Abstract
The α4βδ gamma-aminobutyric acid A receptor (GABAAR) has been proposed to mediate the rewarding effects of low-to-moderate concentrations of alcohol (ethanol) that approximate those achieved by social drinking. If this is true, then this receptor should be necessary for the reinforcing effects of ethanol as assessed in an instrumental self-administration procedure in which rats are trained to lever press for oral ethanol. We used viral-mediated RNA interference to transiently reduce expression of the α4 GABAAR subunit in the shell region of the nucleus accumbens (NAc). We found that responding for ethanol was significantly reduced after α4 reductions in the NAc shell, but not NAc core. This reduction was specific to ethanol, as responding for sucrose was not altered. The presence of ethanol was also required as unreinforced responding for ethanol in subjects previously trained to respond for ethanol (i.e. responding during an extinction test) was not altered. In addition, responding during reinforced sessions was not altered during the initial 5 minutes of the session, but decreased after 5 minutes, following multiple reinforced responses. Together, these findings indicate that the α4 GABAAR subunit in the NAc shell is necessary for the instrumental reinforcing effects of oral ethanol, further supporting a role for α4-containing GABAARs in the rewarding/reinforcing effects of ethanol. Possible pharmacological and non-pharmacological explanations for these effects are considered.
Keywords: Addiction, ethanol, rat, RNA interference, self-administration, ventral striatum
INTRODUCTION
Gamma-aminobutyric acid A receptors (GABAAR) have been proposed to mediate a variety of behavioral effects of ethanol, including ethanol’s reinforcing and rewarding effects (Grobin et al. 1998; Chester & Cunningham 2002; Koob 2004). GABAARs are pentameric complexes that function as ligand-gated chloride channels and are composed from multiple distinct subunits (α 1-6, β 1-3, γ 1-3, δ, ε, θ, π and ρ 1-3). The consequence of this diversity in subunit composition is substantial functional and pharmacological heterogeneity (Macdonald & Olsen 1994; Mody & Pearce 2004).
The α4βδ GABAAR is found at peri- and extrasynaptic sites and has been proposed to mediate the effects of low-to-moderate concentrations of ethanol that approximate those achieved by social drinking (i.e. < 30 mM; Sundstrom-Poromaa et al. 2002; Wallner, Hanchar & Olsen 2003; Hanchar, Wallner & Olsen 2004; Wei, Faria & Mody 2004). Recombinant α4βδ GABAARs are sensitive to low-to-moderate (3–30 mM) concentrations of ethanol (Sundstrom-Poromaa et al. 2002; Wallner et al. 2003, 2006). Recordings made from hippocampal slice have likewise supported a role for α4βδ GABAARs in low-to-moderate dose effects of ethanol (Sundstrom-Poromaa et al. 2002; Wei et al. 2004; Liang et al. 2008). Because of their sensitivity to ethanol at concentrations achieved following oral consumption, α4βδ GABAARs are attractive candidates for the mediation of the reinforcing effects of ethanol. However, not all studies have succeeded in replicating these electrophysiological results (Borghese et al. 2006).
We recently sought to investigate the contribution of the α4βδ GABAARs to ethanol’s behavioral effects. We used viral-mediated RNA interference (RNAi), which allows for region-specific reductions of target gene mRNA levels in adult animals (Hommel et al. 2003; Lasek et al. 2007; Jeanblanc et al. 2009), to selectively down-regulate the expression of the α4 GABAAR subunit in the shell region of the nucleus accumbens (NAc) of rats. We found that ethanol self-administration in the two-bottle preference test was reduced after NAc shell knockdown of the α4 GABAAR subunit, but intake of sucrose was unaffected (Rewal et al. 2009). These findings indicate that α4-containing GABAARs, in a region long considered to mediate processes of reward and reinforcement (Koob, Sanna & Bloom 1998; McBride, Murphy & Ikemoto 1999; Everitt & Robbins 2005), contribute to ethanol intake. Interestingly, a previous study found that mice with a global deletion of the gene encoding the δ GABAAR subunit drink less ethanol than wild-type mice (Mihalek et al. 2001), suggesting a role for δ subunit-containing GABAARs in ethanol intake. Taken together, these findings provide strong evidence that the α4βδ GABAAR contributes to mechanisms underlying ethanol intake.
The current study was undertaken to test if the α4 GABAAR subunit is required for the reinforcing effects of ethanol as measured by instrumental responding; specifically, is the ability of ethanol to maintain lever-press responding dependent upon the α4 GABAAR subunit? Using viral-mediated RNAi (Rewal et al. 2009), we show that reduction of the expression of the α4 GABAAR subunit in the NAc shell reduces responding for ethanol, without altering the initial motivation to perform the lever-press response, and without producing non-selective reductions in reinforcement processes or motor performance.
MATERIALS AND METHODS
Subjects
Male, Long Evans rats (Harlan, Indianapolis, IN) weighing 260–280 g upon arrival were individually housed in ventilated polycarbonate chambers (colony room temperature, 21 ± 1°C; 12-hour light/dark cycle with lights on at 7:00 a.m.). Rats had unrestricted access to standard rat chow and water throughout the study. All procedures were approved by the Gallo Center Institutional Animal Care and Use Committee.
Design and cloning of short-hairpin RNA (shRNA) constructs
Short-hairpin RNAs (shRNAs) directed against the α4 GABAAR subunit were designed, cloned and tested, as in (Rewal et al. 2009), and as described below. A 21-nucleotide (nt) small-interfering RNA (siRNA) sequences of the rat α4 subunit (GenBank accession NM080587) was designed using the GenScript web-based program. Specificity of the siRNA sequence was verified by a BLAST search, termed α4-1, and has the following sequence: 5′-AUAACAUGACAGCUCCAAAUA. A non-related 19-nt sequence (5′-AUGAACGUGAAUU GCUCAA) was used as a negative siRNA control. To employ viral delivery of double-strand siRNA, the adenoviral shuttle vector pRNAT-H1.1 (GenScript Corporation) and the adenoviral vector Adeno-X (Clontech) were used. pRNAT-H1.1 is a vector for vector-based siRNA cloning with an H1 promoter to express shRNA, and contains GFP under control of the CMV promoter. For each siRNA sequence, two complementary DNA oligos, containing sense and antisense siRNA sequences with a stem-loop structure, were synthesized, annealed and ligated into BamHI and HindIII sites of pRNAT-H1.1 vector following the vector cloning protocol. The pRNAT-shRNA recombinants were confirmed by sequencing before subcloning into the cloning sites of I-Ceu I and PI-Sce I of Adeno-X vector.
Adenovirus production
Preparation of adenoviruses, Ad-shα4-1 and Ad-NSS, was initiated by transfection of recombinant adenoviral constructs into HEK293 cells using Lipofectamine 2000 (Invitrogen, Carlsbad, CA). Recombinant viruses were amplified in HEK293 cells, followed by purification using Adeno-X Virus Purification Kit (Clontech).
Home cage drinking
One week after arrival, rats were habituated to the taste and pharmacological effects of EtOH in their home cages. They received concurrent 24-hour access to both water and EtOH (10% v/v) for 2 weeks, followed by water and EtOH (20% v/v) every other day for 6 weeks. This procedure results in relatively high EtOH intakes (Steensland et al. 2007; Carnicella et al. 2008; Simms et al. 2008).
