Abstract
The effectiveness of recombinant Adenovirus serotype 5 (Ad5) vectors to induce immune responses against targeted antigens has been limited by the presence of pre-existing or Ad5 vaccine induced anti-vector immunity. The Ad5 [E1-, E2b-] platform, a recombinant Ad5 with additional deletions, has been previously reported by us to induce immune responses in the presence of Ad5 immunity. In an Ad5 immune non-human primate (NHP) model, an Ad5 [E1-, E2b-] construct expressing HIV-1 Gag induced immune responses in the presence of pre-existing Ad5 immunity. In the present study we expand on these prior observations by comparing the cell mediated immune (CMI) responses induced by Ad5 [E1-, E2b-]-SIV-gag/nef in Ad5 naïve and Ad5 immune NHP. Additionally, NHP were immunized with an Ad5 [E1-, E2b-]-HIV-pol construct following two homologous administrations of Ad5 [E1-, E2b-]-SIV-gag/nef to determine if an immune response could be induced against a third antigen in the presence of vaccine induced Ad5 immunity. Positive CMI responses, as assessed by interferon-gamma (IFN-g) secreting lymphocytes, were induced against all three antigens. These CMI responses increased over a course of multiple immunizations and the response profiles observed in Ad5 naïve and Ad5 immune NHP were similar. No influence of the major histocompatibility complex on CMI responses was observed. These data indicate that the new Ad5 [E1-, E2b-] platform based vaccine could be used for homologous vaccination regimes to induce robust CMI responses in the presence of Ad5 vector immunity.
Keywords: HIV vaccine; Ad5 immunity; adenovirus vector; Ad5 [E1-, E2b-]
Introduction
The use of recombinant viral vector systems is a viable approach to deliver immunogens to prevent infectious diseases (1, 2). Recombinant Ad5 viral vectors have been utilized as vaccine platforms to deliver defined genes that express antigens for the induction of targeted immune responses (3,4–9). Anti-vector immunity is a major limitation to the use of current recombinant viral gene delivery vectors as vaccine platforms (10,11). Pre-existing anti-vector immunity has been demonstrated to reduce the immunogenicity of recombinant Ad5 vector-based vaccines and the generation of anti-vector immunity after primary vaccination has been reported to limit the efficiency of homologous boost immunizations (10,11). In attempts to overcome anti-vector immunity, further studies have revealed novel ways to circumvent pre-existing or vector induced Ad5 immunity (12).
One approach is to reduce the expression of viral vector proteins by removing extensive genetic regions of the viral backbone (13–15). Reduced expression of Ad5 viral genes in a recombinant vector platform may be advantageous in vaccine development for reasons including (a) reduced antigenic competition between the target and Ad5 proteins, (b) greater longevity of target transgene expression providing greater immunologic stimulus, and (c) decreased adverse effects, allowing for higher and more frequent doses of vaccine. Current generation recombinant Ad5 vector platforms (Ad5 [E1-]) are replication defective due to deletions in the early 1 (E1) gene region (16). A next generation Ad5 vector platform has been developed that retains the E1 deletions but has further deletions of the polymerase (pol) and/or preterminal (pTP) protein genes in the early 2 (E2b) gene region (Ad5 [E1-, E2b-]) (15). This new vector is produced in the E.C7 human cell line that contains the E1 and E2b deletions necessary to propagate the recombinant virus (17, 18). The Ad5 [E1-, E2b-] vector platform has an expanded cloning capacity and reduced expression of viral late genes as compared to current generation Ad5 [E1-] vector platforms (19).
We have recently completed comparative studies on the use of the Ad5 [E1-, E2b-] vector platform as a vaccine candidate for HIV and certain cancers. Studies using the tumor associated antigen carcinomembryonic antigen (CEA) demonstrated that the Ad5 [E1-, E2b-]-CEA vector was superior to a first generation Ad5 [E1-]-CEA vector in generating CEA specific CMI responses in a multiple immunization regimen and could be used to generate immune responses in animals with pre-existing Ad5 immunity (20, 21). It was also determined that treatments of Ad5 [E1-, E2b-]-CEA could induce significant anti-tumor responses in Ad5 immune mice with established CEA bearing tumors (21). Studies using HIV-1 antigens Gag, Pol, and Nef demonstrated that the Ad5 [E1-, E2b-] vector was superior to an Ad5 [E1-] vector in generating HIV-1 antigen specific CMI responses employing a multiple immunization protocol and could be used to generate immune responses in mice and non-human primates (NHP) with pre-existing Ad5 immunity (14,22). In the present study, we evaluated the immunogenicity of an Ad5 [E1-, E2b-]-SIV vaccine platform in NHP. We evaluated and compared the CMI responses generated in naïve NHP and NHP with pre-existing immunity to Ad5. In addition, we determined if a CMI response could be induced against a third antigen in the presence of vaccine induced Ad5 immunity. Since major histocompatibility complex (MHC) molecules determine the repertoire of T cell responses that can develop against SIV and/or any other foreign pathogen, all NHP in this study were typed for MHC class I molecules to assess any influence on vaccine-induced CMI responses (23).
Materials and Methods
Animals
Eighteen Chinese-origin rhesus macaques (Macaca mulatta) (6 males and 12 females) were purchased, housed, and handled by BIOQUAL, Inc., Rockville, MD in accordance with their Institutional Animal Care and Use Committee guidelines. Peripheral blood mononuclear cells (PBMC) and tissues from individual animals were collected at BIOQUAL and sent to Etubics Corporation, Seattle, WA for immunogenicity analysis as described below. BIOQUAL performed animal temperature determinations, weights, blood chemistries, and hematology during the course of the study.
