Abstract
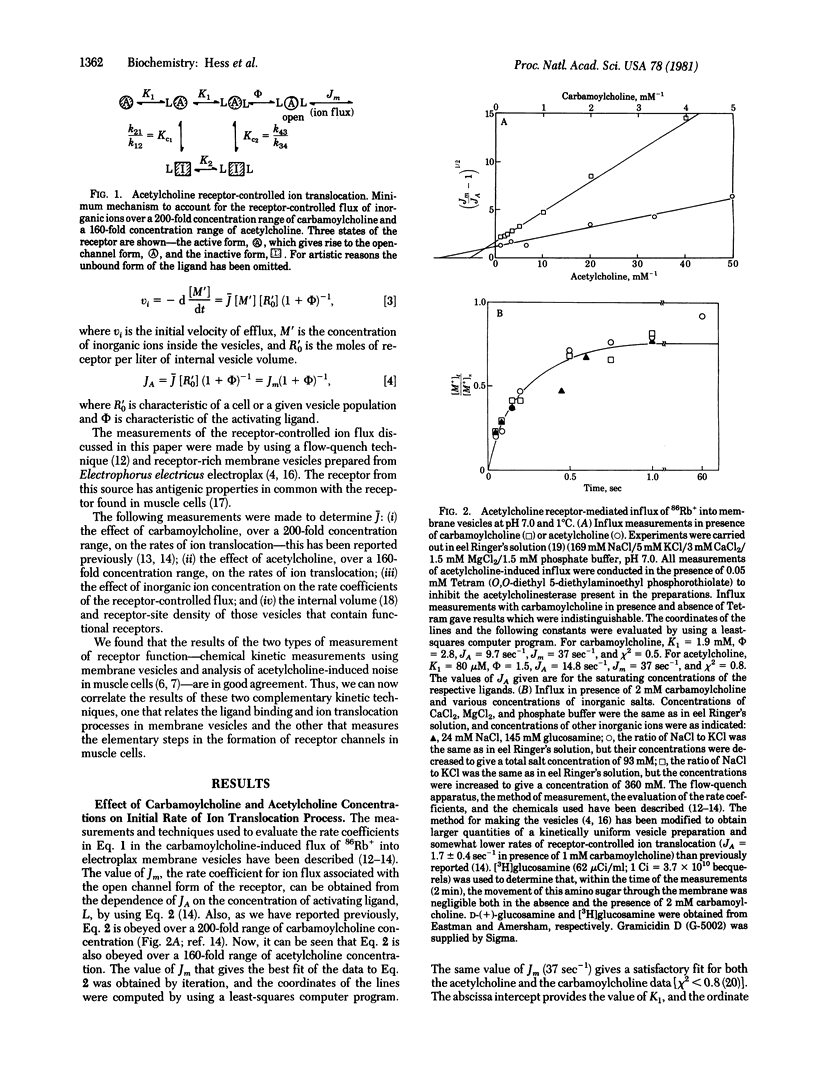
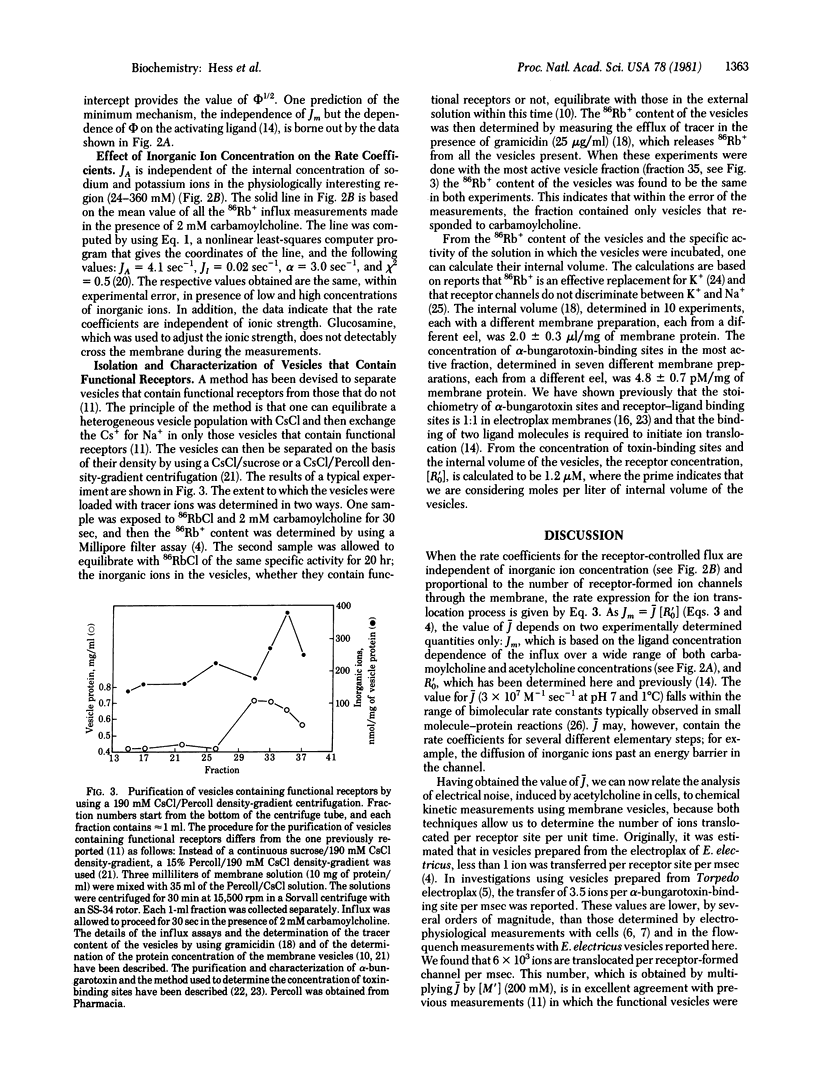
The specific reaction rate (J) of the acetylcholine receptor-controlled ion translocation has been determined. In eel Ringer's solution (pH 7.0) at 1 degrees C, J = 3 X 10(7) M-1 sec-1. J is an intrinsic constant that is characteristic of the receptor and independent of other properties of a receptor-containing cell that also determine the rates of ion translocation. Membrane vesicles (prepared from the electric organ of Electrophorus electricus) and a flow-quench technique that has a millisecond time resolution were used to measure the receptor-controlled ion translocation. Using the value of J and the molar concentrations of receptor sites and inorganic ions, we calculated that 6 X 10(3) ions are translocated per msec per receptor. Analysis of electrical noise in frog muscle cells at temperatures above 8 degrees C [Nether, E. & Stevens, C. F. (1977) Annu. Rev. Biophys. Bioeng. 6, 345-381] gave a value of about 1 X 10(4) ions msec-1 per channel. Thus, each technique gives essentially the same result. It is now possible, therefore, to correlate the results obtained when receptor function is measured in two different ways in membrane vesicles and in muscle cells: (i) chemical kinetic measurements, using membrane vesicles, which relate the ligand binding and ion translocation processes and (ii) analysis of acetylcholine noise in muscle cells [Katz, B. & Miledi, R. (1972) J. Physiol. (London) 224, 665-699], which allows one to measure elementary steps in the formation of ion channels through the cell membrane.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aoshima H., Cash D. J., Hess G. P. Acetylcholine receptor-controlled ion flux in electroplax membrane vesicles: a minimal mechanism based on rate measurements in the millisecond to minute time region. Biochem Biophys Res Commun. 1980 Feb 12;92(3):896–904. doi: 10.1016/0006-291x(80)90787-1. [DOI] [PubMed] [Google Scholar]
- Bulger J. E., Fu J. L., Hindy E. F., Silberstein R. L., Hess G. P. Allosteric interactions between the membrane-bound acetylcholine receptor and chemical mediators. Kinetic studies. Biochemistry. 1977 Feb 22;16(4):684–692. doi: 10.1021/bi00623a020. [DOI] [PubMed] [Google Scholar]
- Cash D. J., Aoshima H., Hess G. P. Acetylcholine-induced receptor-controlled ion flux investigated by flow quench techniques. Biochem Biophys Res Commun. 1980 Aug 14;95(3):1010–1016. doi: 10.1016/0006-291x(80)91573-9. [DOI] [PubMed] [Google Scholar]