Operant ethanol self-administration
Following home cage drinking, operant training began. Operant conditioning chambers (Medical Associates Inc., St. Albans, VT) were located in a different room from where the rats were housed. Chambers were contained within ventilated sound-attenuating cubicles, and comprised of a clear Plexiglas ceiling, front door and back wall, paneled aluminum side walls and a stainless steel bar floor. The right wall featured a central port containing a circular fluid receptacle, into which ethanol was delivered via a 20 ml syringe attached to a pump located outside the sound-attenuating cubicle. Chambers were outfitted with retractable levers flanking the port and a white chamber light (28 V, 100 mA) located centrally near the ceiling on the left wall. Responses on one lever (active lever) resulted in delivery of 0.1 ml of 20% EtOH and responses on the other, inactive, lever resulted in no programmed events. No explicit cues were used at any time during the conditioning sessions. To facilitate acquisition of the lever-press response, rats were placed overnight (12–14 hours) in the operant chambers for two nights in a row and allowed to respond for ethanol on a continuous reinforcement schedule. After overnight training sessions, 30-minute daily training sessions were conducted, with the schedule requirement increasing to FR2 over the course of 2 weeks. Responses on the active and inactive levers, and ethanol port entries, were recorded. After stable responding developed, blood ethanol levels were measured (see below). These data indicated that rats achieved measurable levels of plasma ethanol following a single operant session (mean + SEM: 7.11 + 0.85 mM, range: 5.35–12.34 mM; equivalent to 32.71 + 3.91 mg%; range: 24.62–56.76 mg%; n = 14) and these values were predictive of the estimated g/kg (mean ± SEM: 0.6525 ± 0.066 g/kg, range: 0.4–1.04 g/kg, Pearson correlation, r = 0.944, P < 0.001). After 2 months of training, rats were divided into treatment and control virus groups by attempting to balance the groups for baseline operant responding. Surgery for virus infusion then commenced; after recovery, 30 minutes self-administration sessions occurred daily for 30 days.
Operant sucrose self-administration
Rats were trained to lever press for sucrose after 6 weeks of home cage access to two bottles, one filled with tap water and the other 2% sucrose (w/v). Bottles were placed on subjects’ cages every other day, with water alone available on the intervening days. After these 6 weeks, operant training for sucrose was conducted, using the same chambers as used for operant training for alcohol. Rats were initially trained in two overnight sessions under an FR1 schedule with 0.1 ml of a 2% sucrose solution as the reinforcer. Subsequently, rats were trained 5 days a week (before virus infusions), with the FR schedule progressively increased to FR2 over the course of 2 weeks. After 2 months of training, surgery to infuse viruses occurred. After a 5-day recovery period, 30-minute operant sessions resumed 7 days a week for 30 days.
Intra-NAc infusion of Ad-NSS or Ad-shα4 virus
To infuse viruses into the NAc, subjects were anesthetized with isoflurane, and holes were drilled bilaterally in the skull at the anterior/posterior and medial/lateral (in reference to bregma) coordinates corresponding to the NAc shell (AP: +1.6 mm, ML: +/−0.78 mm, DV: −7 mm) or NAc core (AP: +1.2 mm, ML: +/−1.9 mm, DV: −6.8 mm) using a 0.3-mm carbide drill bit. Stainless steel tubing (30 g) connected to polyvinyl chloride tubing was filled with 1 μl of virus solution (1 × 1010 to 3 × 1010 TU/ml) using a 10-μl Hamilton gastight syringe. Virus (1 μl) was injected at a rate of 0.1 μl per minute using an infusion pump (Harvard Apparatus, Holliston, MA) for 10 minutes and then allowed to diffuse for an additional 10 minutes. The tubing was removed slowly, and the scalp was closed with sutures. Five days later, daily ethanol self-administration sessions (as above) were continued.
Blood ethanol levels measurement
Tail vein blood was collected in heparinized capillary tubes immediately after a typical operant session. Serum was extracted with 3.4% trichloroacetic acid followed by a 5-minute centrifugation at 420 g, and assayed for ethanol content using the NAD-NADH enzyme spectrophotometric method (Weiss et al. 1993; Zapata, Gonzales & Shippenberg 2006). BECs were determined by using a standard calibration curve.
Locomotor activity in an open field
Rats were placed in 43 cm × 43 cm locomotor activity chambers (Medical Associates) and the distance traveled by the animals was recorded for 1 hour. Each subject received two test sessions, one before virus infusion, and one 18 days after virus infusion.
Motor coordination
The rotarod apparatus (manufactured at UCSF machine shop—Department of Physiology) consisted of a rotating stainless steel cylinder divided into a series of 10–15 cm lanes, which provided constant acceleration between 0 and 10 rpm. Baseline performance was determined over 1 day with five trials per session at a fixed speed of 2.5 rpm, three sessions per day. Two more sessions were performed on these rats 18 days after viral infusion with constant acceleration of 0.5 rpm/s for a maximum speed of 5 rpm. The maximum time for each trial was 120 seconds. Rats were monitored during the rotarod procedure and the latency to fall was recorded (cut-off time = 3 minutes). The same animals were used to measure locomotor activity in an open field and latency to fall from rotarod. The open field was conducted in the mornings and the rotarod test during the afternoons with a time difference of 3–4 hours between the two procedures.
Data analysis
Behavioral data were analyzed using one- or two-way analysis of variance (ANOVA), followed by Bonferroni’s post hoc test when indicated by significant (σ = 0.05) main effects or interactions. The ANOVAs for the figures depicting percent control data were conducted on the percent change data. The percent change measure was calculated for each individual subject by dividing each individual rat’s score in the experimental group by the mean score of the control group on that same day, and multiplying by 100.
RESULTS
GABAAR α4 subunit knockdown in the NAc shell decreases instrumental responding for ethanol
To test the effects of NAc knockdown of the α4 GABAAR subunit on instrumental responding for ethanol, adenoviral vectors expressing shRNAs directed against the α4 GABAAR subunit (Ad-shα4-1) or the control adenoviral vector expressing an shRNA predicted to have no effect on mRNA expression (Ad-NSS) were infused into the NAc shell of rats trained to respond on a fixed-ratio 2 schedule for 0.1 ml aliquots of 20% ethanol. We showed previously that the Ad-shα4-1 virus down-regulates the mRNA and protein levels of the α4 subunit transiently, with significant reductions 18 days after infusion and recovery 25 days after infusion (Rewal et al. 2009). Ethanol intake from bottles available on the home cage was also transiently reduced after infusion of Ad-shα4-1 into the NAc shell for about 10 days beginning from ~day 12 (Rewal et al. 2009). Therefore, in the present study, we conducted daily test sessions from 5 to 30 days after virus infusion to encompass the time period during which we expected α4 knockdown.
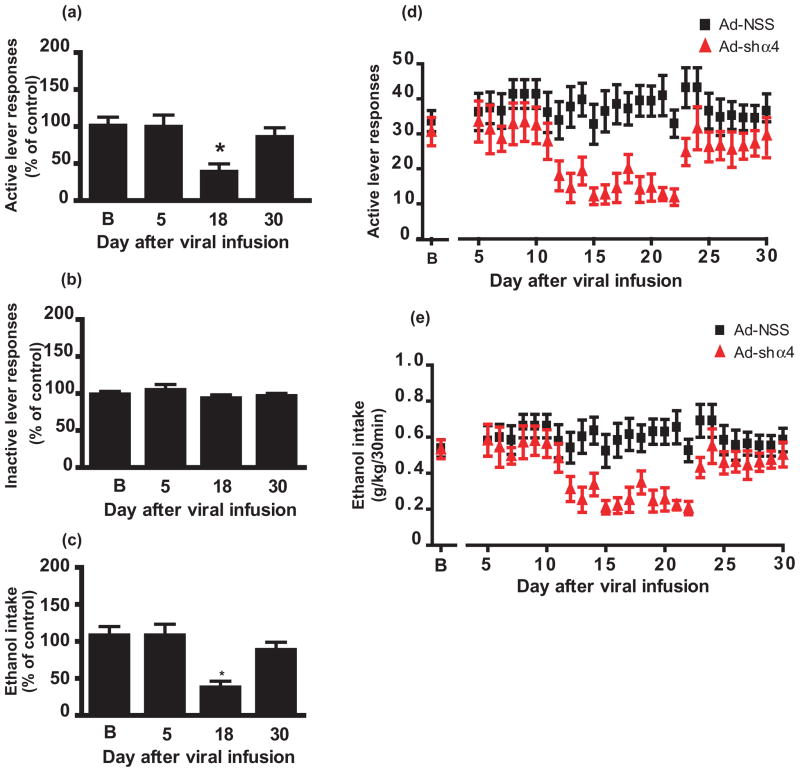
As shown in Fig. 1a, Ad-shα4-1 infusion into the NAc shell significantly decreased active lever presses 18 days after viral infusion (main effect of day, F(3,44) = 5.4, P < 0.0001; P < 0.05, baseline versus day 18). In contrast, there was no significant change in inactive lever responses (Fig. 1b; F(3,44) = 2.17, P = 0.88; range: 0–6 per 30 minutes session). We also observed a reduction in ethanol intake in grams per kilogram, calculated from the number of reinforcers delivered relative to the body weight of the subjects (Fig. 1c; main effect of day, F(3,44) = 4.6, P < 0.0001; P < 0.05, baseline versus day 18) in accordance with the decrease in active lever-press responding.