Vaccine trial design
The eighteen NHP were divided into 3 groups; Group 1: Ad5 naive NHP (n=7, 2 males and 5 females), Group 2: Ad5 immune NHP (n=7, 2 males and 5 females) and Group 3: Ad5 immune controls (n=4, 2 males and 2 females). Ad5 immunity was induced in NHP according to a similar protocol that we have previously published (14, 22). This protocol was reported to induce Ad5 neutralizing antibody (NAb) levels of approximately 1:200, which was classified as “high Ad5 immunity” in previous Ad5 vaccine human trials (24). NHP in Group 2 and 3 were made Ad5 immune by intradermal immunization at 2-week intervals on immunization days −98 and −84 with 1010 viral particles (VP) of a current generation Ad5 [E1-]-null (no transgene) (Table 1). Ad5 immunity was confirmed by the presence of Ad5 NAb when Ad5 [E1-, E2b-] vaccination began (day 0). SIV Gag, Nef, and HIV-1 Pol protein expression by the Ad5 [E1-, E2b-] vector vaccines was confirmed by western blot prior to use in immunizations (data not shown). For this study, we decided to employ an initial 4-injection immunization protocol with Gag and Nef vectors. This strategy enabled us to then employ a secondary 3 injection multiple immunization protocol using a HIV pol vector vaccine. We performed this in order to assess whether or not we could induce a CMI response to a secondary antigen after commencing immunizations with primary antigens. NHP in Group 1 and Group 2 received subcutaneous immunizations of 1010 VP of a 1:1 mixture (2×1010 total VP) of Ad5 [E1-, E2b-]-SIV-gag and Ad5 [E1-, E2b-]-SIV-nef on days 0, 14, 28 and 42. Ad5 immune control animals (Group 3) received injections with buffer solution only. To determine if a CMI response could be induced against a third target antigen in the presence of recently acquired Ad5 vector immunity generated by earlier vaccinations, NHP in Groups 1 and 2 were immunized with 1010 VP of HIV-1 Ad5 [E1-, E2b-]-pol on days 28, 42 and 65. PBMC and serum were collected at baseline, immediately before each vaccination and upon termination.
Table 1.
Immunization and termination schedule
| Study Day | ||||||||
|---|---|---|---|---|---|---|---|---|
| Treatment | -98 | -84 | 0 | 14 | 28 | 42 | 65 | 77 |
| Ad5 [E1-]-null | G2b G3c |
G2 G3 |
||||||
| SIV Ad5 [E1-, E2b-]-gag/nef | G1a G2 |
G1 G2 |
G1 G2 |
G1 G2 |
||||
| HIV Ad5 [E1-, E2b-]-pol | G1 G2 |
G1 G2 |
G1 G2 |
|||||
| Injection Buffer | G3 | G3 | G3 | G3 | G3 | |||
| Termination | G1 G2 G3 |
|||||||
G1. Ad5 naïve NHP (n=7)
G2. Ad5 immune NHP (n=7)
G3. Ad5 immune control NHP (n=4)
MHC class I typing of NHP
The NHP were genotyped for MHC class I alleles by Wisconsin National Primate Research Center, University of Wisconsin, Madison, WI as previously described (25).
Gene synthesis and Ad5 Vector Construction
Ad5 [E1-, E2b-]-SIV-gag, Ad5 [E1-, E2b-]-SIV-nef and Ad5 [E1-, E2b-]-HIV-pol were constructed and produced as previously described (15,22) using a gag insert derived from the complete SIVmac239 genome (GenBank accession M33262), a SIV nef sequence from the nef protein (premature stop)-protein id AAA47638.1 (nt 9333–9611) derived from the complete SIVmac239 genome (GenBank accession M33262) and a HIV-pol gene sequence provided by the Vaccine Research Center, NIAID. Briefly, the Gag, Nef, or Pol cDNA was sub-cloned into the Ad5 [E1-, E2b-] vectors using a homologous recombination based procedure previously described (15). Gag, Nef, or Pol production was placed under the control of a cytomegalovirus (CMV) enhancer/promoter element. The replication deficient viruses were then propagated in the necessary and sufficient E.C7 packaging cell line, CsCl2 purified, and titered as previously described (15). Viral infectious titers were determined as plaques on E.C7 cell monolayers. The viral particle (VP) concentration was determined by sodium dodecyl sulfate (SDS) disruption and spectrophotometry at 260nm and 280nm (15, 26). The ratio of VP to plaque forming units (PFU) was 35:1 VP/PFU or lower per lot.
Western Blot Analysis
Western Blot analysis to determine transgene expression by the Ad5 [E1-, E2b-] vectors was performed as previously described (14). Briefly, 106 human A-549 lung carcinoma cells (ATCC number CCL-185) were infected at a Multiplicity of Infection (MOI) of 100 VP/cell, incubated for 48 hours, followed by cell lysis. A-549 cell lysates were separated on a 10% SDS-polyacrylamide gel and transferred onto a PVDF membrane (GE Heathcare, Piscataway, NJ). The membranes were then blocked with TBS containing 5% (w/v) blocking reagent (GE Healthcare, Piscataway, NJ) for 2 hours at room temperature and sequentially incubated with mouse anti-SIV-gag, anti-SIV-nef or anti-HIV-pol antibody, respectively (1:250 dilution) and goat anti-mouse-HRP conjugated antibody (1:1000 dilution) (Kirkegaard & Perry Laboratories, Gaithersburg, MD) for one hour at room temperature. Antibody reactivity was determined by chemilluminescence using an ECL Western Blotting analysis system (GE Healthcare) according to the manufacturer’s specifications.
Ad5 neutralizing antibody (NAb) assay
Endpoint Ad5 NAb titers of individual NHP were determined as previously described (14, 22). Briefly, dilutions of heat inactivated test sera in 100 μL of DMEM containing 10% fetal calf serum were mixed with 4 × 107 VP of Ad5 [E1-]-null and incubated for 60 minutes. The samples were added to microwells containing HEK-293 cells at 2 × 103 cells/well that had been cultured for 24 hours at 37° in 5% CO2. The mixture was then incubated for an additional 72 hours at 37° in 5% CO2. An MTS tetrazolium bioreduction assay (21, 27) was used to quantify resultant cell killing and endpoint Ad5 NAb titers were determined.
