Summary
Iron, zinc, copper and manganese are essential metals for cellular enzyme functions while cadmium, mercury and the metalloid arsenic lack any biological function. Both, essential and non-essential metals and metalloids are extremely reactive and toxic. Therefore, plants have acquired specialized mechanisms to sense, transport and maintain essential metals within physiological concentrations and to detoxify non-essential metals and metalloids. This review focuses on the recent identification of transporters that sequester cadmium and arsenic in vacuoles and the mechanisms mediating the partitioning of these metal(loid)s between roots and shoots. We further discuss recent models of phloem-mediated long-distance transport, seed accumulation of Cd and As and recent data demonstrating that plants posses a defined transcriptional response that allow plants to preserve metal homeostasis. This research is instrumental for future engineering of reduced toxic metal(loid) accumulation in edible crop tissues as well as for improved phytoremediation technologies.
Keywords: ABC transporters, Heavy Metal ATPase, transcription factors, phytochelatins, phloem transport, hyperaccumulator plants, phytoremediation
Introduction
The trace metals iron (Fe), zinc (Zn), manganese (Mn) and copper (Cu) are essential to all organisms functioning as co-factors in a variety of enzymes and proteins [1–3]. Metals are intrinsically very reactive and, therefore, their intracellular concentrations must be tightly regulated. Other metals, such as cadmium (Cd), lead (Pb), chromium (Cr), mercury (Hg) and the metalloid arsenic (As) are toxic and biologically non-essential, but can enter plants using the same transporters used for essential nutrient uptake [3–6•]. Once inside the cell, these toxic non-essential metals can displace and interfere with the function of essential metals. Therefore, organisms have acquired genetic and biochemical mechanisms to sense, transport and maintain essential metals within a non-toxic physiological range (i.e. metal homeostasis), while detoxifying non-essential metals (Fig. 1) [1–5,7•].
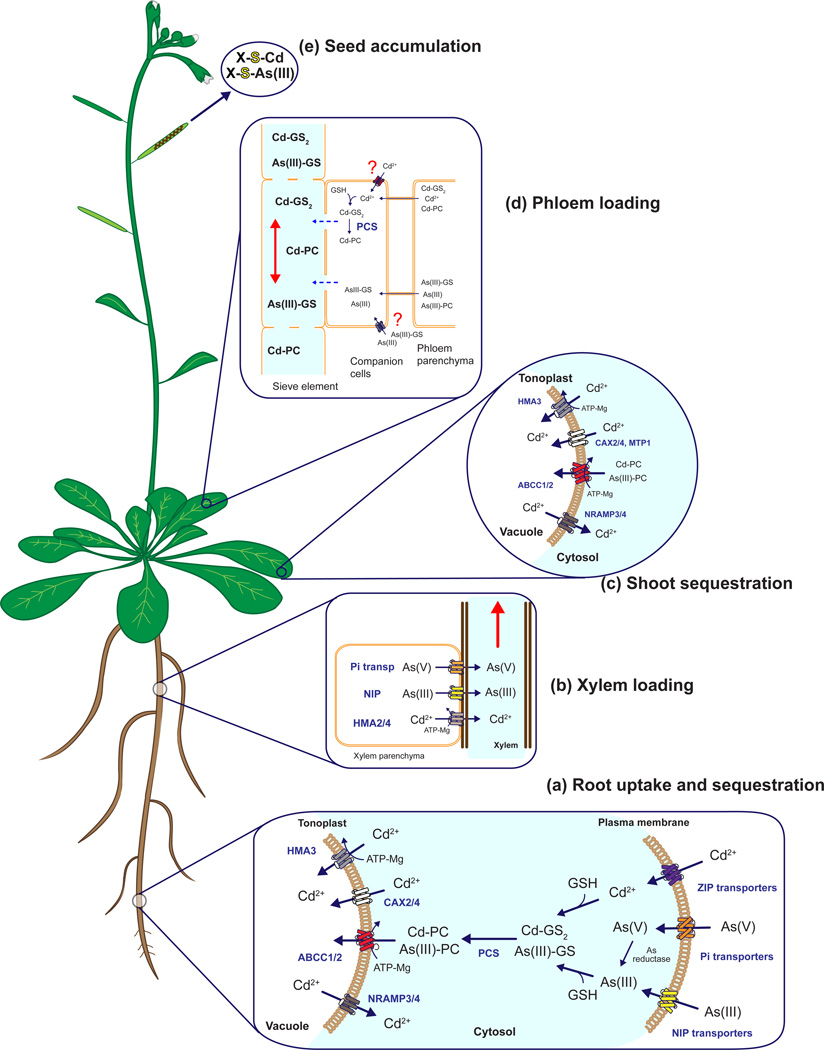
Figure 1.
Uptake, sequestration and long-distance cadmium and arsenic transport mechanisms in plants.
(a) Cadmium (Cd2+) uptake, at the root level, is mediated by ZIP transporters (i.e. IRT1 transporter) and other divalent metal nutrient transporters. Arsenic, depending on the redox state [As(V) or As(III)], may be taken up by phosphate transporters [As(V)] [33] or nodulin 26-like proteins [As(III)] [4–5]. Once inside the cell As(V) is readily reduced to As(III). Cd2+ and As(III) induce phytochelatin (PC) synthesis in the cytosol and PC-metal(loid) complexes are sequestered into vacuoles by ABCC transporters (ABCC1 and ABCC2 in Arabidopsis [9]). ABCC1 and ABCC2 can also transport GS-X conjugates into vacuoles [16]. Cd2+ can also be sequestered into vacuoles by HMA3 and proton/cation exchange transporters (CAX-type transporters). Cadmium can also be released from the vacuole by NRAMP-type transporters [72–73].
(b) Loading of Cd2+ into the xylem for root-to-shoot transport of Cd2+ is mediated by the ATPases HMA2 and HMA4 [39,63]. Similar to root-uptake, NIP-like transporters mediate As(III) loading into the xylem while phosphate transporters mediate loading of As(V) [4–5].
(c) As(V) may also be reduced in leaves to As(III). Phytochelatins induced by Cd2+ or As(III) in leaves are transported into vacuoles by ABCC transporters. Similar to root vacuoles, Cd uptake is also mediated by CAX-type and MTP-type transporters [4–5]. Depending on the species, metal(loid)s may be accumulated in epidermal or mesophyll cells [4,74].
(d) If Cd2+ and As(III) are not fully sequestered in leaf vacuoles, they can reach phloem parenchyma and companion cells through plasmodesmata (symplastic transport). Due to the high permeability of companion cells and sieve element plasmodesmata, GSH, As(III)- or Cd-induced PCs synthesized in companion cells may enter the phloem stream symplastically and be transported from source-to-sink (i.e. to young leaves, seeds and roots) [35].
(e) EXAFS analysis in the Cd hyperaccumulator Thlaspi praecox showed that 60% of Cd in seeds is complexed with thiol-containing compounds [42] and a similar mechanism was also proposed for arsenic accumulation in rice grains [75].
Here we review recent advances in the identification of key genes mediating accumulation and tolerance to cadmium and arsenic, including the identification of the vacuolar phytochelatin transporters that remained elusive for more than 15 years [8••,9••], transporters contributing to metal partitioning between roots and shoots [10••, 11•] and the characterization of vacuolar metal transporters from hyperaccumulator species [12•,13••]. We will discuss recent data demonstrating that the transcriptional changes induced by exposure to non-essential toxic metals are part of a transcriptional response to preserve metal homeostasis [14].
