Abstract
Background
Fragile X syndrome (FXS) is the most common known heritable cause of intellectual disability. Prior studies in FXS have observed a plateau in cognitive and adaptive behavioral development in early adolescence, suggesting that brain development in FXS may diverge from typical development during this period.
Methods
In this study, we examined adolescent brain development using structural magnetic resonance imaging data acquired from 59 individuals with FXS, and 83 typically developing (TD) controls aged 9 to 22, a subset of whom were followed-up longitudinally (1-5 years; TD:17, FXS:19). Regional volumes were modeled to obtain estimates of age-related change.
Results
We found that while structures such as the caudate showed consistent volume differences from controls across adolescence, prefrontal cortex gyri (PFC) showed significantly aberrant maturation. Furthermore, we found that PFC-related measures of cognitive functioning followed a similarly aberrant developmental trajectory in FXS.
Conclusions
Our findings suggest that aberrant maturation of the PFC during adolescence may contribute to persistent or increasing intellectual deficits in FXS.
Keywords: fragile X syndrome, brain development, adolescence, MRI, prefrontal cortex, longitudinal
Introduction
Fragile X syndrome (FXS) is the most common known heritable cause of cognitive and behavioral disability, affecting approximately 1 in 4,000 individuals (1-3). FXS is associated with a repeated CGG sequence on the X chromosome, which results in reduced expression of the protein coded by the FMR1 gene (FMRP). FMRP is required for normal neural development (4) and decreased levels are associated with impaired cognitive, emotional and behavioral outcomes. Females with FXS are typically less affected than males due to the presence of a second unaffected X-chromosome (5). Common problem behaviors observed in FXS include attentional dysfunction and hyperactivity, repetitive-stereotypic behaviors, social anxiety and autistic symptoms (6). Neural systems affecting executive function, spatial cognition and language and communication are also frequently impaired (7-12).
Magnetic resonance imaging (MRI) studies have identified abnormal brain structure in both children and adults with FXS. Regions such as the caudate nucleus and thalamus (13-15) have been found to be enlarged in FXS, perhaps stemming from inadequate pruning of neural connections during brain development. There is also evidence of decreased volume of the cerebellar vermis, amygdala and the superior temporal gyrus (14; 16-19) in FXS. In a recent longitudinal study of brain development in FXS in early childhood (17), our group identified a window of early brain development during which significant deviation from typical neurodevelopment occurred in selected cortical and subcortical brain regions. This early period of brain development coincides with clinical observations of increased cognitive-behavioral impairments in FXS (20). However, in later childhood and adolescence, the trajectory of brain development in FXS, relative to typical development, has not been well characterized on a longitudinal basis.
Adolescence marks a time of profound alterations in cognitive, behavioral and emotional function, accompanied by changes in underlying neurodevelopmental processes (21; 22). Longitudinal studies of cognitive development in FXS have found a slower rate of gain in cognitive development in early adolescence (23; 24), leading to a decline in standardized IQ, as well as a parallel deceleration in the development of adaptive behaviors (25; 26). This slowing in cognitive and behavioral development during adolescence suggests that neurodevelopment may be particularly adversely affected in FXS during this period.
To address the question of how adolescent brain maturation differs in FXS, structural MRI scans were collected from FXS, and typically developing (TD) participants aged 9 to 22; a subset of participants were followed up for a second measurement 1-5 years later (participant characteristics in Table 1). Semi-automated cortical segmentation was performed using FreeSurfer (27; 28), to obtain estimates of cortical and subcortical volumes. Growth trajectories of regions known to be structurally or functionally abnormal in FXS were estimated using linear mixed models; these included: caudate (13; 14; 16; 17; 29), thalamus (13; 17; 29), fusiform gyrus(17), orbitofrontal gyrus (OFG) (14; 17), insula (16), middle and superior frontal gyri (MFG and SFG) (16; 17; 30), hippocampus (15; 16; 31), and superior temporal gyrus (14; 31). We hypothesized that regions that have previously been shown to be structurally or functionally abnormal in FXS would also show differing developmental trajectories relative to controls. We were also interested in characterizing cognitive development in this sample. Therefore, in addition to structural scanning, participants underwent cognitive testing. Specific measures of cognitive functioning in areas of known difficulty in FXS were entered into similar developmental models to test for group differences in cognitive trajectories that parallel those observed in neural development.
Table 1. Participant information.
| Group | Gender | # of time 1 scans | Age at time 1 (yrs) | FSIQ at time 1 | # of time 2 scans | Mean follow-up interval (yrs) |
|---|---|---|---|---|---|---|
| TD | Female | 47 | 15.83 (3.5) | 116.8 (13.1) | 6 | 4.54 (1.6) |
| Male | 36 | 15.47 (4.2) | 116.8 (13.4) | 11 | 2.8 (1.5) | |
|
| ||||||
| FXS | Female | 36 | 16.03 (3.7) | 81.8 (19.2) | 9 | 3.6 (1.6) |
| Male | 23 | 15.2 (3.2) | 58.5 (12.5) | 10 | 2.4 (.3) | |
Mean (S.D.)