Figure 1.
Viral-mediated GABAAR α4 subunit knockdown in NAc shell decreases instrumental responding for ethanol. Rats were infused in the NAc shell with Ad-shα4 (n = 12) or Ad-NSS (n = 12) bilaterally. For panels a–c, data for Ad-shα4-treated rats are expressed as a percentage of Ad-NSS-treated rats at the same timepoint. (a) Responding on the active lever is decreased 18 days after Ad-shα4 infusion. *P < 0.05, compared to baseline (B). (b) Inactive lever responding was not affected. (c) Estimated ethanol intake (g/kg) was decreased 18 days after Ad-shα4 infusion. *P < 0.05, compared to baseline. (d, e) Time course of active lever responses (d) and estimated ethanol intake (e) over days 5–30 after virus infusion depicts the decrease in responding after Ad-shα4, followed by a recovery of responding at day 24. For all panels, ‘B’ refers to baseline, the average of last 3 days prior to virus infusion. Values depict mean +/− SEM
When examined across the 5–30 days after viral infusion, a decrease in active lever responding was observed beginning 12 days after infusion of the Ad-shα4 virus, reaching a maximum 18–19 days after infusion (Fig. 1d), and returning to baseline levels ~23 days after virus infusion. We have found that shRNAs expressed by the adenovirus also reduce target mRNAs over a similar time period, suggesting that the transient suppression of target gene expression is responsible for the transient behavioral effects (Jeanblanc et al. 2009; Rewal et al. 2009). It should be mentioned that a subset of rats (n = 8) received a weekend (two sessions) off between day 22 and day 23, presumably accounting for the rather abrupt recovery of behavior in Fig. 1d and e.
Notably, statistical analysis of the time course of active lever responding after viral infusion found a main effect of time [F(26,572) = 5.7, P < 0.0001] and virus treatment [F(1,22) = 8.36, P < 0.0001], and a significant interaction between treatment and time [F(26,572) = 4.5, P < 0.0001]. Likewise, ethanol intake decreased during a similar time period (Fig. 1e; main effect of time [F(26,572) = 7.34, P < 0.0001], virus treatment [F(1,22) = 12.16, P < 0.05], treatment by time interaction [F(26,572) = 7.34, P < 0.0001]). Baseline ethanol consumed by the Ad-NSS group was 0.54 ± 0.048 g/kg and by the Ad-shα4-1 group was 0.53 ± 0.052 g/kg; ethanol consumed on day 18 after viral infusion was 0.59 ± 0.074 g/kg by the Ad-NSS group and 0.35 ± 0.064 g/kg by the Ad-shα4-1 group, and these values were significantly different (P < 0.02, t-test).
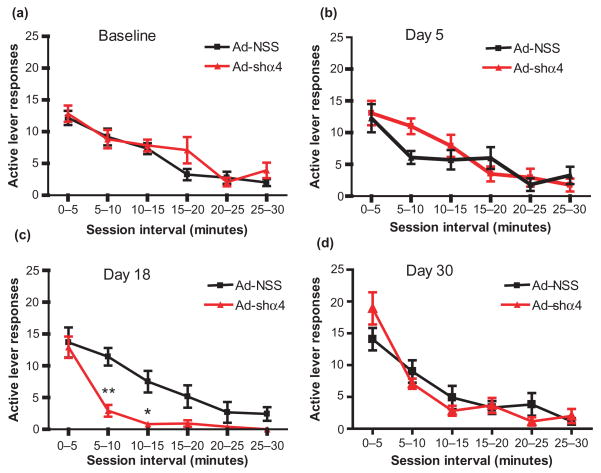
To gain further insight into the observed reduction in responding, we chose four sessions in which to analyze the pattern of responding within the test session in 5-minute bins (Fig. 2). While there were significant main effects of session time for the baseline session (F(5,110) = 24.28, P < 0.001; Fig. 2a), and both day 5 (F(5,110) = 15.94, P < 0.001; Fig. 2b) and day 30 (F(5,110) = 31.75, P < 0.001; Fig. 2d) after virus infusion, there were no main effects of treatment or any treatment by session time interactions (F < 1). However, responding on day 18 was significantly altered after GABAAR α4 subunit knockdown. For day 18, there was a main effect of time (F(5,110) = 25.56, P < 0.0001) and virus treatment (F(1,110) = 19.47, P < 0.001), and a significant interaction between treatment and time (F(5,110) = 2.6, P < 0.05; Fig. 2c). These effects were accounted for by a significant decrease in active lever responding by the Ad-shα4 group 5–10 minutes (P < 0.01) and 10–15 minutes (P < 0.05) after session onset, as compared to responding by Ad-NSS rats. Hence responding was the same for both groups for the first 5 minutes, and as the session progressed, rats with GABAAR α4 subunit knockdown responded less compared to control rats. These findings suggest that responding for ethanol in rats infused with the Ad-shα4 decreases after the rats have the opportunity to experience responding reinforced by ethanol delivery.
Figure 2.
Decreases in instrumental responding for ethanol after GABAAR α4 subunit knockdown in the NAc shell occurs after session initiation. Within-session responding during 30-minute sessions by the same subjects depicted in Fig. 1 during the final session prior to virus infusion (a; baseline) and during day 5 (b), day 18 (c) and day 30 (d). (c) Responding by Ad-shα4-treated rats was significantly decreased on day 18 at the 5–10 and 10–15 minutes intervals (*P < 0.05, **P < 0.01, Ad-shα4 versus Ad-NSS groups) but not during the first 5 minutes of the session. There were no group differences during the baseline session or days 5 and 30 (a, b, d). Values depict mean +/− SEM of active lever responses within 5-minute bins. Data are presented as mean +/− SEM. n = 12 rats per group
Reductions in responding for ethanol after GABAAR α4 subunit knockdown are present when testing occurs at the time of maximal knockdown and require ethanol reinforcement
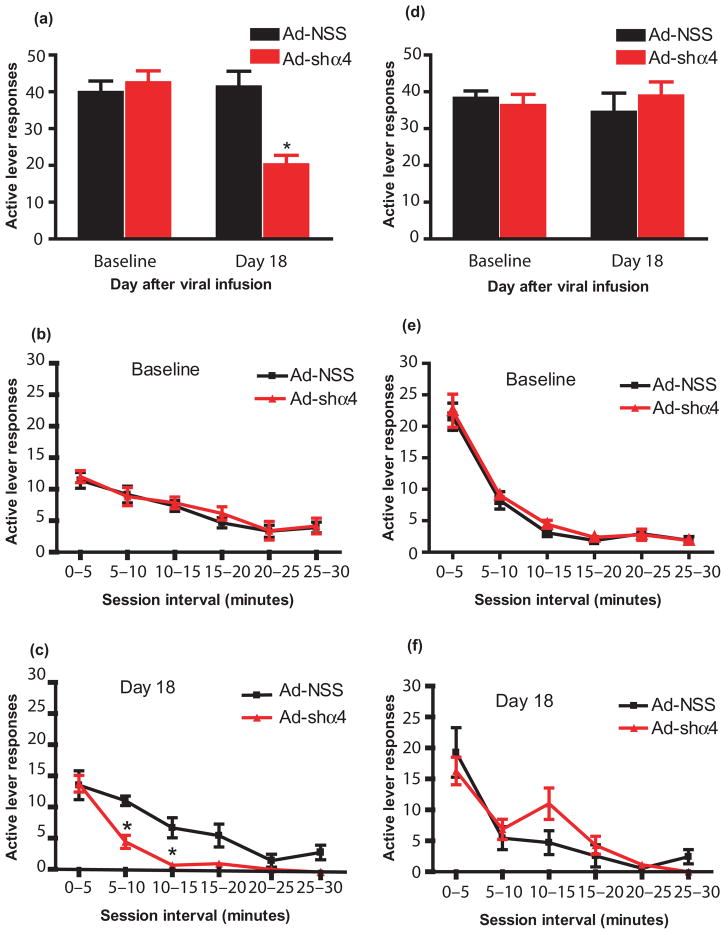
Because the mRNA reduction occurs within a relatively short interval of days, it is possible that ongoing ethanol self-administration during the time period from day 5 to day 18, when the expression of the GABAAR α4 subunit was changing, somehow impacted the results obtained at day 18. To test whether the GABAAR α4 subunit mediates the reinforcing effects of ethanol when testing begins at a point when α4 is at or near its maximal decrease, rats were trained to lever press for 20% ethanol and infused with the Ad-NSS or Ad-shα4 virus as above. However, testing did not commence until the 18th day (instead of the fifth day) after viral infusion, a day at which we have shown maximal reductions in α4 subunit expression occur (Rewal et al. 2009). On the test day, responding by one-half of the rats was reinforced with ethanol; responding by the second half of the rats was not reinforced. If the decrease in responding following Ad-shα4 infusion into the NAc shell depends upon actual delivery of the ethanol reinforcer, then responding should be decreased only in the group receiving ethanol and not in the second cohort in which responding was unreinforced.