Identification of the long-sought vacuolar phytochelatin transporters
Phytochelatin synthesis is perhaps one of the most well studied mechanisms mediating detoxification of Cd, As, Zn, Hg and Cu in plants and some yeast, including Schizosaccharomyces pombe [3,5,15]. Phytochelatins (PCs) are glutathione-derived peptides synthesized in the cytosol where they form PC-metal(loid) complexes that are transported into vacuoles, thus removing these toxic elements from the cytosol [3,5,15–16]. More than 15 years ago, research suggested that vacuolar uptake of PC-metal(loid) complexes was mediated by ATP-binding cassette transporters (ABC transporters) [17–19]. In 1995, Hmt1 (Heavy metal tolerance 1), a half-size ABC transporter was identified as a vacuolar PC transporter required for Cd tolerance in the yeast S. pombe [17–19]. However, attempts to identify Hmt1-like transporters in plants were unsuccessful. In fact, the availability of diverse plant genome sequences revealed that in several cases the plant genes with the highest similarity to S. pombe Hmt1 were the half-size ABC transporters of the mitochondria (ATMs) [16] (Supp. Fig. 1 and Supp. Table 1).
Notably, the precise mechanism by which Hmt1 confers tolerance to Cd was far from understood. Hmt1 confers tolerance to Cd, but not arsenite [As(III)] or arsenate [As(V)] [20–21]. Furthermore, heterologous expression of Hmt1 enhances Cd tolerance in Escherichia coli and Saccharomyces cerevisiae, organisms devoid of phytochelatins (PCs), suggesting that Hmt1 also mediates tolerance in a PC-independent manner [8••, 20–21]. Subsequent analyses in S. pombe demonstrated that vacuoles from the hmt1 mutant still contained a significant amount of PCs, suggesting the existence of additional vacuolar PC transporters [8••,20]. Following a systematic deletion of vacuolar ABC transporters in S. pombe and Arabidopsis, two groups have recently, and independently, identified full-length ABC transporters mediating vacuolar PC uptake in S. pombe (Abc2) and Arabidopsis (MRP1/ABCC1 and MRP2/ABCC2, Fig. 1) [8••,9••]. S. pombe Abc2 is able to rescue the cadmium sensitivity and the capacity to accumulate PCs in vacuoles of a mutant devoid of five vacuolar ABC transporters (hmt1 abc1 abc2 abc3 abc4) [8••]. On the other hand, the Arabidopsis double mutant abcc1 abcc2 is arsenic hypersensitive, and vacuoles isolated from this mutant show a dramatic reduction in vacuolar PC-As uptake compared to vacuoles isolated from wildtype plants [9••]. Interestingly, ABCC1 and ABCC2 were previously described ABC transporters mediating the sequestration of glutathione-conjugates complexes into plant vacuoles [16,22].
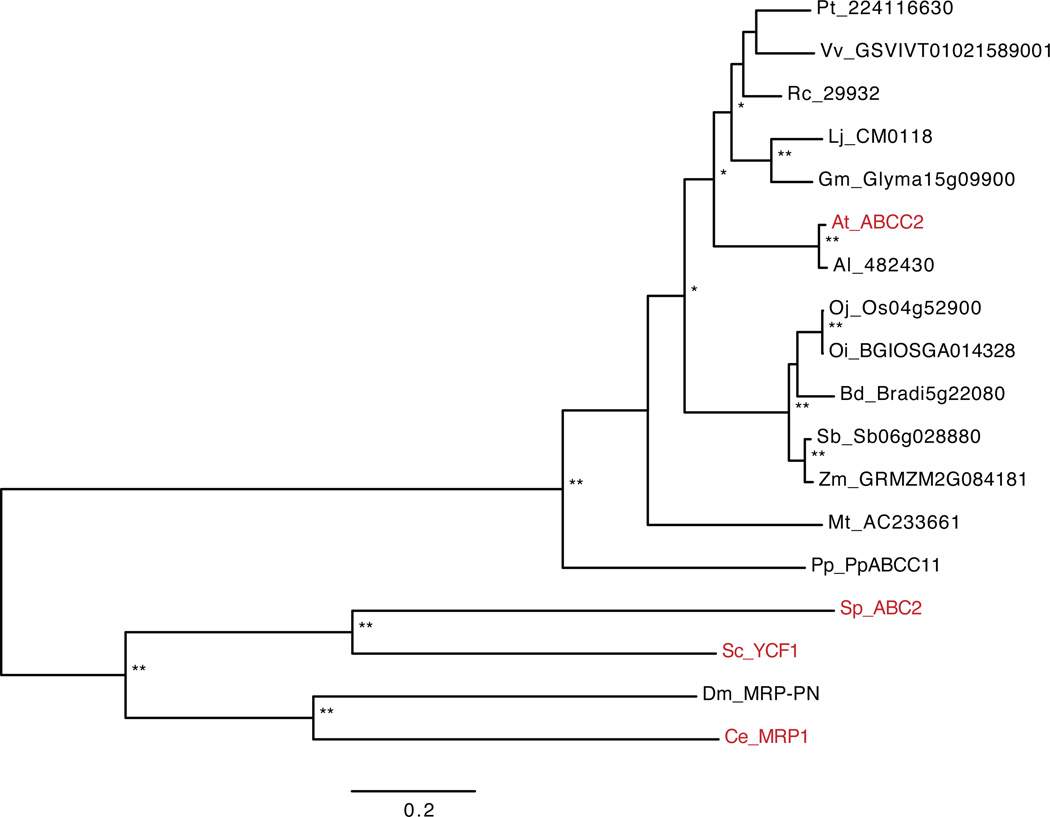
In contrast to Hmt1, a search for related genes in other plant species readily identified ABCC2/Abc2-like proteins throughout the plant kingdom including both terrestrial and aquatic plants (Fig. 2, Table 1). It should be noted, however, that S. pombe Abc2 and Arabidopsis ABCC2 share ≈ 37% identity at the amino acid level, yet they perform similar functions. Functional analysis together with sequence-similarity searches should be used to positively identify vacuolar PC transporters in other organisms. The overlapping function of ABCC1 and ABCC2 in Arabidopsis also explains why forward genetic screens did not identify these transporters. The identification of ABCC1/2 and Abc2 transporters, which belong to a subfamily of ABC transporters different from Hmt1, marks the end of the long-standing search for the genetic identity of vacuolar PC-metal(loid) transporters.
Figure 2.
Maximum likelihood phylogenetic tree of AtABCC2 and ABC-type transporters showing the highest sequence similarity to AtABCC2 in higher plants and selected fungi and animals. ABC transporters fall into two main clades characteristic for plants and animal or fungi [76–78]. The only functionally characterized vacuolar phytochelatin transporters shown are A. thaliana ABCC2 and S. pombe Abc2 [8–9]. A detailed explanation for gene annotations is provided in Supplementary Table 1. Note that the following organisms lack phytochelatins: P. patens, S. cerevisiae and D. melanogaster. The tree was constructed using phyml with 100 bootstrap replicates on a rascal curated mafft alignment of the shown proteins [76–78]. Nodes labeled with two asterisks have a 100% bootstrap support while those with one asterisk have a support in the range of 90% to 100%. The scale bar indicates 0.2 substitutions per site.
The mechanism by which Hmt1 confers Cd tolerance is not fully understood. Hmt1 function is GSH-dependent, but at this point it is unclear whether Cd-GS2 is one of Hmt1 substrates or whether GSH is required to synthesize a more chemically complex substrate [8••,21]. Hmt1 is structurally related to Atm1, which is also a GSH-dependent half-size ABC transporter that exports FeS clusters from the mitochondrial matrix to the cytosol [23–24]. A recent characterization of the cadmium tolerance mediated by Hmt1 in S. cerevisiae and S. pombe suggested that Hmt1 may transport CdS clusters from the cytosol into vacuoles [8••] and that either GSH or PCs help to stabilize these clusters in a similar way that GSH stabilize FeS clusters in plants and bacteria [25–26]. Detoxification of GSH-coated CdS clusters would explain why Hmt1 can confer Cd tolerance in organisms devoid of PCs [8••].