Methods and Materials
Participants
Participants were 68 individuals with FXS, and 95 TD individuals aged 9 to 22 years. FXS participants were recruited through the National Fragile X Foundation and their regional chapters across the United States, as well as through advertisements in local organizations servicing individuals with intellectual disabilities, and local parent groups in the Northern California area. Typically developing participants were recruited as siblings of FXS participants, through local parent organizations in the Palo Alto, CA, area, and as students at Stanford University. While participants were excluded if FSIQ was more than 2 standard deviations above the mean (i.e., ≥130), our sample population may have resulted in a slightly elevated IQ (116) relative to the general population. FSIQ was not significantly different between individuals with and without longitudinal follow-up measurements. All FXS diagnoses were confirmed through DNA analysis using standardized southern blot techniques. Medication information is provided in Table S1 in the Supplement. Potential TD participants were excluded if they had history of seizures, medical problems or psychiatric disorders (schizophrenia, bipolar disorder, major depression), or other known genetic conditions. Informed consent was obtained from all participants 18 years or older; for participants less than 18, informed consent was obtained from the parents, and informed assent was obtained from the participants. The protocols used in this study were approved by the Stanford University Administrative Panel on Human Subjects in Medical Research.
A total of 220 structural MRI images were collected. All images were manually inspected for image quality, and among these, 42 scans were found to be unusable due to artifacts likely induced by subject motion and blood flow, or wraparound artifact (unusable scans: TD = 18; FXS = 24). The 178 remaining usable scans consisted of 142 scans at Time 1, and 36 scans at Time 2 collected 1 to 5 years after the initial scan (mean interval = 2.7 years); one participant was scanned at a third time-point. An additional 8 FXS scans were excluded from analyses of frontal regions due to poor software-based tissue (FreeSurfer) segmentation.
Cognitive and neuropsychological assessments
All participants in this study underwent a battery of cognitive and neuropsychological assessments. The IQs of participants up to 16 years of age were measured with the Wechsler Intelligence Scale for Children—Third Edition (WISC-III)(32). Participants aged 17 and over received the Wechsler Adult Intelligence Scale—Third Edition (WAIS-III)(33). Visual-spatial ability was assessed using the Spatial Relations test, a subtest of the Woodcock–Johnson Tests of Cognitive Ability (34). Cognitive flexibility was assessed using the Verbal fluency “F, A, S” test (35). Executive functions were assessed using the Contingency Naming Test (36). Behavioral measures obtained were the Aberrant Behavior Checklist (37), the Autism Behavior Checklist (38), the Vineland Adaptive Behavior Scales, Second Edition (39), and the Emotionality Activity and Sociability (EAS) temperament scale (40).
MRI Scanning
All images were acquired on the same 1.5 Tesla General Electric Signa scanner (GE Imaging Systems, Milwaukee, WI) at Stanford University, using the same pulse sequence parameters. T1-weighted spoiled gradient echo series were acquired in the coronal direction using the following parameters: TR=35ms, TE=6ms, flip angle=45°, slice thickness: 1.5-1.7mm (adjusted to include the entire brain), in-plane resolution 0.9375×0.9375 mm, and acquisition matrix size = 256×192 mm for 124 contiguous slices.
FreeSurfer processing
Cortical reconstruction and volumetric segmentation were performed with the FreeSurfer 4.5 image analysis suite (27; 28), which is documented and freely available for download online (http://surfer.nmr.mgh.harvard.edu/); see Supplement for full details. Segmented volumes were visually inspected, and where needed, appropriate manual corrections were preformed as per the Freesurfer Tutorial (http://surfer.nmr.mgh.harvard.edu/fswiki/FsTutorial). All raters who performed manual editing of FreeSurfer derived data were trained to achieve inter-rater reliability of ≥0.95 (intraclass correlation coefficient) with gold-standard datasets for all regions of interest.
Modeling regional volume changes
Linear mixed models (41; 42) were used to model age-related growth for total grey and white matter volumes, and each region of interest. For model estimation, we used SPSS 18 (SPSS Inc., Chicago Illinois). Mixed modeling allows inclusion of all available cases, both with single and repeated measurements of the outcome, and individually varying intervals between measurements. Thus, longitudinal measures of study participants assessed at different ages allow for modeling of longitudinal trends over a considerably long period of time. Specifically, in mixed effects modeling, the overall trend is modeled by weaving together individuals’ longitudinal information based on the overlap across individuals in terms of age at assessment points. In other words, each individual contributes to the formation of the overall trend at particular ages when they are actually assessed and borrows information from other individuals for the ages when they are not assessed (e.g., (43; 44)). The key underlying assumption here is that the data are missing at random conditional on observed information (45) such as various baseline characteristics including FXS status. In particular, we assume that individuals with and without missing data at particular age ranges may be different in terms of observed characteristics (e.g., gender, FXS status, IQ, observed outcome data), but not different in terms of unobserved characteristics (e.g., unmeasured variables, missing outcome data). The validity of our analysis method relies on this missing data assumption, sufficient observations across the entire age range, and the overlap across individuals in terms of age at assessment points. Therefore, we interpret the mixed effects model estimates with caution at ages with sparse observations, in particular towards both ends of the age spectrum.