- Cash D. J., Hess G. P. Molecular mechanism of acetylcholine receptor-controlled ion translocation across cell membranes. Proc Natl Acad Sci U S A. 1980 Feb;77(2):842–846. doi: 10.1073/pnas.77.2.842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catterall W. A. Sodium transport by the acetylcholine receptor of cultured muscle cells. J Biol Chem. 1975 Mar 10;250(5):1776–1781. [PubMed] [Google Scholar]
- Dionne V. E., Ruff R. L. Endplate current fluctuations reveal only one channel type at frog neuromuscular junction. Nature. 1977 Mar 17;266(5599):263–265. doi: 10.1038/266263a0. [DOI] [PubMed] [Google Scholar]
- Fu J. L., Donner D. B., Moore D. E., Hess G. P. Allosteric interactions between the membrane-bound acetylcholine receptor and chemical mediators: equilibrium measurements. Biochemistry. 1977 Feb 22;16(4):678–684. doi: 10.1021/bi00623a019. [DOI] [PubMed] [Google Scholar]
- HODGKIN A. L., HUXLEY A. F. A quantitative description of membrane current and its application to conduction and excitation in nerve. J Physiol. 1952 Aug;117(4):500–544. doi: 10.1113/jphysiol.1952.sp004764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HODGKIN A. L., KATZ B. The effect of sodium ions on the electrical activity of giant axon of the squid. J Physiol. 1949 Mar 1;108(1):37–77. doi: 10.1113/jphysiol.1949.sp004310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hess G. P., Andrews J. P. Functional acetylcholine receptor--electroplax membrane microsacs (vesicles): purification and characterization. Proc Natl Acad Sci U S A. 1977 Feb;74(2):482–486. doi: 10.1073/pnas.74.2.482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hess G. P., Andrews J. P., Struve G. E., Goombs S. E. Acetylcholine-receptor-mediated ion flux in electroplax membrane preparations. Proc Natl Acad Sci U S A. 1975 Nov;72(11):4371–4375. doi: 10.1073/pnas.72.11.4371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hess G. P., Cash D. J., Aoshima H. Acetylcholine receptor-controlled ion fluxes in membrane vesicles investigated by fast reaction techniques. Nature. 1979 Nov 15;282(5736):329–331. doi: 10.1038/282329a0. [DOI] [PubMed] [Google Scholar]