As expected, rats with GABAAR α4 subunit reduction showed decreased total active lever responses on day 18 after viral infusion when those responses were reinforced with ethanol (P < 0.001; Fig. 3a). The ethanol intake of the rats with the reduced expression of the GABAAR α4 subunit (0.32 + 0.04 g/kg) was also significantly lower across the entire session than the ethanol intake of control rats (0.66 + 0.08 g/kg; P < 0.001, t-test). Within-session examination of responding indicated that the two groups did not differ during the baseline session [Fig. 3b; significant main effect of time (F(5,70) = 34.11, P < 0.001), but not virus treatment (F(1,70) = 3.6, P = 0.98), nor treatment by time interaction (F(5,70) = 0.12, P = 0.98)]. However, as seen in Fig. 3c, on day 18, rats with GABAAR α4 subunit knockdown in the NAc shell made significantly fewer active lever responses as compared to control rats [main effect of time (F(5,70) = 34.11, P < 0.001), virus treatment (F(1,70) = 18.11, P < 0.001), treatment by time interaction (F(5,70) = 2.67, P < 0.05)]. Responding by Ad-shα4-treated rats did not differ from Ad-NSS-treated rats during the first 5 minutes, but was significantly decreased 5–10 minutes (P < 0.05) and 10–15 minutes (P < 0.05) after the session began. Similarly, no significant difference was observed in ethanol consumption among the Ad-shα4-treated (0.27 ± 0.036 g/kg) and the Ad-NSS (0.27 ± 0.021 g/kg) rats during the first 5 minutes of the session.
Figure 3.
Decreases in instrumental responding for ethanol after GABAAR α4 subunit knockdown in the NAc shell depend upon the concurrent experience of ethanol consumption. Two cohorts of rats were trained to lever press for ethanol and were then infused in the NAc shell with Ad-shα4 or Ad-NSS. On day 18 after viral infusion, rats underwent a single test session in which responding was (a–c) or was not (d–f) reinforced by ethanol delivery. (a) Active lever responses were decreased on day 18 when responding was reinforced (Ad-shα4 versus Ad-NSS groups, *P < 0.001). Panels b and c depict within-session responding during the 30-minute sessions by the same subjects depicted in panel a during the final session prior to virus infusion (b; baseline) and during day 18 (c). (b) There were no group differences during the baseline session. (c)There was a significant decrease in responding by Ad-shα4-treated rats on day 18 at the 5–10 and 10–15 minutes intervals (*P < 0.05, Ad-shα4 versus Ad-NSS groups) but not during the first 5 minutes of the session. (d) When responding was not reinforced on day 18, Ad-shα4-infused rats did not decrease active lever responses. (e) There were no group differences in the pattern of responding during the 30-minute baseline session. (f)There were no group differences in the pattern of responding during the 30-minute session on day 18 when responding was not reinforced. Data are presented as mean +/− SEM. n = 8 rats per group
In contrast, when responding was not reinforced by ethanol at test, there were no significant group differences in active lever responses on day 18 after viral infusion (Fig. 3d; P > 0.05). This lack of effect on unreinforced responding was also apparent in the within-session analysis [Fig. 3e & f; baseline: main effect of time (F(5,70) = 81.26, P < 0.0001); virus treatment (F(1,70) = 0.8, P = 0.38); treatment by time interaction (F(5,70) = 0.11, P = 0.98); day 18: main effect of time (F(5,70) = 25.8, P < 0.0001); virus treatment (F(1,70) = 1.5, P = 0.23); treatment by time interaction (F(5,70) = 1.9, P = 0.1)].
There was no significant difference in inactive lever responding at any timepoint among all groups, both reinforced and non-reinforced (range: 0–6 per 30 minutes session among all groups).
GABAAR α4 subunit knockdown in the NAc core does not affect ethanol self-administration
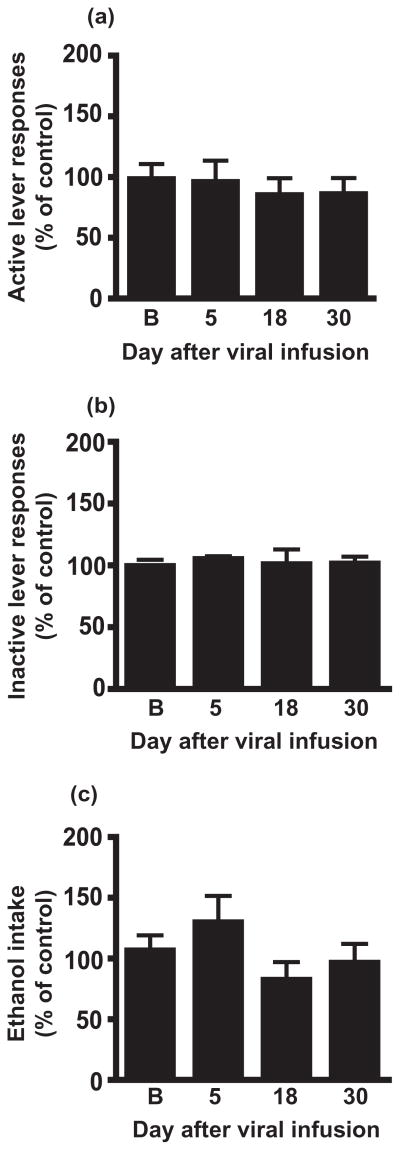
To test for regional specificity, we assessed the effect of Ad-shα4 microinfusion within the NAc core. We found no effect of knocking down the GABAAR α4 subunit in the NAc core on active lever responding for ethanol (Fig. 4a; F(3,44) = 0.2199, P = 0.88), inactive lever responding (Fig. 4b; F(3,44) = 1.023, P = 0.39) or total ethanol intake (Fig. 4c, F(3,44) = 6.08, P = 0.17) at any timepoint tested, suggesting our observed effects are specific to the NAc shell. Baseline ethanol consumed by Ad-NSS group was 0.57 ± 0.052 g/kg and by the Ad-shα4-1 group was 0.57 + 0.037 g/kg, whereas ethanol consumed on day 18 after viral infusion was 0.52 ± 0.039 g/kg by Ad-NSS group and 0.43 ± 0.048 g/kg by the Ad-shα4-1 group.
Figure 4.