Models for total grey and white matter volumes included age as a centered continuous predictor, diagnostic group (FXS vs. TD) gender and a gender x diagnosis interaction term, and interaction terms for diagnosis, gender and gender x diagnosis with age.
Volumes of regions-of-interest were first adjusted for total grey matter; linear mixed models were then estimated using these total grey matter adjusted measures. For ROI models, we included age (centered), diagnosis, gender, age, age x diagnosis, age x gender and age x gender x diagnosis as predictors. Regions-of-interest (based on previously described structural differences in FXS) were grouped into functionally and spatially-related sets for hypothesis testing; see Table S3 in the Supplement. In the frontal regions, we included inferior frontal gyrus, orbitofrontal gyrus, and a dorsal frontal region encompassing middle and superior gyri; in the sub-cortical set we included hippocampus, caudate, pallidus/putamen, and thalamus; in the temporal-occipital set we included superior temporal gyrus, fusiform, and insula. FreeSurfer segmentation of amygdala is known to be unreliable, therefore this region was not modeled, despite known differences in FXS. Similarly, volumes of cerebellar sub-regions are not available through this method, and therefore cerebellar regions were not modeled. Within each set of regions, the null hypothesis that no regions within the set would show a significant interaction between age and diagnosis was tested, using a Holm-Bonferroni-corrected threshold (α=0.05/ number of regions included in the set).
Modeling changes in cognitive measures
We were interested in whether there were parallel differences in cognitive development across this age range in FXS, and therefore we modeled development of verbal fluency (VF) and spatial relations (SR) scores, using a similar linear mixed modeling approach. While it would also have been interesting to model scores on the contingency naming test (CNT), a measure of executive function, a high percentage of FXS participants were unable to complete the test, resulting in a relatively lower sample size. VF and SR scores were modeled in terms of diagnosis (TD, FXS), gender, diagnosis x gender, age (centered), age x diagnosis, age x gender and age x diagnosis x gender. Several participants were assessed behaviorally at a third or fourth time-point, within the specified age range (detailed subject numbers in Table S4 in the Supplement). These additional behavioral data were included in the model, providing a rich picture of cognitive development across the adolescent period. Age-appropriate IQ tests were administered in this study, meaning that roughly half our sample received the WISC-III and the other the WAIS-III; as such developmental trajectory of FSIQ was not modeled.
Results
Total grey and white matter trajectories
Growth trajectories for total grey and white matter volumes were estimated using linear mixed models with the following predictors: diagnosis (TD, FXS), gender, gender x diagnosis, age, age x diagnosis, age x gender, and age x diagnosis x gender. For total grey matter volume (Figure 1A), only gender (t(135) = 6.3, p<0.001) and age (t(146)=-9, p<0.001) were significant predictors; none of the interactions with age were significant. These results indicate that males show greater grey matter volume than females, and that grey matter shows an overall decrease with age; individuals with FXS do not show significantly different grey matter maturation. For total white matter volume (Figure 1B), diagnosis (t(140)=2.6, p<0.05), gender (t(140)=10.5, p<0.001), diagnosis x gender (t(140)=2.9, p<0.01), and age (t(67)=4.9, p<0.001) were significant predictors; none of the interactions with age were significant. These results indicate that males, and individuals with FXS, show greater white matter volume overall, that the gender difference is wider in FXS, and that white matter shows an overall increase with age; individuals with FXS do not show significantly different white matter maturation.
Figure 1. Total grey and white matter volumes.
A) Total grey matter volumes plotted against age, separately for typically developing (TD; left), and Fragile X syndrome (FXS; right). Regression lines estimated from linear mixed models (LMMs) are plotted; slopes were not significantly different between gender or diagnostic groups. B) Total white matter volumes plotted against age for TD (left), and FXS (right). Regression lines estimated from LMMs are plotted.