Long-distance transport of cadmium and arsenic
Distribution of cadmium and arsenic between roots and shoots is a dynamic process driven by root plasma membrane transporters, xylem-loading/unloading and phloem-loading/unloading processes [27–32]. Uptake of metals and arsenic into roots and the xylem have been extensively studied (reviewed by [4–5]). Briefly, Zn and Cd are taken up by ZIP transporters and are loaded into the xylem by the Heavy Metal ATPases HMA2 and HMA4 (Fig. 1). Arsenate [As(V)] is taken up and loaded into the xylem by phosphate transporters [33] while arsenite [As(III)] uptake and translocation is mediated by members of the NIP subfamily of aquaporins [4–5].
Phloem transport, on the other hand, has been less studied, perhaps due to the technical challenges associated with phloem sampling [34–35]. However, transport through the phloem plays a key role in delivering nutrients, including metals, to developing seeds where xylem-mediated transport plays a minor role due to the limited transpiration rate within reproductive tissues [36]. Therefore, understanding phloem transport mechanisms is important to restrict the transport of toxic non-essential metals into (edible) seeds while ensuring the accumulation of essential nutrient metals. Analyses from various plant species have shown that the main metal-ligand molecules found in phloem sap are nicotianamine, GSH and PCs [27,30•,37–38]. Nicotianamine has been shown to form complexes with Fe, Cu, Zn and Mn [38, 39–40], while GSH and PCs have orders of magnitude higher affinities for the metal(loid)s Cd, Zn, Hg and As(III) [27,41]. Furthermore, extended X-ray absorption fine structure (EXAFS) analysis of seeds in the Cd hyperaccumulator Thlaspi praecox shows that 60% of Cd is coordinated with thiol-containing ligands (Fig. 1) [42]. The finding of PCs in phloem sap [27] was unexpected since PCs have long been considered molecules that mediate the transport of metals from the cytosol into vacuoles. However, research revealed long-distance PC transport [43] and liquid chromatography-mass spectrometry analyses revealed high levels of PCs in the phloem sap of Brassica napus [27]. Furthermore, energy-dispersive X-ray microanalysis (EDXMA) in A. thaliana found significant levels of Cd and sulfur-Cd complexes in the cytoplasm of companion cells (phloem-loading cells) [37], suggesting that thiols mediate long-distance transport of metal(loids) through the phloem. The plasma membrane transporters that load PC-metal(loid) complexes into the phloem remain unknown (Fig. 1), but cell-specific transcriptome analyses in A. thaliana show that phytochelatin synthase (PCS1, At5g44070) is most highly expressed in companion cells (phloem-loading cells)([44••], Supp. Fig. 2A). Companion cells and sieve elements (phloem) are connected through highly permeable plasmodesmata [35]. Therefore, compounds synthesized in companion cells, or transported into companion cells such as GSH or PCs, are likely to enter the phloem for further transport into sink tissues (e.g. seeds and roots, Fig. 1)[35,45–47]. We have analyzed low molecular weight thiols in Arabidopsis seeds and found significant levels of GSH but not PCs (Mendoza-Cozatl & Schroeder, unpublished). This suggests that thiol-Cd detection in seeds [42] may result from glutathione-Cd conjugates and that PC-Cd complexes loaded into the phloem are more likely to be sequestered in root (sink) vacuoles by the phytochelatin transporters ABCC1 and ABCC2. Notably, ABCC1 and ABCC2 transcript levels are expressed on average 3-fold higher in roots compared to shoots ([44••], Supp. Fig. 2B).
But why are phytochelatins transported through the phloem? The current model suggests that PCs may contribute to the movement of toxic metals out of the shoots where they could impair photosynthesis [27,37]. The movement of Na+ and K+ from shoots to roots has also been observed [48]. Such re-circulating mechanisms would limit the accumulation of metals in shoots. In rice, it has been suggested that Cd could be transferred directly from the xylem into the phloem at the nodes without being unloaded into leaf blades [31••]. An important consequence of this phloem-mediated transport of toxic elements is that they will be available for accumulation in seeds during the seed-filling stage. Understanding the mechanisms of metal(loid) loading into companion cells, phloem unloading and seed filling and whether these mechanisms are differentially regulated during the vegetative to flowering transition could help to ensure a high nutritional value of fruits, seeds and grains.
Mechanisms contributing to hyperaccumulation of cadmium and arsenic
Plants considered cadmium or arsenic hyperaccumulators have the capacity to tolerate high concentrations of cadmium or arsenic while accumulating a significant fraction of these toxic elements in shoots. Phytochelatins mediate the detoxification of Cd, As, Hg and plants deficient in PC synthesis are highly sensitive to these metal(loids). Hyperaccumulators seem to combine PC-dependent mechanisms with additional mechanisms to enhance their tolerance and accumulation capacity [4,49]. Metal hyperaccumulation has been associated with the following four key processes: 1) enhanced metal(loid) uptake in roots, 2) reduced sequestration of metals in root vacuoles, 3) enhanced root-to-shoot translocation and 4) enhanced sequestration of metals in shoot vacuoles (Fig. 1) [4–5,12•,13••]. The physiology of metal hyperaccumulation has been previously reviewed [4] and several publications have described transcriptional differences between hyperaccumulator and non-hyperaccumulator species [4,49–52]. Of particular interest for this review is the recent identification of ACR3 as a vacuolar As(III) transporter in fern [13••] and the characterization of HMA3 in the Cd hyperaccumulator Noccaea caerulescens (formerly Thlaspi caerulescens) [12•]. The fern Pteris vittata has demonstrated the ability to tolerate and hyper-accumulate arsenic in fronds (reviewed by [53]). The isolation of ACR3, a vacuolar transporter that enhances As(III) tolerance and accumulation in fern was recently reported [13••]. RNAi studies showed that fern with reduced expression of ACR3 were As(III) hypersensitive. On the other hand, HMA3 is a vacuolar Heavy Metal ATPase that was identified as highly expressed in the Cd/Zn hyperaccumulator Arabidopsis halleri compared to its close relative and non-hyperaccumulator A. thaliana [51]. Interestingly, HMA3 in A. thaliana (ecotype Col) is a pseudogene, containing a premature stop codon [54–55]. However, HMA3 is functional in the WS ecotype and its deletion promotes Cd sensitivity [54]. Furthermore, HMA3 over expression enhances tolerance and accumulation of Zn and Cd in the WS background [54]. HMA3 was also recently found to be responsible for differences in Cd accumulation in grains of two varieties of rice [10••]. Loss of HMA3 in roots facilitates root-to-shoot translocation of Cd resulting in an enhanced accumulation of Cd in rice grains. HMA3 was also recently found to be a determinant for Cd hyperaccumulation in Noccaea caerulescens. The ecotype Ganges shows enhanced tolerance and accumulation of Cd compared to the Prayon ecotype and a major difference between these two ecotypes is a sevenfold increase in the expression of HMA3 throughout the plant [12•]. Expression of TcHMA3 in Arabidopsis significantly enhances the accumulation of Cd and to a lesser extent Zn [12•]. Identification of HMA3 as a novel contributor to the hyperaccumulator phenotype offers a new strategy to increase cadmium tolerance and accumulation capacity of non-hyperaccumulating plants to improve current phytoremediation strategies. The engineering of plants with higher content of essential metals to enhance their nutritional value or higher accumulation of toxic metals for phytoremediation purposes will also require a detailed flux analysis of the metabolic pathways mediating tolerance (i.e. thiol biosynthesis), together with the simultaneous expression of thiol-metal and metal transporters to manipulate tolerance, accumulation and allocation of metals throughout the plant [56–57].
Transcriptional regulation mediated by cadmium and arsenic
Plants exposed to toxic metals or to elevated concentrations of essential metals display significant changes in gene expression that allow them to survive sub-optimal growth conditions. The regulatory mechanisms, transcription factors (TFs), and networks mediating these transcriptional responses remain largely unknown. The only transcription factor that has been shown to play a direct role in a Cd-induced transcriptional response in plants is the wheat gene TaHsfA4a [58••]. This heat-shock transcription factor was shown to up-regulate metallothioneins (MT’s) following Cd exposure in both yeast and rice. Specific residues in the DNA binding motif of TaHsfA4a were shown to be critical for Cd-dependent regulation of MT’s and are highly conserved in monocot species but are not present in Arabidopsis and other dicot species [58••]. Thus, the transcriptional regulators important for metal(loid)-induced transcriptional responses in dicots, including Arabidopsis, and metal(loid) hyperaccumulating species remain elusive.