Viral-mediated GABAAR α4 subunit knockdown in the NAc core does not affect instrumental responding for ethanol. Rats were infused in the NAc core with Ad-shα4 (n = 12) or Ad-NSS (n = 12) bilaterally. For panels a–c, data for Ad-shα4-treated rats are expressed as a percentage of Ad-NSS-treated rats at the same timepoint. (a) Responding on the active lever was not altered after Ad-shα4 infusion. (b) Inactive lever responding was not altered. (c) Estimated ethanol intake (g/kg) was not altered.‘B’ refers to baseline, the average of 3 days prior to virus infusion. Data presented as mean +/− SEM
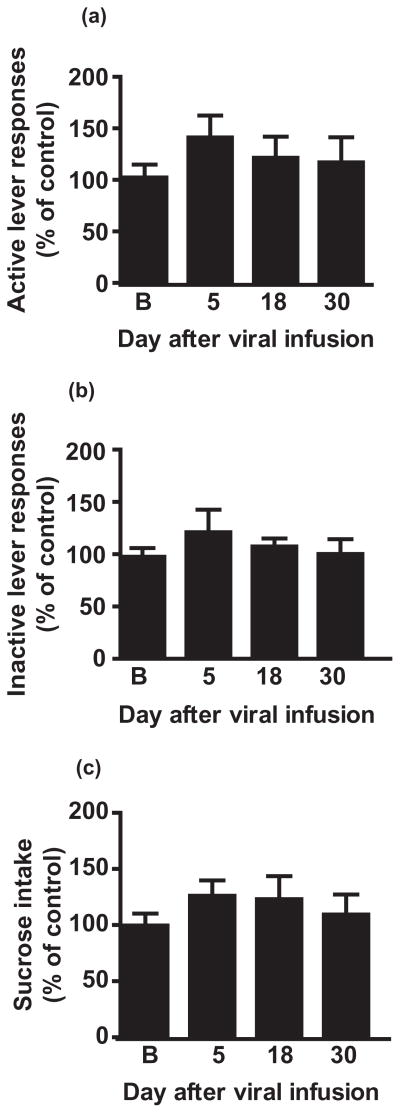
GABAAR α4 subunit knockdown in the NAc shell does not affect responding for sucrose
GABAAR activation in the NAc shell is implicated in the ingestion of preferred substances (Basso & Kelley 1999; Baldo & Kelley 2007); therefore, we tested whether the α4 subunit contributes to intake of the preferred substance, sucrose. Ad-shα4 microinfusion into the NAc shell did not significantly alter active lever responding for a 2% sucrose solution (Fig. 5a; F(3,40) = 0.63, P = 0.59), inactive lever responding (Fig. 5b; F(3,40) = 0.56, P = 0.64) or total sucrose intake (Fig. 5c; F(3,40) = 1.024, P = 0.44), suggesting that the effects of GABAAR α4 subunit reduction are specific to ethanol. Baseline sucrose consumed by the Ad-NSS group was 4.98 ± 0.612 ml and Ad-shα4-1 group was 5.12 ± 0.573 ml whereas sucrose consumed on day 18 after viral infusion was 4.15 ± 0.687 ml by the Ad-NSS group and 5.26 ± 0.97 ml by the Ad-shα4-1 group.
Figure 5.
Viral-mediated GABAAR α4 subunit knockdown in NAc shell does not affect instrumental responding for sucrose. Rats were infused in the NAc shell with Ad-shα4 (n = 11) or Ad-NSS (n = 11) bilaterally. For panels a–c, data for Ad-shα4-treated rats are expressed as a percentage of Ad-NSS-treated rats at the same timepoint. (a) Responding on the active lever for 2% sucrose was not altered after Ad-shα4 infusion. (b) Inactive lever responding was not altered. (c) Estimated sucrose intake (g/kg) was not altered.‘B’ refers to baseline, the average of 3 days prior to virus infusion. Data presented as mean +/− SEM
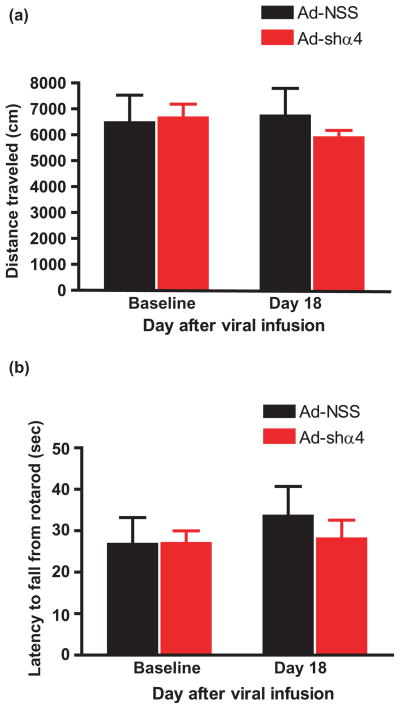
GABAAR α4 subunit knockdown in the NAc shell does not affect motor activity
To examine whether GABAAR α4 subunit knockdown in the NAc shell affects motor activity and coordination, we measured locomotor activity in an open field and on a rotarod in rats infused with Ad-NSS and Ad-shα4. We did not observe significant differences in the mean distance traveled during the open-field test (Fig. 6a). A repeated-measures ANOVA found no effect of test day (baseline versus day 18; F(1,18) = 1.28, P = 0.59) or virus treatment (F(1,18) = 0.122, P = 0.73), and there was no significant treatment by time interaction (F(1,18) = 2.17, P = 0.16). In addition, the latency to fall from the rotarod was not altered (Fig. 6b). A repeated-measures ANOVA found no effect of time (F(1,18) = 0.15, P = 0.70) or virus treatment (F(1,18) = 2.00, P = 0.15) and no treatment by time interaction (F(1,18) = 0.217, P = 0.64). These results suggest that the decrease in the reinforcing effects of ethanol in Ad-shα4 rats is not due to a general alteration in motor activity/coordination.
Figure 6.
Viral-mediated GABAAR α4 subunit knockdown in NAc shell does not affect motor activity. Rats were infused in the NAc shell with Ad-shα4 (n = 10) or Ad-NSS (n = 10) bilaterally. (a) Distance traveled in an open field for Ad-shα4 and Ad-NSS-treated rats prior to viral infusion (baseline) and on day 18 after viral infusion. (b) Latency to fall from the rotarod for Ad-shα4 and Ad-NSS-treated rats prior to viral infusion (baseline) and on day 18 after viral infusion. Data presented as mean +/− SEM
DISCUSSION
The current study provides evidence that the GABAAR α4 subunit in the NAc shell is required for the reinforcing properties of self-administered ethanol. We found that viral-mediated reductions in the expression of the GABAAR α4 subunit in the NAc shell, but not core, reduced lever-press responding for ethanol, but not sucrose. These effects were not apparent at the initiation of the session, indicating that α4 knockdown did not reduce ethanol seeking. Together with our previous results in which α4 reductions in the NAc shell were found to reduce ethanol intake and preference when offered in bottles on the home cage (Rewal et al. 2009), our findings support a critical role for GABAAR α4-containing receptors in the reinforcing effects of orally administered ethanol at the moderate concentrations commonly attained during social drinking.
If the GABAAR α4 subunit were critical for ethanol seeking, then one might expect responding to decrease immediately at session onset. However, this was not what we observed. Examination of the minute-by-minute pattern of instrumental responding for ethanol during the session revealed that decreased responding occurred after subjects were reinforced multiple times. Hence, responding in the first 5 minutes of the session was equal across groups. Because we observed a decrease in responding for ethanol after subjects have the opportunity to consume the reinforcer, these findings are consistent with an effect on the consummatory, not appetitive, phase of the behavior. Hence, we conclude that the GABAAR α4 subunit is important for the reinforcing effects of ethanol. We note that we use the term ‘reinforcing effects’ in the traditional behavioral sense, i.e. the effects that maintain, or reinforce, the operant response. Reinforcing effects of orally consumed ethanol are comprised of both pharmacological effects and more temporally proximal sensory attributes experienced as the subject consumes the ethanol. Hence, the behavioral mechanism(s) underlying the effect of α4 down-regulation on ethanol intake are complicated. Because ethanol ingestion in low ranges (i.e. 0.2–0.3 g/kg) in rats has been shown to significantly alter behavior (Wolffgramm & Heyne 1995), we propose that α4 knockdown reduces self-administration because GABAAR α4 subunit-containing receptors mediate the pharmacological effects of ethanol that arise as the ethanol slowly accumulates across minutes of session time. Consistent with this hypothesis, reductions in the expression of the GABAAR α4 subunit in the NAc shell had no effect on unreinforced responding for ethanol. However, we did not demonstrate that measurable blood levels of ethanol were attained after only a few minutes of responding, which would be required to support this interpretation; it remains to be determined if this is the case. There are other behavioral mechanisms whereby α4 subunit down-regulation in the shell may reduce the reinforcing effects of ethanol. For example, α4 subunit down-regulation may affect taste reactivity, such that acceptance of ethanol is decreased. It is also possible that our findings reflect alterations is neural systems critical for conditioned reinforcing properties of the sensory attributes of ethanol as learned indicators of subsequent pharmacological effects. Finally, it is always possible that decreases in responding reflect increases, rather than decreases, in rewarding properties that may result in faster satiation. Further experimentation is required to address these different possibilities.