Region of interest trajectories
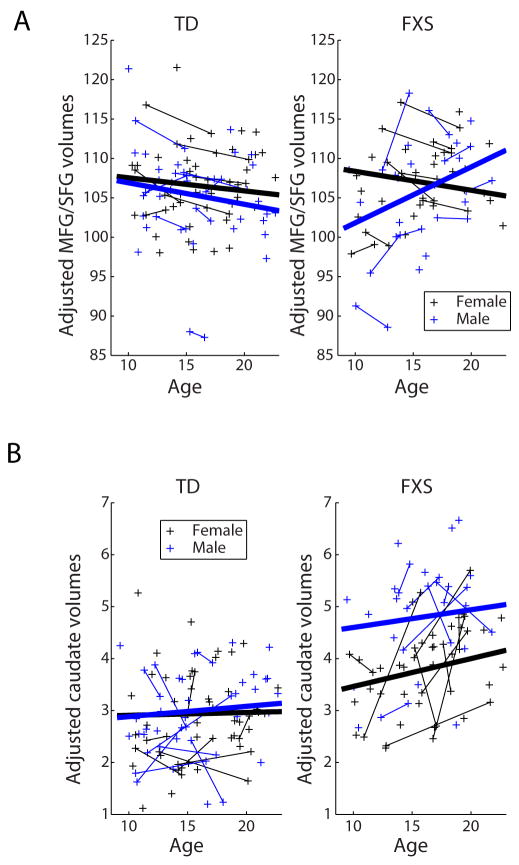
Growth trajectories for volumes of regions known to be structurally or functionally abnormal in FXS were estimated using linear-mixed models, after adjusting for total grey matter (raw values for all ROIs and total grey and white matter are shown in Table S2 in the Supplement). Diagnosis, gender, age, age x gender, age x diagnosis, and age x gender x diagnosis were included as predictors; model results for all ROIs are presented in Table S3 in the Supplement. Only the orbitofrontal gyrus (β=0.17, t(132)=2.5, p<0.05/4; Figure S1 in the Supplement) and superior/middle frontal gyri (β=0.45, t(95)=2.4, p<0.05/3) volumes showed significant age x diagnosis interactions (Figure 2AB), indicating that these prefrontal cortex (PFC) regions develop abnormally in FXS. The SFG/MFG volume also showed a significant gender x diagnosis x age interaction (β=1.1, t(95)=2.8, p=0.006). Specifically, these frontal ROIs show less volume decline, relative to overall grey matter decline, in FXS relative to TD, particularly in males. In contrast, regions such as the caudate (Figure 2C) showed a highly significant effect of diagnosis, but no significant age x diagnosis or age x diagnosis x gender interactions, suggesting that volume differences in these regions emerge early and remain relatively constant throughout adolescence.
Figure 2. Development of PFC and caudate volumes.
A) Bilateral MFG/SFG volumes plotted against age, separately for typically developing (TD; left), and Fragile X syndrome (FXS; right), volumes adjusted for total grey matter. Regression lines estimated from linear mixed models (LMMs) are plotted. The age x diagnosis (p<0.05/3) and age x gender x diagnosis interactions (p=0.006) are significant in this model, indicating a significant group difference in regional development. B) Bilateral caudate volumes plotted against age, typically developing (TD; left), and Fragile X syndrome (FXS; right), volumes adjusted for total grey matter. Regression lines estimated from LMMs are plotted. The interaction between age and diagnosis was not significant in the caudate.
Cognitive trajectories
Having established that specific brain structures mature at a different rate in FXS, we asked whether specific cognitive abilities develop in a similarly aberrant fashion. Participants in this study underwent cognitive and neuropsychological testing, including tests of visual-spatial ability (SR: Spatial Relations test, a subtest of the Woodcock–Johnson Tests of Cognitive Ability (34)) and cognitive flexibility (VF: Verbal Fluency - “F, A, S” test (35)). We hypothesized that the non-standardized SR and VF scores would show slower increases over time in FXS. We used a similar mixed modeling approach to identify differences in age-related change in FXS (Figure 3AB). We found that for both the SR and VF measures, in addition to significant baseline differences (SR: β=-13.1, t(145)=-16.7, p<0.001 ;VF: β=-8, t(134)=13.5, p<0.001), there were significant interactions between age and diagnosis (SR: β=-.49, t(190)=-3.3, p<0.001 ;VF: β=-.6, t(182)=-5.2, p<0.001), indicating that, similar to PFC, cognitive abilities show an aberrant developmental trajectory in FXS.
Figure 3. Development of cognitive measures: Spatial Relations (SR) and Verbal Fluency (VF) scores.
A) Spatial relations scores plotted against age, for typically developing (TD; left), and Fragile X syndrome (FXS; right). B) Verbal fluency scores plotted against age, separately for typically developing (TD; left), and Fragile X syndrome (FXS; right). Regression lines estimated from linear mixed models (LMMs) are plotted. For both measures, the age x diagnosis interaction is significant, indicating significantly aberrant development in FXS.
Discussion
In this study we examined adolescent development in a set of brain structures that have been previously described as structurally and functionally abnormal in FXS. Using semi-longitudinal data, we found that several structures, such as the caudate, showed persistent differences in volume but similar growth trajectories, while gyri in the frontal lobes showed significantly aberrant development, relative to TD controls. Furthermore, we found that specific cognitive functions showed a similarly aberrant developmental trajectory in FXS relative to TD controls. Taken together these findings suggest that abnormal frontal lobe development may contribute to persistent or evolving intellectual deficits in FXS.
Most previous studies of regional volumetric differences in FXS have been conducted across wide age ranges [1-22y(14); 4-19y(13); 2-28y(46);1-43y(47)], controlling for age, and looking for consistent group differences. Notable exceptions are Hoeft et al. 2008, 2010 (16; 17) which looked at FXS males aged 1-3 and 1-5, respectively, and Lee et al. (48) which examined an adolescent sample (15±2y). As such, it is perhaps unsurprising that regions-of-interest taken from previous work would largely show consistent group differences across our sample, rather than interactions between diagnosis and age. However, the longitudinal early childhood study by Hoeft et al. (17) suggests that several regions of the brain show divergent growth trajectories in FXS from an early age, including bilateral thalamus, dorsomedial, dorsolateral, ventromedial, and ventrolateral prefrontal cortices, temporal and occipital regions, including fusiform gyri. The data presented here complement this work by showing that in adolescence, frontal lobe volumes seem to be undergoing differing maturational trajectories in FXS, while regions such as the caudate show stable volume differences, relative to controls.