A limited number of transcriptional profiling studies in Arabidopsis have shed light on the transcriptional networks that are affected by Cd and As exposure. Time course experiments using low (5 µM) and high (50 µM) Cd treatments revealed that rapid Cd-induced gene expression was not correlated with Cd accumulation in the tissues analyzed[14]. These studies also show that Cd-induced gene regulation affects a broad functional range of genes. Within hours of Cd exposure many genes involved in photosynthesis and glucosinolate biosynthesis are downregulated, while genes involved in sulfur metabolism, cell wall metabolism, and phenylpropanoid metabolism are rapidly induced[14,52]. Transcriptional regulators controlling the sulfur-limitation response were not identified in these studies despite a partial overlap between Cd and sulfur deficiency signaling [14,59–61].
As discussed above, Heavy Metal ATPases (HMA) have been identified as key transporters mediating translocation and storage of Cd and Zn (Fig. 1) [54,62–63]. However, it remains unknown how these transporters are regulated at the transcriptional level. Compared to A. thaliana, A. halleri contains two additional copies of the HMA4 gene as well as numerous alterations in the promoter element leading to higher expression compared to A. thaliana[62,64]. In contrast to AtHMA4, AtHMA3 has a very low transcript level and is only slightly up-regulated by Cd exposure in A. thaliana[50–51,65]. However, the A. halleri ortholog AhHMA3 shows higher expression and is up-regulated by Cd exposure[50–51,65]. These differences suggest that HMA genes are regulated at the transcriptional level but the Cd-induced response pathway and the transcription factors mediating this Cd-induced expression have yet to be identified.
Another transporter that is highly regulated under Cd stress is the low-affinity nitrate transporter NRT1.8 (At4g21680). NRT1.8 was identified as a nitrate transporter that mediates nitrate removal from xylem sap [11•]. Loss of NRT1.8 function mutants are Cd sensitive and accumulate nitrate in the xylem sap. Furthermore, the nrt1.8-1 mutant accumulates nitrate in roots during Cd exposure compared to the wild-type control [11•]. Microarray experiments identified NRT1.8 as being one of the most highly up-regulated transporter genes in roots under Cd stress. The transcript level of this transporter was shown to increase in a concentration-dependent and time-dependent manner after cadmium exposure [11•]. The NRT1.8 transporter is the first gene directly linking nitrogen metabolism to Cd-stress response and supports previous models where nitrogen-containing compounds are partially responsible for the translocation of Cd from roots to shoots through the xylem.
It is also important to note that some genes that mediate Cd tolerance are not regulated at the transcript level by exposure to toxic metal(loid)s. AtABCC1 and AtABCC2, the recently identified vacuolar PC transporters, are not induced by As, but are constitutively expressed [9••]. It is thought that this strategy allows for rapid storage and detoxification of these and other toxic xenobiotic compounds in the vacuole [9••].
The signaling pathway responsible for Cd-induced gene expression remains largely unknown. However, it was recently shown that Cd can activate the mitogen-activated protein kinases MAPK3 and MPK6[66]. This is supported by previous studies showing that the MAPK pathway is important for reactive oxygen species (ROS) signaling [67–69]. Both ROS signaling and nitric oxide (NO) have been implicated as early signals in the Cd signal transduction pathway [70–71]. Thus, the question of how Cd and As cause rapid gene expression and which transcription factors mediate early Cd and As transcriptional regulation are open questions.
Concluding Remarks
Metal accumulation and homeostasis require the coordination of several processes working simultaneously to regulate uptake, long-distance transport and distribution of metals to different cells and tissues (Fig. 1). In recent research, key missing transporters mediating metal and arsenic tolerance, vacuolar accumulation and distribution in plants have been identified. Since plants and seeds represent the major source of metal intake for humans and livestock, an in-depth understanding of these processes could help to ensure the accumulation of essential nutrient metals and avoid the retention of toxic metals in the food supply. Furthermore, phytoremediation has received significant attention as an improved alternative to physical removal strategies to restore sites contaminated with metals and metalloids. The recently identified mechanisms for vacuolar detoxification and sequestration of cadmium and arsenic, together with the novel genes discovered in natural metal hyperaccumulator species offer new targets to engineer fast-growing high-biomass producing metal hyperaccumulator organisms. This will likely require the simultaneous increase of metabolic flux through the thiol synthesis pathway [56] together with metal(loid) and thiol-metal(loid) transporters to manipulate tolerance, accumulation and allocation of metals within the plant.
Highlights.
The ABC transporters Abc2 (S. pombe), ABCC1 (A. thaliana), and ABCC2 (A. thaliana) have been identified as the long-sought vacuolar phytochelatin transporters.
The vacuolar ATPase HMA3 affects shoot-root partitioning of cadmium.
Cadmium in seeds is coordinated by thiol-containing compounds.
Heavy metals induce transcriptional responses that allow plants to preserve metal homeostasis.
The transcriptional regulators required for Cd- and As-induced gene expression remain unknown.
Supplementary Material
Maximum likelihood phylogenetic tree of AtABCC2, SpHMT1 and ABC-type transporters showing the highest sequence similarity to AtABCC2 or SpHMT1 in higher plants and selected fungi and animals. The tree was constructed using phyml with 100 bootstrap replicates on a rascal curated mafft alignment of the proteins shown[76–78]. Nodes labeled with two asterisks have a 100% bootstrap support while those with one asterisk have a support in the range of 90% to 100%. The scale bar indicates 0.7 substitutions per site. Information on the gene annotation and sequence source of all proteins is provided in Supplementary Table 1.
Relative expression of SUC2 (sucrose transporter 2, AT1G22710), AtPCS1 (phytochelatin synthase 1, AT5G44070), ABCC1 (AT1G30400) and ABCC2 (AT2G34660) from type-specific cell transcriptome analyses [44]. (A) The SUC2 is preferentially expressed in companion cells (phloem) and analysis of the transcriptional data show that AtPCS1 is also highly expressed in companion cells. (B) Analysis of the expression profile of ABCC1 and ABCC2 shows that the expression of both ABC-type transporters is higher in roots compared to shoots [44]. The following lines were used for the expression analysis (x-axis): (Tot) total RNA, (p35S) Near constitutive Cauliflower mosaic virus 35S, (pRPL11C) Root proliferating cells Ribosomal protein L11C, (pSHR) Root vasculature SHORTROOT, (pWOL) Root vasculature WOODENLEG, (pGL2) Root atrichoblast epidermis, shoot trichomes GLABRA2, (pSUC2) Root and shoot phloem companion cells Sucrose transporter2, (pSULTR2;2) Root phloem companion cells, shoot bundle sheath, Sulfate transporter, (SCR) Root endodermis, quiescent center SCARECROW, (CO2) Root cortex meristematic zone Cortex specific transcript, (PEP) Root cortex elongation and maturation zone Endopeptidase and (KAT1) Cotyledon and leaf guard cells K+ channel. The letter in front of the cell type (R or S) denotes whether the tissue origin, roots or shoots, and the C at the end of the cell type refers to data taken form control (unstressed) conditions.
