Abstract
A series of alkylated (bis)urea and (bis)thiourea polyamine analogues were synthesized and screened for antimalarial activity against chloroquine-sensitive and -resistant strains of Plasmodium falciparum in vitro. All analogues showed growth inhibitory activity against P. falciparum at less than 3 μM, with the majority having effective IC50 values in the 100–650 nM range. Analogues arrested parasitic growth within 24 hours of exposure due to a block in nuclear division and therefore asexual development. Moreover, this effect appears to be cytotoxic and highly selective to malaria parasites (>7000-fold lower IC50 against P. falciparum) and is not reversible by the exogenous addition of polyamines. With this first report of potent antimalarial activity of polyamine analogues containing 3-7-3 or 3-6-3 carbon backbones and substituted terminal urea- or thiourea moieties, we propose that these compounds represent a structurally novel class of antimalarial agents.
Keywords: Malaria, Plasmodium, polyamine analogue, antimalarial drugs, (bis)urea, (bis)thiourea
INTRODUCTION
Malaria remains one of the most deadly parasitic diseases, with nearly 250 million new cases each year, resulting in approximately one million deaths (www.who.int). The spreading resistance of Plasmodium falciparum to existing antimalarials including chloroquine, antifolates and artemisinin has resulted in a pressing need to discover new chemotherapeutic agents against this disease.1 One class of promising antiparasitic agents include inhibitors of polyamine biosynthesis,2 as well as polyamine analogues,3 with ample evidence indicating that these rapidly dividing cells have an exquisite need for the presence of polyamines for a myriad of cellular functions during cell growth and division.4,5
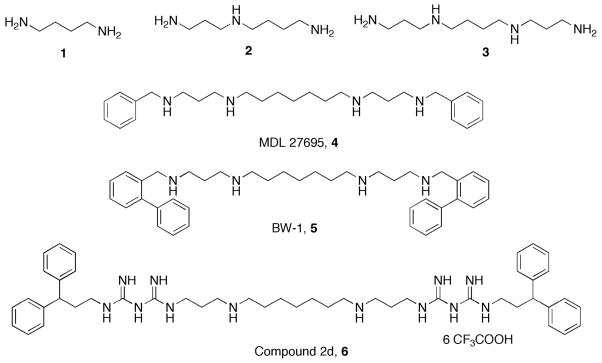
The naturally occurring polyamines putrescine (1), spermidine (2) and spermine (3) (Figure 1) interact with a variety of cellular effector sites due to their highly cationic nature and specific spatial orientation of positive charge, and are therefore able to stabilize DNA, RNA and other acidic cellular constituents.4 Polyamine analogues are structurally similar to the naturally occurring polyamines and act as either polyamine antimetabolites that deplete intracellular polyamine pools, or polyamine mimetics that displace the natural polyamines from their binding sites, without substituting for their cellular functions.6 Particularly, terminal alkylation of polyamines and polyamine analogues results in a change of pKa of the amine groups of these molecules, resulting in non-functional polyamine characteristics.7,8 Moreover, these analogues may compete for polyamine uptake, and in mammalian cells in particular, can induce polyamine catabolism.3
Figure 1.
The natural polyamines putrescine (1), spermidine (2) and spermine 3) and antimalarial polyamine analogs MDL 27695 (4), BW-1 (5) and compound 6.
The first generation of antiparasitic alkylpolyamines, typified by the N, N′-bis(benzyl)-substituted polyamine analogue MDL 27695 (4, Figure 1), exhibited antitrypanosomal and antiplasmodial activity in the μM range.9–11 A bis[(2-phenyl)benzyl)]spermine analogue of 4 known as BW-1 (5, Figure 1) was subsequently shown to have inhibitory activity against various strains of Trypanosoma and the microsporidial, Encephalitozoon cuniculi, particularly by blocking polyamine uptake and inhibiting polyamine oxidase activity9,11,12 and additionally being curative in a rodent model of infection with the microsporidial organism.9 Derivatives of 5 include analogues of the substituted (bis)biguanide known as 2d (6, Figure 1) that, in addition to depleting the polyamine pool, inhibits trypanothione reductase (a spermidine-glutathione conjugate) activity in trypanosomes.8 Compound 6 and its derivatives are highly active antiparasitic agents, with in vitro IC50’s against T. brucei as low as 90 nM. Several urea- and thiourea-based isosteres of 6 have subsequently been shown to be effective epigenetic modulators in mammalian cells by influencing selective chromatin marks in tumor cell lines through inhibition of lysine specific demethylase 1, thereby decreasing cancerous cell growth.13
Based on the success of terminally (bis)alkylated polyamine analogues against other parasites, several analogues of 6, as well as a new generation of (bis)urea and (bis)thiourea alkylated isosteres of 6, were synthesized and evaluated for their ability to inhibit the proliferation of malaria parasites. These compounds contain a variety of carbon backbones, and terminal urea/thiourea substituents that are symmetrically substituted aralkyl substituents, and as such present a structurally novel class of scaffolds, unrelated to any known antimalarials. This study reports the antimalarial activity against drug sensitive and resistant P. falciparum strains in vitro, their effects on the parasites’ DNA replication and polyamine-specific events.
RESULTS AND DISCUSSION
Chemical syntheses of urea and thiourea polyamine analogues
To access a library of urea and thiourea analogues isosteric to 6 (7–20, Table 1; and 25–39, Table 2) and analogous amidine analogs (21–24, Table 1), we employed our previously published synthesis8,14 of precursor molecules 43, as shown in Scheme 1. The appropriate diamine 40 (n = 1, 2, 4, 5) was (bis)cyanoethylated (acrylonitrile, EtOH, reflux) to afford the corresponding (bis)cyano intermediates 41. The central nitrogens in 41 were then N-Boc protected ((Boc)2O, CH2Cl2/Aq. NaHCO3)15 to form 42, and the cyano groups were reduced (Raney Ni) to yield the desired diamines 43.8,16 Compounds 43 (n = 1, 2, 4, 5) were then reacted with the 2 equivalent of appropriate alkyl- or aryl-substituted isocyanates or isothiocyanates 44 in anhydrous CH2Cl2, followed by acid removal of the N-Boc protection groups (HCl in EtOAc)15 to afford the desired urea or thiourea products (7–20 and 25–39). The amidine analogs compounds 21–24 were prepared (Scheme 2) by reacting diamines 43 with 2 equivalent of S-naphthylmethyl thioacetimidate hydrobromide 46 (prepared by refluxing 2-bromomethylnaphthalene with thiacetamide in CHCl3 according to literature procedure14) using absolute ethanol, and the Boc protecting groups were subsequently removed with HCl in EtOAc.
