Abstract
MAGUKs are proteins that act as key scaffolds in surface complexes containing receptors, adhesion proteins, and various signaling molecules. These complexes evolved prior to the appearance of multicellular animals and play key roles in cell-cell intercommunication. A major example of this is the neuronal synapse, which contains several presynaptic and postsynaptic MAGUKs including PSD-95, SAP102, SAP97, PSD-93, CASK, and MAGIs. Here, they play roles in both synaptic development and in later synaptic plasticity events. During development, MAGUKs help to organize the postsynaptic density via associations with other scaffolding proteins, such as Shank, and the actin cytoskeleton. They affect the clustering of glutamate receptors and other receptors, and these associations change with development. MAGUKs are involved in long-term potentiation and depression (e.g., via their phosphorylation by kinases and phosphorylation of other proteins associated with MAGUKs). Importantly, synapse development and function are dependent on the kind of MAGUK present. For example, SAP102 shows high mobility and is present in early synaptic development. Later, much of SAP102 is replaced by PSD-95, a more stable synaptic MAGUK; this is associated with changes in glutamate receptor types that are characteristic of synaptic maturation.
Keywords: SAP97, MAGI, CASK, PSD-95, SAP102
Introduction: MAGUKs and Synaptic Junctional Complexes
From sponges to people, the cells of multicellular eukaryotes (metazoans) sense their environment and communicate with that environment through junctional complexes. Junctional complexes contain various adhesion and receptor proteins on the cell membrane that are integrated into a protein scaffold ultimately associated with an actin framework and that regulate numerous intracellular signaling molecules. These signaling molecules modulate the developmental/functional state of the cell (i.e., plasticity) following initial signal input from the cell’s environment. Junctional complexes are often tied to and help regulate endocytosis/exocytosis regions by controlling vesicle movement along actin and/or microtubule-based pathways. Classic examples of junctional complex associations include the neuronal postsynaptic density (PSD) and presynaptic active zone, as will be discussed in this article, as well as epithelial cell apical junctional complexes (e.g., tight junctions, adherens junctions, desmosomes) and apical specializations of sensory cells (e.g., mechanosensory hair cells in ears and photoreceptor cells in retinas).
PDZ (PSD-95, Dlg [discs large homolog], and ZO-1 [zona occludens or tight junction]) domain–containing proteins, which directly bind to the PDZ-binding region of the C-termini of many adhesion and receptor proteins, are key to the organization of junctional complexes (Sheng and Sala 2001; Funke and others 2005; Alie and Manuel 2010) (Figs. 1 and 2). These PDZ domain–containing proteins often contain additional types of domains, which can bind to each other and to various signaling proteins in the junctional complex. Two of the most common groups of PDZ domain–containing proteins that often form the main scaffold of the junctional complex are 1) those with proline-rich domains, such as Shank and Whirlin (Brown and others 2008; Alie and Manuel 2010), and 2) those with a guanylate kinase (GK) domain, called the MAGUKs (membrane-associated guanylate kinases). We will concentrate on the latter proteins in this review. In many cases, these 2 types of proteins join together in a scaffold, either directly or via connecting proteins such as GKAP or IRSp53, as found in the postsynaptic density (Figs. 2 and 3). Interestingly, both of these types of PDZ domain–containing proteins are found in single-celled choanoflagellate protists from which the metazoans may be derived, as well as in simple metazoans that lack a nervous system; these include sponges and placozoans (Sakarya and others 2007; Ryan and Grant 2009; Alie and Manuel 2010; de Mendoza and others 2010). These MAGUK-containing junctional complexes evolved very early, perhaps for sensing the environment and regulating exocytosis/endocytosis. Presumably, as the metazoans evolved, these junctional complexes were modified to take on the functions of neuronal synapses and other specialized junctions.
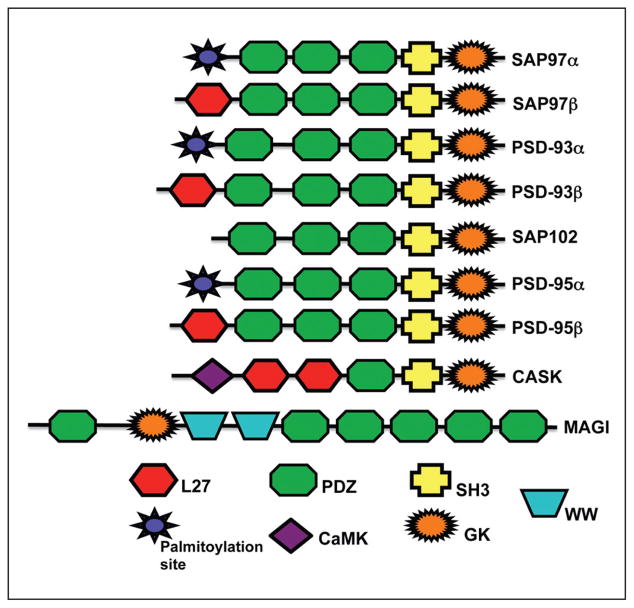
Figure 1.
Diagram shows protein domains of major synaptic MAGUKs, as discussed in the text. These include DLG1-4 or SAP97, PSD-93, SAP102, and PSD-95, as well as CASK and MAGI, which include MAGI1-3. Note the 2 domains that define the MAGUKs, that is, PDZ and guanylate kinase (GK) domains. The GK domain may be associated with an SH3 domain. Only CASK can function as an active protein kinase, that is, via its CaMK domain, while MAGI has 2 WW domains. SAP97, PSD-93, and PSD-95 are shown with alternative N-terminal domains, that is, L27 or a palmitoylation site. Adapted from Funke and others (2005). L27 = domain in LIN-2 and LIN-7; PDZ = PSD-95/Dlg/ZO-1; SH3 = Src-homology-3; CaMK = calcium-calmodulin kinase; GK = guanylate kinase; WW = 2 conserved Trp residues.
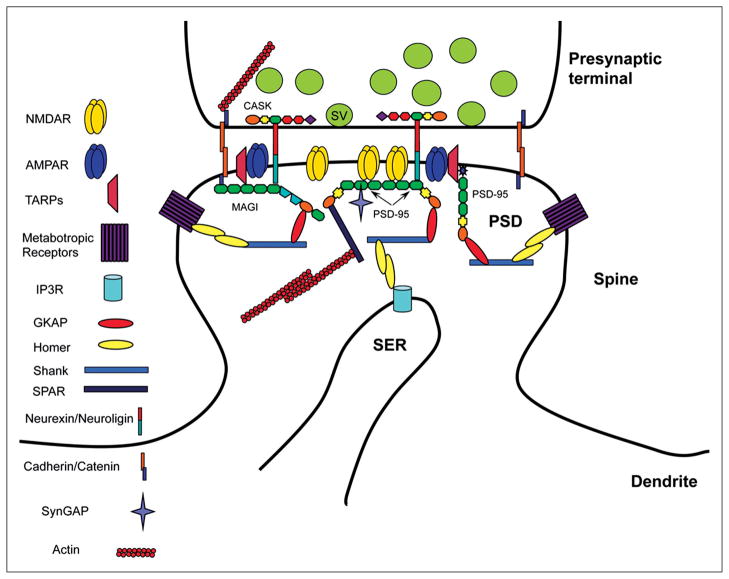
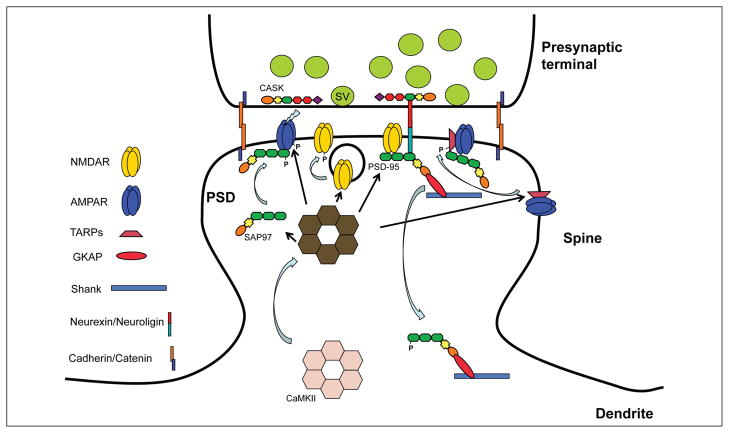
Figure 2.
Diagram illustrates the position of MAGUKs in junctional complexes at the neuronal synapse, including both the postsynaptic density and presynaptic active zone. As described in the text, surface receptors and adhesion proteins and other membrane proteins in the postsynaptic membrane can bind via PDZ domains to MAGUKs that also interact with various signaling proteins and bind through intermediate proteins like GKAP to proline-rich domain–containing proteins like Shank. Similar arrangements may be present in the presynaptic active zone, especially with the MAGUK, CASK. Adhesion proteins can link in the cleft to join presynaptic and postsynaptic complexes via their MAGUKs. Note that the exact orientation of MAGUKs in the PSD is not known (and not discussed in this review) and would be affected by their interactions with glutamate receptors, adhesion proteins, other MAGUKs, etc.; for example, there could be different orientations depending on binding partners and position in the scaffold. Putative orientations are shown in this figure and in Figures 9 and 10. SER = smooth endoplasmic reticulum.
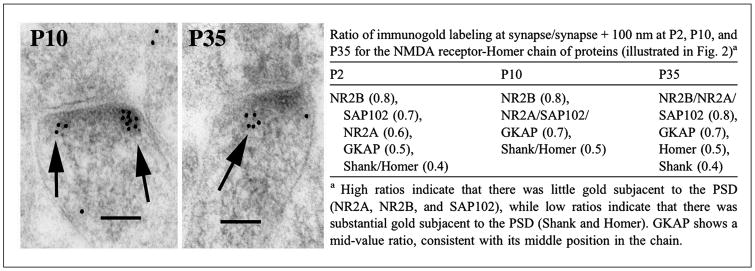
Figure 3.
Immunogold localization in synapses of the hippocampus during postnatal development reveals that the organization of the postsynaptic junctional complex is configured shortly after synaptogenesis, so that it contains a regular sequence of joined proteins at all ages studied, in this case, with glutamate receptors at the surface (NR2A/B), followed by lower MAGUKs (SAP102), then by GKAP, with Shank at the bottom of the postsynaptic density. Micrographs are examples of the immunogold labeling at postnatal day 10 (P10) and P35 for Shank. The table compares the distribution of gold particles at P2, P10, and P35. Reprinted from part of Figure 9 and Table 1 in Petralia and others (2005).
Classification of MAGUKs is complex and cannot be discussed here in detail. A recent phylogenetic study (de Mendoza and others 2010) divides MAGUKs into 5 groups, all originating from choanoflagellates: 1) DLG group including DLG 1-5, ZO 1-3, and CARMA (caspase recruitment domain); 2) MPP (palmitoylated membrane protein) group including MPP1-7 and CASK (calcium/calmodulin-dependent protein kinase; the only MAGUK that can function as an active protein kinase); 3) CACNB (calcium channel β subunit); 4) MAGI (membrane associated guanylate kinase inverted) group including MAGI 1-3 (MAGI-2 = S-SCAM); and 5) the DLG-like group including unusual types of MAGUKs with numerous PDZ domains or with a tyrosine phosphatase domain and found only in choanoflagellates. In this review, we will concentrate on MAGUKs that play well-studied roles in synaptic development and plasticity, such as DLG 1-4 (more commonly known as SAP97, PSD-93 [Chapsyn 110], SAP102, and PSD-95 [SAP90], respectively), MAGIs, and CASK (Figs. 1 and 2). The PSD-95 group (DLG 1-4) and MAGIs are major components of the postsynaptic density of glutamatergic synapses, but in some cases, these MAGUKs can be localized to the presynaptic terminal or the inhibitory postsynaptic compartment (Funke and others 2005; Sheng and Hoogenraad 2007; Alie and Manuel 2010). The MAGI, S-SCAM, is found at both excitatory and inhibitory synapses (Sumita and others 2007). Most evidence supports a presynaptic localization for CASK at excitatory synapses, but it has also been observed in postsynaptic compartments (Hsueh 2009). MAGUKs are also distributed to nonsynaptic sites, such as the cytoplasm or associated with extrasynaptic glutamate receptors (Petralia and others 2002, 2010; Zheng and others 2010) (Figs. 4 and 5).
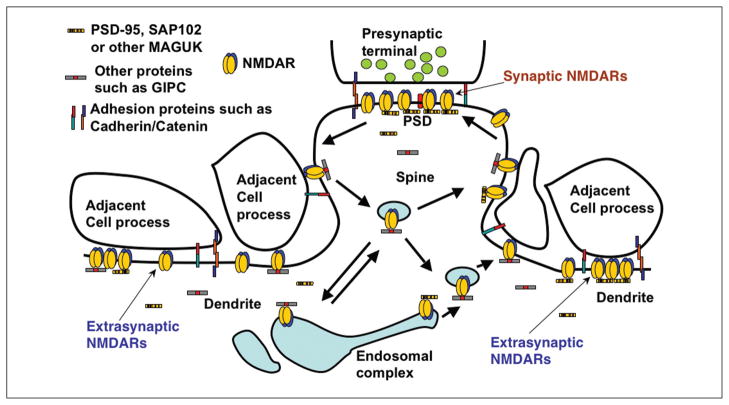
Figure 4.