Although the specific mechanisms whereby GABAAR α4 subunit knockdown in the NAc shell decreased responding for ethanol remains to be delineated, knockdown did not affect instrumental responding for the natural reward, sucrose. These results are consistent with our previous findings in which reducing the expression of GABAAR α4 subunit did not influence sucrose intake or preference in the home cage (Rewal et al. 2009). This further strengthens the hypothesis that α4 subunit-containing receptors within the NAc shell specifically mediate the reinforcing effects of ethanol, and not preferred substances in general. A decrease in the responding for ethanol and not sucrose also suggests that sweet taste, at least, is not altered by reducing the expression of GABAAR α4 subunit.
We did not find any evidence to suggest that the decreases in expression of the GABAAR α4 subunit altered motor activity or coordination, ruling out this interpretation of the decreases in responding for ethanol. Specifically, in subjects with reduced expression of the GABAAR α4 subunit and with no ethanol exposure, we did not observe any effects on locomotor activity in the open field test or on the rotarod. In addition, we found no changes in inactive lever responding, although it is difficult to observe decreases when responding is already near floor. However, we observed no changes in the relatively high levels of sucrose-reinforced responding, further indicating that non-specific motoric changes are not responsible for the decreases in responding for ethanol.
Prior research has implicated the GABA system in the NAc in the mediation of ethanol self-administration. For example, Hodge, Chappelle and Samson (1995) found that both the GABAAR agonist, muscimol and the GABAAR antagonist, bicuculline, decreased operant responding for ethanol, primarily by producing an earlier termination of responding within the session. However, the site of drug infusion for this study was in the core region of the NAc, rather than the shell. Two other studies have found that infusion of the GABAAR antagonist, SR 95531, decreased ethanol self-administration when infused into the NAc shell (Hyytia & Koob 1995; Eiler & June 2007), a finding that is congruent with the present findings. However, recently, Stratford and Wirtshafter (2011) reported that infusion of muscimol into the medial shell decreased ethanol intake at doses that increase sucrose and chow intake. Hence, the manipulation used here, a down-regulation of α4 subunits, which would be predicted to reduce inhibition in some neurons within the shell, produces a similar behavioral effect as a general inhibition of GABAARs (and the neurons expressing these receptors). This difference suggests that the effects of down-regulating the GABAAR α4 subunit in particular on ethanol intake may not result from the disinhibition of the circuits identified by Kelley and colleagues in control of consumption of palatable substances in general (Baldo & Kelley 2007). It is possible that these differences could arise from differences in the effects on cellular function when altering both extrasynaptic and synaptic GABA transmission, rather than just extrasynaptic. Alternatively, α4 down-regulation and muscimol infusion could exert distinct effects on ethanol intake if α4-containing receptors were expressed only on a subset of neurons within the NAc shell, e.g. in only the direct or indirect pathway neurons. Future studies are required to determine the specific physiological effects of α4-containing GABAARs in the NAc medial shell.
While only a handful of studies have examined GABA transmission in particular in the NAc shell, other studies have implicated this region in the control of ethanol self-administration. For example, alterations in glutamatergic transmission (Kapasova & Szumlinski 2008) and serotonergic transmission (Jankowska & Kostowski 1995; Hoplight, Sandygren & Neumaier 2006) in the shell region are reported to modulate ethanol intake. In addition, electrolytic lesions of the medial shell in mice reduced ethanol self-administration (Dhaher et al. 2009). Together, these studies point to a role of the medial shell in regulation of ethanol intake.
Why might the NAc shell and core play distinct roles in ethanol self-administration? Although both the shell and the core express GABAAR α4 subunits, we observed that GABAAR α4 subunit reductions in the NAc core had no effect on instrumental responding for ethanol (present study) or on home cage ethanol intake (Rewal et al. 2009). Rather, the NAc shell appears to be the critical site for the decreases in ethanol self-administration after α4 reductions. The shell and core have distinct patterns of afferent inputs and efferent outputs, defining distinct circuits that likely underlie distinct behaviors (Brog et al. 1993; Robbins & Everitt 2002). The shell projects to the lateral hypothalamus and this connection may be especially important for consummatory behaviors; the shell and core also project to distinct areas of the ventral pallidum (Groenewegen et al. 1999). The core receives input from more dorsal areas of the medial prefrontal cortex, while the shell receives input from more ventral regions (Groenewegen et al. 1999). These anatomical differences may contribute to behavioral differences. Whereas the core is hypothesized to contribute to stimulus-appropriate instrumental responding (Ikemoto 2007), the shell is hypothesized to contribute to stimulus-outcome learning (Ikemoto 2007) and the direct reinforcing effects drugs (Everitt & Robbins 2005). Prior studies have found that cocaine (Rodd-Henricks et al. 2002; Ikemoto 2003), amphetamine (Ikemoto, Qin & Liu 2005) and Δ9THC (Zangen et al. 2006) are self-administered directly into the medial shell. Most relevant to the present report, a recent paper by Engelman and colleagues found that ethanol is also self-administered directly into the medial shell (Engleman et al. 2009). Therefore, the shell and not the core may be a region in which drugs, including ethanol, produce (or contribute to) reinforcing effects by engaging a naturally occurring circuit that promotes contact and ingestion of substances. It is tempting to speculate that pharmacological interactions with α4-containing GABAARs may be the initial and specific means by which ethanol interacts with that circuit, although this remains to be determined.
The observation that the maximum effect of the virus infusion on the behavior occurs on 18th day of viral infusion correlates with our work where we showed that the Ad-shα4-1 virus down-regulates mRNA and protein levels of the α4 subunit transiently, with significant reductions 18 days after infusion and recovery 25 days after infusion (Rewal et al. 2009). In addition, we previously demonstrated that decreases in α4 expression after in vivo infusion of the Ad-sh4-1 virus into the NAc shell were not accompanied by compensatory changes in the expression of the mRNA or protein for the δ, α1, β2 and γ2 subunits (Rewal et al. 2009). Although we cannot rule out cellular changes that may serve to compensate for changes in the general level of inhibition of affected neurons, it is likely that our manipulation produced minimal changes in other GABAergic receptors given the lack of evidence for changes in many of the subunits.
In conclusion, we found that transient reductions in the expression of the GABAAR α4 subunit in the NAc shell, but not the core, reduced instrumental responding for oral ethanol. The present results demonstrate that the GABAAR α4 subunit in the NAc shell is required for the reinforcing properties of ethanol, further supporting a prominent role for α4-containing GABAARs in the self-administration of ethanol.
Acknowledgments
Supported by R01 AA016835 (PHJ) and by funds from the State of California for medical research on alcohol and substance abuse through UCSF (PHJ and DR). We would like to thank Myra Engalla for technical assistance.
Footnotes
Authors Contribution
PHJ, DR and MR were responsible for the study concept and design. MR, RD, TMG and HN carried out the experiments, and performed data analysis and interpretation of findings. MR and PHJ drafted the manuscript. All authors provided critical revision of the manuscript, and critically reviewed content, and approved the final version that was submitted for publication.