Enzyme-linked immunospot (ELISpot) assay for IFN-γ secreting cells
SIV Gag, Nef, or HIV-1 Pol specific IFN-γ secretion in PBMC isolated from in NHP throughout the course of vaccination and in splenocytes and PBMC upon termination was detected using an ELISpot assay as previously described (22). Cells were stimulated with SIV Gag, Nef, or HIV-1 Pol peptides provided by the AIDS Research and Reference Reagent Program, Division of AIDS, NIAID, NIH: SIVmac 239 Gag (15-mer Peptides-Complete Set, SIVmac239 Full length Nef (15-mer) Peptides-Complete Set and HIV-1 Consensus B Pol (15-mer) peptides-Complete Set. PBMC were used at a concentration of 2 × 105 cells/well and reported as the number of spot forming cells (SFC) per 106 cells per well. All 125 SIVmac239 Gag peptides were combined and tested as a single pool. Similarly, all 64 SIVmac239 Nef peptides, and all 249 HIV-1 Pol peptides were combined and tested as a single pool, respectively. Each peptide pool was tested in duplicate. Ad5 [E1-]-null virus was used at 108 VP/well to test the immune response against adenovirus. Peptides were utilized at 0.1 μg of each peptide/well. To determine the breath of the CMI induced by vaccination, Gag, Nef, and Pol peptide pools consisting of 15 amino acid peptides with an 11-mer overlap were used to map the breadth of SIV or HIV-1 specific CMI responses. Four SIVmac239 gag peptide pools (31 peptides/pool for pool # 1, #2, and #3; 32 peptides/pool for pool # 4) spanning the whole Gag protein, five HIV-1 pol pools (50 peptides/pool for pools #1–4; 49 peptides/pool for pool #5) spanning the whole Pol protein and 2 SIVmac239 Nef peptide pools spanning the whole Nef protein (32 peptides/pool) were used as the stimulating antigen in IFN-γ ELISpot analysis. In all ELISpot assays, cells stimulated with concanavalin A (ConA) at a concentration of 1 μg/well served as a positive control. Colored SFC were counted using an Immunospot ELISpot plate reader (Cellular Technology, Shaker Heights, OH) and responses were considered to be positive if, 1) 50 SFC were detected/106 cells after subtraction of the negative control and, 2) the number of SFC were ≥2-fold the number of SFC in the negative control wells.
Statistical analysis
Analyses were performed in individual NHP in each group. Statistically significant differences in the mean immune responses between groups of animals were determined by Student’s t-test with a P-value of 0.05 or lower being considered significant, using GraphPad Prism® (GraphPad Software, Inc.).
Results
Vaccine-elicited CMI responses
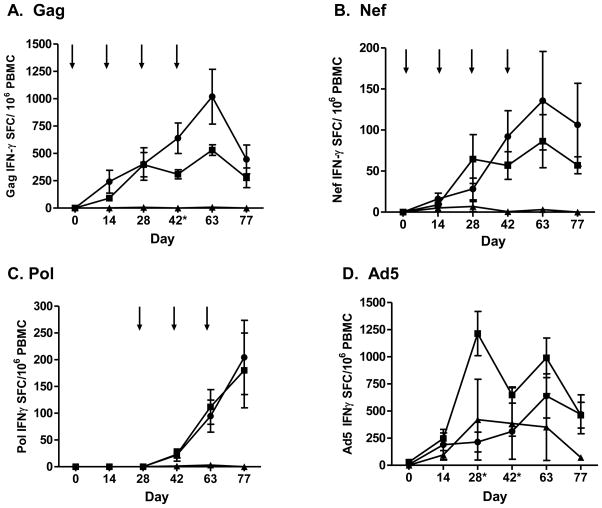
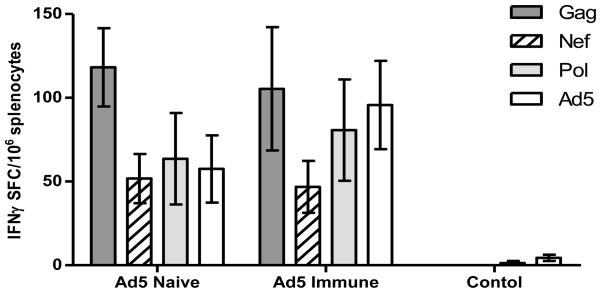
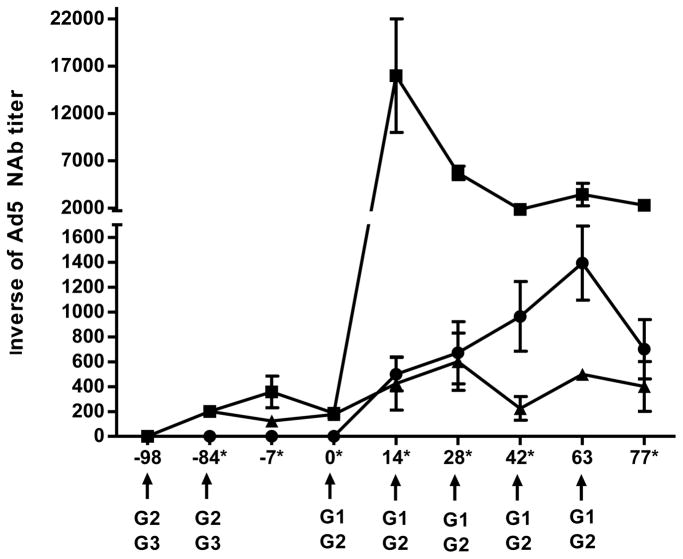
CMI response induced by Ad5 [E1-, E2b-] constructs expressing SIV and HIV-1 antigens in the NHP were determined in PBMC and splenocytes of individual NHP by IFN-γ ELISpot assay as previously described (22). In Ad5 naïve NHP (Group 1), the mean CMI response to Gag and Nef in PBMC increased above baseline values throughout the immunization course, reaching the highest level two weeks following the last immunization (day 63). At only one time point (day 42) was there a significant difference (P<0.05) in the CMI response observed between Ad5 naïve and Ad5 immune NHP (Figure 1A, B). A CMI response was mounted to Pol in the presence of recently induced vaccine associated Ad5 immunity. Pol specific IFN-γ secretion was detected two weeks after the first Ad5 [E1-, E2b-]-HIV-pol immunization on day 42, which increased on days 63 and day 77 (Figure 1C). Ad5 specific CMI was induced by vaccination and gradually increased throughout the study until peaking on day 63 (Figure 1D). At termination, splenocytes were assessed by ELISpot assay for target and vector specific responses. CMI was detected against all three target antigens as well as Ad5 (Figure 2). Ad5 naïve NHP in Group 1 had no detectable Ad5 NAb titers on day 0. The Ad5 NAb titers increased by day 63 and subsequently decreased by termination of the study on day 77 (Figure 3).