Diagram illustrating the synaptic and extrasynaptic distributions of NMDA-type glutamate receptors and associated scaffolding and adhesion proteins and especially the associations of extrasynaptic NMDARs with adjacent cell processes. Reprinted from Figure 9 in Petralia and others (2010). GIPC contains a PDZ domain that binds NMDARs (Yi and others 2007); other structures and interactions are described in the text, or see Petralia and others (2010). See also review by Hardingham and Bading (2010).
Figure 5.
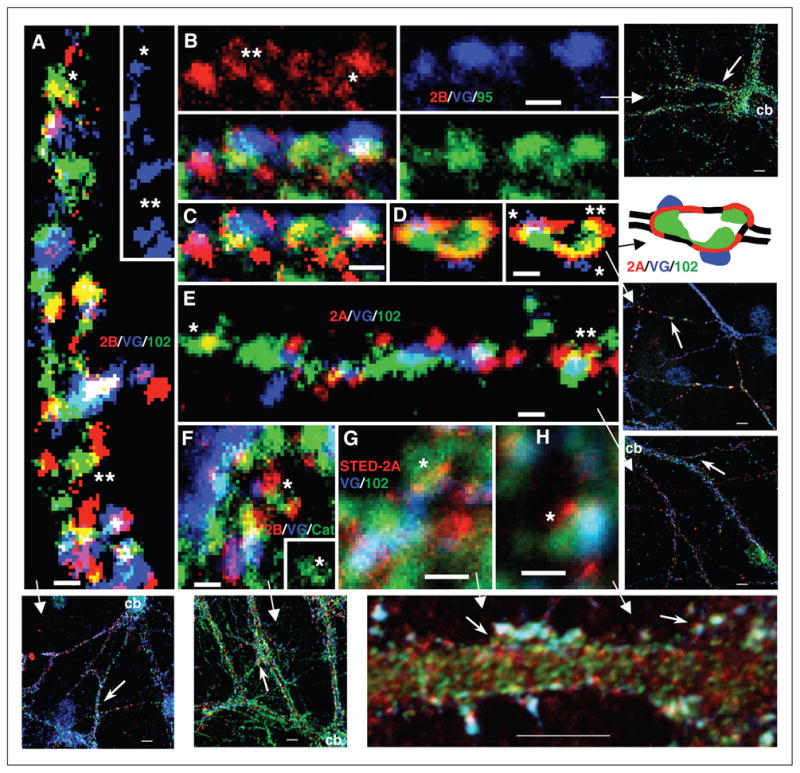
PSD-95 group MAGUKs can be localized both at synapses and associated with NMDARs in extrasynaptic locations. Triple immunofluorescence labeling of hippocampal cultures with 1) live surface labeling of NR2A (red; 555 for D and E; 647 [STED] for G and H) or NR2B (red; 555; A–C and F), 2) the presynaptic marker VGLUT (blue; 647 for A,D–F; 488 for B,C,G,H), and 3) a third protein (green; 488 for A,D–F; 555 for G,H; 647 for B,C) including PSD-95/93 (B and C), SAP102 (A, D, E, G, and H), or catenin (F). (A) NR2B/SAP102: note colocalization in areas devoid of VGLUT-labeled terminals (shown also alone in the inset); some appear to be real associations (*), while others may be coincidental (**). (B and C) NR2B/PSD-95/93: NR2B forms in a perisynaptic ring (**) around synaptic PSD-95/93 and the terminal and forms a ring of 3 puncta around an extrasynaptic punctum of PSD-95/93 (*); (C) the same image, with the NR2B labeling shown in higher contrast. (D) NR2A/SAP102: this is an enlarged region found along a thin, distal dendrite. Note how NR2A labeling is spread in the perisynaptic regions surrounding 2 synapses (*); SAP102 forms around the enlargement in conjunction with both the synaptic and extrasynaptic (**) NR2A; the right image is a high-contrast version of the left one. In the diagram, the outline of the thin dendrite is shown as black lines. (E) NR2A/SAP102: NR2A forms a partial perisynaptic ring around synaptic SAP102 (**); in another example (*), the colocalization of NR2A with an elongate punctum of SAP102 may be coincidental. (F) NR2B/catenin: 2 NR2B puncta, one synaptic and one extrasynaptic, associate with an elongate punctum of catenin (*; seen alone in the inset). (G and H) NR2A[STED]/SAP102: synaptic or perisynaptic puncta (*) of NR2A can be seen at a slightly higher resolution than in the confocal images since the NR2A labeling was imaged using a Leica TSC Stimulated Emission Depletion Microscope (STED). Scale bars are 500 nm. For more information, see Petralia and others (2010). Reprinted from Figure 2 of Petralia and others (2010).
In addition to the PDZ and GK domains, MAGUK molecules contain various combinations of protein domains, including SH3 (Src homology 3), WW (2 tryptophan residues), L27 (MAGUK LIN-2 + LIN-7), and CaMK (calcium/calmodulin-dependent protein kinase) (Funke and others 2005) (Fig. 1). As noted above, many surface receptors and adhesion proteins bind to MAGUK PDZ domains via C-terminal PDZ-binding domains (but note that one adhesion protein, Occludin, binds to the GK domain of the MAGUK, ZO-1, in tight junctions) (Fig. 2). A variety of cytoplasmic proteins also bind to MAGUK PDZ domains, including signaling proteins such as SynGAP, CRIPT, NOS, and Citron (Funke and others 2005; Alie and Manuel 2010), and proteins that form scaffolding links, such as IRSp53.1 that binds to the PDZ domain of PSD-95 and to the proline-rich domain of Shank (Soltau and others 2004). A better known link between PSD-95 and Shank is GKAP, which connects the GK domain of PSD-95 with the PDZ domain of Shank; together, these components form the main scaffold of the postsynaptic density of neuronal glutamatergic synapses. This scaffold is linked indirectly to the actin cytoskeleton, for example, via Spar, which binds to the GK domain of PSD-95. SH3 domains, absent only in the MAGI group of MAGUKs, may be linked to the function of the usually adjacent GK domain, forming a Hook domain between them where the molecule may bend (Funke and others 2005; Feng and Zhang 2009). SH3-GK domains may be important for oligomerization of MAGUKs (Funke and others 2005; Alie and Manuel 2010). MAGIs, lacking SH3 domains, may use WW domains for oligomerization; also like SH3 domains, WW domains bind to proline-rich domains (Nguyen and others 1998; te Velthuis and others 2007). The WW domains of the MAGI, S-SCAM, also bind to dystroglycan at inhibitory synapses (Sumita and others 2007). The L27 domain is named from 2 PDZ proteins from the nematode worm, Caenorhabditis elegans (the MAGUK LIN-2 + LIN-7). L27 domains can heteromulti-merize, and the 2 best-known L27-containing MAGUKs, SAP97 and CASK, can bind together to regulate development. The CASK CaMK domain can phosphorylate itself and CASK’s binding partner, the adhesion protein, neurexin (apparently without the magnesium binding normally required for kinase activation) (Mukherjee and others 2008).
Role of Alternative Splicing
The PSD-95 family members (PSD-95, PSD-93, SAP97, and SAP102) are among the best-characterized MAGUKs. All members of this family are alternatively spliced. For example, SAP97 is alternatively spliced in 3 distinct regions. First, the far N-terminus of SAP97 is composed of either an L27 domain (β-isoform) or a region containing a palmitoylation motif (α-isoform); similar isoforms were also described for PSD-95 and PSD-93 (Funke and others 2005; Schlüter and others 2006) (Fig. 1). This N-terminal splicing of MAGUK proteins has dramatic functional consequences for trafficking of MAGUKs and for their roles in synaptic functioning. The α-isoforms of PSD-95 and SAP97 tend to be less mobile in the dendritic spines than β-isoforms (Waites and others 2009) and influence excitatory synaptic strength independent of synaptic activity, whereas the β-isoforms require synaptic activity (Schlüter and others 2006). Second, there is a region in the N-terminus close to PDZ1 in SAP97 that contains insertions, called I1A or I1B (Funke and others 2005). These insertions facilitate multimerization of SAP97 and mediate interactions with SH3 domains of tyrosine kinases via proline-rich regions (Nakagawa and others 2004; Funke and others 2005). A similar alternatively spliced region in close proximity to PDZ1 was described for SAP102 (Müller and others 1996). Third, the Hook region of SAP97 contains 4 alternatively spliced insertions called I2, I3, I4, and I5. Interestingly, the I3 insert is recognized by the actin/spectrin-associated protein, 4.1N, and this interaction is responsible for synaptic targeting of SAP97 and AMPA receptors (AMPARs) (Rumbaugh and others 2003). The Hook region is also alternatively spliced in PSD-93 and SAP102 (Rumbaugh and others 2003; Müller and others 1996).
Developmental Plasticity
The development of neuronal synapses is dependent on several signaling molecules that are involved in guiding growth cones to their proper location and the formation of contacts between axons and dendrites. Several cell adhesion molecules (Figs. 2 and 4) are thought to be involved in the initial contacts, which then lead to the recruitment of presynaptic and postsynaptic proteins involved in the organization of presynaptic components, such as active zone proteins and synaptic vesicles, and postsynaptic components like postsynaptic density proteins and various receptors (reviewed in Li and Sheng 2003; Ziv and Garner 2004; Petralia and others 2005; Wenthold and others 2008).
Development of the neuromuscular junction (NMJ) in Drosophila is one example of the role of the MAGUK DLG, the Drosophila homolog of PSD-95 family members, in synapse formation. DLG can be easily detected postsynaptically using immunofluorescence but also can be detected presynaptically with electron microscopy (EM) (reviewed in Ataman and others 2006). The clustering of DLG at synapses begins with the contact of motor nerve terminals (presynaptic boutons) with the muscle, followed by the formation of the postsynaptic subsynaptic reticulum (SSR), a complex specialization of muscle membrane that forms around the boutons during larval development. These boutons release glutamate, which is the primary neurotransmitter at the NMJ. Glutamate receptors localize in the SSR membrane layer directly across from the presynapse, while DLG can localize in all layers of the SSR, as well as other areas of the muscle. In contrast to PSD-95 family members, DLG does not interact directly with glutamate receptors and is excluded from the glutamate receptor fields during initial stages of development (Ataman and others 2006; Thomas and others 2010).
During postembryonic development, DLG is first recruited to the muscle membrane and becomes more concentrated at the SSR as the NMJ matures. It is recruited to the edge of synaptic sites and may be involved in regulating the width of the contact sites (reviewed in Packard and others 2003; Ataman and others 2006; Thomas and others 2010). DLG interacts with the homophilic cell adhesion molecule Fascilin II (FasII), which has a PDZ-binding domain (PDZ-BD) that binds to DLG. Initially, FasII is enriched in growth cones and is only expressed at low levels in target muscles. However, as contacts form, FasII clusters together on both sides of the synapse, along with DLG (Kohsaka and others 2007). The amount of FasII present on either side of the junction regulates synaptic connectivity. Overexpression of FasII on one side of the junction reduces the number of active sites, while equal expression restores normal activity (Packard and others 2003). At mature synapses, changes in DLG distribution may regulate synaptic levels of FasII; mutant DLG results in decreased FasII at boutons. In the absence of FasII, synapses retract, suggesting that FasII stabilizes mature contacts. When FasII (or DLG) levels are decreased rather than completely removed, new boutons sprout, increasing neurotransmitter release (Packard and others 2003; Ataman and others 2006; Thomas and others 2010). Interestingly, synaptic accumulation of the glutamate receptor subunits GluRIIA and GluRIIB is also decreased in FasII mutants (Kohsaka and others 2007).
During neuronal development in mammals, expression of PSD-95 family members occurs in a regulated manner (Figs. 3 and 6). SAP102 is highly expressed before post-natal day 10 (P10) in the rodent brain and then gradually decreases. In contrast, little PSD-95, PSD-93, and SAP97 are found in the brain of the newborn rat, but their expression dramatically increases during postnatal development. PSD-95 is the dominant MAGUK in the hippocampus of adult rats (Sans and others 2000,2001). Interestingly, there is a correspondence between the expression pattern of SAP102 or PSD-95 and NR2B- or NR2A-containing NMDA receptors (NR2B- or NR2A-NMDARs), respectively (Sans and others 2000). Thus, similar to the pattern for SAP102, NR2B is the dominant NR2 subunit that is detected in the hippocampus during early postnatal development, while NR2A has a similar pattern to PSD-95 and is not abundant until late postnatal development (Sans and others 2000; Washbourne and others 2004; Petralia and others 2005). One MAGUK that may influence NR2B expression levels is CASK. Translocation of CASK to the nucleus has been reported to down-regulate the expression of NR2B through its interaction with the transcription factor T-brain-1 (Tbr-1) (reviewed in Hsueh 2009). The trafficking and clustering of NMDARs at synapses will be discussed in more detail in a later section.