A recent study in an animal model of FXS, the Fmr1 KO mouse, specifically investigated the effects of reduced FMRP on prefrontal synaptic function, and concomitant effects of behavior (49). Interestingly, this study identified impairments in visual-spatial discrimination and cognitive flexibility in post-pubertal young adult male Fmr1 KO mice (2-4 months) that were linked with reduced expression of synaptic proteins in the PFC, and also identified a marker of reduced neuronal activity. These findings provide a compelling analogy to the present work in which we demonstrate in humans that visual-spatial skills and cognitive flexibility, as assessed by the spatial relations and verbal fluency tasks, respectively, develop abnormally in FXS in the same developmental period in which aberrant PFC trajectories are observed.
Abnormal generation and pruning of synapses has been shown in animal models of FXS (50), indicating that plasticity is affected, and loss of FMRP affects the timing of the critical period of synaptic development (51). FMRP has been shown to be important for long-term potentiation in amygdala (52), hippocampus (53) and sensory regions (54; 55). Several studies have also found abnormal experience-dependent plasticity in sensory regions (50; 56). A study of FMRP expression in adult monkey brains found that this protein is expressed in many brain regions related to the cognitive deficits in FXS: cerebellar cortex, medial temporal lobe structures (including the hippocampal formation), frontal cortical regions, the striatum and the anterior portion of the cingulate gyrus (57). Taken together, these findings in animal models suggest that developmental differences in adolescence could be due to abnormal synaptic activity and experience-dependent plasticity and pruning, caused by FMRP deficiency.
Whole and regional brain volumes and thicknesses have been correlated with IQ in the typically developing population (58-60). In the present study, we assessed whether particular domains of cognitive function would demonstrate aberrant developmental trajectories that paralleled abnormal frontal lobe development in adolescents and young adults with FXS. We found that measures of cognitive flexibility and visual-spatial skills, cognitive domains known to be particularly problematic for individuals with FXS, showed developmental trajectories that differed significantly from TD controls. This suggests that aberrant frontal lobe maturation may be related to the well described slowing of cognitive development in FXS in pre-adolescence (24; 25). Due to the relatively small number of longitudinal scan participants, it was impractical to perform within group correlations between regional volume changes and changes in cognitive measures in the present study. Future studies with dense longitudinal sampling will be required to elucidate the precise link between frontal volumetric changes and cognitive development in FXS (e.g. (60)).
While the data presented here offer novel insight into abnormal brain development in adolescents with FXS, this study has several limitations. Due to limitations associated with sample size, a linear trend was assumed to capture age-related change, rather than a non-linear trend as has been employed in other studies of cortical development (22; 43; 60). As developmental studies have typically found decreasing grey matter volume after approximately 12 years of age, we assume that a linear approximation is reasonable in our age range. The use of a mixed cross-sectional and longitudinal design is less powerful than a fully longitudinal design. We used semi-automated cortical segmentation procedures, and while segmentations were manually inspected for errors, this procedure may be less precise than manual delineation of brain structures. Finally, though we observed differences in grey matter development, with MRI we cannot determine the specific causes that lead to this difference.
In conclusion, we have presented here, to our knowledge, the first longitudinal investigation of brain development in adolescents with FXS. We found that while the volume of several structures, such as the caudate, showed persistent differences between FXS and TD participants, volumes of prefrontal gyri showed significantly aberrant maturation in FXS. Furthermore, we found a parallel developmental abnormality in scores on tests of cognitive flexibility and visual-spatial reasoning, indicating that aberrant frontal cortex maturation may be related to abnormal intellectual development in FXS. These findings are important, both for understanding the effects of FMRP deficits on brain development, and more generally for understanding how synaptic activity and plasticity shapes the gross development of neural structures. These results also suggest that the age at which disease-specific therapeutic interventions are introduced in individuals with FXS may be critical. In particular, such interventions might be more effective if introduced before, or during early adolescence to promote optimal maturation and function of the PFC prior to transition to adulthood.
Supplementary Material
Acknowledgments
We gratefully acknowledge the assistance of Mira Raman with data processing, and Arianna Martin, Rianne Hastie, Christa Watson, Sudharshan Parthasarathy, Lauren Penniman, Asya Karchemsky, Jessica Ringel, Natalee Maynes, and Ellen van Stone with data collection. This work was supported by the US National Institute of Health [R01 MH050047 to ALR], and a National Alliance for Research on Schizophrenia and Depression Young Investigator Award.
Footnotes
The authors report no biomedical financial interests or potential conflicts of interest.