Aknowledgments
This research was supported by National Institute of Environmental Health Sciences grant number P42 ES010337 and the Chemical Sciences, Geosciences, and Biosciences Division of the Office of Basic Energy Sciences at the US Department of Energy (DE-FG02-03ER15449) (J.I.S.). TOJ was supported by the UCSD-Salk IGERT Plant Systems Biology Interdisciplinary Graduate Training Program (Grant No. 0504645) DGMC is recipient of a PEW Latin American Fellowship.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References and recommended reading
• of special interest
•• of outstanding interest
- 1.Palmer CM, Guerinot ML. Facing the challenges of Cu, Fe and Zn homeostasis in plants. Nat Chem Biol. 2009;5:333–340. doi: 10.1038/nchembio.166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Morrissey J, Guerinot ML. Iron uptake and transport in plants: the good, the bad, and the ionome. Chem Rev. 2009;109:4553–4567. doi: 10.1021/cr900112r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mendoza-Cozatl D, Loza-Tavera H, Hernandez-Navarro A, Moreno-Sanchez R. Sulfur assimilation and glutathione metabolism under cadmium stress in yeast, protists and plants. FEMS Microbiol Rev. 2005;29:653–671. doi: 10.1016/j.femsre.2004.09.004. [DOI] [PubMed] [Google Scholar]
- 4.Milner MJ, Kochian LV. Investigating heavy-metal hyperaccumulation using Thlaspi caerulescens as a model system. Ann Bot. 2008;102:3–13. doi: 10.1093/aob/mcn063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Verbruggen N, Hermans C, Schat H. Mechanisms to cope with arsenic or cadmium excess in plants. Curr Opin Plant Biol. 2009;12:364–372. doi: 10.1016/j.pbi.2009.05.001. [DOI] [PubMed] [Google Scholar]
- 6. Duffus JH. "Heavy metals" a meaningless term? Pure Appl. Chem. 2002;74:793–807. Metals with a density higher than five (5 g/mL) have been historically called “heavy metals”. There is some discussion about whether this term should be replaced by other terms such as trace metals or trace metallic elements. This IUPAC report explains some of the reasons why the term "heavy metal" may be misleading or even inaccurate. For simplicity and to avoid further confusion, our review refers only to metals and metalloids and emphasizes which of them are essential for biological functions and which ones are not.
- 7. Buescher E, Achberger T, Amusan I, Giannini A, Ochsenfeld C, Rus A, Lahner B, Hoekenga O, Yakubova E, Harper JF, et al. Natural genetic variation in selected populations of Arabidopsis thaliana is associated with ionomic differences. PLoS One. 2010;5 doi: 10.1371/journal.pone.0011081. e11081. The authors use inductively coupled plasma - mass spectroscopy to establish the ‘ionome’ of 12 different Arabidopsis accessions and 3 recombinant inbred lines. They performed elemental analysis on 17 different elements and compared the results to identify QTLs linked to differences in the ionomic profiles of these accessions. The ionomic data has been made publically available and is an excellent resource for comparing the impact of both genetics and various environmental conditions on the elemental composition of Arabidopsis.
- 8. Mendoza-Cozatl DG, Zhai Z, Jobe TO, Akmakjian GZ, Song WY, Limbo O, Russell MR, Kozlovskyy VI, Martinoia E, Vatamaniuk OK, et al. Tonoplast-localized Abc2 transporter mediates phytochelatin accumulation in vacuoles and confers cadmium tolerance. J Biol Chem. 2010;285:40416–40426. doi: 10.1074/jbc.M110.155408. The authors identify a novel vacuolar phytochelatin-cadmium uptake transporter in a subfamily different from the previously identified phytochelatin transporter Hmt1. In contrast to the hmt1 mutant, which still accumulates a significant amount of phytochelatin in vacuoles, the hmt1abc2 double mutant largely removes phytochelatin accumulation in vacuoles. A new function for HMT1 in Cd-S cluster transport is also proposed.
- 9. Song WY, Park J, Mendoza-Cozatl DG, Suter-Grotemeyer M, Shim D, Hortensteiner S, Geisler M, Weder B, Rea PA, Rentsch D, et al. Arsenic tolerance in Arabidopsis is mediated by two ABCC-type phytochelatin transporters. Proc Natl Acad Sci U S A. 2010;107:21187–21192. doi: 10.1073/pnas.1013964107. Plant vacuolar phytochelatin transporters were sought for more than 15 years. In this paper the authors identified two Arabidopsis vacuolar ABC transporters required for arsenic tolerance. In vitro experiments show that ABCC1 and ABCC2 mediate the uptake of PC and PC-As(III) complexes into plants vacuoles.
- 10. Ueno D, Yamaji N, Kono I, Huang CF, Ando T, Yano M, Ma JF. Gene limiting cadmium accumulation in rice. Proc Natl Acad Sci U S A. 2010;107:16500–16505. doi: 10.1073/pnas.1005396107. By using QTL mapping, the authors identify a gene responsible for reducing cadmium accumulation in grains of the Nipponbare rice cultivar. OsHMA3 is expressed at the root tonoplast and the cultivar showing enhanced expression of functional HMA3 in roots significantly reduces accumulation of cadmium in seeds and shoots.
- 11. Li J-Y, Fu Y-L, Pike SM, Bao J, Tian W, Zhang Y, Chen C-Z, Zhang Y, Li H-M, Huang J, et al. The Arabidopsis Nitrate Transporter NRT1.8 Functions in Nitrate Removal from the Xylem Sap and Mediates Cadmium Tolerance. Plant Cell. 2010;22:1633–1646. doi: 10.1105/tpc.110.075242. The authors report the functional characterization of NRT1.8 as a sought after gene mediating nitrate unloading from the xylem. The mutant was also found to be Cd hypersensitive suggesting that nitrate levels in the xylem and distribution in plants plays a role in cadmium tolerance.
- 12. Ueno D, Milner MJ, Yamaji N, Yokosho K, Koyama E, Clemencia Zambrano M, Kaskie M, Ebbs S, Kochian LV, Ma JF. Elevated expression of TcHMA3 plays a key role in the extreme Cd tolerance in a Cd-hyperaccumulating ecotype of Thlaspi caerulescens. Plant J. 2011;66:852–862. doi: 10.1111/j.1365-313X.2011.04548.x. Thlaspi caerulescens is considered a Cd/Zn hyperaccumulator. The authors show that the enhanced cadmium accumulation a hyperaccumulating ecotype of T. caerulescens correlates well with an enhanced gene copy and expression of TaHMA3. TaHM3 is functional in both ecotypes, but in the ecotype showing higher tolerance and cadmium accumulation, TaHMA3 exhibits constitutive expression at higher levels.
- 13. Indriolo E, Na G, Ellis D, Salt DE, Banks JA. A vacuolar arsenite transporter necessary for arsenic tolerance in the arsenic hyperaccumulating fern Pteris vittata is missing in flowering plants. Plant Cell. 2010;22:2045–2057. doi: 10.1105/tpc.109.069773. In this paper, the authors isolate and characterize two genes from Pteris vittata that are similar to a yeast arsenite efflux transporter. Genetic analysis shows that one of these genes, ACR3, is essential for arsenite detoxification in the gametophyte and localizes to the tonoplast. While orthologues of this gene are shown to exist in other gymnosperms, it is absent in angiosperms hinting at a possible mechanism for arsenic tolerance in gymnosperms.