Figure 6.
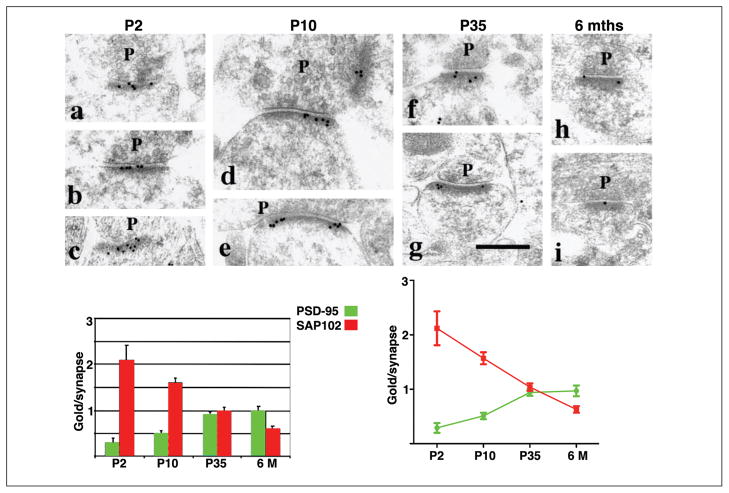
Immunogold labeling of SAP102 and PSD-95 in spines in the CA1 stratum radiatum of the hippocampus during development. Electron micrographs show immunogold labeling for SAP102 at P2, P10, P35, and 6 months. P = presynaptic terminal. Postsynaptic structures include dendrite shafts (b), protuberances (a and c), and spines (d–i). Scale bar is 300 nm. Representative micrographs were chosen to illustrate the decrease in SAP102 with age. Summary histogram and chart show developmental changes in the number of gold particles per synapse for SAP102 versus PSD-95; note the reciprocal pattern evident. Modified from Figure 4 and Table 2 of Sans and others (2000; see that paper for more details).
It is not clear if MAGUKs are essential elements for synapse formation. As discussed previously, SAP102 is present in early excitatory synaptic contacts, while the other MAGUK family members are less common at these early stages (Sans and others 2000). Knockout of PSD-93, believed to be the only MAGUK in cerebellar Purkinje neurons, does not affect synaptogenesis or PSD formation in these neurons. This finding suggests that MAGUKs are not essential for synaptogenesis in Purkinje cells (McGee and others 2001); in contrast, overexpression of PSD-95 significantly increases spine number in hippocampal neurons (El-Husseini and others 2000b). After the earliest synaptic contacts are initiated by various adhesion factors (discussed later in this review), presynaptic and postsynaptic components are recruited to the synaptic terminal in packets. One preformed postsynaptic complex includes PSD-95, GKAP, and Shank (Gerrow and others 2006). In young cortical neurons, SAP102 is associated with NMDAR packets traveling along microtubules (Washbourne and others 2002,2004). The recruitment process occurs rapidly; a synapse with a PSD is formed within 2 hours (Friedman and others 2000). By postnatal day 2 (P2), hippocampal excitatory synapses contain the majority of key PSD components involved in synaptic function and/or development (Petralia and others 2005) (Fig. 3), including mainly NR2B-NMDARs and SAP102, as well as GKAP, Shank, Homer, and Syn-GAP (Petralia and others 2005; Carlisle and others 2008; Foa and Gasperini 2009).
MAGUKs, especially PSD-95, form the key skeleton of the PSD structure (Blanpied and others 2008), and the number of PSD-95 molecules determines the size of the PSD (Gray and others 2006). In mature hippocampal neurons, the MAGUKs are largely concentrated at the PSD; 69.4% of SAP102 and 94.2% of PSD-95 in spines were found in the PSD area (Zheng and others 2010) (Figs. 2, 7, and 8). The number of MAGUKs within the PSDs is tightly regulated since they play critical roles in synaptic functioning. Various mechanisms are likely involved in the degradation of MAGUKs. PSD-95 is degraded by the ubiquitin-proteasome system (UPS) after its direct ubiquitination or indirectly through the degradation of other synaptic components (Colledge and others 2003; Pak and Sheng 2003). Activation of the UPS system in spines involves CaMKIIα-dependent recruitment of proteasomes to dendritic spines (Bingol and others 2010).
Figure 7.
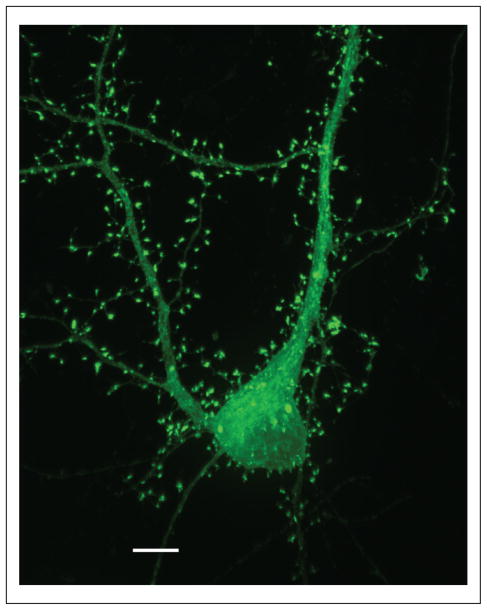
A hippocampal neuron expressing GFP-SAP102. Hippocampal neurons were cultured at embryonic day 18, transfected on 18 days in vitro (DIV), and then fixed on 22 DIV. Note prominent labeling throughout synaptic spines as well as throughout the dendrites and soma. Scale bar is 5 μm.
Figure 8.
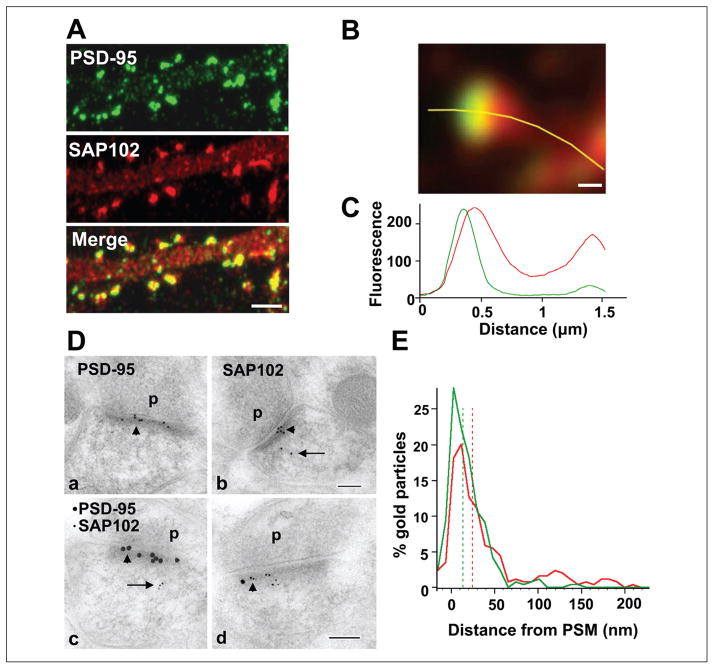
Distribution of SAP102 and PSD-95 in spines. (A) Double staining of endogenous SAP102 (red) and PSD-95 (green) in hippocampal neurons (21 DIV). Both SAP102 and PSD-95 are enriched in spines relative to dendrites and are highly colocalized in spines but not in dendrites. Scale bar is 2 μm. (B) High-magnification image of a mushroom spine that was double stained for endogenous SAP102 (red) and PSD-95 (green). Scale bar is 200 nm. (C) Fluorescence intensity of SAP102 (red line) and PSD-95 (green line) along the yellow marker in panel B. The x-axis shows the distance from the left end of the yellow marker, and the y-axis is the fluorescence intensity. (D) Immunogold labeling of SAP102 and PSD-95 in spines from CA1 stratum radiatum of the 37-day-old rat hippocampus. SAP102 is localized at the PSD (arrowhead) as well as in the cytoplasm (arrow), while PSD-95 is concentrated at the PSD (arrowhead). (a and b) Single immunogold labeling of PSD-95 (a) or SAP102 (b) using 10 nm gold (2 animals for each). (c and d) Sections that were double labeled for PSD-95 (15 nm gold) and SAP102 (5 nm gold) show distributions indistinguishable from those that were single labeled (2 animals). p = presynaptic terminal. Scale bar is 100 nm. (E) Distribution of gold particles in spines from CA1 stratum radiatum of P37 rat hippocampus. There is an overlap in the localization of PSD-95 and SAP102. The median values (dash lines) are 13.5 nm from the postsynaptic membrane for PSD-95 and 24.2 nm from the postsynaptic membrane for SAP102. SAP102 is more generally distributed in the cytoplasm between 50 to 250 nm from the postsynaptic membrane. Data are from both single and double labeling of SAP102 or PSD-95 (n = 294 for PSD-95, including n = 192 from single labeling and n = 102 from double labeling; n = 283 for SAP102, including n = 144 from single labeling and n = 139 from double labeling). PSM = postsynaptic membrane. Reprinted from Figure 1 of Zheng and others (2010).
MAGUK Interactions with Cell Adhesion Molecules during Development
The MAGUKs interact with several cell adhesion molecules (CAMs) that are involved in regulating neurite outgrowth, axon formation, synaptogenesis, and spine maturation (Figs. 2, 4, and 5). The cadherins were among the first CAMs linked to the regulation of synaptic contacts. They are homophilic, calcium-dependent CAMs, and their presence at the active zone and PSD help stabilize axodendritic contacts (reviewed in Dalva and others 2007). Interactions through their C-terminal domains with the catenins (α, β, and δ) link cadherins to the actin cytoskeleton and MAGUK proteins, such as MAGI. In addition to cadherins, several other CAMs recently have been reported to interact with MAGUKS. Neuroligin, SALMs 1-3 (synaptic adhesion-like molecules), and NGL (netrin-G ligand) interact with PSD-95 family members through a class I binding domain (X-S/T-X-V/L), while SynCAMs (synaptic cell adhesion molecules) and neurexins (Nrxs) bind CASK through a class II binding domain (X-ϕ-X-ϕ; ϕ = hydrophobic amino acid) (Sheng and Sala 2001). A number of CAMs can induce presynaptic and/or postsynaptic differentiation including neuroligins (NLs) with neurexins, EphBs with ephrinBs, SynCAMs, and netrin-G ligands (NGL-2 and NGL-3) (reviewed in Dalva and others 2007). Recently, both the SALMs (SALMs 3 and 5) (Mah and others 2010) and LRRTMs (leucine-rich repeat transmembrane neuronal proteins) (Ko and others 2009; de Wit and others 2009; Siddiqui and others 2010) were also reported to regulate synapse formation by inducing presynaptic differentiation.
MAGUKs can play a role in regulating the location of CAMs that are involved in axogenesis and/or dendrite formation. In the chick retina, MAGIs regulate the localization of the homophilic CAMs Sidekick and Dscam (Down syndrome cell adhesion molecule) to specific sub-laminae layers. This allows neurons to target processes to these regions of the retina during development. MAGI-1 regulates Sidekick2 localization in photoreceptor cells of the outer plexiform layer, while MAGI-2 and MAGI-3 interact with Sidekick2 and Dscam, respectively, in the inner plexiform layer. Knockdown of MAGI-2 or deletion of the PDZ-BD of Sidekick2 disrupts the localization of Sidekick2 to its proper target. This loss of Sidekick2 causes defasciculation of axonal processes and mistargeting of axons to other layers, disrupting laminar specificity in retinal ganglion cells (Yamagata and Sanes 2010). On the other hand, several CAMs interacting with MAGUKs are involved in the formation of dendrites and recruitment of receptors to synapses. Dasm1 (dendrite arborization and synapse maturation 1), which binds Shank and S-SCAM but not GRIP (a PDZ protein that binds to several glutamate receptors) or PSD-95 family members (Shi and others 2004a), influences dendrite morphology and spine maturation (Shi and others 2004b). Expression of RNAi or Dasm1 dominant-negative constructs, such as Dasm1 lacking the C-tail, results in a decrease in dendritic arborization (Shi and others 2004b). Also, overexpression of the SALMs in cultured hippocampal neurons at early stages (4-7DIV) enhances neurite outgrowth (Wang and others 2006; Wang and others 2008).