Supplementary material cited in this article is available online.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Crawford DC, Acuna JM, Sherman SL. FMR1 and the fragile X syndrome: human genome epidemiology review. Genet Med. 2001;3:359–71. doi: 10.1097/00125817-200109000-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Turner G, Webb T, Wake S, Robinson H. Prevalence of fragile X syndrome. Am J Med Genet. 1996;64:196–7. doi: 10.1002/(SICI)1096-8628(19960712)64:1<196::AID-AJMG35>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 3.Hagerman PJ. The fragile X prevalence paradox. J Med Genet. 2008;45:498–9. doi: 10.1136/jmg.2008.059055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Till SM. The developmental roles of FMRP. Biochem Soc Trans. 2010;38:507–10. doi: 10.1042/BST0380507. [DOI] [PubMed] [Google Scholar]
- 5.Reiss AL, Dant CC. The behavioral neurogenetics of fragile X syndrome: analyzing gene-brain-behavior relationships in child developmental psychopathologies. Dev Psychopathol. 2003;15:927–68. doi: 10.1017/s0954579403000464. [DOI] [PubMed] [Google Scholar]
- 6.Reiss AL, Hall SS. Fragile X syndrome: assessment and treatment implications. Child Adolesc Psychiatr Clin N Am. 2007;16:663–75. doi: 10.1016/j.chc.2007.03.001. [DOI] [PubMed] [Google Scholar]
- 7.Mazzocco MM, Singh Bhatia N, Lesniak-Karpiak K. Visuospatial skills and their association with math performance in girls with fragile X or Turner syndrome. Child Neuropsychol. 2006;12:87–110. doi: 10.1080/09297040500266951. [DOI] [PubMed] [Google Scholar]
- 8.Mazzocco MM, Pennington BF, Hagerman RJ. The neurocognitive phenotype of female carriers of fragile X: additional evidence for specificity. J Dev Behav Pediatr. 1993;14:328–35. [PubMed] [Google Scholar]
- 9.Fisch GS. Cognitive-behavioral profiles of females with the fragile X mutation. Am J Med Genet A. 2006;140:673–7. doi: 10.1002/ajmg.a.31113. [DOI] [PubMed] [Google Scholar]
- 10.Kuo AY, Reiss AL, Freund LS, Huffman LC. Family environment and cognitive abilities in girls with fragile-X syndrome. J Intellect Disabil Res. 2002;46:328–39. doi: 10.1046/j.1365-2788.2002.00396.x. [DOI] [PubMed] [Google Scholar]
- 11.Bennetto L, Pennington BF, Porter D, Taylor AK, Hagerman RJ. Profile of cognitive functioning in women with the fragile X mutation. Neuropsychology. 2001;15:290–299. [PubMed] [Google Scholar]
- 12.Freund LS, Reiss AL. Cognitive profiles associated with the fra(X) syndrome in males and females. Am J Med Genet. 1991;38:542–7. doi: 10.1002/ajmg.1320380409. [DOI] [PubMed] [Google Scholar]
- 13.Eliez S, Blasey CM, Freund LS, Hastie T, Reiss AL. Brain anatomy, gender and IQ in children and adolescents with fragile X syndrome. Brain. 2001;124:1610–8. doi: 10.1093/brain/124.8.1610. [DOI] [PubMed] [Google Scholar]
- 14.Gothelf D, Furfaro JA, Hoeft F, Eckert MA, Hall SS, O’Hara R, et al. Neuroanatomy of fragile X syndrome is associated with aberrant behavior and the fragile X mental retardation protein (FMRP) Ann Neurol. 2008;63:40–51. doi: 10.1002/ana.21243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kates WR, Abrams MT, Kaufmann WE, Breiter SN, Reiss AL. Reliability and validity of MRI measurement of the amygdala and hippocampus in children with fragile X syndrome. Psychiatry Res. 1997;75:31–48. doi: 10.1016/s0925-4927(97)00019-x. [DOI] [PubMed] [Google Scholar]
- 16.Hoeft F, Lightbody AA, Hazlett HC, Patnaik S, Piven J, Reiss AL. Morphometric spatial patterns differentiating boys with fragile X syndrome, typically developing boys, and developmentally delayed boys aged 1 to 3 years. Arch Gen Psychiatry. 2008;65:1087–97. doi: 10.1001/archpsyc.65.9.1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hoeft F, Carter JC, Lightbody AA, Cody Hazlett H, Piven J, Reiss AL. Region-specific alterations in brain development in one- to three-year-old boys with fragile X syndrome. Proc Natl Acad Sci U S A. 2010;107:9335–9. doi: 10.1073/pnas.1002762107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Reiss AL, Aylward E, Freund LS, Joshi PK, Bryan RN. Neuroanatomy of fragile X syndrome: the posterior fossa. Ann Neurol. 1991;29:26–32. doi: 10.1002/ana.410290107. [DOI] [PubMed] [Google Scholar]
- 19.Reiss AL, Freund L, Tseng JE, Joshi PK. Neuroanatomy in fragile X females: the posterior fossa. Am J Hum Genet. 1991;49:279–88. [PMC free article] [PubMed] [Google Scholar]