Table 1.
In vitro antimalarial activity of the compounds 6–24, against P. falciparum strains 3D7, HB3 and W2.
| Antimalarial activity against P. falciparum IC50, nMa | |||||||
|---|---|---|---|---|---|---|---|
| Cpd | Chemical Structure | Back bone | Type | 3D7b | HB3c | W2d | RIe |
| 7 |
|
3-3-3 | Urea | 653 ± 44 | - | - | - |
| 8 |
|
3-4-3 | Urea | 657 ± 90 | 517 ± 43 | - | - |
| 9 |
|
3-7-3 | Urea | 144 ± 31 | 200 ± 9 | 162.6 ± 3.2 | 1.13 |
| 10 |
|
3-4-3 | Urea | 3475 ± 600 | >1000 | - | - |
| 11 |
|
3-3-3 | Thiourea | 1434 ± 22 | 1641 ± 104 | - | - |
| 12 |
|
3-4-3 | Thiourea | 528 ± 9 | 1031 ± 83 | - | - |
| 13 |
|
3-7-3 | Thiourea | 253 ± 3 | 256 ± 17 | 147 ± 4 | 0.58 |
| 14 |
|
3-3-3 | Thiourea | 1316 ± 10 | 715 ± 76 | - | - |
| 15 |
|
3-4-3 | Thiourea | 329 ± 9 | 170 ± 32 | 200 ± 17 | 0.61 |
| 16 |
|
3-7-3 | Thiourea | 405 ± 8 | - | 183.1 ± 2.9 | 0.45 |
| 17 |
|
3-3-3 | Thiourea | 1081 ± 15 | 766 ± 24 | - | - |
| 18 |
|
3-4-3 | Thiourea | 433 ± 9 | 317 ± 37 | - | - |
| 19 |
|
3-7-3 | Thiourea | 641 ± 8 | 138 ± 17 | - | - |
| 20 |
|
3-4-3 | Thiourea | 355 ± 90 | - | 66 ± 14 | 0.19 |
| 21 |
|
3-3-3 | Amidine | 147 ± 23f | 109.5 ± 13f | - | - |
| 22 |
|
3-4-3 | Amidine | 80 ± 75f | 14.2 ± 4.9f | - | - |
| 23 |
|
3-6-3 | Amidine | - | - | - | - |
| 24 |
|
3-7-3 | Amidine | - | 70.3 ± 25f | - | - |
| 6 |
|
3-7-3 | Amidine | 298 ± 27 | 224 ± 80 | - | - |
| CQg |

|
- | 9 ± 0.04 | - | 70 ± 1 | - | |
Values are the means ± S.E. of at least 3 independent experiments (n≥3).
P. falciparum drug sensitive strain 3D7.
P. falciparum chloroquine resistant strain HB3.
P. falciparum antifolate resistant strain W2.
Resistance index (RI) defined as the ratio of the IC50 values of the resistance to sensitive strains, W2/3D7.
Indicates IC50 values in μM.
Control drug, chloroquine
Table 2.
In vitro antimalarial activity of the second-generation compounds (25–39), against P. falciparum strains 3D7 and W2
| Antimalarial activity against P. falciparum IC50, nMa | ||||||
|---|---|---|---|---|---|---|
| Cpd | Chemical Structure | Backbone | Type | 3D7b | W2c | RId |
| 25 |
|
3-6-3 | Thiourea | 211 ± 7 | 256 ± 4 | 1.2 |
| 26 |
|
3-6-3 | Thiourea | 436 ± 11 | - | - |
| 27 |
|
3-6-3 | Thiourea | 310 ± 8 | - | - |
| 28 |
|
3-6-3 | Thiourea | 846 ± 25 | - | - |
| 29 |
|
3-6-3 | Thiourea | 106 ± 11 | 198 ± 15 | 1.9 |
| 30 |
|
3-6-3 | Urea | 88 ± 7 | 26 ± 1 | 0.30 |
| 31 |
|
3-6-3 | Urea | 339 ± 70 | - | - |
| 32 |
|
3-6-3 | Thiourea | 1353 ± 60 | - | - |
| 33 |
|
3-6-3 | Thiourea | 425 ± 40 | - | - |
| 34 |
|
3-6-3 | Urea | >15e | - | - |
| 35 |
|
3-6-3 | Urea | 14084 ± 600 | - | - |
| 36 |
|
3-4-3 | Urea | 691 ± 9 | - | - |
| 37 |
|
3-4-3 | Urea | 408 ± 5 | - | - |
| 38 |
|
3-7-3 | Urea | 130.9 ± 1.9 | 221 ± 7 | 1.7 |
| 39 |
|
3-7-3 | Urea | 100.1 ± 2.7 | 140 ± 5 | 1.4 |
| CQf |

|
- | 9 ± 0.04 | 70 ± 1 | 8 | |
Values are the means ± S.E. of at least 3 independent experiments (n≥3).
P. falciparum drug sensitive strain 3D7.
P. falciparum antifolate resistan strain W2.
Resistance index (RI) defined as the ratio of the IC50 values of the resistance to sensitive strains, W2/3D7.
Indicates IC50 values in μM.
Control drug, chloroquine
Scheme 1.
Scheme 2.
In vitro activity of polyamine analogues against P. falciparum
The first diverse library of isoteric (bis)urea and (bis)thiourea alkylated polyamine analogues was tested for possible growth inhibitory affect against intraerythrocytic P. falciparum in vitro (Table 1). The majority of these compounds showed in vitro inhibitory activity against both drug resistant (W2 chloroquine resistant strain, HB3 antifolate resistant strain) and sensitive P. falciparum (strain 3D7) at concentrations below 3 μM (Table 1). Compound 6, containing terminal (bis)diphenylpropylguanidine moieties, is active against P. falciparum at 298 nM. Conversely, it is clear that amidine substituted analogues that lacked terminal alkyl groups were not active against the malaria parasite. Compound 21, an amidine analogue containing a 3-3-3 carbon backbone, which lacks any (bis)urea and (bis)thiourea substituents, was the least effective compound (IC50 = 147 μM, Table 1). Of the 19 compounds tested, 15 have potent in vitro antimalarial activity (<1 μM) with the six most active compounds (6, 9, 13, 15, 16 and 20) displaying IC50 values in the range of 144–405 nM against the 3D7 strain of P. falciparum (Table 1). Moreover, all of these compounds had IC50 values in the nM range against drug resistant P. falciparum with compounds 13, 15, 16 and 20 being more active against chloroquine-resistant P. falciparum (strain W2) displaying low resistance factors (ranging from 0.19–0.61) compared to chloroquine against this strain. This suggests that these analogues are minimally affected by the resistance mechanisms of e.g. chloroquine with a mechanistically distinct mode of action (Table 1).