For many of these CAMs, interactions with MAGUKs may regulate their localization at a particular type of synapse. For example, interactions between neuroligins and MAGUKs regulate the balance between excitatory and inhibitory synapses. Neuroligin 2 (NL2) interacts with β-dystroglycan and S-SCAM/MAGI-2 at inhibitory synapses (Sumita and others 2007). However, overexpressed PSD-95 can recruit NL2 from inhibitory synapses to excitatory synapses and increase the ratio of excitatory to inhibitory synaptic responses (reviewed in Cline 2005). Recently, several MAGUKs were found to localize to neuronal nicotinic synapses with neuroligin 1 (NL1). S-SCAM, PSD-93, and PSD-95 are all enriched at synapses containing nicotinic acetylcholine receptors (nAChRs), and all 3 can bind to NL1; however, only S-SCAM influences the surface expression of NL1 (Rosenberg and others 2010). S-SCAM links NL1 to the postsynaptic adenomatous polyposis coli (APC) complex via β-catenin. NL1 binds to both the PDZ1 and WW domains of S-SCAM, and β-catenin associates with its PDZ5 domain (reviewed in Rosenberg and others 2010). The APC complex interacts with nAChRs, via the protein 14-3-3, and with cytoskeletal proteins. Peptides that inhibit S-SCAM association with β-catenin and APC decrease the surface clustering of S-SCAM and NL1. Loss of NL1 also has a presynaptic effect; synaptic localization of its binding partner neurexin and associated active zone proteins is decreased, resulting in less mature terminals in avian ciliary ganglion neurons (Rosenberg and others 2010). Therefore, the S-SCAM/β-catenin/APC complex may serve to localize NL1 at nicotinic synapses and promote the maturation of presynaptic terminals.
Synaptic levels of CAMs may also be regulated by MAGUK interactions. One example, involving the adhesion molecule FasII and DLG at the NMJ of Drosophila, was described in the previous section. In CASK knockout (KO) mice, spontaneous synaptic events are increased at excitatory synapses but decreased at inhibitory synapses (Atasoy and others 2007). These mice have decreased protein levels of β-neurexins but increased expression of neuroligins (Atasoy and others 2007). Since CASK has been reported to regulate the gene expression of proteins, by forming a complex with transcription factors in the nucleus (Hsueh 2009), perhaps the changes in synaptic activity seen in CASK KO mice are due to changes in the synaptic levels of key proteins up-regulated by this gene expression. The CASK KO mice have no major physical abnormalities, aside from a cleft palate, and their synaptic structures appear normal; however, they die shortly after birth (Atasoy and others 2007). Modifications of CASK by SUMOylation, or its interaction with Syndecans, can also modulate dendritic spinogenesis but will not be discussed in detail here (reviewed in Hsueh 2009).
Finally, MAGUKs can interact with CAMs involved in the regulation of surface expression of postsynaptic receptors at synaptic spines. Dasm1 dominant-negative constructs, including the overexpression of the Dasm1 C-tail with a single point mutation in the PDZ-BD, decrease AMPAR-mediated synaptic transmission, resulting in an increase in silent synapses (Shi and others 2004a). S-SCAM interacts with the PDZ-BD of Stargazin and other TARPs (transmembrane AMPA regulatory proteins) (Deng and others 2006) and may serve as a possible link between Dasm1 and AMPARs. LRRTM2 associates with PSD-95 when overexpressed in heterologous cells, but it has not been shown to coimmunoprecipitate with PSD-95 from the rat brain. However, it can regulate the recruitment of PSD-95 to dendritic spines in hippocampal neurons (Ko and others 2009; de Wit and others 2009; Siddiqui and others 2010). Similar to Dasm1, in vitro expression of LRRTM2 shRNA in hippocampal neurons decreases surface expression of AMPARs, and in vivo injection of lentivirus/LRRTM2 shRNA into the dentate gyrus decreases evoked excitatory synaptic currents recorded in granule cells (de Wit and others 2009). Overexpression of SALM1 at later stages of development (14DIV) increases surface detection of NR2A-GFP (green fluorescent protein) and recruits endogenous NMDARs to dendritic spines in hippocampal neurons (Wang and others 2006). Overexpression of SALM1 lacking the PDZ-BD shows less surface expression in dendrites and less colocalization with endogenous PSD-95 and NMDARs, compared with expression of wild-type SALM1 (Wang and others 2006). Bead-induced aggregation of SALM2 and SALM3 in dendrites also causes clustering of PSD-95 (Ko and others 2006; Mah and others 2010). In contrast, SALM5, which does not have a PDZ-BD, does not cluster PSD-95 (Mah and others 2010).
Modulating Synaptic Plasticity: Spine Dynamics
Synaptic plasticity is a term used to describe any process in the mammalian CNS that leads to changes in the strength of synaptic transmission between neurons. One way to modulate synaptic plasticity is via changes in spine dynamics. MAGUKs play a central role in molecular mechanisms involved in the regulation of spine morphogenesis, centered on actin-regulating proteins that play major roles in controlling spine size and shape (Tada and Sheng 2006). In hippocampal brain slices, induction of long-term potentiation (LTP) at individual spines leads to an increase in actin-based spine volume that depends on the expression of PSD-95 (Steiner and others 2008). Knockdown of endogenous PSD-95 results in reduced spine volume during LTP, while overexpression of PSD-95 following knockdown rescues the effect. PSD-95 is relatively stable in spines. During basal conditions, the mobility of PSD-95 is not affected by the mobility of actin filaments (Fig. 9). In contrast, the mobility of SAP102 in spines depends on actin filaments (Zheng and others 2010). During synaptic plasticity, PSD-95 can regulate the mobility of actin filaments. One possible explanation is that PSD-95 directly binds to glutamate receptors and links the receptors to the actin-based cytoskeleton complex. However, it is not known if other MAGUKs play a similar role in regulating spine volume or if they form similar complexes with actin in the PSD. It is possible that other MAGUKs interact with actin through similar protein complexes as PSD-95 does since the MAGUK family members have similar SH3/GK domains, through which they bind to a variety of scaffolding proteins.
Figure 9.
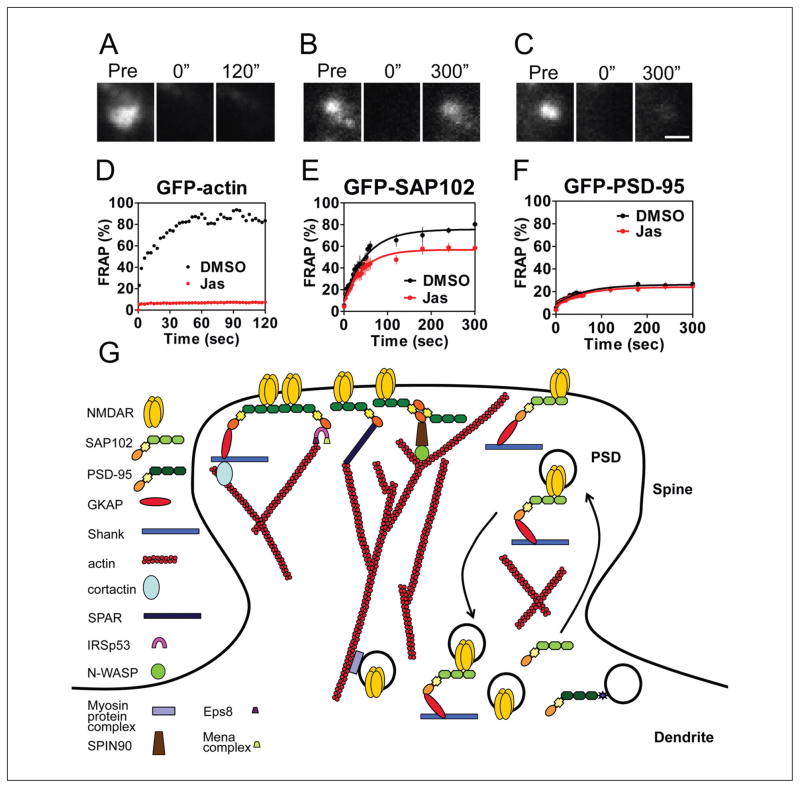
Actin and MAGUKs in spines. (A–C) The images represent FRAP of neurons expressing GFP-actin (A), GFP-SAP102 (B), and GFP-PSD-95 (C) in the presence of Jasplakinolide. The fluorescence of the same areas before (Pre) and at 0 and 120 (or 300) seconds after photobleaching is shown. Scale bar is 1 μm. (D) FRAP sample of transfected GFP-actin in spines. The recovery was totally blocked by incubating the neurons with actin stabilizer Jasplakinolide (10 μM) for 30 minutes before FRAP measurement. The solution also contained 10 μM Jasplakinolide during imaging. Untreated cultures (black) and Jasplakinolide-treated cultures (red) are shown over a 120-second recording. Jas = Jasplakinolide. (E) FRAP of GFP-SAP102 was partly blocked by pretreatment of Jasplakinolide for 30 minutes. n = 5 for DMSO control, and n = 7 for Jasplakinolide treatment. (F) FRAP of GFP-PSD-95 was not affected by pretreatment of Jasplakinolide. n = 8 for each. (G) Some proteins are quite stable at synapses under basal conditions, typically involving NMDARs and PSD-95–based scaffolding proteins. PSD-95 protein complexes interact with actin filaments in multiple ways, including PSD-95–GKAP–shank–cortactin–actin, PSD-95–IRSp53–Eps8 or Mena protein complex–actin, PSD-95–SPAR–actin, and PSD-95–SPIN90/WISH–N-WASP–actin. Some synaptic proteins exchange between synaptic and extrasynaptic regions frequently, for example, SAP102 protein complexes and a small portion of NMDA receptors and PSD-95. Panels A through F are reprinted from Figure 5 in Zheng and others (2010).
Because dendritic spines are actin-rich protrusions, the size and shape of spines depend upon the regulation of actin filaments in the spine. PSD-95 is a critical element of the receptor-scaffolding-actin complex (Fig. 9). Overexpression of PSD-95 increases the size of the spine head (El-Husseini and others 2000b; Nikonenko and others 2008), and a high abundance of actin has been found near PSD-95 in spines (Frost and others 2010). Many PSD-95–actin complexes have been identified in the PSD fraction of the rat brain, including PSD-95–IRSp53–Eps8 (or Mena protein complex)–actin, PSD-95–SPAR–actin, PSD-95–GKAP–shank–cortactin–actin, PSD-95–SPIN90/WISH–N-WASP–actin, and SAP97–MyosinVI–actin (reviewed in Petralia and others 2009; Choi and others 2005) (Fig. 9). Some proteins that are downstream of PSD-95, for example, SPIN90/WISH and SPAR, may be important in regulating spine morphology, suggesting that multiple pathways are involved during glutamate receptor and PSD-95–dependent actin remodeling (Pak and others 2001; Lee and others 2006).
Glutamate Receptor Trafficking and Clustering at Synapses
The NMDAR is a tetrameric complex that assembles with 2 NR1 subunits and 2 NR2 subunits. The first 2 PDZ domains of all 4 MAGUKs can directly bind to the C2’ cassettes of NR1 subunits and/or the distal C-terminus of NR2 subunits (reviewed by Wenthold and others 2008) (Fig. 2). MAGUK-NMDAR complexes could form early in the secretory pathway, such as in the endoplasmic reticulum (ER) and Golgi apparatus. For example, SAP102, NMDAR, and mPins form clusters in the ER (Sans and others 2005), and SAP97 associates with the NMDAR-CASK-KIF17 complex in ER-derived transport vesicles (Jeyifous and others 2009). Synaptic delivery of the NMDAR-MAGUK complex might require its association with an exocytosis-related octameric protein complex called the exocyst. For example, the exocyst component Sec8 is required for delivery of the exocyst-SAP102-NMDAR complex to the postsynaptic membrane (Sans and others 2003; Jeyifous and others 2009). However, NMDARs can still be delivered to the extrasynaptic membrane even when their ability to bind PDZ domain– containing proteins is blocked, suggesting that MAGUKs are not required for the extrasynaptic localization of NMDARs (Sans and others 2003). On the other hand, deletion of the PDZ-BD of NR2B subunits leads to a significant decrease in synaptic NMDARs, and NR2B with a point mutation that blocks its interaction with MAGUKs does not enter the synapse (Yi and others 2007; Wenthold and others 2008). These findings suggest that NR2B-NMDARs require a MAGUK interaction for synapse localization. It is not clear whether NR2A-NMDARs require a MAGUK interaction for synaptic localization. Some studies find that NR2A-NMDARs do not require a PDZ interaction for synapse localization (Prybylowski and others 2005; Park and others 2008), whereas others demonstrate that maturational synaptic delivery of NR2A- NMDARs requires the presence of MAGUKs (Elias and others 2008). NMDARs containing either NR2A or NR2B also can be found in various extrasynaptic regions of spines and dendrites, often at points of close contact with adjacent cell processes; this has been shown with a combination of in vivo and in vitro light and electron microscope methodologies (Petralia and others 2010). In some cases, MAGUKs are associated closely with these extra-synaptic NMDARs (Figs. 4 and 5), even though there is usually little evidence of a dense structure as seen with electron microscopy. Thus, these extrasynaptic NMDARs usually would not be bound in a complex scaffold, but MAGUKs still may be involved in these extrasynaptic localizations.