Figure 1. CMI responses of PBMC from NHP.
Ad5 naïve (n=7, circles), Ad5 immune (n=7, squares) and control NHP (n=4, triangles) were vaccinated with 1010 VP of SIV Ad5 [E1-, E2b-]gag/nef and HIV-1 Ad5 [E1-, E2b-]-pol at time points indicated by arrows. NHP PBMC responses to Gag (A), Nef (B), Pol (C) and Ad5 (D) were evaluated by IFN-γ ELISpot assay 14 days after each vaccine immunization. The symbol * indicates days that there was a significant difference (P<0.05) in the average CMI response between Ad5 naïve and Ad5 immune Ad5 [E1-, E2b-] vaccinated animals. For positive controls, splenocytes were exposed to Concanavalin A (Con A) (data not shown). Error bars depict ± SEM.
Figure 2. CMI responses of splenocytes from NHP.
Upon termination of the NHP on Day 77 post initiation of Ad5 [E1-, E2b-] vaccinations, splenocytes from Ad5 naïve (n=7), Ad5 immune (n=7) and control NHP (n=4) were assessed for responses to Gag (black), Nef (striped), Pol (grey) and Ad5 (white) by IFN-γ ELISpot assay. There were no statistically significant differences detected in the Gag, Nef, Pol or Ad5 specific responses between the Ad5 naïve and Ad5 immune NHP. Error bars depict ± SEM.
Figure 3. Ad5 neutralizing antibody titers in sera of vaccinated NHP.
The kinetics of Ad5 immune induction was determined by Ad5 NAb titer assay at various time points during the study. Serum from Ad5 naïve, (G1, circles, n=7), Ad5 immune (G2, squares, n=7) and control (Ad5 immune) NHP (G3, triangles, n=4) was evaluated for Ad5 NAb throughout the course of immunizations. Arrows indicate study days that the NHP were exposed to Ad5 through immunizations with Ad5 [E1-]-null (days −98 and −84), SIV Ad5 [E1-, E2b-]-gag/nef (days 0, 14, 28, 42) and HIV-1 Ad5 [E1-, E2b-]-pol (days 28, 42 and 63). The groups that received the vaccinations are listed below the arrows. The symbol * indicates days that there was a significant difference (P<0.05) of the average Ad5 NAb titers between Ad5 naïve and Ad5 immune Ad5 [E1-, E2b-] vaccinated animals. Error bars depict ± SEM.
Since pre-existing immunity to Ad5 has been reported to mitigate the effectiveness of Ad5 vectored vaccination, we next evaluated the CMI immune response induced by the Ad5 [E1-, E2b-] constructs expressing SIV and HIV antigens in Ad5 immune NHP. The NHP in Group 2 were made Ad5 immune by two intradermal immunizations at a two-week interval with 1010 VP of Ad5-null, a recombinant Ad5 platform with no inserted transgene, on study days −98 and −84. To induce Ad5 specific immune responses in a memory phase, similar to Ad5 directed immune responses that may been found in the human population, Ad5 [E1-, E2b-] vaccination was initiated 12 weeks post Ad5-null exposure (28,29). Ad5 NAb titers averaged 1:185 ± 14.29 on study day 0 when the NHP were administered their first Ad5 [E1-, E2b-]-SIV-gag and Ad5 [E1-, E2b-]-SIV-nef vaccination (Figure 3). The Gag specific CMI response in these Ad5 immune monkeys had over a 4-fold increase in IFN-γ secretion from day 14 to day 28 (P=0.018), and also significantly increased from day 42 to day 63 (P=0.005) (Figure 1A). As compared with Group 1, a similar CMI response was induced against Nef in Group 2 that was first detected on day 14 and increased by day 63 (Figure 1B). Again, as observed with Group 1, an immune response was mounted against Pol in Group 2 despite the presence of Ad5 hyper immunity. Pol specific CMI was initially detected two weeks after the first Ad5 [E1-, E2b-]-HIV-pol immunization on day 42 and increased until termination on day 77 (Figure 1C). Both target and vector specific CMI responses were detected in splenocytes from the Ad5 immune NHP (Figure 2). Ad5 specific CMI responses increased above baseline levels throughout the immunization protocol (Figure 1D). The Ad5 NAb titers peaked on day 14 in the Ad5 immune Group 2 with an average Ad5 NAb titer of 1:16000 ± 6000 and then began to decrease throughout the course of the study (Figure 3).
MHC class I typing revealed that two of the NHP in the Ad5 immune Group 2 were positive for Mamu-B*003, an allele which shares a high degree of amino acid homology in peptide binding domains with Mamu-B*008 and HLA-B*2705 which have been associated with the control of immunodeficiency virus replication (Supplementary Table 1) (30). Although two NHP had this protective allele, there was no correlation or trend between the magnitude of vector or insert CMI response and MHC I typing.
