yu tang nai liang shou sun huan zhe de lin chuang guan cha] Chinese Journal of Clinical Healthcare April. 2007;10(2):183. Ongoing studies
Other references
Additional references
- ADA 1997.American Diabetic Association Report of the Expert Committee on the Diagnosis and Classification of Diabetes Mellitus. Diabetes Care. 1997;20:1183–97. doi: 10.2337/diacare.20.7.1183. [DOI] [PubMed] [Google Scholar]
- ADA 1999.American Diabetes Association The Expert Committee on the Diagnosis and Classification of Diabetes Mellitus. Report of The Expert Committee on the Diagnosis and Classification of Diabetes Mellitus. Diabetes Care. 1999;22(Suppl 1):S5–19. doi: 10.2337/diacare.26.2007.s5. [DOI] [PubMed] [Google Scholar]
- ADA 2003.American Diabetes Association The prevention or delay of type 2 diabetes. Diabetes Care. 2003;26(Suppl 1):S62–9. doi: 10.2337/diacare.26.2007.s62. [DOI] [PubMed] [Google Scholar]
- ADA 2009.American Diabetes Association American Diabetes Association Clinical Practice Recommendations. Diabetes Care. 2009;32(Suppl 1):S13–S61. [PubMed] [Google Scholar]
- AHFS 1999.AHFS . American Hospital Formulary Service Drug Information. Vol. 1. American Society of Health-System Pharmacists, Inc.; Bethesda, USA: 1999. Metformin Hydrochloride; pp. 2755–63. [Google Scholar]
- Bensoussan 1996.Bensoussan A, Myers SP. Towards a safer choice: The practice of traditional Chinese medicine in Australia. Faculty of Health, University of Western Sydney (Macarthur); Sydney: 1996. [Google Scholar]
- Davies 2004.Davies MJ, Tringham JR. Prevention of Type 2 diabetes mellitus. A review of the evidence and its application in a UK setting. Diabetic Medicine. 2004;21(5):403–14. doi: 10.1111/j.1464-5491.2004.01176.x. [DOI] [PubMed] [Google Scholar]
- DPP Research Gp 2002.DPP Research Group Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. The New England Journal of Medicine. 2002;346(6):393. doi: 10.1056/NEJMoa012512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du 1994.Du XM, Wu Y, Zhou YS. The measurement of quality of life in patients with hypertension. Zhong Guo Kang Fu. 1994;9(3):129–32. [Google Scholar]
- Engberg 2009.Engberg S, Vistisen D, Lau C, Glümer C, Jørgensen T, Pedersen O, et al. Progression to Impaired Glucose Regulation and Diabetes in the Population-Based Inter99 Study. Diabetes Care. 2009;32(4):606–11. doi: 10.2337/dc08-1869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forrest 1988.Forrest RD, Jackson CA, Yudkin JS. The abbreviated glucose tolerance test in screening for diabetes: The Islington Diabetes Survey. Diabetic Medicine. 1988;5(6):557–561. doi: 10.1111/j.1464-5491.1988.tb01051.x. [DOI] [PubMed] [Google Scholar]
- Gagnier 2006.Gagnier J, et al. Reporting Randomized, Controlled Trials of Herbal Interventions: An Elaborated CONSORT Statement. Annals of Internal Medicine. 2006;144(5):364. doi: 10.7326/0003-4819-144-5-200603070-00013. [DOI] [PubMed] [Google Scholar]
- Haller 1998.Haller H. The clinical importance of postprandial glucose. Diabetes Research and Clinical Practice. 1998;40(Supplement 1):S21–S25. doi: 10.1016/s0168-8227(98)00042-4. [DOI] [PubMed] [Google Scholar]
- Higgins 2002.Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Statistics in Medicine. 2002;21(11):1539–58. doi: 10.1002/sim.1186. [DOI] [PubMed] [Google Scholar]
- Higgins 2003.Higgins JPT, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analysis. BMJ. 2003;327:557–60. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins 2008.Higgins JPT, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions. Version 5.0.0. The Cochrane Collaboration; [updated February 2008]. 2008. Available from www.cochrane-handbook.org. [Google Scholar]
- IDF 2008.International Diabetes Federation . Diabetes Atlas. 3rd edition International Diabetes Federation; 2008. [Google Scholar]
- Jia 2003.Jia W, Gao WY, Xiao PG. Antidiabetic drugs of plant origin used in China: compositions, pharmacology, and hypoglycaemic mechanisms. Zhongguo Zhong Yao Za Zhi. 2003;28(2):108–13. [PubMed] [Google Scholar]