Analysis of the antimalarial effects of this first series of (bis)urea and (bis)thiourea alkylated polyamine analogues suggests that the most potent compounds contain either a 3-7-3 or 3-4-3 carbon backbones, with the 3-7-3 carbon backbone delivering the best activity against the parasite. Compounds with (bis)urea substituents exhibited the most potent antimalarial activity, followed by the (bis)thiourea substituted compounds. The diphenylpropyl substituents proved more effective than the diphenylethyl substituents as terminal groups of these compounds. Analysis of the amidine alkylated polyamine analogues suggests that the 3-3-3 and 3-4-3 carbon backbones are not effective against the parasite and that analogues with a 3-7-3 carbon backbone are active in the nM range, particularly when they have terminal diphenylpropyl substituents. Based on results observed with this first evaluation of the antimalarial activity of polyamine analogues, the selective design and synthesis of a second series of compounds, predicted to have a higher antimalarial capacity, was attempted. This second series of 15 compounds contained 3-6-3, 3-7-3 and 3-4-3 backbones but with a variety of terminal substituents.
Of the second series of analogues, 13 had potent antimalarial activity with IC50 values in the range of 88–846 nM (Table 2). The five most potent compounds (25, 29, 30, 38 and 39), with IC50 values ranging between 88–211 nM, were also active against chloroquine resistant P. falciparum (W2 strain). These compounds all have a 3-7-3 or 3-6-3 carbon architecture, the majority with (bis)urea and terminal aralkyl substituents. Compounds 34 and 35 were the least effective against the parasite, with 34 not active in the μM range, and 35 with an IC50 = 14.08 μM (Table 2). Both of these contain 3-6-3 carbon backbones with (bis)urea substituents, similar to the structures of the most effective compounds, with the exception that they feature alkyl rather than aralkyl substituent rings. This second generation of synthesized (bis)urea and (bis)thiourea alkylated polyamine analogues suggests that the most potent compounds contain either a 3-6-3 or 3-7-3 carbon backbones, with the 3-6-3 carbon backbone delivering the best activity against the parasite. Compounds with (bis)urea substituents exhibited the most potent antimalarial activity, followed by the (bis)thiourea substituted compounds. The selection of terminal substituents of these compounds are of vital importance in enhancing their antimalarial activity, compounds with terminal phenyl rings had the best activity, followed by diphenylethyl substituents, benzyl rings and lastly diphenylmethyl substituents. Compounds with no terminal aralkyl groups were the least effective against the parasite with IC50 values in the high μM range, thus demonstrating the importance of bulky terminal substituents for antimalarial activity.
Some terminally, symmetrically substituted polyamine analogues target DNA and exert a cytotoxic effect through DNA aggregation.7 In most instances, the polyamine analogues (compounds 9, 13, 15, 16 and 25, 29, 30, 38, 39) elicited a significant cytotoxic response in the parasite (P<0.05), as measured by a decrease in viable cell numbers (% parasitemia, Figure 2). However, even through compound 20 was active against the parasite, it was not able to decrease viable cell numbers in P. falciparum over time, indicating a cytostatic action on in vitro growth. During its asexual intraerythrocytic development, P. falciparum replicates its DNA as it develops from single-nucleated ring (1N) and trophozoite stages (1N) to multinucleated schizont (2N or >2N) stages that result in up to 32 daughter merozoites (mono-nucleated) being formed from a single parent parasite.17,18 Polyamines have been shown to be important for DNA replication and therefore implied life cycle development in Plasmodia.19 The effects of the polyamine analogues (compounds 9, 13, 15, 16 and 25, 29, 30, 38, 39) on the ability of the parasite to replicate its DNA were monitored. An untreated parasite population contained 28% of parasites with single nuclei (1N), whereas 38% and 34% were in multinucleated schizonts forms of 2N or >2N, respectively (Figure 3), demonstrating complete intraerytrocytic asexual development as expected after 72 h of development as measured by flow cytometry. Treatment of P. falciparum with compound 20 produced similar cytometric profiles to untreated parasites and was not able to prevent DNA replication, confirming its cytostatic nature. However, for compound 16, a dramatic halt in schizogony and associated nuclear division was observed with parasites containing 78% 1N rings/trophozoites, 20% 2N and 2% >2N schizonts after treatment with this compound. Compounds 9, 13, 15, 25, 29, 30, revealed similar profiles to that of compound 16 (results not shown). Compounds 16, 38 and 39 had the greatest inhibitory affect on DNA replication and nuclear division with the majority of the parasites being confined to the ring stage (Figure 3).
Figure 2.
Viable cell count of P. falciparum (3D7) treated with compounds 16 or 20 (2xIC50). Parasites were treated for 72 h, after which parasitemia was determined microscopically with Giemsa stains (counting 100 parasites per slide 10x, n=3) and DNA levels were quantified using SYBR Green I incorporation. Black bars: % parasitemia at 0 h; grey bars: after 72 h. Results are the mean of three independent experiments, performed in triplicate, ± S.E. Significance is indicated at P<0.05 (*) as determined with a student-T test.
Figure 3.
Flow cytometric analysis of nuclear division of P. falciparum treated with compounds 16, 38 and 39 (2xIC50). Ring or trophozoite stage parasites contain 1 nucleus (1N), followed by nuclear division in late trophozoites (2N) and multinucleated schizonts (>2N), represented as the % parasites in each population. Flow cytometric measurement of nuclear content was performed with SYBR Green I staining of DNA tracked in the FITC channel. Data are represented as the mean of three independent experiments, performed in duplicate, ± S.E.
Treatment of P. falciparum with polyamine biosynthesis inhibitors like the substrate analogues α-difluoromethylornithine and 3-aminooxy-1-aminopropane has been shown to be reversible and therefore the inhibitory effect is alleviated with the addition of exogenous polyamines to the parasite.20,21 In order to investigate the influence of exogenous polyamines on P. falciparum growth inhibition observed with the current series of polyamine analogues, polyamine reversal studies were performed to determine if treated parasites could recover when supplemented with exogenous putrescine. The inhibitory effect observed with the polyamine compounds could not be reversed with exogenous polyamines for any of the most potent compounds (Figure 4). The decreased cell viability observed for compound 16 was again visible over a 72 hr time period, and this was already evident after the first 24 h, during which the parasite needed to start nuclear division. Therefore, the cytotoxic action of these polyamine analogues on P. falciparum seems to be independent of changes in the polyamine pool. This may be due to the analogues’ ability to block the intracellular binding sites of the natural polyamines, or to displace intracellular polyamines from their binding sites.10 Alternatively, the mode of action of these polyamine analogues against P. falciparum may be independent of the polyamine pathway. In trypanosomes, polyamine analogues act as competitive inhibitors of enzymes not directly related to polyamine biosynthesis.22 It remains to be seen if the polyamine analogues manifest their effect on P. falciparum through targeting epigenetic control mechanisms, as has been observed in mammalian cells.13 The parasite seems to be exquisitely sensitive towards epigenetic regulatory mechanisms, particularly for the control of expression of variant gene families.23
Figure 4.