Breadth of CMI response
We determined the epitopic breadth of the CMI response induced by vaccination with Ad5 [E1-, E2b-]-SIV-gag, Ad5 [E1-, E2b-]-SIV-nef and Ad5 [E1-, E2b-]-HIV-pol in 2–3 representative NHP from each group that had robust IFN-γ ELISpot responses against the entire SIV or HIV-1 antigen. A broad immune response was induced to SIV Gag and HIV-1 Pol in both groups of Ad5 naïve and Ad5 immune NHP recognizing on average 4 of 4 Gag, 1 of 2 Nef and at least 2 of five Pol peptide pools (Supplementary Figure 1). The genomic portion of Nef that was contained in the immunizing vector was represented in Nef peptide pool #1. No immune response was induced to the non-immunizing portion of Nef represented in Nef peptide pool #2. No significant differences (p>0.05) were observed in the magnitude of the CMI responses to each peptide pool between Ad5 naïve and Ad5 immune NHP except that Ad5 immune NHP exhibited a significantly greater (P=0.008) CMI response against Pol pool #1. This may be a result of a small sample size. Ad5 naïve NHP trended to have a greater magnitude of response against Gag peptide pools #1 and 2 although the difference was not significant.
The CMI responses to Gag, Pol and Nef were similar in magnitude and breadth between Ad5 naïve and Ad5 immune NHP in both PBMC and splenocytes. The only time point in which the Gag, Nef or Pol CMI response induced in Ad5 naive NHP was significantly higher than in Ad5 immune NHP was in Gag specific IFN-γ secretion from PBMC on day 42 (P=0.04). At termination, the target and vector specific CMI responses in splenocytes were similar between the two groups and no statistically significant differences were observed against any of the target antigens or Ad5. The Ad5 specific CMI responses in PBMC detected following immunizations differed in magnitude and the kinetics of the responses were different between Groups 1 and 2, confirming that pre-exposure to Ad5 was successful. Ad5 specific CMI responses increased more rapidly in Ad5 immune Group 2 animals, peaking at day 28 as compared to day 64 in the Ad5 naïve Group 1 (Figure 1D). The Ad5 CMI responses were significantly increased in Group 2 Ad5 immune NHP on day 28 (P<0.001) and 42 (P=0.002). This difference diminished by day 63 (P=0.22) and, by day 77, the responses were similar (P=0.97). Ad5 NAb titers also differed between the two groups throughout the course of immunizations. The Ad5 immune NHP in Group 2 had significantly (P<0.05) higher levels of Ad5 NAb than Group 1 Ad5 naïve animals for every time point assessed except day 63 (Figure 3).
Discussion
Recombinant viral vectors have been investigated for their use as a delivery platform for HIV-1 genes (1). Of the viral vectors investigated, Ad5 based vaccines have been repeatedly reported to outperform other vaccine platforms expressing identical antigens, including other Ad serotypes, poxvirus based vectors and DNA-based vaccines, although the quality of the induced immune responses necessary to protect against HIV-1 may define which vector is most suitable for HIV-1 vaccine development (1, 31). Recombinant Ad5 vectors are the most widely used viral vector in clinical applications including gene therapy and vaccines, most likely due to a number of positive attributes including an extensive safety profile, a broad tropism of infecting a variety of cell types and the ability to grow high titers of Ad5 vectors under GMP conditions (13). Unfortunately, the widespread utility of Ad5 recombinant viral vectors is questionable because antigen-specific immune responses induced by the platform are negatively impacted by anti-vector immunity (32–35). Pre-existing immunity to Ad5 has been reported to reduce both the magnitude and frequency of responses to the targeted genes in human clinical trials (32). This is a challenge for Ad5 vector platforms in particular because Ad5 pre-existing immunity in humans is widespread (24).
We have previously described the Ad5 [E1-, E2b-] platform as an Ad5 vector containing deletions in the E1, E2b and E3 regions (14, 15, 20–22). This vector has been reported to overcome pre-existing Ad5 immunity in murine and NHP models (14, 20–22). Previously, we reported that HIV-1 transgene CMI responses could be induced by multiple homologous administrations of Ad5 [E1-, E2b-]-gag in Ad5 immune NHP (22). In the current study, we expanded on these observations and determined the magnitude of CMI responses that could be induced by three Ad5 [E1-, E2b-] constructs expressing SIV Gag, SIV Nef or HIV Pol in Ad5 immune and Ad5 naïve NHP. We found that not only were the Ad5 [E1-, E2b-] constructs successful at inducing immune responses to each antigen, but these responses were increased by subsequent administration of the vaccines. The immune response induced by the Ad5 [E1-, E2b-] vectored transgenes occurred despite pre-existing Ad5 immunity within the limitations of the statistical comparisons preformed. Ad5 naïve and Ad5 immune animals mounted similar CMI responses to the target antigens despite various levels of Ad5 immunity. These findings correspond with our results from other studies using HIV-1 and tumor associated antigens as targets (14, 21–22). In a similar NHP model, Tatsis et al reported that the CMI response induced by an Ad5 [E1-] vector, the same vector used in the Merck STEP trial, were significantly affected (p<0.001) by pre-exposure to Ad5 (37). The mitigating effect of pre-existing Ad5 immunity when vaccinating with Ad5 [E1-] platforms has also been reported in human immunogenicity studies (24, 31). Clinical trials are needed to determine if the Ad5 [E1-, E2b-] platform can break through the barrier of Ad5 immunity in humans as it has in animal models.