- 14.Herbette S, Taconnat L, Hugouvieux V, Piette L, Magniette MLM, Cuine S, Auroy P, Richaud P, Forestier C, Bourguignon J, et al. Genome-wide transcriptome profiling of the early cadmium response of Arabidopsis roots and shoots. Biochimie. 2006;88:1751–1765. doi: 10.1016/j.biochi.2006.04.018. [DOI] [PubMed] [Google Scholar]
- 15.Clemens S. Toxic metal accumulation, responses to exposure and mechanisms of tolerance in plants. Biochimie. 2006;88:1707–1719. doi: 10.1016/j.biochi.2006.07.003. [DOI] [PubMed] [Google Scholar]
- 16.Rea PA. Plant ATP-binding cassette transporters. Annu Rev Plant Biol. 2007;58:347–375. doi: 10.1146/annurev.arplant.57.032905.105406. [DOI] [PubMed] [Google Scholar]
- 17.Ortiz DF, Kreppel L, Speiser DM, Scheel G, McDonald G, Ow DW. Heavy metal tolerance in the fission yeast requires an ATP-binding cassette-type vacuolar membrane transporter. EMBO J. 1992;11:3491–3499. doi: 10.1002/j.1460-2075.1992.tb05431.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ortiz DF, Ruscitti T, McCue KF, Ow DW. Transport of metal-binding peptides by HMT1, a fission yeast ABC-type vacuolar membrane protein. J Biol Chem. 1995;270:4721–4728. doi: 10.1074/jbc.270.9.4721. [DOI] [PubMed] [Google Scholar]
- 19.Salt DE, Rauser WE. MgATP-Dependent Transport of Phytochelatins Across the Tonoplast of Oat Roots. Plant Physiol. 1995;107:1293–1301. doi: 10.1104/pp.107.4.1293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sooksa-Nguan T, Yakubov B, Kozlovskyy VI, Barkume CM, Howe KJ, Thannhauser TW, Rutzke MA, Hart JJ, Kochian LV, Rea PA, et al. Drosophila ABC transporter, DmHMT-1, confers tolerance to cadmium. DmHMT-1 and its yeast homolog, SpHMT-1, are not essential for vacuolar phytochelatin sequestration. J Biol Chem. 2009;284:354–362. doi: 10.1074/jbc.M806501200. [DOI] [PubMed] [Google Scholar]
- 21.Preveral S, Gayet L, Moldes C, Hoffmann J, Mounicou S, Gruet A, Reynaud F, Lobinski R, Verbavatz JM, Vavasseur A, et al. A common highly conserved cadmium detoxification mechanism from bacteria to humans: heavy metal tolerance conferred by the ATP-binding cassette (ABC) transporter SpHMT1 requires glutathione but not metal-chelating phytochelatin peptides. J Biol Chem. 2009;284:4936–4943. doi: 10.1074/jbc.M808130200. [DOI] [PubMed] [Google Scholar]
- 22.Liu G, Sanchez-Fernandez RA, Li Z-S, Rea PA. Enhanced Multispecificity of Arabidopsis Vacuolar Multidrug Resistance-associated Protein-type ATP-binding Cassette Transporter, AtMRP2. J Biol Chem. 2001;276:8648–8656. doi: 10.1074/jbc.M009690200. [DOI] [PubMed] [Google Scholar]
- 23.Broderick JB. Assembling iron-sulfur clusters in the cytosol. Nat Chem Biol. 2007;3:243–244. doi: 10.1038/nchembio0507-243. [DOI] [PubMed] [Google Scholar]
- 24.Iwaki T, Giga-Hama Y, Takegawa K. A survey of all 11 ABC transporters in fission yeast: two novel ABC transporters are required for red pigment accumulation in a Schizosaccharomyces pombe adenine biosynthetic mutant. Microbiology. 2006;152:2309–2321. doi: 10.1099/mic.0.28952-0. [DOI] [PubMed] [Google Scholar]
- 25.Iwema T, Picciocchi A, Traore DA, Ferrer JL, Chauvat F, Jacquamet L. Structural basis for delivery of the intact [Fe2S2] cluster by monothiol glutaredoxin. Biochemistry. 2009;48:6041–6043. doi: 10.1021/bi900440m. [DOI] [PubMed] [Google Scholar]
- 26.Rouhier N, Unno H, Bandyopadhyay S, Masip L, Kim SK, Hirasawa M, Gualberto JM, Lattard V, Kusunoki M, Knaff DB, et al. Functional, structural, and spectroscopic characterization of a glutathione-ligated [2Fe-2S] cluster in poplar glutaredoxin C1. Proc Natl Acad Sci U S A. 2007;104:7379–7384. doi: 10.1073/pnas.0702268104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mendoza-Cozatl DG, Butko E, Springer F, Torpey JW, Komives EA, Kehr J, Schroeder JI. Identification of high levels of phytochelatins, glutathione and cadmium in the phloem sap of Brassica napus. A role for thiol-peptides in the long-distance transport of cadmium and the effect of cadmium on iron translocation. Plant J. 2008;54:249–259. doi: 10.1111/j.1365-313X.2008.03410.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ma JF, Yamaji N, Mitani N, Xu XY, Su YH, McGrath SP, Zhao FJ. Transporters of arsenite in rice and their role in arsenic accumulation in rice grain. Proc Natl Acad Sci U S A. 2008;105:9931–9935. doi: 10.1073/pnas.0802361105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liu WJ, Wood BA, Raab A, McGrath SP, Zhao FJ, Feldmann J. Complexation of arsenite with phytochelatins reduces arsenite efflux and translocation from roots to shoots in Arabidopsis. Plant Physiol. 2010;152:2211–2221. doi: 10.1104/pp.109.150862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Ye WL, Wood BA, Stroud JL, Andralojc PJ, Raab A, McGrath SP, Feldmann J, Zhao FJ. Arsenic speciation in phloem and xylem exudates of castor bean. Plant Physiol. 2010;154:1505–1513. doi: 10.1104/pp.110.163261. Using high-resolution inductively coupled plasma-mass spectrometry and xylem/phloem exudates from Ricinus communis the authors determine that the predominant chemical form of arsenic in phloem sap was As(III). The authors also found that glutathione and phytochelatins were consistently higher in phloem sap compared to xylem sap.
- 31. Fujimaki S, Suzui N, Ishioka NS, Kawachi N, Ito S, Chino M, Nakamura S. Tracing cadmium from culture to spikelet: noninvasive imaging and quantitative characterization of absorption, transport, and accumulation of cadmium in an intact rice plant. Plant Physiol. 2010;152:1796–1806. doi: 10.1104/pp.109.151035. Using a positron-emitting tracer imaging system, the authors show in real-time the dynamics of cadmium uptake from roots to leaves and grains. The accumulation pattern in nodes and further movement of cadmium into the grain suggest that the nodes are the location where cadmium is transferred from the xylem to the phloem.
- 32.Ishimaru Y, Masuda H, Bashir K, Inoue H, Tsukamoto T, Takahashi M, Nakanishi H, Aoki N, Hirose T, Ohsugi R, et al. Rice metal-nicotianamine transporter, OsYSL2, is required for the long-distance transport of iron and manganese. Plant J. 2010;62:379–390. doi: 10.1111/j.1365-313X.2010.04158.x. [DOI] [PubMed] [Google Scholar]
- 33.Catarecha P, Segura MD, Franco-Zorrilla JM, Garcia-Ponce B, Lanza M, Solano R, Paz-Ares J, Leyva A. A mutant of the Arabidopsis phosphate transporter PHT1;1 displays enhanced arsenic accumulation. Plant Cell. 2007;19:1123–1133. doi: 10.1105/tpc.106.041871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nelson T, Gandotra N, Tausta SL. Plant cell types: reporting and sampling with new technologies. Curr Opin Plant Biol. 2008;11:567–573. doi: 10.1016/j.pbi.2008.06.006. [DOI] [PubMed] [Google Scholar]
- 35.Turgeon R, Wolf S. Phloem transport: cellular pathways and molecular trafficking. Annu Rev Plant Biol. 2009;60:207–221. doi: 10.1146/annurev.arplant.043008.092045. [DOI] [PubMed] [Google Scholar]
- 36.Bauer P, Hell R. Translocation of Iron in Plant Tissues. In: Barton LL, Abadia J, editors. Iron Nutrition in Plants and Rhizospheric Microorganisms. Netherlands: Springer; 2006. pp. 279–288. [Google Scholar]
- 37.Van Belleghem F, Cuypers A, Semane B, Smeets K, Vangronsveld J, d'Haen J, Valcke R. Subcellular localization of cadmium in roots and leaves of Arabidopsis thaliana. New Phytol. 2007;173:495–508. doi: 10.1111/j.1469-8137.2006.01940.x. [DOI] [PubMed] [Google Scholar]
- 38.Curie C, Cassin G, Couch D, Divol F, Higuchi K, Le Jean M, Misson J, Schikora A, Czernic P, Mari S. Metal movement within the plant: contribution of nicotianamine and yellow stripe 1-like transporters. Ann Bot. 2009;103:1–11. doi: 10.1093/aob/mcn207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Haydon MJ, Cobbett CS. Transporters of ligands for essential metal ions in plants. New Phytol. 2007;174:499–506. doi: 10.1111/j.1469-8137.2007.02051.x. [DOI] [PubMed] [Google Scholar]
- 40.Trampczynska A, Kupper H, Meyer-Klaucke W, Schmidt H, Clemens S. Nicotianamine forms complexes with Zn(II) in vivo. Metallomics. 2010;2:57–66. doi: 10.1039/b913299f. [DOI] [PubMed] [Google Scholar]
- 41.Dorcak V, Krezel A. Correlation of acid-base chemistry of phytochelatin PC2 with its coordination properties towards the toxic metal ion Cd(ii) Dalton Transactions. 2003:2253–2259. [Google Scholar]
- 42.Vogel-Mikuš K, Arčon I, Kodre A. Complexation of cadmium in seeds and vegetative tissues of the cadmium hyperaccumulator Thlaspi praecox as studied by X-ray absorption spectroscopy. Plant and Soil. 2010;331:439–451. [Google Scholar]
- 43.Gong JM, Lee DA, Schroeder JI. Long-distance root-to-shoot transport of phytochelatins and cadmium in Arabidopsis. Proc Natl Acad Sci U S A. 2003;100:10118–10123. doi: 10.1073/pnas.1734072100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Mustroph A, Zanetti ME, Jang CJ, Holtan HE, Repetti PP, Galbraith DW, Girke T, Bailey-Serres J. Profiling translatomes of discrete cell populations resolves altered cellular priorities during hypoxia in Arabidopsis. Proc Natl Acad Sci U S A. 2009;106:18843–18848. doi: 10.1073/pnas.0906131106. Using ribosome immunopurification, the authors generate a cell-specific transcriptome atlas of 13 cell-types in Arabidopsis under control and hypoxic conditions. This paper provides a high-quality dataset to identify genes expressed in particular cells within a specific organs or plant tissues.