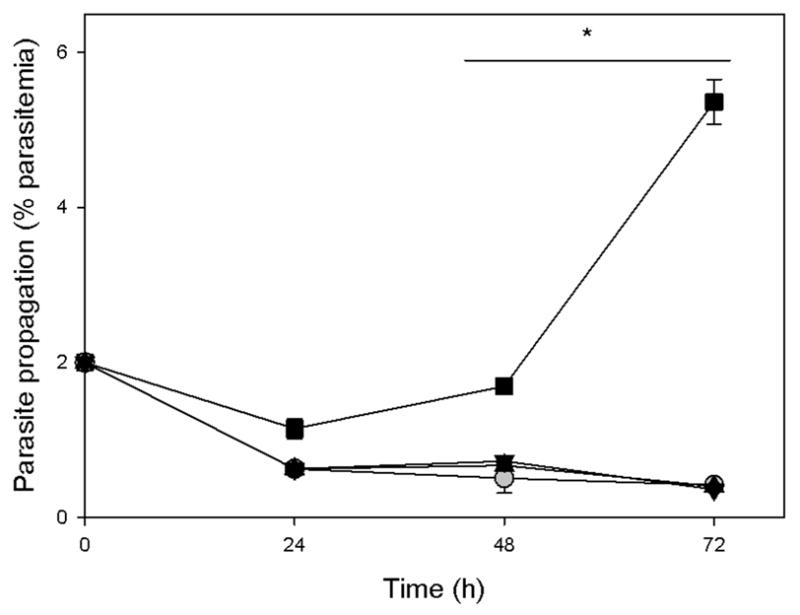
Influence of exogenous addition of polyamines to P. falciparum (3D7) treated with polyamine analogues. Parasites were treated with compound 16 alone (2xIC50, grey circles), or supplemented with 1 mM putrescine after either 24 h (upward triangles) or 48 h (downward triangles). Parasitemia was monitored for 72 h using SYBR Green I. Untreated parasites are indicated with squares. Data are represented as the mean of three independent experiments, performed in duplicate, ± S.E. Error bars fall within symbols where not shown. Significance is indicated at P<0.05 (*) as determined with two-way ANOVA.
P. falciparum appear to be highly sensitive to the polyamine analogues described above, with the majority showing parasite IC50 values below 500 nM. In order to ensure that this effect was not merely due to general toxicity of the compounds, in vitro cytotoxicity testing was performed in a sensitive mammalian cell line. A subset of the compounds described in this manuscript, notably 14, 15 and 16, have been evaluated as potential antitumor agents in the Calu-6 non-small cell human lung carcinoma cell line.13 It is important to note that these compounds exert antitumor effects through re-expression of aberrantly silenced tumor suppressor genes, and as such are not inherantly cytotoxic in mammalian cells when used as single agents. Maximal cytotoxicity to tumor cells in vitro and in vivo can only be achieved through synergistic effects with a traditional agents such as 5-azacytidine. The GI50 values in the Calu-6 cell line for 14–16 alone range between 10 and 40 μM, and thus they are not generally cytotoxic agents. The most active compounds against P. falciparum (compounds 9, 13, 15, 16, 20 and 25, 29, 30, 38, 39) showed remarkable selectivity towards the parasite compared to a mammalian cell line (HepG2 human hepatocellular liver carcinoma, Table 3), with the majority of the compounds (particularly compounds 20, 25, 29, 30, 38, 39) showing >500-fold selectivity towards the parasite. Remarkably, the most active compound (30) is ~7000-fold more selective towards the parasite. Comparatively, compounds 14, 15 and 16 were not the most effective against P. falciparum and inhibited cell growth in Calu-6 cells at μM concentrations (Table 3), but even these compounds showed more than ~10-fold selectivity against the malaria parasite.13 The results presented here indicate that the compounds do not show general cytotoxicity in mammalian cells, and that P. falciparum seems to show selective sensitivity to these polyamine analogues. These results are encouraging in implicating this series of polyamine analogues as highly selective antimalarial agents. Moreover, the ability of polyamine analogues to cure in vivo infections of malaria in the murine model of P. berghei should provide clues as to the ultimate antimalarial potential of this structurally distinct class of compounds, and these studies are currently underway.
Table 3.
Selectivity of polyamine analogues for growth inhibition of P. falciparum (3D7) compared to selected mammalian cells.
| Cpd | P. falciparum (IC50, μM) | Calu-6 (GI50, μM)a | HepG2 (GI50, μM) | SIc (Calu-6/Pf) | SIc (HepG2/Pf) |
|---|---|---|---|---|---|
| 9 | 0.144 ± 0.031 | 23.92 ± 0.4 | 166 | ||
| 13 | 0.253 ± 0.003 | 24.52 ± 1.4 | 97 | ||
| 14 | 1.316 ± 0.01 | 9.4 | nd | 7 | - |
| 15 | 0.329 ± 0.009 | 38.3 | 18.92 ± 1.39 | 38 | 58 |
| 16 | 0.405± 0.008 | 10.3 | 24.67 ± 0.3 | 25 | 61 |
| 20 | 0.355 ± 0.09 | >200 | >500 | ||
| 25 | 0.211 ± 0.007 | >200 | >500 | ||
| 29 | 0.106 ± 0.011 | >200 | >1500 | ||
| 30 | 0.088 ± 0.007 | 619.3 ± 25.8d | 7038 | ||
| 38 | 0.130 ± 0.002 | >200 | >1500 | ||
| 39 | 0.1 ± 0.003 | 33.8 ± 3.24 | 338 | ||
| CQ | 0.009 | 22.16 ± 0.39 | 2462 |
Data obtained from Sharma et al.13 Calu-6 are human nonsmall cell lung carcinoma cells
Values are the means ± S.E. of at least 2 independent experiments performed in duplicate. HepG2 are human hepatocellular liver carcinoma cells.
Selectivity indices were determined as the compound GI50 mammalian cell/IC50 P. falciparum
Values are the means ± S.E. of 3 independent experiments performed in duplicate.