Although immune correlates of protection from HIV-1 have not been identified, CD8 T cell responses in long-term HIV infected non-progressors and elite controllers have been reported to protect against disease progression and have resulted in some efficacy in NHP SIV challenge models (38, 39). It has been reported that CD8 T cell and NK depletion in African green monkeys infected with SIV results in a dramatic increase in SIV viremia, implicating their role in viral control (40). Recent data from Mullins, et al suggests that although subtle, the MRKAd5 vaccine used in the Merck STEP trial, which was designed to induce HIV-specific T cell responses, did successfully block acquisition of some strains of HIV-1 that had genetically similar Gag, Pol and Nef genes as the vaccine (41). While CMI responses have resulted in benefits to humans and NHP, their correlates of protection against immunodeficiency viruses remain unclear and their effectiveness most likely will depend on several factors including HLA type, what immunizing antigens are used, and route of immunization.
Although the mechanism of how the Ad5 [E1-, E2b-] platform eludes anti-Ad5 immunity has not been completely defined, it is believed that the unique deletions of the pol and pTP regions results in decreased immunogenicity of the vector. It has been demonstrated that deletion of the pol region in Ad5 vectors results in at least a 10,000-fold reduction in the production of potent antigenic Ad5 proteins, such as fiber, following infection of human cells (15). Ad5 vectors deleted for E1 and pol have also been reported to exhibit extended transgene expression in vivo as compared to Ad5 [E1-] vectors expressing the same transgene (42). Deletion of the pTP region results in a complete viral replication blockade, which is believed to reduce an inflammatory immune response directed toward the Ad5 [E1-, E2b-] vector (15). Ad5 [E1-] vectors induce an inflammatory response that leads to the destruction of transduced cells, thus eliminating expression of the transgene target. These data suggest that pre-existing Ad5 immunity in a vaccinee does not readily cause destruction of cells transfected by Ad5 [E1-, E2b-] vectors allowing for extended transgene expression. We believe that the increased length of time that vector infected cells exist in the vaccinee can result in a heightened immunity to the transgene target. Of interest in the present study is the observation that anti-Ad5 vector immune responses appeared to peak and then wane over time in Ad5 pre-immune NHP despite continued immunizations. In this group, Ad5 NAb titers peaked at 14 days after the first immunization and then declined to a newly established baseline level 28 days after the first immunization then trended downward thereafter. In Ad5 naïve NHP, Ad5 NAb titers and Ad5 directed CMI gradually increased throughout the course of immunization; however, the Ad5 NAb titers were lower than those observed in Ad5 immune animals. This may result from reduced antigenicity of the Ad5 [E1-, E2b-] platform as compared to Ad5 [E1-] vectors.
An important issue to consider when developing a commercial Ad5 vaccine platform is the effectiveness of the platform to be used to immunize against heterologous disease targets. It has been reported that repeated Ad5 vectored vaccination in humans with low or no pre-existing Ad5 immunity fails to boost immunity to the transgene following the first immunization (32). This indicates acquired Ad5 immunity induced by the primary immunization is sufficient to suppress subsequent vaccination even if expressing a different trasgene target. To determine if the Ad5 [E1-, E2b-] platform could be utilized in homologous vaccination protocols we introduced a third target, HIV Pol, after multiple administrations of the Ad5 [E1-, E2b-] platform expressing SIV Gag and Nef. A CMI response was induced against the HIV Pol antigen even in the presence of vector induced Ad5 immunity of animals in Group 1 resulting from two prior administrations of Ad5 [E1-, E2b-]-SIV-gag and Ad5 [E1-, E2b-]-SIV-nef and in Ad5-hyperimmune Group 2 that had an average Ad5 NAb titer of 1:5700 on the day they received their fist Ad5 [E1-, E2b-]-HIV-pol vaccination. These observations indicate that despite the presence of anti-vector immunity, an immune response can be generated to the same or a different target.
The pre-clinical studies herein, which compare the immunization of Ad5 naïve and Ad5 pre-immunized NHP, demonstrate that the Ad5 [E1-, E2b-] platform can induce immunity in either setting. As a result of these data, Ad5 immunity may no longer be an inhibitory factor with respect to immunization and encourage the continued development of the Ad5 [E1-, E2b-] vector as a platform for vaccination against multiple disease targets.
Supplementary Material
Acknowledgments
This work was supported by NIH-NIAID grant number 2R44A1071733. The authors would like to thank Drs. Andrea Amalfitano and Kent J. Weinhold for their assistance. The authors would also like to thank Ms. Carol Jones for her management of grant activities, Julie A. Karl for her assistance in this study, and Drs. Anthony Cook and Salih Muhammad and Matt Collins for veterinary and technical services. The authors would like to acknowledge ViraQuest, North Liberty, IA, USA for virus vector production.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Robert-Guroff M. Replicating and non-replicating viral vectors for vaccine development. Curr Opin Biotechnol. 2007;18:546–56. doi: 10.1016/j.copbio.2007.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Woolard SN, Kumaraguru U. Viral vaccines and CTL response. J Biomedicine and Biotechnology. 2010:141657. doi: 10.1155/2010/141657. (Epub 2010 Mar 31) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tatsis N, Ertl HC. Adenoviruses as vaccine vectors. Mol Ther. 2004;10:616–29. doi: 10.1016/j.ymthe.2004.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gómez-Román VR, Robert-Guroff M. Adenoviruses as vectors for HIV vaccines. AIDS Rev. 2003;5:178–185. [PubMed] [Google Scholar]