- 45.Chen A, Komives EA, Schroeder JI. An improved grafting technique for mature Arabidopsis plants demonstrates long-distance shoot-to-root transport of phytochelatins in Arabidopsis. Plant Physiol. 2006;141:108–120. doi: 10.1104/pp.105.072637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Li Y, Dankher OP, Carreira L, Smith AP, Meagher RB. The shoot-specific expression of gamma-glutamylcysteine synthetase directs the long-distance transport of thiol-peptides to roots conferring tolerance to mercury and arsenic. Plant Physiol. 2006;141:288–298. doi: 10.1104/pp.105.074815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Li Y, Dhankher OP, Carreira L, Lee D, Chen A, Schroeder JI, Balish RS, Meagher RB. Overexpression of phytochelatin synthase in Arabidopsis leads to enhanced arsenic tolerance and cadmium hypersensitivity. Plant Cell Physiol. 2004;45:1787–1797. doi: 10.1093/pcp/pch202. [DOI] [PubMed] [Google Scholar]
- 48.Tian H, Baxter IR, Lahner B, Reinders A, Salt DE, Ward JM. Arabidopsis NPCC6/NaKR1 is a phloem mobile metal binding protein necessary for phloem function and root meristem maintenance. Plant Cell. 2010;22:3963–3979. doi: 10.1105/tpc.110.080010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Plaza S, Tearall KL, Zhao FJ, Buchner P, McGrath SP, Hawkesford MJ. Expression and functional analysis of metal transporter genes in two contrasting ecotypes of the hyperaccumulator Thlaspi caerulescens. J Exp Bot. 2007;58:1717–1728. doi: 10.1093/jxb/erm025. [DOI] [PubMed] [Google Scholar]
- 50.Talke IN, Hanikenne M, Krämer U. Zinc-Dependent Global Transcriptional Control, Transcriptional Deregulation, and Higher Gene Copy Number for Genes in Metal Homeostasis of the Hyperaccumulator Arabidopsis halleri. Plant Physiology. 2006;142:148–167. doi: 10.1104/pp.105.076232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Becher M, Talke IN, Krall L, Krämer U. Cross-species microarray transcript profiling reveals high constitutive expression of metal homeostasis genes in shoots of the zinc hyperaccumulator Arabidopsis halleri. Plant Journal. 2004;37:251–268. doi: 10.1046/j.1365-313x.2003.01959.x. [DOI] [PubMed] [Google Scholar]
- 52.Weber M, Harada E, Vess C, Roepenack-Lahaye Ev, Clemens S. Comparative microarray analysis of Arabidopsis thaliana and Arabidopsis halleri roots identifies nicotianamine synthase, a ZIP transporter and other genes as potential metal hyperaccumulation factors. Plant Journal. 2004;37:269–281. doi: 10.1046/j.1365-313x.2003.01960.x. [DOI] [PubMed] [Google Scholar]
- 53.Zhao FJ, Ma JF, Meharg AA, McGrath SP. Arsenic uptake and metabolism in plants. New Phytol. 2009;181:777–794. doi: 10.1111/j.1469-8137.2008.02716.x. [DOI] [PubMed] [Google Scholar]
- 54.Morel M, Crouzet J, Gravot A, Auroy P, Leonhardt N, Vavasseur A, Richaud P. AtHMA3, a P1B-ATPase Allowing Cd/Zn/Co/Pb Vacuolar Storage in Arabidopsis. Plant Physiology. 2009;149:894–904. doi: 10.1104/pp.108.130294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hussain D, Haydon MJ, Wang Y, Wong E, Sherson SM, Young J, Camakaris J, Harper JF, Cobbett CS. P-Type ATPase Heavy Metal Transporters with Roles in Essential Zinc Homeostasis in Arabidopsis. Plant Cell. 2004;16:1327–1339. doi: 10.1105/tpc.020487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Mendoza-Cozatl DG, Moreno-Sanchez R. Control of glutathione and phytochelatin synthesis under cadmium stress. Pathway modeling for plants. J Theor Biol. 2006;238:919–936. doi: 10.1016/j.jtbi.2005.07.003. [DOI] [PubMed] [Google Scholar]
- 57.Naqvi S, Farre G, Sanahuja G, Capell T, Zhu C, Christou P. When more is better: multigene engineering in plants. Trends Plant Sci. 2010;15:48–56. doi: 10.1016/j.tplants.2009.09.010. [DOI] [PubMed] [Google Scholar]
- 58. Shim D, Hwang J-U, Lee J, Lee S, Choi Y, An G, Martinoia E, Lee Y. Orthologs of the Class A4 Heat Shock Transcription Factor HsfA4a Confer Cadmium Tolerance in Wheat and Rice. Plant Cell. 2009;21:4031–4043. doi: 10.1105/tpc.109.066902. A wheat gene that confers tolerance to yeast is discovered in an elegant screen. The gene, TaHSF4A, and orthologs from other monocots were shown to play a role in upregulating metallothionines during cadmium exposure. However, orthologs from dicots were shown to lack the structural domains important for this function.