EXPERIMENTAL
All reagents and dry solvents were purchased from Aldrich Chemical Co. (Milwaukee, WI), Sigma Chemical Co. (St. Louis, MO) or Acros Chemical (Chicago, IL) and were used without further purification except as noted below. Triethylamine was distilled from potassium hydroxide and stored in a nitrogen atmosphere. Methanol was distilled from magnesium and iodine under a nitrogen atmosphere and stored over molecular sieves. Methylene chloride was distilled from phosphorus pentoxide and chloroform was distilled from calcium sulfate. Tetrahydrofuran was purified by distillation from sodium and benzophenone. Dimethyl formamide was dried by distillation from anhydrous calcium sulfate and was stored under nitrogen. Preparative scale chromatographic procedures were carried out using E. Merck silica gel 60, 230–440 mesh. Thin layer chromatography was conducted on Merck precoated silica gel 60 F-254. Ion exchange chromatography was conducted on Dowex 1X8-200 anion exchange resin. All 1H- and 13C-NMR spectra were recorded on a Varian Mercury 400 MHz spectrometer, and all chemical shifts are reported as d values referenced to TMS. In all cases, 1H-NMR, 13C-NMR and IR spectra were consistent with assigned structures. Mass spectra were recorded on a Kratos MS 80 RFA (EI and CI) or Kratos MS 50 TC (FAB) mass spectrometer. Prior to biological testing, target molecules 7–39 were determined to be 95% pure or greater by HPLC chromatography using an Agilent Series 1100 high-performance liquid chromatograph fitted with a C18 reversed-phase column. Compounds 7–20 were previously synthesized and their 1H and 13C NMR spectra have been reported.13
General Procedure for the Synthesis of Urea and Thiourea Analogs
Step-A
In a 100 mL round-bottom flask, the centrally Boc substituted diamino compounds 43 (0.5 mol) were dissolved in 10 mL of HPLC grade CH2Cl2, and added a solution of alkyl-, benzyl-, or phenyl- substituted isocyanate or isothiocyanate, 44 (1.0 mmol, 2 equiv) in 5 mL of CH2Cl2 under cold condition. The flask was protected with N2 atmosphere and the reaction mixture was allowed to stir at room temperature for 18–24 h, the progress for formation of product was monitored by TLC using CH2Cl2/MeOH/NH4OH (94.4:5.0:0.5 or 89:10:1). After completion of the reaction, CH2Cl2 was removed under reduced pressure on a rotary evaporator to produce a viscous colorless material. The product was transferred into next step without further purification.
Step-B
The above crude product was dissolved in anhydrous MeCO2Et (6.0 mL), and added 1M HCl in MeCO2Et (6.0 mL), the reaction mixture becomes cloudy. The flask was protected with N2 atmosphere and the reaction mixture was allowed to stir at room temperature for 24–48 h, the progress for formation of product was monitored by TLC using CH2Cl2:MeOH:NH4OH (89:10:1 and 78:20:2). After completion of the reaction as confirmed by TLC, and the nature of the product (white crystalline materials separated from the solution), MeCO2Et was removed under reduced pressure on a rotary evaporator to produce a white powder. The solid product was well stirred with 20 mL of fresh MeCO2Et, and decanted the soluble part, the solid so obtained was vacuum dried to give pure product as a white solid.
1,14-Bis-{3-[1-(1′,1′-diphenylmethyl)thioureado]}-4,11-diazatetradecane Hydrochloride, 25 (RJB-92-09C)
1H NMR (DMSO-d6): d 8.83 (bs, 6H, NH), 8.26 (bs, 2H, NH), 7.28 (bs, 16H, Ar-H), 7.21 (bs, 4H, Ar-H), 6.71 (b, 2H, CHPh2), 3.39 (bs, 4H, NCH2), 2.87 (bs, 4H, NCH2), 2.82 (bs, 4H, NCH2), 1.85 (bs, 4H, CH2CH2), 1.58 (bs, 4H, CH2CH2), 1.28 (bs, 4H, CH2CH2); 13C NMR (DMSO-d6): d183.21 (C=S), 143.41, 129.07, 127.89, 127.57 (ArC), 61.28, 47.22, 45.23, 41.39, 26.38, 26.08, 25.85 (CH2).
1,14-Bis-{3-[1-(2′,2′-diphenylethyl)thioureado]}-4,11-diazatetradecane Hydrochloride, 26 (RJB-92-09)
1H NMR (DMSO-d6): d 9.00 (bs, 4H, NH), 7.80 (bs, 2H, NH), 7.54 (b, 2H, NH), 7.28 (bs, 16H, Ar-H), 7.17 (bs, 4H, Ar-H), 4.37 (b, 2H, CHPh2), 4.01 (b, 4H, NCH2), 3.43 (bs, 4H, NCH2), 2.79 (bs, 8H, NCH2), 1.80 (bs, 4H, CH2CH2), 1.60 (bs, 4H, CH2CH2), 1.30 (bs, 4H, CH2CH2); 13C NMR (DMSO-d6): d 183.00 (C=S), 143.40, 129.17, 128.65, 127.08 (ArC), 50.46, 48.71, 47.22, 45.12, 41.22, 26.25, 26.14, 25.84 (CH2).
1,14-Bis-{3-[1-(3′,3′-diphenylpropyl)thioureado]}-4,11-diazatetradecane Hydrochloride, 27 (RJB-92-11C)
1H NMR (DMSO-d6): d 8.86 (bs, 4H, NH), 7.87 (bs, 4H, NH), 7.31-7.24 (m, 16H, Ar-H), 7.14 (t, 4H, J = 7.2Hz, Ar-H), 4.01 (t, 2H, J = 7.2 Hz, CHPh2), 3.43 (b, 4H, NCH2), 3.22 (b, 4H, NCH2), 2.83 (b, 8H, NCH2), 2.23 (b, 4H, NCH2), 1.82 (bs, 4H, CH2CH2), 1.52 (bs, 4H, CH2CH2), 1.29 (bs, 4H, CH2CH2); 13C NMR (DMSO-d6): d145.37, 129.11, 128.30, 126.79 (ArC), 48.62, 47.22, 45.17, 41.00, 34.92, 26.36, 26.11, 25.86 (CH2).