- Knowler 2005.Knowler WC, Hamman RF, Edelstein SL, Barrett-Connor E, Ehrmann DA, Walker EA, et al. Diabetes Prevention Program Research Group Prevention of Type 2 Diabetes With Troglitazone in the Diabetes Prevention Program. Diabetes. 2005;54(4):1150–56. doi: 10.2337/diabetes.54.4.1150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lau 2006.Lau J, Ioannidis JPA, Terrin N, Schmid CH, Olkin I. The case of the misleading funnel plot. BMJ. 2006;333:597–600. doi: 10.1136/bmj.333.7568.597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liberati 2009.Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JPA, et al. The PRISMA Statement for Reporting Systematic and Meta-Analyses of Studies That Evaluate Interventions: Explanation and Elaboration. PLoS Med. 1999;6(7):1–28. doi: 10.1371/journal.pmed.1000100. [10.1371/journal.pmed.1000100] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu 2004.Liu JP, Zhang M, Wang WY, Grimsgaard S. Chinese herbal medicines for type 2 diabetes. Cochrane Database of Systematic Reviews. 2004;(Issue 3) doi: 10.1002/14651858.CD003642.pub2. Art. No.: CD003642 DOI: 10.1002/14651858.CD003642.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Padwal 2005.Padwal R, Majumdar SR, Johnson JA, Varney J, McAlister FA. A systematic review of drug therapy to delay or prevent type 2 diabetes. Diabetes Care. 2005;28(3):736–44. doi: 10.2337/diacare.28.3.736. [DOI] [PubMed] [Google Scholar]
- Polonsky 1996.Polonsky KS, Sturis J, et al. Seminars in Medicine of the Beth Israel Hospital, Boston. Non-insulin dependent diabetes mellitus - a genetically programmed failure of the beta cell to compensate for insulin resistance. New England Journal of Medicine. 1996;334(12):777–83. doi: 10.1056/NEJM199603213341207. [DOI] [PubMed] [Google Scholar]
- Rambod 2009.Rambod M, et al. Fine-tuning of prediction of isolated impaired glucose tolerance: A quantitative clinical prediction model. Diabetes Research and Clinical Practice. 2009;83(1):61–8. doi: 10.1016/j.diabres.2008.09.040. [DOI] [PubMed] [Google Scholar]
- Riccardi 1985.Riccardi G, Vaccaro O, Rivellese A. Reproducibility of the new diagnostic criteria for impaired glucose tolerance. American Journal of Epidemiology. 1985;121(3):422–9. doi: 10.1093/oxfordjournals.aje.a114014. [DOI] [PubMed] [Google Scholar]
- Robinson 2002.Robinson KA, Dickersin K. Development of a highly sensitive search strategy for the retrieval of reports of controlled trials using PubMed. International Journal of Epidemiology. 2002;31(1):150–3. doi: 10.1093/ije/31.1.150. [DOI] [PubMed] [Google Scholar]
- Testa 1989.Testa MA, Sudilovsky A, Rippey RM, Williams GH. A short form for clinical assessment of quality of life among hypertensive patients. American Journal of Preventive Medicine. 1989;5(2):82–9. [PubMed] [Google Scholar]
- Unwin 2002.Unwin N, Shaw J, Zimmet P, Alberti KG. Impaired glucose tolerance and impaired fasting glycaemia: the current status on definition and intervention. Diabetic Medicine. 2002;19(9):708–23. doi: 10.1046/j.1464-5491.2002.00835.x. [DOI] [PubMed] [Google Scholar]
- Van de Laar 2006.Van de Laar Floris A, Lucassen PLBJ, Akkermans RP, Van de Lisdonk EH, De Grauw WJC. Alpha-glucosidase inhibitors for people with impaired glucose tolerance or impaired fasting blood glucose. Cochrane Database of Systematic Reviews. 2006;(Issue 4) doi: 10.1002/14651858.CD005061.pub2. Art. No.: CD005061 DOI: 10.1002/14651858.CD005061.pub2. [DOI] [PubMed] [Google Scholar]
- WHO 1985.World Health Organisation . Report of a WHO study group. WHO; Geneva: 1985. Diabetes Mellitus. (Technical report series 727). [PubMed] [Google Scholar]
- WHO 1999.World Health Organisation . Report of a WHO Consultation. Part 1: Diagnosis and Classification of Diabetes Mellitus. WHO; 1999. Definition, Diagnosis and Classification of Diabetes Mellitus and its Complications; pp. 1–50. [Google Scholar]
- Wu 2007.Wu T, Zhang J, Yan Q, Xie L, Liu G. Chinese medicinal herbs for the common cold. Cochrane Database of Systematic Reviews. 2007;(Issue 1) doi: 10.1002/14651858.CD004782.pub2. [DOI] [PubMed] [Google Scholar]
- Yamaoka 2005.Yamaoka K, Tango T. Efficacy of lifestyle education to prevent type 2 diabetes: a meta-analysis of randomized controlled trials. Diabetes Care. 2005;28(11):2780–6. doi: 10.2337/diacare.28.11.2780. [DOI] [PubMed] [Google Scholar]
- Yu 2006.Yu J, Zhang Y, Sun S, Shen J, Qiu J, Yin X, et al. Inhibitory effects of astragaloside IV on diabetic peripheral neuropathy in rats. Canadian journal of physiology and pharmacology. 2006;84(6):579–87. doi: 10.1139/y06-015. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.