- Hess G. P., Lipkowitz S., Struve G. E. Acetylcholine-receptor-mediated ion flux in electroplax membrane microsacs (vesicles): change in mechanism produced by asymmetrical distribution of sodium and potassium ions. Proc Natl Acad Sci U S A. 1978 Apr;75(4):1703–1707. doi: 10.1073/pnas.75.4.1703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KEYNES R. D., MARTINS-FERREIRA H. Membrane potentials in the electroplates of the electric eel. J Physiol. 1953 Feb 27;119(2-3):315–351. doi: 10.1113/jphysiol.1953.sp004849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaback H. R. Transport. Annu Rev Biochem. 1970;39:561–598. doi: 10.1146/annurev.bi.39.070170.003021. [DOI] [PubMed] [Google Scholar]
- Katz B., Miledi R. The statistical nature of the acetycholine potential and its molecular components. J Physiol. 1972 Aug;224(3):665–699. doi: 10.1113/jphysiol.1972.sp009918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim P. S., Hess G. P. Acetylcholine receptor-controlled ion flux in electroplax membrane vesicles: identification and characterization of membrane properties that affect ion flux measurements. J Membr Biol. 1981 Feb 28;58(3):203–211. doi: 10.1007/BF01870906. [DOI] [PubMed] [Google Scholar]
- Kohanski R. A., Andrews J. P., Wins P., Eldefrawi M. E., Hess G. P. A simple quantitative assay of 125I-labeled alpha-bungarotoxin binding to soluble and membrane-bound acetylcholine receptor protein. Anal Biochem. 1977 Jun;80(2):531–539. doi: 10.1016/0003-2697(77)90676-5. [DOI] [PubMed] [Google Scholar]
- Neher E., Sakmann B. Single-channel currents recorded from membrane of denervated frog muscle fibres. Nature. 1976 Apr 29;260(5554):799–802. doi: 10.1038/260799a0. [DOI] [PubMed] [Google Scholar]
- Neher E., Stevens C. F. Conductance fluctuations and ionic pores in membranes. Annu Rev Biophys Bioeng. 1977;6:345–381. doi: 10.1146/annurev.bb.06.060177.002021. [DOI] [PubMed] [Google Scholar]
- Neubig R. R., Cohen J. B. Permeability control by cholinergic receptors in Torpedo postsynaptic membranes: agonist dose-response relations measured at second and millisecond times. Biochemistry. 1980 Jun 10;19(12):2770–2779. doi: 10.1021/bi00553a036. [DOI] [PubMed] [Google Scholar]
- Palfrey C., Littauer U. Z. Sodium-dependent efflux of K+ and Rb+ through the activated sodium channel of neuroblastoma cells. Biochem Biophys Res Commun. 1976 Sep 7;72(1):209–215. doi: 10.1016/0006-291x(76)90981-5. [DOI] [PubMed] [Google Scholar]
- Patrick J., Stallcup W. B. Immunological distinction between acetylcholine receptor and the alpha-bungarotoxin-binding component on sympathetic neurons. Proc Natl Acad Sci U S A. 1977 Oct;74(10):4689–4692. doi: 10.1073/pnas.74.10.4689. [DOI] [PMC free article] [PubMed] [Google Scholar]