Interestingly, SAP102 and PSD-95 seem to play different roles in regulating the expression and trafficking of NMDA receptors (Elias and Nicoll 2007; Wenthold and others 2008). Although both of them have the ability to bind to either NR2A- or NR2B-NMDARs (Wenthold and others 2008), overexpression of SAP102, but not PSD-95, leads to an increase of functional synaptic NMDARs in immature neurons. During synaptogenesis, PSD-95 but not SAP102 regulates the maturational switch of NR2B-NMDARs to NR2A-NMDARs (Elias and others 2008). Also, knockout of PSD-95 increases NR2A- but not NR2B-NMDARs in lipid rafts (Delint-Ramirez and others 2010). Although MAGUKs have distinct expression patterns and abilities to recruit receptors, individual family members can functionally compensate when the others are knocked down (Elias and Nicoll 2007). In addition to different expression levels during development (as discussed earlier), SAP102 and PSD-95 have different mobility at synapses. The majority of SAP102 is highly mobile in spines, whereas the majority of PSD-95 is immobile in spines (Zheng and others 2010) (Fig. 9). Regulation of MAGUK mobility is tied to actin mobility, as discussed in the previous section. NR2B-NMDARs are highly mobile, whereas NR2A-NMDARs are stable at synapses (Groc and others 2006). It is not clear if the mobility of NR2B-NMDARs is related to the mobility of SAP102. Nevertheless, SAP102 and other PSD scaffolding proteins, such as Shank and Homer, turn over rapidly during synaptogenesis. The different mobility of PSD-95 and SAP102 may indicate that they play distinct roles in regulating expression and trafficking of NMDARs.
Although MAGUKs cluster NMDARs at synapses, the NMDAR-MAGUK complex is not required for the clustering of MAGUKs at the PSD. Specifically, point mutations that disrupt NMDAR-SAP102 binding do not alter the synaptic localization of SAP102 in cultured hippocampal neurons (Zheng and others 2010). Instead, synaptic clustering of SAP102 requires its SH3/GK domains, and synaptic clustering of PSD-95 requires its N-terminal palmitoylation motif and a tyrosine-based trafficking signal in its C-terminus (Craven and Bredt 2000; El-Husseini and others 2000a; Zheng and others 2010). Thus, clustering of SAP102 at synapses could depend on its interaction with PSD cytoskeleton proteins indirectly via the GK domain (noted above), while clustering of PSD-95 at synapses could depend mainly on its membrane association through palmitoylation sites. Presumably, this clustering of MAGUKs at synapses also is affected by their multimerization via either their SH3/GK domains or N-terminals (Morabito and others 2004; Feng and Zhang 2009), as discussed elsewhere in this review. In Drosophila, the SH3/GK region of DLG is also important for the proper trafficking of DLG to synaptic contact sites of the NMJ (reviewed by Packard and others 2003; Thomas and others 2010). As mentioned above, DLG is first targeted to the muscle membrane and then moves to the SSR at the NMJ. Deletion of the Hook region between the SH3 and GK domains of DLG disrupts membrane association with the subcortical network of the muscle and thus results in inefficient synaptic targeting. On the other hand, synaptic targeting of DLG lacking the GK domain can occur in the presence of endogenous DLG, suggesting that DLG multimers can form to traffic the truncated protein to the proper location. Finally, removal of the first 2 PDZ domains disrupts the second step, targeting to the SSR, and results in localization of DLG outside of the NMJ. However, the presence of either PDZ domain allows DLG recruitment to the synaptic region of the NMJ (Thomas and others 2000).
The members of the PSD-95 family of proteins differently regulate basal synaptic activity (Elias and Nicoll 2007). Many electrophysiological reports show that overexpression of PSD-95 enhances amplitudes of AMPAR synaptic currents but not amplitudes of NMDAR synaptic currents. These data were unexpected since NMDARs coimmunoprecipitate with PSD-95 in biochemical experiments (Kornau and others 1995). PSD-95, which interacts with AMPARs indirectly through TARPs, such as Stargazin, is required for the synaptic expression of AMPARs (Bats and others 2007). Deletion of the PSD-95 binding motif of Stargazin disrupts synaptic localization of AMPARs (Chen and others 2000). Also, AMPAR function at synapses is regulated by the phosphorylation of Stargazin (Sumioka and others 2010), which is probably via CaMKII (discussed below; Opazo and others 2010). Similarly, overexpression of PSD-93 and, to a lesser extent, SAP102 selectively enhances AMPAR synaptic currents (Elias and Nicoll 2007). SAP97 is the only MAGUK that directly binds to the C-terminus of the GluR1 subunit of AMPARs (Leonard and others 1998). The first 2 PDZ domains of SAP97 also bind to the C-terminus of NMDARs. In organotypic hippocampal slice cultures, overexpression of SAP97 increases the number of synaptic AMPARs and NMDARs (Howard and others 2010). Interestingly, the AMPAR-SAP97 complex forms early in the secretory pathway (Sans and others 2001).
At excitatory synapses, AMPARs undergo rapid endocytosis, while NMDARs are relatively stable under basal conditions. Studies show that PSD-95 is involved in the endocytosis of both AMPA and NMDA receptors. Endocytosis occurs at an endocytic zone, which is on the spine membrane adjacent to the postsynaptic density (Lu and others 2007). The physical link between PSD-95–GKAP– Shank–Homer–Dynamin-3 may help determine the position of the endocytic zone (Lu and others 2007). Although PSD-95 does not directly interact with AMPARs, knockdown of PSD-95 inhibits NMDAR-triggered AMPAR endocytosis at synapses (Bhattacharyya and others 2009). On the other hand, PSD-95 directly interacts with NMDARs, but how it regulates the endocytosis of NMDARs remains unclear. The endocytosis of NR2A is dependent on a dileucine motif (Lavezzari and others 2004). In the NR2B subunit, the endocytic motif YEKL is located only 3 amino acids away from the PDZ-binding motif ESDV, where PSD-95 binds to the NR2 subunits. Expression of PSD-95 disrupts NR2B-mediated endocytosis, and deletion of the PDZ-binding domain promotes endocytosis of NR2B (Roche and others 2001). It is possible that disruption of the PDZ interaction is the first step of NMDAR endocytosis at the synapse, followed by binding of the clathrin adaptor protein AP-2 to the YEKL motif of NR2B-NMDARs (Lavezzari and others 2003; Prybylowski and others 2005). This will be discussed in further detail in the next section.
MAGUKs also link together glutamate receptors and other proteins from different regions of the PSD. They interact via Shank with members of the Homer protein family that have important functional and developmental roles in synapses (Foa and Gasperini 2009). In addition to structural roles, Homer proteins are key regulators of calcium concentration in dendritic spines. They link metabotropic glutamate receptors (mGluRs) and various calcium channels (Fig. 2), both on nearby smooth ER (IP3 or ryanodine receptors) and on the surface membrane (including calcium-permeable glutamate receptors), and this arrangement may precisely regulate calcium levels in subregions of postsynaptic spines (Petralia and others 2001; Foa and Gasperini 2009).
Modulating Synaptic Plasticity: Kinases and Phosphorylation
Two well-studied examples of synaptic plasticity are LTP and long-term depression (LTD) (reviewed by Elias and Nicoll 2007). Both of these are triggered by calcium influx through NMDARs, followed by the redistribution of CaMKII (Fig. 10) from the dendritic shaft to spines and its activation by autophosphorylation (Merrill and others 2005). CaMKII is the most abundant protein in the PSD, and in addition to its kinase activity, it is also thought to play a structural role at the synapse. Activated CAMKII interacts with and/or phosphorylates many proteins in the PSD and thus regulates synaptic plasticity and spine growth (Fig. 10). Depending on the state of phosphorylation of CaMKII, an increase, in the case of LTP, or decrease, in the case of LTD, of the number of synaptic AMPARs is observed (Pi and others 2010). Furthermore, phosphorylation of PSD-95 at serine 73 (S73) by CaM-KII inhibits both LTP and LTP-associated spine growth, controlling activity-dependent trafficking of PSD-95 and Shank2 out of spines (Steiner and others 2008). Similarly, in Drosophila, phosphorylation of DLG by CAM-KII at serine 48 (S48) regulates the movement of DLG out of the synaptic region of the NMJ, resulting in a phenotype similar to that of DLG mutants, which have enlarged boutons and abnormal NMJ arbors (reviewed in Thomas and others 2010). Recent data also suggest that phosphorylation of a specific splice variant of SAP97, in the Hook region, by CaMKII disrupts its interaction with AKAP79/150, resulting in a decrease of GluR1-mediated AMPAR currents (Nikandrova and others 2010).
Figure 10.
CaMKII and synaptic plasticity in dendritic spines. Upon synaptic excitation, CaMKII is redistributed from the dendritic shaft to spines and becomes constitutively active. Activated CAMKII phosphorylates Ser39 of SAP97, increasing the clustering of SAP97 and AMPARs at postsynaptic sites (Merrill and others 2005). CaMKII also contributes to LTP by fostering phosphorylation of Ser831 of the GluR1 subunit of the AMPAR, resulting in an increase in AMPAR ion channel activity. Activated CAMKII also specifically interacts with NR2B-containing NMDARs, and this is crucial for expression of LTP in the CA1 region of the hippocampus (Barria and Malinow 2005). In the PSD, CaMKII phosphorylates Stargazin, facilitating its binding to PSD-95 and its synaptic retention; indirectly also, AMPARs bound to Stargazin are stabilized (Opazo and others 2010). Activated CaMKII further stimulates the formation of a spine growth-promotion complex (Steiner and others 2008). Interestingly, CaMKII phosphorylation of Ser73 of PSD-95 terminates the spine growth by inducing the translocation of PSD-95 and SHANK out of the active spine. CaMKII translocation to synapses is also required for activity-induced proteasome accumulation in spines that is essential for maintenance of LTP (Bingol and others 2010).
Tyrosine kinase activity also affects synaptic plasticity and MAGUK-NMDAR associations. PSD-95 regulates NMDAR localization and its signaling cascades upon activation. Deletion of the NR2B PDZ-BD decreases synaptic localization of NR2B-containing NMDARs (Steigerwald and others 2000; Prybylowski and others 2002). Mutating this domain blocks incorporation of NR2B into active synapses in cerebellar granule cell cultures (Prybylowski and others 2005). However, blocking internalization of the receptors through AP-2–dependent endocytosis using dominant-negative μ2, which binds to a tyrosine-based motif, overcomes this deficiency. The Src tyrosine kinase family member Fyn interacts with the PDZ3 of PSD-95 and regulates tyrosine phosphorylation of Y1472 of NR2B, part of the AP-2 binding site (YEKL) in the NR2B C-terminal tail (Tezuka and others 1999; Prybylowski and others 2005; reviewed in Wenthold and others 2008). Constitutively active Fyn or a peptide inhibiting AP-2 binding increases NMDAR-mediated synaptic activity, suggesting that the association of Fyn with PSD-95 helps regulate synaptic localization of NMDARs by phosphorylation of NR2B. This phosphorylation maintains the receptor at the PSD and prevents its extrasynaptic localization, where it can be internalized via its association with AP-2. Similar to Fyn, Src also can regulate the phosphorylation of Y1472 of NR2B and prevent NMDAR internalization in an activity-dependent manner (Zhang and others 2008). In support of these findings, Y1472F knockin mice generate an increase in perisynaptic NR2B-containing NMDAR receptors (Nakazawa and others 2006). Finally, PSD-93 also has been reported to interact with Fyn. Biochemical fractionation of brain tissue from PSD-93 KO mice indicates that the loss of PSD-93 decreases Fyn localization to the synaptosomal membrane fraction and reduces tyrosine phosphorylation of the NMDARs in that fraction (Sato and others 2008). However, whether this Fyn-mediated phosphorylation regulates NMDAR synaptic localization is yet to be determined.
Pyk2 (proline-rich tyrosine kinase) is another tyrosine kinase associated with PSD-95; it regulates NMDAR activity and is involved in synaptic plasticity. Pyk2 binds to the SH3 domain of PSD-95, and its association with PSD-95 is enhanced by calcium and calmodulin (Seabold and others 2003; Bartos and others 2010). Similar to CAMKII, calcium influx through the NMDAR promotes Pyk2 clustering and activation through trans-autophosphorylation, as well as enhancing Pyk2 localization at the postsynapse in hippocampal neurons. A PKC-Pyk2-Src– signaling cascade regulates LTP in the CA1 region of the hippocampus (reviewed in Salter and Kalia 2004) and requires Pyk2 association with PSD-95. LTP is blocked if the interaction between Pyk2 and PSD-95 is prevented by the infusion of glutathione S-transferase (GST) proteins expressing the SH3 domain of PSD-95 or the proline-rich region of Pyk2; this does not affect basal NMDAR responses (Bartos and others 2010). The serine/threonine kinase, Cdk5 (cyclin-dependent kinase 5), has recently been reported to regulate this NMDAR/PSD-95/tyrosine kinase complex. Cdk5 can phosphorylate the N-terminus of PSD-95 and thereby decreases the ability of PSD-95 to form multimers in hippocampal cultures and cluster ion channels overexpressed in heterologous cells (Morabito and others 2004). Cdk5 also influences the association of PSD-95 with the tyrosine kinase Src, which binds to the N-terminal domain of PSD-95 (Kalia and Salter 2003; Kalia and others 2006). Inhibition of Cdk5 results in an increased association of PSD-95 with Src but not with Fyn (Zhang and others 2008). The use of p35 KO mice or inhibition of Cdk5 in cortical neurons was also shown to increase the association between PSD-95 and Pyk2 (Bianchetta and Morabito 2009), suggesting that Cdk5 may regulate the formation of a Src/Pyk2/PSD-95 complex.