References
- Ariwodola OJ, Crowder TL, Grant KA, Daunais JB, Friedman DP, Weiner JL. Ethanol modulation of excitatory and inhibitory synaptic transmission in rat and monkey dentate granule neurons. Alcohol Clin Exp Res. 2003;27:1632–1639. doi: 10.1097/01.ALC.0000089956.43262.17. [DOI] [PubMed] [Google Scholar]
- Ariwodola OJ, Weiner JL. Ethanol potentiation of GABAergic synaptic transmission may be self-limiting: role of presynaptic GABA(B) receptors. J Neurosci. 2004;24:10679–10686. doi: 10.1523/JNEUROSCI.1768-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baldo BA, Kelley AE. Discrete neurochemical coding of distinguishable motivational processes: insights from nucleus accumbens control of feeding. Psychopharmacology (Berl) 2007;191:439–459. doi: 10.1007/s00213-007-0741-z. [DOI] [PubMed] [Google Scholar]
- Basso AM, Kelley AE. Feeding induced by GABA(A) receptor stimulation within the nucleus accumbens shell: regional mapping and characterization of macronutrient and taste preference. Behav Neurosci. 1999;113:324–336. doi: 10.1037//0735-7044.113.2.324. [DOI] [PubMed] [Google Scholar]
- Borghese CM, Harris RA. Studies of ethanol actions on recombinant delta-containing gamma-aminobutyric acid type A receptors yield contradictory results. Alcohol. 2007;41:155–162. doi: 10.1016/j.alcohol.2007.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borghese CM, Storustovu S, Ebert B, Herd MB, Belelli D, Lambert JJ, Marshall G, Wafford KA, Harris RA. The delta subunit of gamma-aminobutyric acid type A receptors does not confer sensitivity to low concentrations of ethanol. J Pharmacol Exp Ther. 2006;316:1360–1368. doi: 10.1124/jpet.105.092452. [DOI] [PubMed] [Google Scholar]
- Brog JS, Salyapongse A, Deutch AY, Zahm DS. The patterns of afferent innervation of the core and shell in the ‘accumbens’ part of the rat ventral striatum: immunohistochemical detection of retrogradely transported fluorogold. J Comp Neurol. 1993;338:255–278. doi: 10.1002/cne.903380209. [DOI] [PubMed] [Google Scholar]
- Brown N, Kerby J, Bonnert TP, Whiting PJ, Wafford KA. Pharmacological characterization of a novel cell line expressing human alpha(4)beta(3)delta GABA(A) receptors. Br J Pharmacol. 2002;136:965–974. doi: 10.1038/sj.bjp.0704795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carnicella S, Kharazia V, Jeanblanc J, Janak PH, Ron D. GDNF is a fast-acting potent inhibitor of alcohol consumption and relapse. Proc Natl Acad Sci USA. 2008;105:8114–8119. doi: 10.1073/pnas.0711755105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chester JA, Cunningham CL. GABA(A) receptor modulation of the rewarding and aversive effects of ethanol. Alcohol. 2002;26:131–143. doi: 10.1016/s0741-8329(02)00199-4. [DOI] [PubMed] [Google Scholar]
- Criswell HE, Breese GR. A conceptualization of integrated actions of ethanol contributing to its GABAmimetic profile: a commentary. Neuropsychopharmacology. 2005;30:1407–1425. doi: 10.1038/sj.npp.1300750. [DOI] [PubMed] [Google Scholar]
- Dhaher R, Finn DA, Oberbeck DL, Yoneyama N, Snelling CC, Wu W, Hitzemann RJ. Electrolytic lesions of the medial nucleus accumbens shell selectively decrease ethanol consumption without altering preference in a limited access procedure in C57BL/6J mice. Pharmacol Biochem Behav. 2009;92:335–342. doi: 10.1016/j.pbb.2008.12.024. [DOI] [PubMed] [Google Scholar]
- Eiler WJ, 2nd, June HL. Blockade of GABA(A) receptors within the extended amygdala attenuates D(2) regulation of alcohol-motivated behaviors in the ventral tegmental area of alcohol-preferring (P) rats. Neuropharmacology. 2007;52:1570–1579. doi: 10.1016/j.neuropharm.2007.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engleman EA, Ding ZM, Oster SM, Toalston JE, Bell RL, Murphy JM, McBride WJ, Rodd ZA. Ethanol is self-administered into the nucleus accumbens shell, but not the core: evidence of genetic sensitivity. Alcohol Clin Exp Res. 2009;33:2162–2171. doi: 10.1111/j.1530-0277.2009.01055.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Everitt BJ, Robbins TW. Neural systems of reinforcement for drug addiction: from actions to habits to compulsion. Nat Neurosci. 2005;8:1481–1489. doi: 10.1038/nn1579. [DOI] [PubMed] [Google Scholar]
- Grobin AC, Matthews DB, Devaud LL, Morrow AL. The role of GABA(A) receptors in the acute and chronic effects of ethanol. Psychopharmacology (Berl) 1998;139:2–19. doi: 10.1007/s002130050685. [DOI] [PubMed] [Google Scholar]
- Groenewegen HJ, Wright CI, Beijer AV, Voorn P. Convergence and segregation of ventral striatal inputs and outputs. Ann N Y Acad Sci. 1999;877:49–63. doi: 10.1111/j.1749-6632.1999.tb09260.x. [DOI] [PubMed] [Google Scholar]
- Hanchar HJ, Chutsrinopkun P, Meera P, Supavilai P, Sieghart W, Wallner M, Olsen RW. Ethanol potently and competitively inhibits binding of the alcohol antagonist Ro15-4513 to {alpha}4/6{beta}3{delta} GABAA receptors. Proc Natl Acad Sci USA; 2006. pp. ••–••. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanchar HJ, Wallner M, Olsen RW. Alcohol effects on gamma-aminobutyric acid type A receptors: are extrasynaptic receptors the answer? Life Sci. 2004;76:1–8. doi: 10.1016/j.lfs.2004.05.035. [DOI] [PubMed] [Google Scholar]
- Hodge CW, Chappelle AM, Samson HH. GABAergic transmission in the nucleus accumbens is involved in the termination of ethanol self-administration in rats. Alcohol Clin Exp Res. 1995;19:1486–1493. doi: 10.1111/j.1530-0277.1995.tb01012.x. [DOI] [PubMed] [Google Scholar]
- Hommel JD, Sears RM, Georgescu D, Simmons DL, DiLeone RJ. Local gene knockdown in the brain using viral-mediated RNA interference. Nat Med. 2003;9:1539–1544. doi: 10.1038/nm964. [DOI] [PubMed] [Google Scholar]
- Hoplight BJ, Sandygren NA, Neumaier JF. Increased expression of 5-HT1B receptors in rat nucleus accumbens via virally mediated gene transfer increases voluntary alcohol consumption. Alcohol. 2006;38:73–79. doi: 10.1016/j.alcohol.2006.04.003. [DOI] [PubMed] [Google Scholar]
- Hyytia P, Koob GF. GABAA receptor antagonism in the extended amygdala decreases ethanol self-administration in rats. Eur J Pharmacol. 1995;283:151–159. doi: 10.1016/0014-2999(95)00314-b. [DOI] [PubMed] [Google Scholar]
- Ikemoto S. Involvement of the olfactory tubercle in cocaine reward: intracranial self-administration studies. J Neurosci. 2003;23:9305–9311. doi: 10.1523/JNEUROSCI.23-28-09305.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikemoto S. Dopamine reward circuitry: Two projection systems from the ventral midbrain to the nucleus accumbensolfactory tubercle complex. Brain Res Rev. 2007;••:••–••. doi: 10.1016/j.brainresrev.2007.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikemoto S, Qin M, Liu ZH. The functional divide for primary reinforcement of D-amphetamine lies between the medial and lateral ventral striatum: is the division of the accumbens core, shell, and olfactory tubercle valid? J Neurosci. 2005;25:5061–5065. doi: 10.1523/JNEUROSCI.0892-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jankowska E, Kostowski W. The effect of tropisetron injected into the nucleus accumbens septi on ethanol consumption in rats. Alcohol. 1995;12:195–198. doi: 10.1016/0741-8329(94)00082-o. [DOI] [PubMed] [Google Scholar]