- 59.Maruyama-Nakashita A, Nakamura Y, Tohge T, Saito K, Takahashi H. Arabidopsis SLIM1 is a central transcriptional regulator of plant sulfur response and metabolism. Plant Cell. 2006;18:3235–3251. doi: 10.1105/tpc.106.046458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Van De Mortel JE, Schat H, Moerland PD, Van Themaat EVL, Van Der Ent S, Blankestijn H, Ghandilyan A, Tsiatsiani S, Aarts MGM. Expression differences for genes involved in lignin, glutathione and sulphate metabolism in response to cadmium in Arabidopsis thaliana and the related Zn/Cd-hyperaccumulator Thlaspi caerulescens. Plant, Cell & Environment. 2008;31:301–324. doi: 10.1111/j.1365-3040.2007.01764.x. [DOI] [PubMed] [Google Scholar]
- 61.Weber M, Trampczynska A, Clemens S. Comparative transcriptome analysis of toxic metal responses in Arabidopsis thaliana and the Cd2+-hypertolerant facultative metallophyte Arabidopsis halleri. Plant, Cell & Environment. 2006;29:950–963. doi: 10.1111/j.1365-3040.2005.01479.x. [DOI] [PubMed] [Google Scholar]
- 62.Hanikenne M, Talke IN, Haydon MJ, Lanz C, Nolte A, Motte P, Kroymann J, Weigel D, Kramer U. Evolution of metal hyperaccumulation required cis-regulatory changes and triplication of HMA4. Nature. 2008;453:391–395. doi: 10.1038/nature06877. [DOI] [PubMed] [Google Scholar]
- 63.Verret F, Gravot A, Auroy P, Preveral S, Forestier C, Vavasseur A, Richaud P. Heavy metal transport by AtHMA4 involves the N-terminal degenerated metal binding domain and the C-terminal His11 stretch. FEBS Lett. 2005;579:1515–1522. doi: 10.1016/j.febslet.2005.01.065. [DOI] [PubMed] [Google Scholar]
- 64.Courbot M, Willems G, Motte P, Arvidsson S, Roosens N, Saumitou-Laprade P, Verbruggen N. A major quantitative trait locus for cadmium tolerance in Arabidopsis halleri colocalizes with HMA4, a gene encoding a heavy metal ATPase. Plant Physiol. 2007;144:1052–1065. doi: 10.1104/pp.106.095133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Gravot A, Lieutaud A, Verret F, Auroy P, Vavasseur A, Richaud P. AtHMA3, a plant P1B-ATPase, functions as a Cd/Pb transporter in yeast. FEBS Letters. 2004;561:22–28. doi: 10.1016/S0014-5793(04)00072-9. [DOI] [PubMed] [Google Scholar]
- 66.Liu X-M, Kim KE, Kim K-C, Nguyen XC, Han HJ, Jung MS, Kim HS, Kim SH, Park HC, Yun D-J, et al. Cadmium activates Arabidopsis MPK3 and MPK6 via accumulation of reactive oxygen species. Phytochemistry. 2010;71:614–618. doi: 10.1016/j.phytochem.2010.01.005. [DOI] [PubMed] [Google Scholar]
- 67.Pitzschke A, Djamei A, Bitton F, Hirt H. A Major Role of the MEKK1-MKK1/2-MPK4 Pathway in ROS Signalling. Molecular Plant. 2009;2:120–137. doi: 10.1093/mp/ssn079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Nakagami H, Pitzschke A, Hirt H. Emerging MAP kinase pathways in plant stress signalling. Trends in plant science. 2005;10:339–346. doi: 10.1016/j.tplants.2005.05.009. [DOI] [PubMed] [Google Scholar]
- 69.Jammes F, Song C, Shin D, Munemasa S, Takeda K, Gu D, Cho D, Lee S, Giordo R, Sritubtim S, et al. MAP kinases MPK9 and MPK12 are preferentially expressed in guard cells and positively regulate ROS-mediated ABA signaling. Proc Natl Acad Sci U S A. 2009;106:20520–20525. doi: 10.1073/pnas.0907205106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Rodríguez-Serrano M, Romero-Puertas MC, Pazmiño DM, Testillano PS, Risueño MC, del Río LA, Sandalio LM. Cellular Response of Pea Plants to Cadmium Toxicity: Cross Talk between Reactive Oxygen Species, Nitric Oxide, and Calcium. Plant Physiology. 2009;150:229–243. doi: 10.1104/pp.108.131524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Arasimowicz-Jelonek M, Floryszak-Wieczorek J, Gwózdz EA. The message of nitric oxide in cadmium challenged plants. Plant Science. doi: 10.1016/j.plantsci.2011.03.019. in press. [DOI] [PubMed] [Google Scholar]
- 72.Thomine S, Wang R, Ward JM, Crawford NM, Schroeder JI. Cadmium and iron transport by members of a plant metal transporter family in Arabidopsis with homology to Nramp genes. Proc Natl Acad Sci U S A. 2000;97:4991–4996. doi: 10.1073/pnas.97.9.4991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Oomen RJ, Wu J, Lelievre F, Blanchet S, Richaud P, Barbier-Brygoo H, Aarts MG, Thomine S. Functional characterization of NRAMP3 and NRAMP4 from the metal hyperaccumulator Thlaspi caerulescens. New Phytol. 2009;181:637–650. doi: 10.1111/j.1469-8137.2008.02694.x. [DOI] [PubMed] [Google Scholar]
- 74.Kupper H, Lombi E, Zhao FJ, McGrath SP. Cellular compartmentation of cadmium and zinc in relation to other elements in the hyperaccumulator Arabidopsis halleri. Planta. 2000;212:75–84. doi: 10.1007/s004250000366. [DOI] [PubMed] [Google Scholar]
- 75.Lombi E, Scheckel KG, Pallon J, Carey AM, Zhu YG, Meharg AA. Speciation and distribution of arsenic and localization of nutrients in rice grains. New Phytol. 2009;184:193–201. doi: 10.1111/j.1469-8137.2009.02912.x. [DOI] [PubMed] [Google Scholar]
- 76.Guindon S, Gascuel O. A simple, fast, and accurate algorithm to estimate large phylogenies by maximum likelihood. Syst Biol. 2003;52:696–704. doi: 10.1080/10635150390235520. [DOI] [PubMed] [Google Scholar]
- 77.Thompson JD, Thierry JC, Poch O. RASCAL: rapid scanning and correction of multiple sequence alignments. Bioinformatics. 2003;19:1155–1161. doi: 10.1093/bioinformatics/btg133. [DOI] [PubMed] [Google Scholar]
- 78.Katoh K, Misawa K, Kuma K, Miyata T. MAFFT: a novel method for rapid multiple sequence alignment based on fast Fourier transform. Nucleic Acids Research. 2002;30:3059–3066. doi: 10.1093/nar/gkf436. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Maximum likelihood phylogenetic tree of AtABCC2, SpHMT1 and ABC-type transporters showing the highest sequence similarity to AtABCC2 or SpHMT1 in higher plants and selected fungi and animals. The tree was constructed using phyml with 100 bootstrap replicates on a rascal curated mafft alignment of the proteins shown[76–78]. Nodes labeled with two asterisks have a 100% bootstrap support while those with one asterisk have a support in the range of 90% to 100%. The scale bar indicates 0.7 substitutions per site. Information on the gene annotation and sequence source of all proteins is provided in Supplementary Table 1.
Relative expression of SUC2 (sucrose transporter 2, AT1G22710), AtPCS1 (phytochelatin synthase 1, AT5G44070), ABCC1 (AT1G30400) and ABCC2 (AT2G34660) from type-specific cell transcriptome analyses [44]. (A) The SUC2 is preferentially expressed in companion cells (phloem) and analysis of the transcriptional data show that AtPCS1 is also highly expressed in companion cells. (B) Analysis of the expression profile of ABCC1 and ABCC2 shows that the expression of both ABC-type transporters is higher in roots compared to shoots [44]. The following lines were used for the expression analysis (x-axis): (Tot) total RNA, (p35S) Near constitutive Cauliflower mosaic virus 35S, (pRPL11C) Root proliferating cells Ribosomal protein L11C, (pSHR) Root vasculature SHORTROOT, (pWOL) Root vasculature WOODENLEG, (pGL2) Root atrichoblast epidermis, shoot trichomes GLABRA2, (pSUC2) Root and shoot phloem companion cells Sucrose transporter2, (pSULTR2;2) Root phloem companion cells, shoot bundle sheath, Sulfate transporter, (SCR) Root endodermis, quiescent center SCARECROW, (CO2) Root cortex meristematic zone Cortex specific transcript, (PEP) Root cortex elongation and maturation zone Endopeptidase and (KAT1) Cotyledon and leaf guard cells K+ channel. The letter in front of the cell type (R or S) denotes whether the tissue origin, roots or shoots, and the C at the end of the cell type refers to data taken form control (unstressed) conditions.