MAGUK Regulation of Other Receptors
In addition to glutamate and acetylcholine receptors, MAGUKs show various functional associations with other receptors and are even found at inhibitory synapses, as noted above. PSD-95 associates with the D1 dopamine receptors through its N-terminal (NT) domain (Zhang and others 2007). This interaction regulates dopamine receptor signaling by reducing the surface expression of D1 receptors and causing a decrease in D1-mediated cAMP production (Zhang and others 2007). PSD-95 KO mice show increased locomotor activity in response to dopamine agonists and amphetamine (Zhang and others 2007). PSD-95 also influences the ability of dopamine receptors to interact with glutamate receptors. D1 receptors and the NR1 subunit of NMDARs associate through their C-terminal tails, and this regulates the surface expression and activity of D1 receptors (reviewed in Lee and Liu 2004). Activation of NMDARs inhibits D1 receptor internalization and increases the production of cAMP. However, the presence of PSD-95 negatively regulates these signaling cascades by inhibiting the interaction between these receptor types (Zhang and others 2009). Although PSD-95 interacts with D1 receptors through its N-terminus, the ability for it to facilitate D1 receptor endocytosis, and block NMDAR-mediated inhibition of endocytosis, requires both its N-terminal and PDZ1-2 domains (Zhang and others 2009). This interaction may serve to regulate the excitability of neurons and prevent overactivation of neurons by dopamine and NMDARs.
The interaction between β2-adrenergic receptors (β2ARs) and AMPARs is also mediated by PSD-95 but indirectly via Stargazin. The C-terminus of the β2AR binds to the PDZ3 domain (Joiner and others 2010), while Stargazin interacts with PDZ1-2 domains and can regulate surface expression of AMPARs (Chen and others 2000). Stimulation of β2AR in hippocampal cultures results in increased phosphorylation and surface expression of the GluR1 population associated with the adrenergic receptor, as well as an increase in AMPAR-mediated synaptic activity in prefrontal cortical slices (Joiner and others 2010). These effects are dependent on the intermediary interactions of Stargazin and PSD-95 with the receptors because membrane-permeable peptides that disrupt the interaction of β2AR and Stargazin with PSD-95 inhibit β2AR-mediated increases in activity (Joiner and others 2010). In addition, β2AR can interact with the fifth PDZ domain of MAGI-3; this association inhibits β2AR-mediated activation of ERK1/2 (Yang and others 2010). Similarly, the interaction of β1AR with PDZ1 of MAGI-3 inhibits β1AR-mediated activation of ERK1/2 but does not influence cAMP production or agonist-induced internalization of the receptor (He and others 2006).
Summary and Future Directions
MAGUKs form the core of protein complexes that mediate synaptic development, plasticity, and function. These protein complexes make up both the postsynaptic density and the presynaptic active zone. MAGUKs act as part of a scaffold for these structures, linking cell adhesion molecules, receptors, ion channels, kinases, phosphatases, etc. These typically generate multiprotein signaling complexes (with the prime role of MAGUKs indicated by the term MAGUK-associated signaling complex or MASC) (Ryan and Grant 2009). Our knowledge of the complexity of these complexes is expanding rapidly, with more than 1500 proteins identified already in the postsynaptic proteome (and hundreds as well in the presynaptic active zone) (Ryan and Grant 2009). Combinations of proteomic, genomic, and functional studies will continue to reveal and expand on the knowledge of MAGUK associations with newly discovered and known synaptic proteins and on how these complexes interact and function in novel ways. A better understanding of MAGUK function may be essential to discovering the nature of learning and memory as well as the etiology of neurological disorders. Major questions still need to be answered and concepts clarified about MAGUKs and their protein associations: 1) We need to understand better the physical nature of MAGUKs, that is, their shape, orientation, and arrangement at synapses, as this may help define their interactions in protein complexes. 2) There are still numerous questions about the function of MAGUKs and their associations with other proteins, including their interplay with different kinds of glutamate receptors as well as other kinds of receptors and how these change in development and in adult plasticity. 3) Much research is needed to understand the complex interplay of so many kinds of adhesion proteins with them (including new types of adhesion proteins that continue to be discovered). 4) We need to clarify their role in determining the differentiation of synapses into excitatory versus inhibitory types. Thus, MAGUK research is just getting started!
Acknowledgments
The authors thank Drs. Katherine W. Roche and Elizabeth Webber for critical review of the article. This work was supported by the Intramural Program of the National Institute on Deafness and Other Communication Disorders (NIDCD) at the National Institutes of Health (NIH).
Funding
The author(s) received no financial support for the research and/or authorship of this article.
Footnotes
Reprints and permission: http://www.sagepub.com/journalsPermissions.nav
Declaration of Conflicting Interests
The author(s) declared no potential conflicts of interest with respect to the authorship and/or publication of this article.
References
- Alie A, Manuel M. The backbone of the post-synaptic density originated in a unicellular ancestor of choanoflagel-lates and metazoans. BMC Evol Biol. 2010;10:34. doi: 10.1186/1471-2148-10-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ataman B, Budnik V, Thomas U. Scaffolding proteins at the Drosophila neuromuscular junction. Int Rev Neurobiol. 2006;75:181–216. doi: 10.1016/S0074-7742(06)75009-7. [DOI] [PubMed] [Google Scholar]
- Atasoy D, Schoch S, Ho A, Nadasy KA, Liu X, Zhang W, et al. Deletion of CASK in mice is lethal and impairs synaptic function. Proc Natl Acad Sci U S A. 2007;104(7):2525–30. doi: 10.1073/pnas.0611003104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barria A, Malinow R. NMDA receptor subunit composition controls synaptic plasticity by regulating binding to CaMKII. Neuron. 2005;48(2):289–301. doi: 10.1016/j.neuron.2005.08.034. [DOI] [PubMed] [Google Scholar]
- Bartos JA, Ulrich JD, Li H, Beazely MA, Chen Y, Macdonald JF, et al. Postsynaptic clustering and activation of Pyk2 by PSD-95. J Neurosci. 2010;30(2):449–63. doi: 10.1523/JNEUROSCI.4992-08.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bats C, Groc L, Choquet D. The interaction between Stargazin and PSD-95 regulates AMPA receptor surface trafficking. Neuron. 2007;53(5):719–34. doi: 10.1016/j.neuron.2007.01.030. [DOI] [PubMed] [Google Scholar]
- Bhattacharyya S, Biou V, Xu W, Schluter O, Malenka RC. A critical role for PSD-95/AKAP interactions in endocytosis of synaptic AMPA receptors. Nat Neurosci. 2009;12(2):172–81. doi: 10.1038/nn.2249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bianchetta MJ, Morabito MA. 2009 Neuroscience Meeting Planner. Chicago, IL: Society for Neuroscience; 2009. PSD-95 tyrosine phosphorylation and Pyk2 binding are regulated by Cdk5. [Google Scholar]
- Bingol B, Wang CF, Arnott D, Cheng D, Peng J, Sheng M. Autophosphorylated CaMKIIalpha acts as a scaffold to recruit proteasomes to dendritic spines. Cell. 2010;140(4):567–78. doi: 10.1016/j.cell.2010.01.024. [DOI] [PubMed] [Google Scholar]
- Blanpied TA, Kerr JM, Ehlers MD. Structural plasticity with preserved topology in the postsynaptic protein network. Proc Natl Acad Sci U S A. 2008;105(34):12587–92. doi: 10.1073/pnas.0711669105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown SD, Hardisty-Hughes RE, Mburu P. Quiet as a mouse: dissecting the molecular and genetic basis of hearing. Nat Rev Genet. 2008;9(4):277–90. doi: 10.1038/nrg2309. [DOI] [PubMed] [Google Scholar]
- Carlisle HJ, Manzerra P, Marcora E, Kennedy MB. Syn-GAP regulates steady-state and activity-dependent phosphorylation of cofilin. J Neurosci. 2008;28(50):13673–83. doi: 10.1523/JNEUROSCI.4695-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L, Chetkovich DM, Petralia RS, Sweeney NT, Kawasaki Y, Wenthold RJ, et al. Stargazin regulates synaptic targeting of AMPA receptors by two distinct mechanisms. Nature. 2000;408(6815):936–43. doi: 10.1038/35050030. [DOI] [PubMed] [Google Scholar]
- Choi J, Ko J, Racz B, Burette A, Lee JR, Kim S, et al. Regulation of dendritic spine morphogenesis by insulin receptor substrate 53, a downstream effector of Rac1 and Cdc42 small GTPases. J Neurosci. 2005;25(4):869–79. doi: 10.1523/JNEUROSCI.3212-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cline H. Synaptogenesis: a balancing act between excitation and inhibition. Curr Biol. 2005;15(6):R203–5. doi: 10.1016/j.cub.2005.03.010. [DOI] [PubMed] [Google Scholar]
- Colledge M, Snyder EM, Crozier RA, Soderling JA, Jin Y, Langeberg LK, et al. Ubiquitination regulates PSD-95 degradation and AMPA receptor surface expression. Neuron. 2003;40(3):595–607. doi: 10.1016/s0896-6273(03)00687-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craven SE, Bredt DS. Synaptic targeting of the postsynaptic density protein PSD-95 mediated by a tyrosine-based trafficking signal. J Biol Chem. 2000;275(26):20045–51. doi: 10.1074/jbc.M910153199. [DOI] [PubMed] [Google Scholar]
- Dalva MB, McClelland AC, Kayser MS. Cell adhesion molecules: signalling functions at the synapse. Nat Rev Neurosci. 2007;8(3):206–20. doi: 10.1038/nrn2075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Mendoza A, Suga H, Ruiz-Trillo I. Evolution of the MAGUK protein gene family in premetazoan lineages. BMC Evol Biol. 2010;10:93. doi: 10.1186/1471-2148-10-93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Wit J, Sylwestrak E, O’Sullivan ML, Otto S, Tiglio K, Savas JN, et al. LRRTM2 interacts with Neurexin1 and regulates excitatory synapse formation. Neuron. 2009;64(6):799–806. doi: 10.1016/j.neuron.2009.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delint-Ramirez I, Fernandez E, Bayes A, Kicsi E, Komiyama NH, Grant SG. In vivo composition of NMDA receptor signaling complexes differs between membrane subdomains and is modulated by PSD-95 and PSD-93. J Neurosci. 2010;30(24):8162–70. doi: 10.1523/JNEUROSCI.1792-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng F, Price MG, Davis CF, Mori M, Burgess DL. Stargazin and other transmembrane AMPA receptor regulating proteins interact with synaptic scaffolding protein MAGI-2 in brain. J Neurosci. 2006;26(30):7875–84. doi: 10.1523/JNEUROSCI.1851-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Husseini AE, Craven SE, Chetkovich DM, Firestein BL, Schnell E, Aoki C, et al. Dual palmitoylation of PSD-95 mediates its vesiculotubular sorting, postsynaptic targeting, and ion channel clustering. J Cell Biol. 2000a;148(1):159–72. doi: 10.1083/jcb.148.1.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Husseini AE, Schnell E, Chetkovich DM, Nicoll RA, Bredt DS. PSD-95 involvement in maturation of excitatory synapses. Science. 2000b;290(5495):1364–8. [PubMed] [Google Scholar]
- Elias GM, Elias LA, Apostolides PF, Kriegstein AR, Nicoll RA. Differential trafficking of AMPA and NMDA receptors by SAP102 and PSD-95 underlies synapse development. Proc Natl Acad Sci U S A. 2008;105(52):20953–8. doi: 10.1073/pnas.0811025106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elias GM, Nicoll RA. Synaptic trafficking of glutamate receptors by MAGUK scaffolding proteins. Trends Cell Biol. 2007;17(7):343–52. doi: 10.1016/j.tcb.2007.07.005. [DOI] [PubMed] [Google Scholar]