1,14-Bis-{3-[1-(phenyl)thioureado]}-4,11-diazatetradecane Hydrochloride 28 (RJB-92-06)
1H NMR (DMSO-d6): d10.09 (bs, 2H, NH), 8.90 (b, 4H, NH), 8.33 (bs, 2H, NH), 7.45 (d, 4H, J = 8.0 Hz, Ar-H), 7.28 (t, 4H, J = 8.0 Hz, Ar-H), 7.06 (t, 2H, J = 7.6 Hz, Ar-H), 3.54 (b, 4H, NHCH2), 2.84 (b, 8H, NCH2), 1.96 (bs, 4H, CH2CH2), 1.60 (bs, 4H, CH2CH2), 1.35 (bs, 4H, CH2CH2); 13C NMR (DMSO-d6): d181.29 (C=S), 140.10, 129.16, 124.61, 123.38, 47.21, 45.25, 41.34, 28.74, 26.12, 25.88.
1,14-Bis-{3-[1-(benzyl)thioureado]}-4,11-diazatetradecane Hydrochloride 29 (RJB-92-11)
1H NMR (DMSO-d6): d 8.96 (bs, 4H, NH), 8.25 (b, 2H, NH), 8.05 (bs, 2H, NH), 7.36-7.20 (m, 10H, Ar-H), 4.64 (s, 4H, ArCH2NH), 3.48 (bs, 4H, NHCH2), 2.82 (bs, 8H, NCH2), 1.85 (bs, 4H, CH2CH2), 1.60 (bs, 4H, CH2CH2), 1.30 (bs, 4H, CH2CH2); 13C NMR (DMSO-d6): d182.00 (C=S), 140.01, 128.90, 127.91, 127.44, 47.23, 45.15, 41.30, 31.98, 26.34, 26.14, 25.85;
1,14-Bis-{3-[1-(phenyl)ureado]}-4,11-diazatetradecane Hydrochloride 30 (RJB-92-04)
1H NMR (DMSO-d6): d 8.99 (s, 2H, NH), 8.88 (bs, 4H, NH), 7.37 (d, 4H, J = 8.0 Hz, Ar-H), 7.19 (t, 4H, J = 7.2 Hz, Ar-H), 6.84 (t, 2H, J = 7.2 Hz, Ar-H), 6.66 (bs, 2H, NH), 3.14 (b, 4H, NHCH2), 2.85 (b, 8H, NCH2), 1.77 (t, 4H, J = 6.8 Hz, CH2CH2), 1.59 (bs, 4H, CH2CH2), 1.29 (bs, 4H, CH2CH2); 13C NMR (DMSO-d6): d156.36 (C=O), 141.23, 129.28, 121.62, 118.20, 47.22, 45.28, 36.83, 27.29, 26.09, 25.86;
1,14-Bis-{3-[1-(benzyl)ureado]}-4,11-diazatetradecane Hydrochloride 31 (RJB-92-13)
1H NMR (DMSO-d6): d 9.06 (bs, 4H, NH), 7.30-7.16 (m, 14H, Ar-H, and NH), 4.18 (s, 4H, ArCH2NH), 3.08 (b, 4H, NHCH2), 2.79 (b, 8H, NCH2), 1.73 (b, 4H, CH2CH2), 1.58 (bs, 4H, CH2CH2), 1.27 (bs, 4H, CH2CH2); 13C NMR (DMSO-d6): d159.33 (C=O), 141.50, 128.88, 127.60, 127.20, 47.16, 45.12, 43.56, 36.99, 27.44, 26.10, 25.82;
1,14-Bis-{3-[1-(ethyl)thioureado]}-4,11-diazatetradecane Hydrochloride 32 (RJB-92-08)
1H NMR (DMSO-d6): d 9.92 (bs, 4H, NH), 7.79 (s, 2H, NH), 7.79 (s, 2H, NH), 3.42 (bs, 4H, NHCH2), 3.31 (bs, 4H, NCH2), 2.82 (bs, 8H, NCH2), 1.82 (b, 4H, CH2CH2), 1.59 (b, 4H, CH2CH2), 1.30 (b, 4H, CH2CH2), 1.02 (t, 6H, J = 7.20 Hz, CH3); 13C NMR (DMSO-d6): d 47.21, 45.13, 41.04, 39.00, 26.36, 26.11, 25.84, 15.11.
1,14-Bis-{3-[1-(n-propyl)thioureado]}-4,11-diazatetradecane Hydrochloride 33 (RJB-92-14C)
1H NMR (DMSO-d6): d 8.93 (bs, 4H, NH), 7.83 (bs, 2H, NH), 7.76 (bs, 2H, NH), 3.43 (bs, 4H, NHCH2), 3.25 (bs, 4H, NCH2), 2.83 (bs, 8H, NCH2), 1.82 (bs, 4H, CH2CH2), 1.59 (bs, 4H, CH2CH2), 1.44 (q, 4H, J = 7.2 Hz, CH2CH3), 1.29 (b, 4H, CH2CH2), 0.82 (t, 6H, J = 7.20 Hz, CH3); 13C NMR (DMSO-d6): d 47.20, 46.00, 45.13, 41.03, 26.36, 26.12, 25.84, 22.70,12.08.
1,14-Bis-{3-[1-(ethyl)ureado]}-4,11-diazatetradecane Hydrochloride 34 (RJB-92-15C)
1H NMR (DMSO-d6): d 9.09 (bs, 4H, NH), 7.27 (b, NH), 7.79 (s, 2H, NH), 3.04-2.95 (m, 8H, NHCH2), 2.80 (bs, 8H, NCH2), 1.71 (b, 4H, CH2CH2), 1.60 (b, 4H, CH2CH2), 1.29 (b, 4H, CH2CH2), 0.95 (t, 6H, J = 6.8 Hz, CH3); 13C NMR (DMSO-d6): d 159.22 (C=O), 47.15, 45.10, 37.00, 34.92, 27.34, 26.11, 25.81, 16.23.
1,14-Bis-{3-[1-(n-propyl)ureado]}-4,11-diazatetradecane Hydrochloride 35 (RJB-92-13C)
1H NMR (DMSO-d6): d 9.07 (bs, 4H, NH), 7.77 (b, 4H, NH), 3.04 (t, 4H, J = 6.0 Hz, NHCH2), 2.90 (t, 4H, J = 7.2 Hz, NCH2), 2.79 (b, 8H, NCH2), 1.71 (q, 4H, J = 6.8 Hz, CH2CH2), 1.60 (b, 4H, CH2CH2), 1.36-1.28 (m, 8H, CH2CH2), 0.79 (t, 6H, J = 7.6 Hz, CH3); 13C NMR (DMSO-d6): d 159.36 (C=O), 47.14, 45.10, 41.87, 36.96, 27.38, 26.10, 25.80, 23.77, 12.02.