- 5.Shiver JW, Fu TM, Chen L, Casimiro DR, Davies ME, Evans RK, et al. Replication-incompetent adenoviral vaccine vector elicits effective anti-immunodeficiency-virus immunity. Nature. 2002;415:331–5. doi: 10.1038/415331a. [DOI] [PubMed] [Google Scholar]
- 6.Appaiahgari MB, Saini M, Rauthan M, Jyoti, Vrati S. Immunization with recombinant adenovirus synthesizing the secretory form of Japanese encephalitis virus envelope protein protects adenovirus-exposed mice against lethal encephalitis. Microbes Infect. 2006;8:92–104. doi: 10.1016/j.micinf.2005.05.023. [DOI] [PubMed] [Google Scholar]
- 7.Barratt-Boyes SM, Soloff AC, Gao W, Nwanegbo E, Liu X, Rajakumar PA, et al. Broad cellular immunity with robust memory responses to simian immunodeficiency virus following serial vaccination with adenovirus 5- and 35-based vectors. J Gen Virol. 2006;87:139–149. doi: 10.1099/vir.0.81445-0. [DOI] [PubMed] [Google Scholar]
- 8.Cantanzaro AT, Koup RA, Roederrer M, Bailer RT, Enama ME, Moodic Z, et al. Phase 1 safety and immunogenicity evaluation of a multiclade HIV-1 candidate vaccine delivered by a replication-defective recombinant adenovirus vector. J Infect Dis. 2006;194:1638–49. doi: 10.1086/509258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tims T, Briggs DJ, Davis RD, Xiang Z, Ertl HC, Fu ZF. Adult dogs receiving a rabies booster dose with a recombinant adenovirus expressing rabies virus glycoprotein develop high titers of neutralizing antibodies. Vaccine. 2000;18:2804–7. doi: 10.1016/s0264-410x(00)00088-8. [DOI] [PubMed] [Google Scholar]
- 10.Roberts DM, Nanda A, Havenga MJE, Abbink P, Lynch DM, Ewald BA, et al. Hexon-chimaeric adenovirus serotype 5 vectors circumvent pre-existing anti-vector immunity. Nature. 2006;441:239–43. doi: 10.1038/nature04721. [DOI] [PubMed] [Google Scholar]
- 11.Varnavski AN, Zhang Y, Schnell M, Tazelaar J, Louboutin JP, Yu QC, et al. Preexisting Immunity to Adenovirus in Rhesus Monkeys fails to prevent vector-induced Toxicity. J Virol. 2002;76:5711–19. doi: 10.1128/JVI.76.11.5711-5719.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bangari DS, Mittal SK. Development of non-human adenoviruses as vaccine vectors. Vaccine. 2006;24:849–62. doi: 10.1016/j.vaccine.2005.08.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Seregin SS, Amalfitano A. Overcoming pre-existing adenovirus immunity by genetically engineering of adenovirus-based vectors. Expert Opin Biol Ther. 2009;9:1521–31. doi: 10.1517/14712590903307388. [DOI] [PubMed] [Google Scholar]
- 14.Gabitzsch ES, Xu Y, Yoshida LH, Balint J, Gayle RB, Amalfitano A, et al. A preliminary and comparative evaluation of a novel Ad5 [E1-, E2b-] recombinant based vaccine used to induce cell mediated immune responses. Immunol Lett. 2009;122:44–51. doi: 10.1016/j.imlet.2008.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Amalfitano A, Hauser MA, Hu H, Serra D, Begy CR, Chamberlain JS. Production and characterization of improved adenovirus vectors with the E1, E2b, and E3 genes deleted. J Virol. 1998;72:926–33. doi: 10.1128/jvi.72.2.926-933.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Campos SK, Barry MA. Current advances and future challenges in Adenoviral vector biology and targeting. Curr Gene Ther. 2007;7:189–204. doi: 10.2174/156652307780859062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Amalfitano A, Begy CR, Chamberlain JS. Improved adenovirus packaging cell lines to support the growth of replication-defective gene-delivery vectors. Proc Natl Acad Sci U S A. 1996;93:3352–6. doi: 10.1073/pnas.93.8.3352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Amalfitano A, Chamberlain JS. Isolation and characterization of packaging cell lines that co-express the adenovirus E1, DNA Polymerase, and preterminal proteins: Implications for gene therapy. Gene Therapy. 1997;4:258–263. doi: 10.1038/sj.gt.3300378. [DOI] [PubMed] [Google Scholar]
- 19.Hodges BL, Serra D, Hu H, Begy CA, Chamberlain JS, Amalfitano A. Multiply deleted [E1-, polymerase-, and pTP-] adenovirus vector persists despite deletion of the preterminal protein. J Gene Medicine. 2000;2:250–9. doi: 10.1002/1521-2254(200007/08)2:4<250::AID-JGM113>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 20.Osada T, Yang XY, Hartman ZC, Glass O, Hodges BL, Niedzwiecki D, et al. Optimization of vaccine responses with an E1, E2b and E3-deleted Ad5 vector circumvents pre-existing anti-vector immunity. Can Gene Ther. 2009;16:673–82. doi: 10.1038/cgt.2009.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gabitzsch ES, Xu Y, Balint JP, Hartman ZC, Lyerly HK, Jones FR. Anti-tumor immunotherapy despite immunity to adenovirus using a novel adenoviral vector Ad5 [E1-, E2b-]-CEA. Cancer Immunol Immunother. 2010;59:1131–5. doi: 10.1007/s00262-010-0847-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gabitzsch ES, Xu Y, Yoshida LH, Balint J, Amalfitano A, Jones FR. Novel Adenovirus type 5 vaccine platform induces cellular immunity against HIV-1 Gag, Pol, Nef despite the presence of Ad5 immunity. Vaccine. 2009;27:6394–8. doi: 10.1016/j.vaccine.2009.06.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Parham P. MHC class I molecules and KIRs in human history, health and survival. Nat Rev Immunol. 2005;5:201–14. doi: 10.1038/nri1570. [DOI] [PubMed] [Google Scholar]