- Jeanblanc J, He DY, Carnicella S, Kharazia V, Janak PH, Ron D. Endogenous BDNF in the dorsolateral striatum gates alcohol drinking. J Neurosci. 2009;29:13494–13502. doi: 10.1523/JNEUROSCI.2243-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapasova Z, Szumlinski KK. Strain differences in alcohol-induced neurochemical plasticity: a role for accumbens glutamate in alcohol intake. Alcohol Clin Exp Res. 2008;32:617–631. doi: 10.1111/j.1530-0277.2008.00620.x. [DOI] [PubMed] [Google Scholar]
- Koob GF. A role for GABA mechanisms in the motivational effects of alcohol. Biochem Pharmacol. 2004;68:1515–1525. doi: 10.1016/j.bcp.2004.07.031. [DOI] [PubMed] [Google Scholar]
- Koob GF, Sanna PP, Bloom FE. Neuroscience of addiction. Neuron. 1998;21:467–476. doi: 10.1016/s0896-6273(00)80557-7. [DOI] [PubMed] [Google Scholar]
- Lasek A, Janak PH, He L, Whistler J, Heberlein U. Down-regulation of mu opioid receptor by RNAi in the VTA reduces ethanol consumption in mice. Genes Brain Behav. 2007;6:728–735. doi: 10.1111/j.1601-183X.2007.00303.x. [DOI] [PubMed] [Google Scholar]
- Liang J, Suryanarayanan A, Chandra D, Homanics GE, Olsen RW, Spigelman I. Functional consequences of GABAA receptor alpha 4 subunit deletion on synaptic and extrasynaptic currents in mouse dentate granule cells. Alcohol Clin Exp Res. 2008;32:19–26. doi: 10.1111/j.1530-0277.2007.00564.x. [DOI] [PubMed] [Google Scholar]
- McBride WJ, Murphy JM, Ikemoto S. Localization of brain reinforcement mechanisms: intracranial self-administration and intracranial place-conditioning studies. Behav Brain Res. 1999;101:129–152. doi: 10.1016/s0166-4328(99)00022-4. [DOI] [PubMed] [Google Scholar]
- Macdonald RL, Olsen RW. GABAA receptor channels. Annu Rev Neurosci. 1994;17:569–602. doi: 10.1146/annurev.ne.17.030194.003033. [DOI] [PubMed] [Google Scholar]
- Mihalek RM, Bowers BJ, Wehner JM, Kralic JE, VanDoren MJ, Morrow AL, Homanics GE. GABA(A)-receptor delta subunit knockout mice have multiple defects in behavioral responses to ethanol. Alcohol Clin Exp Res. 2001;25:1708–1718. [PubMed] [Google Scholar]
- Mody I, Pearce RA. Diversity of inhibitory neurotransmission through GABA(A) receptors. Trends Neurosci. 2004;27:569–575. doi: 10.1016/j.tins.2004.07.002. [DOI] [PubMed] [Google Scholar]
- Olsen RW, Hanchar HJ, Meera P, Wallner M. GABAA receptor subtypes: the ‘one glass of wine’ receptors. Alcohol. 2007;41:201–209. doi: 10.1016/j.alcohol.2007.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rewal M, Jurd R, Gill TM, He DY, Ron D, Janak PH. Alpha4-containing GABAA receptors in the nucleus accumbens mediate moderate intake of alcohol. J Neurosci. 2009;29:543–549. doi: 10.1523/JNEUROSCI.3199-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robbins TW, Everitt BJ. Limbic-striatal memory systems and drug addiction. Neurobiol Learn Mem. 2002;78:625–636. doi: 10.1006/nlme.2002.4103. [DOI] [PubMed] [Google Scholar]
- Roberto M, Madamba SG, Moore SD, Tallent MK, Siggins GR. Ethanol increases GABAergic transmission at both pre- and postsynaptic sites in rat central amygdala neurons. Proc Natl Acad Sci USA. 2003;100:2053–2058. doi: 10.1073/pnas.0437926100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberto M, Madamba SG, Stouffer DG, Parsons LH, Siggins GR. Increased GABA release in the central amygdala of ethanol-dependent rats. J Neurosci. 2004;24:10159–10166. doi: 10.1523/JNEUROSCI.3004-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodd-Henricks ZA, McKinzie DL, Li TK, Murphy JM, McBride WJ. Cocaine is self-administered into the shell but not the core of the nucleus accumbens of Wistar rats. J Pharmacol Exp Ther. 2002;303:1216–1226. doi: 10.1124/jpet.102.038950. [DOI] [PubMed] [Google Scholar]
- Simms JA, Steensland P, Medina B, Abernathy KE, Chandler LJ, Wise R, Bartlett SE. Intermittent access to 20% ethanol induces high ethanol consumption in Long-Evans and Wistar rats. Alcohol Clin Exp Res. 2008;32:1816–1823. doi: 10.1111/j.1530-0277.2008.00753.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steensland P, Simms JA, Holgate J, Richards JK, Bartlett SE. Varenicline, an alpha4beta2 nicotinic acetylcholine receptor partial agonist, selectively decreases ethanol consumption and seeking. Proc Natl Acad Sci USA. 2007;104:12518–12523. doi: 10.1073/pnas.0705368104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stratford TR, Wirtshafter D. Opposite effects on the ingestion of ethanol and sucrose solutions after injections of muscimol into the nucleus accumbens shell. Behav Brain Res. 2011;216:514–518. doi: 10.1016/j.bbr.2010.08.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sundstrom-Poromaa I, Smith DH, Gong QH, Sabado TN, Li X, Light A, Wiedmann M, Williams K, Smith SS. Hormonally regulated alpha(4)beta(2)delta GABA(A) receptors are a target for alcohol. Nat Neurosci. 2002;5:721–722. doi: 10.1038/nn888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallner M, Hanchar HJ, Olsen RW. Ethanol enhances alpha 4 beta 3 delta and alpha 6 beta 3 delta gamma-aminobutyric acid type A receptors at low concentrations known to affect humans. Proc Natl Acad Sci USA. 2003;100:15218–15223. doi: 10.1073/pnas.2435171100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallner M, Hanchar HJ, Olsen RW. Low-dose alcohol actions on {alpha}4{beta}3{delta} GABAA receptors are reversed by the behavioral alcohol antagonist Ro15-4513. Proc Natl Acad Sci USA. 2006;103(22):8540–5. doi: 10.1073/pnas.0600194103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei W, Faria LC, Mody I. Low ethanol concentrations selectively augment the tonic inhibition mediated by delta subunit-containing GABAA receptors in hippocampal neurons. J Neurosci. 2004;24:8379–8382. doi: 10.1523/JNEUROSCI.2040-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiner JL, Valenzuela CF. Ethanol modulation of GABAergic transmission: the view from the slice. Pharmacol Ther. 2006;111:533–554. doi: 10.1016/j.pharmthera.2005.11.002. [DOI] [PubMed] [Google Scholar]
- Weiss F, Lorang MT, Bloom FE, Koob GF. Oral alcohol self-administration stimulates dopamine release in the rat nucleus accumbens: genetic and motivational determinants. J Pharmacol Exp Ther. 1993;267:250–258. [PubMed] [Google Scholar]
- Wolffgramm J, Heyne A. From controlled drug intake to loss of control: the irreversible development of drug addiction in the rat. Behav Brain Res. 1995;70:77–94. doi: 10.1016/0166-4328(95)00131-c. [DOI] [PubMed] [Google Scholar]
- Zangen A, Solinas M, Ikemoto S, Goldberg SR, Wise RA. Two brain sites for cannabinoid reward. J Neurosci. 2006;26:4901–4907. doi: 10.1523/JNEUROSCI.3554-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zapata A, Gonzales RA, Shippenberg TS. Repeated ethanol intoxication induces behavioral sensitization in the absence of a sensitized accumbens dopamine response in C57BL/6J and DBA/2J mice. Neuropsychopharmacology. 2006;31:396–405. doi: 10.1038/sj.npp.1300833. [DOI] [PMC free article] [PubMed] [Google Scholar]