1,12-Bis-{3-[1-(1′,1′-diphenylmethyl)ureado]}-4,9-diazadodecane Hydrochloride, 36 (SKS-96-02)
1H NMR (DMSO-d6): d 8.95 (b, 4H, NH), 8.19 (b, 2H, NH), 7.39-7.18 (m, 20H, Ar-H), 6.48 (b, 2H, NH), 5.86 (d, 2H, J = 8.4 Hz, CHPh2), 3.07 (t, 4H, J = 5.6 Hz, NCH2), 2.75 (b, 8H, NCH2), 1.79 (b, 4H, CH2CH2), 1.58 (b, 4H, CH2CH2); 13C NMR (DMSO-d6): d 158.64 (C=O), 144.45, 129.04, 127.59, 127.37 (ArC), 57.78, 46.55, 45.09, 36.86, 27.47, 23.20 (CH2).
1,12-Bis-{3-[1-(2′,2′-diphenylethyl)ureado]}-4,9-diazadodecane Hydrochloride, 37 (SKS-96-01)
1H NMR (DMSO-d6): d 9.11 (bs, 4H, NH), 7.34-7.15 (m, 20H, Ar-H), 4.10 (t, 2H, J = 6.0 Hz, CHPh2), 3.64 (m, 4H, NCH2), 3.01 (bs, 4H, NCH2), 2.81 (bs, 4H, NCH2), 2.75 (bs, 4H, NCH2), 1.68 (bs, 8H, CH2CH2); 13C NMR (DMSO-d6): d 159.05 (C=O), 143.77, 129.10, 128.58, 126.92 (ArC), 51.72, 46.51, 45.06, 44.53, 36.83, 27.39, 23.25 (CH2).
1,15-Bis-{3-[1-(1′,1′-diphenylmethyl)ureado]}-4,12-diazapentadecane Hydrochloride, 38 (SKS-96-02C)
1H NMR (DMSO-d6): d 8.93 (bs, 4H, NH), 7.28-7.14 (m, 20H, Ar-H), 5.88 (bs, 2H, CHPh2), 3.08 (bs, 4H, NCH2), 2.77 (bs, 4H, NCH2), 2.69 (bs, 4H, NCH2), 1.71 (bs, 4H, CH2CH2), 1.53 (bs, 4H, CH2CH2), 1.20 (b, 6H, CH2CH2CH2); 13C NMR (DMSO-d6): d 159.30 (C=O), 144.02, 129.60, 127.38, 127.60 (ArC), 57.70, 42.26, 45.07, 36.81, 28.56, 27.47, 26.37, 25.88 (CH2).
1,15-Bis-{3-[1-(2′,2′-diphenylethyl)ureado]}-4,12-diazapentadecane Hydrochloride, 39 (SKS-96-01C)
1H NMR (DMSO-d6): d 9.06 (bs, 4H, NH), 7.31-7.14 (m, 20H, Ar-H), 4.10 (t, 2H, J = 7.6 Hz, CHPh2), 3.64 (m, 4H, NCH2), 3.01 (t, 4H, J = 5.6 Hz, NCH2), 2.74 (bs, 8H, NCH2), 1.68 (m, 4H, CH2CH2), 1.64 (m, 4H, CH2CH2), 1.16 (bs, 6H, CH2CH2CH2); 13C NMR (DMSO-d6): d 159.11 (C=O), 143.75, 129.11, 128.58, 126.94 (ArC), 51.71, 47.26, 45.06, 44.55, 36.82, 28.62, 27.39, 26.42, 25.91 (CH2).
S-2-Naphthylmethyl Thioacetimidate Hydrobromide 46 (SKS-84-31)
To a stirred solution of thioacetamide (1.127 g, 15 mmol) in anhydrous CHCl3 (40 mL) was added 2-bromomethylnaphthalene 45 (3.40 g, 15 mmol) by cooling the reaction flask. The reaction mixture was allowed to stir at room temperature for 5 min and then heated to reflux for 2 h, cooled to back to room temperature and placed in an ice bath. The resulting solid was filtered off, washed with 50 mL CHCl3 and dried in vacuum for 3h to afford 46 (3.83 g, 86%) as a white solid, 1H NMR (DMSO-d6): d 8.00 (s, 1H Ar-H), 7.96 (m, 3H, Ar-H), 7.56 (m, 3H, Ar-H), 3.80 (s, 2H, SCH2), 2.62 (s, 3H, CH3); 13C NMR (DMSO-d6): d 193.48 (C=S), 133.48, 133.15, 131.42, 129.48, 128.83, 128.40, 128.37, 127.51, 127.42, 127.34 (ArC), 36.74 (CH2), 24.86 (CH3).
General Procedure for the Synthesis of Amidine Analogs
The diamino compound 43 (0.50 mmol) was dissolved in 12 mL of absolute ethanol, and added a solution of S-2-Naphthylmethyl Thioacetimidate Hydrobromide 46 (310 mg, 1.0 mmol, 2 equv.) under cold condition. The flask was protected with N2 atmosphere and the resulting suspension that eventually becomes homogenous was allowed to stir at room temperature for 48–72 h, the progress for formation of product was monitored by TLC using CH2Cl2/MeOH/NH4OH (89:10:1). After completion of the reaction, the ethanol was removed under reduced pressure on a rotary evaporator to produce a viscous colorless material which was purified by stirring the mixture with dry ether, discarded ether soluble part, and insoluble material was again stirred with fresh ether (25 mL). The white solid so obtained was dried in vacuum.
1,11-Bis-(acetamidinyl)-4,8-di(tert-butyloxycarbonyl)-4,8-diazoundecane Hydrobromide 47a (SKS-84-40)
1H NMR (DMSO-d6): d 9.38 (bs, 1H, NH), 9.06 (bs, 1H, NH), 8.55 (bs, 1H, NH), 8.27 (bs, 1H, NH), 7.19 (t, 2H, NH), 3.20-3.05 (m, 12H, NCH2), 2.11 (s, 6H, CH3), 1.72-1.60 (m, 6H, CH2), 1.33 (s, 18H, C[CH3]3).
1,12-Bis-(acetamidinyl)-4,9-di(tert-butyloxycarbonyl)-4,9-diazododecane Hydrobromide 47b (SKS-84-31C)
1H NMR (DMSO-d6): d 9.18 (b, 1H, NH), 9.04 (b, 1H, NH), 8.50 (b, 1H, NH), 7.91 (b, 1H, NH), 7.08 (t, 2H, NH), 3.1o (b, 12H, NCH2), 2.11 (s, 6H, CH3), 1.71 (m, 4H, CH2), 1.42-1.30 (bs, 22H, CH2 and C[CH3]3); 1H NMR (CD3OD): d 3.30 (t, 4H, J = 7.2 Hz, NCH2), 3.24 (bs, 8H, NCH2), 2.24 (s, 6H, CH3), 1.90 (m, 4H, CH2), 1.53 (m, 4H, CH2), 1.45 (s, 18H, C[CH3]3).