- 24.Priddy FH, Brown D, Kublin J, Monahan K, Wright DP, Lalezari J, et al. Safety and immunogenicity of a replication-incompetent Adenovirus type 5 HIV-1 clade B gag/pol/nef vaccine in healthy adults. Clin Infect Dis. 2008;46:1769–81. doi: 10.1086/587993. [DOI] [PubMed] [Google Scholar]
- 25.Wiseman RW, Karl JA, Bimber BN, O’Leary CE, Lank SM, Tuscher JT, et al. Major histocompatibility complex genotyping with massively parallel pyrosequencing. Nat Med. 2009;15:1322–6. doi: 10.1038/nm.2038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mittereder N, March KL, Trapnell BC. Evaluation of the concentration and bioactivity of adenovirus vectors for gene therapy. J Virol. 1996;70:7498–7509. doi: 10.1128/jvi.70.11.7498-7509.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cory AH, Owen TC, Barltrop JA, Cory JG. Use of an aqueous soluble tetrazolium/formazan assay for cell growth assays in culture. Cancer Commun. 1991;3:207–12. doi: 10.3727/095535491820873191. [DOI] [PubMed] [Google Scholar]
- 28.Hutnick NA, Carnathan D, Ertl H, Betts MR. Adenovirus-specfic human T cells are pervasive, polyfuntional, and cross reactive. Vaccine. 2010;28:1932–41. doi: 10.1016/j.vaccine.2009.10.091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Miller JD, van der Most RG, Akindy RS, Glidewell JT, Albott S, et al. Human effector and memory CD8+ T cell responses to smallpox and yellow fever vaccines. Immunity. 2008;28:710–22. doi: 10.1016/j.immuni.2008.02.020. [DOI] [PubMed] [Google Scholar]
- 30.Loffredo JT, Sidney J, Bean AT, Beal DR, Bardet W, Wahl A, et al. Two MHC Class I molecules associated with elite control of immunodeficiency virus replication, Mamu-B*08 and HLA-B*2705, bind peptides with sequence similarity. J Immunol. 2009;182:7763–75. doi: 10.4049/jimmunol.0900111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hansen SG, Vieville C, Whizin N, Coyne-Johnson L, Siess DC, Drummond DD, et al. Effector-memory T cell responses are associated with protection of rhesus monkeys from mucosal SIV challenge. Nat Med. 2009;15:293–299. doi: 10.1038/nm.1935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.McElrath MJ, DeRosa SC, Moodie Z, Dubey S, Kierstead L, Janes H, et al. HIV-1 vaccine-induced immunity in the test-of-concept Step Study: a case-cohort analysis. Lancet. 2008;372:1894–1905. doi: 10.1016/S0140-6736(08)61592-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.McCoy K, Tatsis N, Korioth-Schmitz B, Lasaro MO, Hensley SE, Lin Sw, et al. Effect of preexisting immunity to adenovirus human serotype 5 antigens on the immune responses of nonhuman primates to vaccine regimens based on human- or chimpanzee-derived adenovirus vectors. J Virol. 2007;81:6594–604. doi: 10.1128/JVI.02497-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fitzgerald JC, Gao GP, Reyes-Sandoval A, Pavlakis GN, Xiang ZQ, Wlazlo AO, et al. A simian replication-defective adenoviral recombinant vaccine to HIV-1 gag. J Immunol. 2003;170:1416–22. doi: 10.4049/jimmunol.170.3.1416. [DOI] [PubMed] [Google Scholar]
- 35.Casimiro DR, Chen L, Fu TM, Evans RK, Caulfield MJ, Davies ME, et al. Comparative immunogenicity in rhesus monkeys of DNA plasmid, recombinant vaccinia virus, and replication-defective adenovirus vectors expressing a human immunodeficiency virus type 1 gag gene. J Virol. 2003;77:6305–13. doi: 10.1128/JVI.77.11.6305-6313.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mast TC, Kierstead L, Gupta SB, Nikas AA, Kallas EG, Novitsky V, et al. International epidemiology of human pre-existing adenovirus (Ad) type-5, type-6, type-26 and type-36 neutralizing antibodies: Correlates of high Ad5 titers and implications for potential HIV vaccine trials. Vaccine. 2010;28:950–7. doi: 10.1016/j.vaccine.2009.10.145. [DOI] [PubMed] [Google Scholar]
- 37.Tatsis N, Lasaro MO, Lin SW, Xiang ZQ, Zhou D, DiMenna L, et al. Adenovirus vector-induced immune responses in nonhuman primates: responses to prime boost regimens. J Immunol. 2009;182:6587–6599. doi: 10.4049/jimmunol.0900317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Harrer T, Harrer E, Kalams SA, Elbeik T, Staprans SI, Feinberg MB, et al. Strong cytotoxic T cell and weak neutralizing antibody responses in a subset of persons with stable nonprogressing HIV type 1 infection. AIDS Res Hum Retroviruses. 1996;12:585–92. doi: 10.1089/aid.1996.12.585. [DOI] [PubMed] [Google Scholar]
- 39.Giraldo-Vela JP, Bean AT, Rudersdorf R, Wallace LT, Loffredo JT, Erickson P, et al. Simian immunodeficiency virus-specific CD4+ T cells from successful vaccinees target the SIV Gag capsid. Immunogenetics. 2010;62:701–707. doi: 10.1007/s00251-010-0473-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gaufin T, Ribeiro RM, Gautam R, Dufour J, Mandell D, Apetrei C, et al. Experimental depletion of CD8+ cells in acutely SIVagm-infected African Green Monkeys results in increased viral replication. Retrovirology. 2010;7:42. doi: 10.1186/1742-4690-7-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mullins RM, Tovanabutra S, deCamp AC, Frahm N, Gilbert PB, Sanders-Buell E, et al. Genetic impact of vaccination on breakthrough HIV-1 sequences from the STEP trial. Nat Med. 2011;17:366–71. doi: 10.1038/nm.2316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hu H, Serra D, Amalfitano A. Persistence of an [E1-, polymerase-] adenovirus vector despite transduction of a neoantigen into immune-competent mice. Hum Gene Ther. 1999;10:355–364. doi: 10.1089/10430349950018805. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.