- 20.Roberts JE, Mankowski JB, Sideris J, Goldman BD, Hatton DD, Mirrett PL, et al. Trajectories and predictors of the development of very young boys with fragile X syndrome. J Pediatr Psychol. 2009;34:827–36. doi: 10.1093/jpepsy/jsn129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Blakemore SJ, Burnett S, Dahl RE. The Role of Puberty in the Developing Adolescent Brain. Human Brain Mapping. 2010;31:926–933. doi: 10.1002/hbm.21052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gogtay N, Giedd JN, Lusk L, Hayashi KM, Greenstein D, Vaituzis AC, et al. Dynamic mapping of human cortical development during childhood through early adulthood. Proc Natl Acad Sci U S A. 2004;101:8174–9. doi: 10.1073/pnas.0402680101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dykens EM, Hodapp RM, Ort S, Finucane B, Shapiro LR, Leckman JF. The trajectory of cognitive development in males with fragile X syndrome. J Am Acad Child Adolesc Psychiatry. 1989;28:422–6. doi: 10.1097/00004583-198905000-00020. [DOI] [PubMed] [Google Scholar]
- 24.Hall SS, Burns DD, Lightbody AA, Reiss AL. Longitudinal changes in intellectual development in children with Fragile X syndrome. J Abnorm Child Psychol. 2008;36:927–39. doi: 10.1007/s10802-008-9223-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dykens E, Ort S, Cohen I, Finucane B, Spiridigliozzi G, Lachiewicz A, et al. Trajectories and profiles of adaptive behavior in males with fragile X syndrome: multicenter studies. J Autism Dev Disord. 1996;26:287–301. doi: 10.1007/BF02172475. [DOI] [PubMed] [Google Scholar]
- 26.Dykens EM, Hodapp RM, Ort SI, Leckman JF. Trajectory of adaptive behavior in males with fragile X syndrome. J Autism Dev Disord. 1993;23:135–45. doi: 10.1007/BF01066423. [DOI] [PubMed] [Google Scholar]
- 27.Dale AM, Fischl B, Sereno MI. Cortical surface-based analysis - I. Segmentation and surface reconstruction. Neuroimage. 1999;9:179–194. doi: 10.1006/nimg.1998.0395. [DOI] [PubMed] [Google Scholar]
- 28.Fischl B, Sereno MI, Dale AM. Cortical surface-based analysis - II: Inflation, flattening, and a surface-based coordinate system. Neuroimage. 1999;9:195–207. doi: 10.1006/nimg.1998.0396. [DOI] [PubMed] [Google Scholar]
- 29.Reiss AL, Mazzocco MM, Greenlaw R, Freund LS, Ross JL. Neurodevelopmental effects of X monosomy: a volumetric imaging study. Ann Neurol. 1995;38:731–8. doi: 10.1002/ana.410380507. [DOI] [PubMed] [Google Scholar]
- 30.Hallahan BP, Craig MC, Toal F, Daly EM, Moore CJ, Ambikapathy A, et al. In vivo brain anatomy of adult males with Fragile X syndrome: an MRI study. Neuroimage. 2011;54:16–24. doi: 10.1016/j.neuroimage.2010.08.015. [DOI] [PubMed] [Google Scholar]
- 31.Reiss AL, Lee J, Freund L. Neuroanatomy of Fragile-X syndrome - The temporal-lobe. Neurology. 1994;44:1317–1324. doi: 10.1212/wnl.44.7.1317. [DOI] [PubMed] [Google Scholar]
- 32.Wechsler D. Wechsler Intelligence Scale for Children. 3. San Antonio, TX: The Psychological Corporation; 1991. [Google Scholar]
- 33.Wechsler D. The Wechsler Adult Intelligence Scale-III: Administration and scoring manual. San Antonio, TX: The Psychological Corporation; 1997. [Google Scholar]
- 34.Woodcock RW, J . Woodcock–Johnson psycho-educationalbattery—Revised. Chicago: Riverside Publishing; 1990. [Google Scholar]
- 35.Spreen O, Benton A. Manual of instructions. Victoria, BC, Canada: University of Victoria; 1977. Neurosensory centre comprehensive examination for aphasia. [Google Scholar]
- 36.Taylor H. Learning disabilities. In: Mash E, editor. Behavioral assessment of childhood disorders. New York: Guilford Press; 1988. pp. 402–405. [Google Scholar]
- 37.Aman MG, Singh NN, Stewart AW, Field CJ. The aberrant behavior checklist: a behavior rating scale for the assessment of treatment effects. Am J Ment Defic. 1985;89:485–91. [PubMed] [Google Scholar]
- 38.Wadden NP, Bryson SE, Rodger RS. A closer look at the Autism Behavior Checklist: discriminant validity and factor structure. J Autism Dev Disord. 1991;21:529–41. doi: 10.1007/BF02206875. [DOI] [PubMed] [Google Scholar]
- 39.Sparrow S, Balla D, Cicchetti D. Vineland Adaptive Behavior Scales. CirclePines, MN: American Guidance Service; 1984. [Google Scholar]
- 40.Buss A, Plomin R. Temperament: Early developing personality traits. Hillsdale, New Jersey: Erlbaum; 1984. [Google Scholar]
- 41.Singer J, Willett J. Applied longitudinal data analysis: modeling change and event occurrence. New York, NY: Oxford University Press; 2003. [Google Scholar]
- 42.Raudenbusch S, Bryk A. Hierarchical linear models: applications and data analysis methods. 2. Thousand Oaks, CA: Sage Publications Ltd; 2002. [Google Scholar]