- Feng W, Zhang M. Organization and dynamics of PDZ-domain-related supramodules in the postsynaptic density. Nat Rev Neurosci. 2009;10(2):87–99. doi: 10.1038/nrn2540. [DOI] [PubMed] [Google Scholar]
- Foa L, Gasperini R. Developmental roles for Homer: more than just a pretty scaffold. J Neurochem. 2009;108(1):1–10. doi: 10.1111/j.1471-4159.2008.05726.x. [DOI] [PubMed] [Google Scholar]
- Friedman HV, Bresler T, Garner CC, Ziv NE. Assembly of new individual excitatory synapses: time course and temporal order of synaptic molecule recruitment. Neuron. 2000;27(1):57–69. doi: 10.1016/s0896-6273(00)00009-x. [DOI] [PubMed] [Google Scholar]
- Frost NA, Shroff H, Kong H, Betzig E, Blanpied TA. Single-molecule discrimination of discrete perisynaptic and distributed sites of actin filament assembly within dendritic spines. Neuron. 2010;67(1):86–99. doi: 10.1016/j.neuron.2010.05.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Funke L, Dakoji S, Bredt DS. Membrane-associated guanylate kinases regulate adhesion and plasticity at cell junctions. Annu Rev Biochem. 2005;74:219–45. doi: 10.1146/annurev.biochem.74.082803.133339. [DOI] [PubMed] [Google Scholar]
- Gerrow K, Romorini S, Nabi SM, Colicos MA, Sala C, El-Husseini A. A preformed complex of postsynaptic proteins is involved in excitatory synapse development. Neuron. 2006;49(4):547–62. doi: 10.1016/j.neuron.2006.01.015. [DOI] [PubMed] [Google Scholar]
- Gray NW, Weimer RM, Bureau I, Svoboda K. Rapid redistribution of synaptic PSD-95 in the neocortex in vivo. PLoS Biol. 2006;4(11):e370. doi: 10.1371/journal.pbio.0040370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groc L, Heine M, Cousins SL, Stephenson FA, Lounis B, Cognet L, et al. NMDA receptor surface mobility depends on NR2A-2B subunits. Proc Natl Acad Sci U S A. 2006;103(49):18769–74. doi: 10.1073/pnas.0605238103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardingham GE, Bading H. Synaptic versus extrasynaptic NMDA receptor signalling: implications for neurodegenerative disorders. Nat Rev Neurosci. 2010;11:682–96. doi: 10.1038/nrn2911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He J, Bellini M, Inuzuka H, Xu J, Xiong Y, Yang X, et al. Proteomic analysis of beta1-adrenergic receptor interactions with PDZ scaffold proteins. J Biol Chem. 2006;281(5):2820–7. doi: 10.1074/jbc.M509503200. [DOI] [PubMed] [Google Scholar]
- Howard MA, Elias GM, Elias LA, Swat W, Nicoll RA. The role of SAP97 in synaptic glutamate receptor dynamics. Proc Natl Acad Sci U S A. 2010;107(8):3805–10. doi: 10.1073/pnas.0914422107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsueh YP. Calcium/calmodulin-dependent serine protein kinase and mental retardation. Ann Neurol. 2009;66(4):438–43. doi: 10.1002/ana.21755. [DOI] [PubMed] [Google Scholar]
- Jeyifous O, Waites CL, Specht CG, Fujisawa S, Schubert M, Lin EI, et al. SAP97 and CASK mediate sorting of NMDA receptors through a previously unknown secretory pathway. Nat Neurosci. 2009;12(8):1011–9. doi: 10.1038/nn.2362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joiner ML, Lise MF, Yuen EY, Kam AY, Zhang M, Hall DD, et al. Assembly of a beta2-adrenergic receptor-- GluR1 signalling complex for localized cAMP signalling. EMBO J. 2010;29(2):482–95. doi: 10.1038/emboj.2009.344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalia LV, Pitcher GM, Pelkey KA, Salter MW. PSD-95 is a negative regulator of the tyrosine kinase Src in the NMDA receptor complex. EMBO J. 2006;25(20):4971–82. doi: 10.1038/sj.emboj.7601342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalia LV, Salter MW. Interactions between Src family protein tyrosine kinases and PSD-95. Neuropharmacology. 2003;45(6):720–8. doi: 10.1016/s0028-3908(03)00313-7. [DOI] [PubMed] [Google Scholar]
- Ko J, Fuccillo MV, Malenka RC, Südhof TC. LRRTM2 functions as a neurexin ligand in promoting excitatory synapse formation. Neuron. 2009;64(6):791–8. doi: 10.1016/j.neuron.2009.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ko J, Kim S, Chung HS, Kim K, Han K, Kim H, et al. SALM synaptic cell adhesion-like molecules regulate the differentiation of excitatory synapses. Neuron. 2006;50(2):233–45. doi: 10.1016/j.neuron.2006.04.005. [DOI] [PubMed] [Google Scholar]
- Kohsaka H, Takasu E, Nose A. In vivo induction of post-synaptic molecular assembly by the cell adhesion molecule Fasciclin2. J Cell Biol. 2007;179(6):1289–300. doi: 10.1083/jcb.200705154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kornau HC, Schenker LT, Kennedy MB, Seeburg PH. Domain interaction between NMDA receptor subunits and the postsynaptic density protein PSD-95. Science. 1995;269(5231):1737–40. doi: 10.1126/science.7569905. [DOI] [PubMed] [Google Scholar]
- Lavezzari G, McCallum J, Dewey CM, Roche KW. Subunit-specific regulation of NMDA receptor endocytosis. J Neurosci. 2004;24(28):6383–91. doi: 10.1523/JNEUROSCI.1890-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavezzari G, McCallum J, Lee R, Roche KW. Differential binding of the AP-2 adaptor complex and PSD-95 to the C-terminus of the NMDA receptor subunit NR2B regulates surface expression. Neuropharmacology. 2003;45(6):729–37. doi: 10.1016/s0028-3908(03)00308-3. [DOI] [PubMed] [Google Scholar]
- Lee FJ, Liu F. Direct interactions between NMDA and D1 receptors: a tale of tails. Biochem Soc Trans. 2004;32(Pt 6):1032–6. doi: 10.1042/BST0321032. [DOI] [PubMed] [Google Scholar]
- Lee S, Lee K, Hwang S, Kim SH, Song WK, Park ZY, et al. SPIN90/WISH interacts with PSD-95 and regulates dendritic spinogenesis via an N-WASP-independent mechanism. EMBO J. 2006;25(20):4983–95. doi: 10.1038/sj.emboj.7601349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leonard AS, Davare MA, Horne MC, Garner CC, Hell JW. SAP97 is associated with the alpha-amino-3-hydroxy-5-methylisoxazole-4-propionic acid receptor GluR1 subunit. J Biol Chem. 1998;273(31):19518–24. doi: 10.1074/jbc.273.31.19518. [DOI] [PubMed] [Google Scholar]
- Li Z, Sheng M. Some assembly required: the development of neuronal synapses. Nat Rev Mol Cell Biol. 2003;4(11):833–41. doi: 10.1038/nrm1242. [DOI] [PubMed] [Google Scholar]
- Lu J, Helton TD, Blanpied TA, Racz B, Newpher TM, Weinberg RJ, et al. Postsynaptic positioning of endocytic zones and AMPA receptor cycling by physical coupling of dynamin-3 to Homer. Neuron. 2007;55(6):874–89. doi: 10.1016/j.neuron.2007.06.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mah W, Ko J, Nam J, Han K, Chung WS, Kim E. Selected SALM (synaptic adhesion-like molecule) family proteins regulate synapse formation. J Neurosci. 2010;30(16):5559–68. doi: 10.1523/JNEUROSCI.4839-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGee AW, Topinka JR, Hashimoto K, Petralia RS, Kakizawa S, Kauer FW, et al. PSD-93 knock-out mice reveal that neuronal MAGUKs are not required for development or function of parallel fiber synapses in cerebellum. J Neurosci. 2001;21(9):3085–91. doi: 10.1523/JNEUROSCI.21-09-03085.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merrill MA, Chen Y, Strack S, Hell JW. Activity-driven postsynaptic translocation of CaMKII. Trends Pharmacol Sci. 2005;26(12):645–53. doi: 10.1016/j.tips.2005.10.003. [DOI] [PubMed] [Google Scholar]
- Morabito MA, Sheng M, Tsai LH. Cyclin-dependent kinase 5 phosphorylates the N-terminal domain of the post-synaptic density protein PSD-95 in neurons. J Neurosci. 2004;24(4):865–76. doi: 10.1523/JNEUROSCI.4582-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukherjee K, Sharma M, Urlaub H, Bourenkov GP, Jahn R, Südhof TC, et al. CASK functions as a Mg2+-independent neurexin kinase. Cell. 2008;133(2):328–39. doi: 10.1016/j.cell.2008.02.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller BM, Kistner U, Kindler S, Chung WJ, Kuhlendahl S, Fenster SD, et al. SAP102, a novel postsynap-tic protein that interacts with NMDA receptor complexes in vivo. Neuron. 1996;17(2):255–65. doi: 10.1016/s0896-6273(00)80157-9. [DOI] [PubMed] [Google Scholar]
- Nakagawa T, Futai K, Lashuel HA, Lo I, Okamoto K, Walz T, et al. Quaternary structure, protein dynamics, and synaptic function of SAP97 controlled by L27 domain interactions. Neuron. 2004;44(3):453–67. doi: 10.1016/j.neuron.2004.10.012. [DOI] [PubMed] [Google Scholar]
- Nakazawa T, Komai S, Watabe AM, Kiyama Y, Fukaya M, Arima-Yoshida F, et al. NR2B tyrosine phosphorylation modulates fear learning as well as amygdaloid synaptic plasticity. EMBO J. 2006;25(12):2867–77. doi: 10.1038/sj.emboj.7601156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen JT, Turck CW, Cohen FE, Zuckermann RN, Lim WA. Exploiting the basis of proline recognition by SH3 and WW domains: design of N-substituted inhibitors. Science. 1998;282(5396):2088–92. doi: 10.1126/science.282.5396.2088. [DOI] [PubMed] [Google Scholar]
- Nikandrova YA, Jiao Y, Baucum AJ, Tavalin SJ, Colbran RJ. Ca2+/calmodulin-dependent protein kinase II binds to and phosphorylates a specific SAP97 splice variant to disrupt association with AKAP79/150 and modulate alpha-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid-type glutamate receptor (AMPAR) activity. J Biol Chem. 2010;285(2):923–34. doi: 10.1074/jbc.M109.033985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikonenko I, Boda B, Steen S, Knott G, Welker E, Muller D. PSD-95 promotes synaptogenesis and multiinnervated spine formation through nitric oxide signaling. J Cell Biol. 2008;183(6):1115–27. doi: 10.1083/jcb.200805132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Opazo P, Labrecque S, Tigaret CM, Frouin A, Wiseman PW, De Koninck P, et al. CaMKII triggers the diffusional trapping of surface AMPARs through phosphorylation of stargazin. Neuron. 2010;67(2):239–52. doi: 10.1016/j.neuron.2010.06.007. [DOI] [PubMed] [Google Scholar]
- Packard M, Mathew D, Budnik V. FASt remodeling of synapses in Drosophila. Curr Opin Neurobiol. 2003;13(5):527–34. doi: 10.1016/j.conb.2003.09.008. [DOI] [PubMed] [Google Scholar]
- Pak DT, Sheng M. Targeted protein degradation and synapse remodeling by an inducible protein kinase. Science. 2003;302(5649):1368–73. doi: 10.1126/science.1082475. [DOI] [PubMed] [Google Scholar]
- Pak DT, Yang S, Rudolph-Correia S, Kim E, Sheng M. Regulation of dendritic spine morphology by SPAR, a PSD-95-associated RapGAP. Neuron. 2001;31(2):289–303. doi: 10.1016/s0896-6273(01)00355-5. [DOI] [PubMed] [Google Scholar]
- Park CS, Elgersma Y, Grant SG, Morrison JH. alpha-Isoform of calcium-calmodulin-dependent protein kinase II and postsynaptic density protein 95 differentially regulate synaptic expression of NR2A- and NR2B-containing N-methyl-D-aspartate receptors in hippocampus. Neuroscience. 2008;151(1):43–55. doi: 10.1016/j.neuroscience.2007.09.075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petralia RS, Al-Hallaq RA, Wenthold RJ. Trafficking and targeting of NMDA receptors. In: Van Dongen AM, editor. Biology of the NMDA receptor. New York: Taylor and Francis Group; 2009. pp. 149–200. [PubMed] [Google Scholar]
- Petralia RS, Sans N, Wang YX, Wenthold RJ. Ontogeny of postsynaptic density proteins at glutamatergic synapses. Mol Cell Neurosci. 2005;29(3):436–52. doi: 10.1016/j.mcn.2005.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petralia RS, Wang YX, Hua F, Yi Z, Zhou A, Ge L, et al. Organization of NMDA receptors at extrasynaptic locations. Neuroscience. 2010;167(1):68–87. doi: 10.1016/j.neuroscience.2010.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petralia RS, Wang YX, Sans N, Worley PF, Hammer JA, 3rd, Wenthold RJ. Glutamate receptor targeting in the postsynaptic spine involves mechanisms that are independent of myosin Va. Eur J Neurosci. 2001;13(9):1722–32. doi: 10.1046/j.0953-816x.2001.01553.x. [DOI] [PubMed] [Google Scholar]