- 43.Giedd JN, Blumenthal J, Jeffries NO, Castellanos FX, Liu H, Zijdenbos A, et al. Brain development during childhood and adolescence: a longitudinal MRI study. Nat Neurosci. 1999;2:861–3. doi: 10.1038/13158. [DOI] [PubMed] [Google Scholar]
- 44.Castellanos FX, Lee PP, Sharp W, Jeffries NO, Greenstein DK, Clasen LS, et al. Developmental trajectories of brain volume abnormalities in children and adolescents with attention-deficit/hyperactivity disorder. JAMA. 2002;288:1740–8. doi: 10.1001/jama.288.14.1740. [DOI] [PubMed] [Google Scholar]
- 45.Little R, Rubin D. Statistical analysis with missing data. New York: John Wiley & Sons; 2002. [Google Scholar]
- 46.Reiss AL, Abrams MT, Greenlaw R, Freund L, Denckla MB. Neurodevelopmental effects of the FMR-1 full mutation in humans. Nat Med. 1995;1:159–67. doi: 10.1038/nm0295-159. [DOI] [PubMed] [Google Scholar]
- 47.Mostofsky SH, Mazzocco MM, Aakalu G, Warsofsky IS, Denckla MB, Reiss AL. Decreased cerebellar posterior vermis size in fragile X syndrome: correlation with neurocognitive performance. Neurology. 1998;50:121–30. doi: 10.1212/wnl.50.1.121. [DOI] [PubMed] [Google Scholar]
- 48.Lee AD, Leow AD, Lu A, Reiss AL, Hall S, Chiang MC, et al. 3D pattern of brain abnormalities in Fragile X syndrome visualized using tensor-based morphometry. NeuroImage. 2007;34:924–38. doi: 10.1016/j.neuroimage.2006.09.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Krueger DD, Osterweil EK, Chen SP, Tye LD, Bear MF. Cognitive dysfunction and prefrontal synaptic abnormalities in a mouse model of fragile X syndrome. Proceedings of the National Academy of Sciences of the United States of America. 2011 doi: 10.1073/pnas.1013855108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Pan F, Aldridge GM, Greenough WT, Gan WB. Dendritic spine instability and insensitivity to modulation by sensory experience in a mouse model of fragile X syndrome. Proc Natl Acad Sci U S A. 2010;107:17768–73. doi: 10.1073/pnas.1012496107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Harlow EG, Till SM, Russell TA, Wijetunge LS, Kind P, Contractor A. Critical Period Plasticity Is Disrupted in the Barrel Cortex of Fmr1 Knockout Mice. Neuron. 2010;65:385–398. doi: 10.1016/j.neuron.2010.01.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Suvrathan A, Hoeffer CA, Wong H, Klann E, Chattarji S. Characterization and reversal of synaptic defects in the amygdala in a mouse model of fragile X syndrome. Proc Natl Acad Sci U S A. 2010;107:11591–6. doi: 10.1073/pnas.1002262107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Huber KM, Gallagher SM, Warren ST, Bear MF. Altered synaptic plasticity in a mouse model of fragile X mental retardation. Proc Natl Acad Sci U S A. 2002;99:7746–50. doi: 10.1073/pnas.122205699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Desai NS, Casimiro TM, Gruber SM, Vanderklish PW. Early postnatal plasticity in neocortex of Fmr1 knockout mice. J Neurophysiol. 2006;96:1734–45. doi: 10.1152/jn.00221.2006. [DOI] [PubMed] [Google Scholar]
- 55.Wilson BM, Cox CL. Absence of metabotropic glutamate receptor-mediated plasticity in the neocortex of fragile X mice. Proc Natl Acad Sci U S A. 2007;104:2454–9. doi: 10.1073/pnas.0610875104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bureau I, Shepherd GM, Svoboda K. Circuit and plasticity defects in the developing somatosensory cortex of FMR1 knock-out mice. J Neurosci. 2008;28:5178–88. doi: 10.1523/JNEUROSCI.1076-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zangenehpour S, Cornish KM, Chaudhuri A. Whole-brain expression analysis of FMRP in adult monkey and its relationship to cognitive deficits in fragile X syndrome. Brain Res. 2009;1264:76–84. doi: 10.1016/j.brainres.2009.01.059. [DOI] [PubMed] [Google Scholar]
- 58.Reiss AL, Abrams MT, Singer HS, Ross JL, Denckla MB. Brain development, gender and IQ in children. A volumetric imaging study. Brain. 1996;119:1763–74. doi: 10.1093/brain/119.5.1763. [DOI] [PubMed] [Google Scholar]
- 59.Frangou S, Chitins X, Williams SCR. Mapping IQ and gray matter density in healthy young people. Neuroimage. 2004;23:800–805. doi: 10.1016/j.neuroimage.2004.05.027. [DOI] [PubMed] [Google Scholar]
- 60.Shaw P, Greenstein D, Lerch J, Clasen L, Lenroot R, Gogtay N, et al. Intellectual ability and cortical development in children and adolescents. Nature. 2006;440:676–9. doi: 10.1038/nature04513. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.