- Petralia RS, Wang YX, Wenthold RJ. NMDA receptors and PSD-95 are found in attachment plaques in cerebellar granular layer glomeruli. Eur J Neurosci. 2002;15(3):583–7. doi: 10.1046/j.1460-9568.2002.01896.x. [DOI] [PubMed] [Google Scholar]
- Pi HJ, Otmakhov N, Lemelin D, De Koninck P, Lisman J. Autonomous CaMKII can promote either long-term potentiation or long-term depression, depending on the state of T305/T306 phosphorylation. J Neurosci. 2010;30(26):8704–9. doi: 10.1523/JNEUROSCI.0133-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prybylowski K, Chang K, Sans N, Kan L, Vicini S, Wenthold RJ. The synaptic localization of NR2B-containing NMDA receptors is controlled by interactions with PDZ proteins and AP-2. Neuron. 2005;47(6):845–57. doi: 10.1016/j.neuron.2005.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prybylowski K, Fu Z, Losi G, Hawkins LM, Luo J, Chang K, et al. Relationship between availability of NMDA receptor subunits and their expression at the synapse. J Neurosci. 2002;22(20):8902–10. doi: 10.1523/JNEUROSCI.22-20-08902.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roche KW, Standley S, McCallum J, Dune Ly C, Ehlers MD, Wenthold RJ. Molecular determinants of NMDA receptor internalization. Nat Neurosci. 2001;4(8):794–802. doi: 10.1038/90498. [DOI] [PubMed] [Google Scholar]
- Rosenberg MM, Yang F, Mohn JL, Storer EK, Jacob MH. The postsynaptic adenomatous polyposis coli (APC) multiprotein complex is required for localizing neuroligin and neurexin to neuronal nicotinic synapses in vivo. J Neurosci. 2010;30(33):11073–85. doi: 10.1523/JNEUROSCI.0983-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rumbaugh G, Sia GM, Garner CC, Huganir RL. Synapse-associated protein-97 isoform-specific regulation of surface AMPA receptors and synaptic function in cultured neurons. J Neurosci. 2003;23(11):4567–76. doi: 10.1523/JNEUROSCI.23-11-04567.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan TJ, Grant SG. The origin and evolution of synapses. Nat Rev Neurosci. 2009;10(10):701–12. doi: 10.1038/nrn2717. [DOI] [PubMed] [Google Scholar]
- Sakarya O, Armstrong KA, Adamska M, Adamski M, Wang IF, Tidor B, et al. A post-synaptic scaffold at the origin of the animal kingdom. PLoS One. 2007;2(6):e506. doi: 10.1371/journal.pone.0000506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salter MW, Kalia LV. Src kinases: a hub for NMDA receptor regulation. Nat Rev Neurosci. 2004;5(4):317–28. doi: 10.1038/nrn1368. [DOI] [PubMed] [Google Scholar]
- Sans N, Petralia RS, Wang YX, Blahos J, 2nd, Hell JW, Wenthold RJ. A developmental change in NMDA receptor-associated proteins at hippocampal synapses. J Neurosci. 2000;20(3):1260–71. doi: 10.1523/JNEUROSCI.20-03-01260.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sans N, Prybylowski K, Petralia RS, Chang K, Wang YX, Racca C, et al. NMDA receptor trafficking through an interaction between PDZ proteins and the exocyst complex. Nat Cell Biol. 2003;5(6):520–30. doi: 10.1038/ncb990. [DOI] [PubMed] [Google Scholar]
- Sans N, Racca C, Petralia RS, Wang YX, McCallum J, Wenthold RJ. Synapse-associated protein 97 selectively associates with a subset of AMPA receptors early in their biosynthetic pathway. J Neurosci. 2001;21(19):7506–16. doi: 10.1523/JNEUROSCI.21-19-07506.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sans N, Wang PY, Du Q, Petralia RS, Wang YX, Nakka S, et al. mPins modulates PSD-95 and SAP102 trafficking and influences NMDA receptor surface expression. Nat Cell Biol. 2005;7(12):1179–90. doi: 10.1038/ncb1325. [DOI] [PubMed] [Google Scholar]
- Sato Y, Tao YX, Su Q, Johns RA. Post-synaptic density-93 mediates tyrosine-phosphorylation of the N-methyl-D-aspartate receptors. Neuroscience. 2008;153(3):700–8. doi: 10.1016/j.neuroscience.2008.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlüter OM, Xu W, Malenka RC. Alternative N-terminal domains of PSD-95 and SAP97 govern activity-dependent regulation of synaptic AMPA receptor function. Neuron. 2006;51(1):99–111. doi: 10.1016/j.neuron.2006.05.016. [DOI] [PubMed] [Google Scholar]
- Seabold GK, Burette A, Lim IA, Weinberg RJ, Hell JW. Interaction of the tyrosine kinase Pyk2 with the N-methyl-D-aspartate receptor complex via the Src homology 3 domains of PSD-95 and SAP102. J Biol Chem. 2003;278(17):15040–8. doi: 10.1074/jbc.M212825200. [DOI] [PubMed] [Google Scholar]
- Sheng M, Hoogenraad CC. The postsynaptic architecture of excitatory synapses: a more quantitative view. Annu Rev Biochem. 2007;76:823–47. doi: 10.1146/annurev.biochem.76.060805.160029. [DOI] [PubMed] [Google Scholar]
- Sheng M, Sala C. PDZ domains and the organization of supramolecular complexes. Annu Rev Neurosci. 2001;24:1–29. doi: 10.1146/annurev.neuro.24.1.1. [DOI] [PubMed] [Google Scholar]
- Shi SH, Cheng T, Jan LY, Jan YN. The immunoglobulin family member dendrite arborization and synapse maturation 1 (Dasm1) controls excitatory synapse maturation. Proc Natl Acad Sci U S A. 2004a;101(36):13346–51. doi: 10.1073/pnas.0405371101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi SH, Cox DN, Wang D, Jan LY, Jan YN. Control of dendrite arborization by an Ig family member, dendrite arborization and synapse maturation 1 (Dasm1) Proc Natl Acad Sci U S A. 2004b;101(36):13341–5. doi: 10.1073/pnas.0405370101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siddiqui TJ, Pancaroglu R, Kang Y, Rooyakkers A, Craig AM. LRRTMs and neuroligins bind neurexins with a differential code to cooperate in glutamate synapse development. J Neurosci. 2010;30(22):7495–506. doi: 10.1523/JNEUROSCI.0470-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soltau M, Berhorster K, Kindler S, Buck F, Richter D, Kreienkamp HJ. Insulin receptor substrate of 53 kDa links postsynaptic shank to PSD-95. J Neurochem. 2004;90(3):659–65. doi: 10.1111/j.1471-4159.2004.02523.x. [DOI] [PubMed] [Google Scholar]
- Steigerwald F, Schulz TW, Schenker LT, Kennedy MB, Seeburg PH, Kohr G. C-terminal truncation of NR2A subunits impairs synaptic but not extrasynaptic localization of NMDA receptors. J Neurosci. 2000;20(12):4573–81. doi: 10.1523/JNEUROSCI.20-12-04573.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steiner P, Higley MJ, Xu W, Czervionke BL, Malenka RC, Sabatini BL. Destabilization of the postsynaptic density by PSD-95 serine 73 phosphorylation inhibits spine growth and synaptic plasticity. Neuron. 2008;60(5):788–802. doi: 10.1016/j.neuron.2008.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sumioka A, Yan D, Tomita S. TARP phosphorylation regulates synaptic AMPA receptors through lipid bilayers. Neuron. 2010;66(5):755–67. doi: 10.1016/j.neuron.2010.04.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sumita K, Sato Y, Iida J, Kawata A, Hamano M, Hirabayashi S, et al. Synaptic scaffolding molecule (S-SCAM) membrane-associated guanylate kinase with inverted organization (MAGI)-2 is associated with cell adhesion molecules at inhibitory synapses in rat hippocampal neurons. J Neurochem. 2007;100(1):154–66. doi: 10.1111/j.1471-4159.2006.04170.x. [DOI] [PubMed] [Google Scholar]
- Tada T, Sheng M. Molecular mechanisms of dendritic spine morphogenesis. Curr Opin Neurobiol. 2006;16(1):95–101. doi: 10.1016/j.conb.2005.12.001. [DOI] [PubMed] [Google Scholar]
- te Velthuis AJ, Admiraal JF, Bagowski CP. Molecular evolution of the MAGUK family in metazoan genomes. BMC Evol Biol. 2007;7:129. doi: 10.1186/1471-2148-7-129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tezuka T, Umemori H, Akiyama T, Nakanishi S, Yamamoto T. PSD-95 promotes Fyn-mediated tyrosine phosphorylation of the N-methyl-D-aspartate receptor subunit NR2A. Proc Natl Acad Sci U S A. 1999;96(2):435–40. doi: 10.1073/pnas.96.2.435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas U, Ebitsch S, Gorczyca M, Koh YH, Hough CD, Woods D, et al. Synaptic targeting and localization of discs-large is a stepwise process controlled by different domains of the protein. Curr Biol. 2000;10(18):1108–17. doi: 10.1016/s0960-9822(00)00696-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas U, Kobler O, Gundelfinger ED. The Drosophila larval neuromuscular junction as a model for scaffold complexes at glutamatergic synapses: benefits and limitations. J Neurogenet. 2010;24(3):109–19. doi: 10.3109/01677063.2010.493589. [DOI] [PubMed] [Google Scholar]
- Waites CL, Specht CG, Hartel K, Leal-Ortiz S, Genoux D, Li D, et al. Synaptic SAP97 isoforms regulate AMPA receptor dynamics and access to presynaptic glutamate. J Neurosci. 2009;29(14):4332–45. doi: 10.1523/JNEUROSCI.4431-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang CY, Chang K, Petralia RS, Wang YX, Seabold GK, Wenthold RJ. A novel family of adhesion-like molecules that interacts with the NMDA receptor. J Neurosci. 2006;26(8):2174–83. doi: 10.1523/JNEUROSCI.3799-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang PY, Seabold GK, Wenthold RJ. Synaptic adhesion-like molecules (SALMs) promote neurite outgrowth. Mol Cell Neurosci. 2008;39(1):83–94. doi: 10.1016/j.mcn.2008.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Washbourne P, Bennett JE, McAllister AK. Rapid recruitment of NMDA receptor transport packets to nascent synapses. Nat Neurosci. 2002;5(8):751–9. doi: 10.1038/nn883. [DOI] [PubMed] [Google Scholar]
- Washbourne P, Liu XB, Jones EG, McAllister AK. Cycling of NMDA receptors during trafficking in neurons before synapse formation. J Neurosci. 2004;24(38):8253–64. doi: 10.1523/JNEUROSCI.2555-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wenthold RJ, Al-Hallaq RA, Swanwick CC, Petralia RS. Molecular properties and cell biology of the NMDA receptor. In: Hell JW, Ehlers MD, editors. Structural and functional organization of the synapse. New York: Springer; 2008. pp. 317–67. [Google Scholar]
- Yamagata M, Sanes JR. Synaptic localization and function of Sidekick recognition molecules require MAGI scaffolding proteins. J Neurosci. 2010;30(10):3579–88. doi: 10.1523/JNEUROSCI.6319-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang X, Zheng J, Xiong Y, Shen H, Sun L, Huang Y, et al. Beta-2 adrenergic receptor mediated ERK activation is regulated by interaction with MAGI-3. FEBS Lett. 2010;584(11):2207–12. doi: 10.1016/j.febslet.2010.03.039. [DOI] [PubMed] [Google Scholar]
- Yi Z, Petralia RS, Fu Z, Swanwick CC, Wang YX, Prybylowski K, et al. The role of the PDZ protein GIPC in regulating NMDA receptor trafficking. J Neurosci. 2007;27(43):11663–75. doi: 10.1523/JNEUROSCI.3252-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Vinuela A, Neely MH, Hallett PJ, Grant SG, Miller GM, et al. Inhibition of the dopamine D1 receptor signaling by PSD-95. J Biol Chem. 2007;282(21):15778–89. doi: 10.1074/jbc.M611485200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Xu TX, Hallett PJ, Watanabe M, Grant SG, Isacson O, et al. PSD-95 uncouples dopamine-glutamate interaction in the D1/PSD-95/NMDA receptor complex. J Neurosci. 2009;29(9):2948–60. doi: 10.1523/JNEUROSCI.4424-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang S, Edelmann L, Liu J, Crandall JE, Morabito MA. Cdk5 regulates the phosphorylation of tyrosine 1472 NR2B and the surface expression of NMDA receptors. J Neurosci. 2008;28(2):415–24. doi: 10.1523/JNEUROSCI.1900-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng CY, Petralia RS, Wang YX, Kachar B, Wenthold RJ. SAP102 is a highly mobile MAGUK in spines. J Neurosci. 2010;30(13):4757–66. doi: 10.1523/JNEUROSCI.6108-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziv NE, Garner CC. Cellular and molecular mechanisms of presynaptic assembly. Nat Rev Neurosci. 2004;5(5):385–99. doi: 10.1038/nrn1370. [DOI] [PubMed] [Google Scholar]