1,14-Bis-(acetamidinyl)-4,11-di(tert-butyloxycarbonyl)-4,11-diazotetradecane Hydrobromide 47c (SKS-99-05C)
1H NMR (CD3OD): d 3.30-3.19 (m, 12H, NCH2), 2.24 (s, 6H, CH3), 1.87 (b, 4H, CH2), 1.55 (b, 4H, CH2), 1.45 (s, 18H, C[CH3]3), 1.32 (b, 4H, CH2).
1,15-Bis-(acetamidinyl)-4,12-di(tert-butyloxycarbonyl)-4,12-diazopentadecane Hydrobromide 47d (SKS-84-33C)
1H NMR (CD3OD): d 3.28-3.19 (m, 12H, NCH2), 2.22 (s, 6H, CH3), 1.86 (m, 4H, CH2), 1.54 (m, 4H, CH2), 1.45 (s, 18H, C[CH3]3), 1.29 (m, 6H, CH2).
Cleavage of Boc group
The compound 47a–d (0.43–0.45 mmol) was stirred with 6 mL of anhydrous MeCO2Et for 5 min, and added 1M HCl in MeCO2Et (5 mL). The flask was protected with N2 atmosphere and the reaction mixture was allowed to stir at room temperature for 24–48 h, the progress for formation of product was monitored by tlc (CH2Cl2:MeOH:NH4OH 78:20:2). After completion of the reaction, MeCO2Et was removed under reduced pressure on a rotary evaporator to produce a white powder. The solid product was well stirred with 20 mL of fresh MeCO2Et, and decanted the soluble part, the solid so obtained was vacuum dried to give pure product 21–24 as a white solid.
1,11-Bis-(acetamidinyl)-4,8-diazoundecane Hydrochloride 21 (SKS-84-40C)
1H NMR (DMSO-d6): d 9.75 (s, 2H, NH), 9.29 (s, 2H, NH), 9.23 (s, 4H, NH), 8.79 (s, 2H, NH), 2.98 (b, 12H, NCH2), 2.13 (s, 6H, CH3), 2.07 (b, 2H, CH2), 1.89 (b, 4H, CH2); 13C NMR (DMSO-d6): d 164.79 (=CH), 44.83, 44.61, 39.54 (NCH2), 24.73, 22.85(CH2), 19.27 (CH3); 1H NMR (D2O): d 3.35 (t, 4H, J = 6.4 Hz, NCH2), 3.18-3.11 (m, 8H, NCH2), 2.19 (s, 6H, CH3), 2.12-2.09 (m, 2H, CH2), 2.08-2.01 (m, 2H, CH2); 13C NMR (D2O): d 164.80 (=CH), 45.35, 44.83, 39.37 (NCH2), 29.86, 24.07(CH2), 18.70 (CH3).
1,12-Bis-(acetamidinyl)-4,9-diazododecane Hydrochloride 22 (SKS-84-35)
1H NMR (DMSO-d6): d 9.88 (s, 2H, NH), 9.10 (s, 6H, NH), 8.81 (s, 2H, NH), 3.34 (m, 4H, NCH2), 2.94 (b, 4H, NCH2), 2.87 (b, 4H, NCH2), 2.14 (s, 6H, CH3), 1.90 (m, 4H, CH2), 1.71 (b, 4H, CH2); 13C NMR (DMSO-d6): d 164.79 (=CH), 46.62, 44.72, 39.84 (NCH2), 24.71, 23.21 (CH2), 19.26 (CH3); 1H NMR (D2O): d 3.34 (t, 4H, J = 7.2 Hz, NCH2), 3.13-3.06 (m, 8H, NCH2), 2.20 (s, 6H, CH3), 2.02 (m, 4H, CH2), 1.76 (m, 4H, CH2); 13C NMR (D2O): d 47.26, 45.19, 39.40 (NCH2), 24.09, 23.07 (CH2), 18.69 (CH3). HR-MS m/z 285.4
1,14-Bis-(acetamidinyl)-4,11-diazotetradecane Hydrochloride, 23 (SKS-99-06)
1H NMR (DMSO-d6): d 9.79 (s, 2H, NH), 9.27 (s, 2H, NH), 9.13 (s, 4H, NH), 8.82 (s, 2H, NH), 3.33 (b, 4H, NCH2), 2.93 (b, 4H, NCH2), 2.83 (b, 4H, NCH2), 2.14 (s, 6H, CH3), 1.90 (b, 4H, CH2), 1.63 (b, 4H, CH2) 1.30 (b, 4H, CH2); 13C NMR (DMSO-d6): d 164.77 (=CH), 47.28, 44.77, 39.98 (NCH2), 26.11, 25.74, 24.73(CH2), 19.22 (CH3); 1H NMR (D2O): d 3.21 (t, 4H, J = 6.4 Hz, NCH2), 2.96 (t, 4H, J = 7.2 Hz, NCH2), 2.90 (t, 4H, J = 7.6 Hz, NCH2), 2.06 (s, 6H, CH3), 1.89 (m, 4H, CH2), 1.54 (b, 4H, CH2) 1.25 (b, 4H, CH2); 13C NMR (D2O): d 165.30 (=CH), 47.81, 44.97, 39.36 (NCH2), 25.45, 25.34, 23.99(CH2), 19.10(CH3).
1,15-Bis-(acetamidinyl)-4,12-diazopentadecane Hydrochloride 24 (SKS-84-34)
1H NMR (DMSO-d6): d 9.78 (s, 2H, NH), 9.25 (s, 2H, NH), 9.12 (s, 4H, NH), 8.81 (s, 2H, NH), 3.33 (m, 4H, NCH2), 2.93 (b, 4H, NCH2), 2.82 (b, 4H, NCH2), 2.14 (s, 6H, CH3), 1.89 (m, 4H, CH2), 1.62 (b, 4H, CH2) 1.27 (b, 6H, CH2); 13C NMR (DMSO-d6): d 164.77 (=CH), 47.35, 44.75, 39.84 (NCH2), 28.58, 26.39, 25.86, 24.75 (CH2), 19.26 (CH3); 1H NMR (D2O): d 3.34 (t, 4H, J = 6.4 Hz, NCH2), 3.09 (t, 4H, J = 8.0 Hz, NCH2), 3.03 (t, 4H, J = 8.0 Hz, NCH2), 2.20 (s, 6H, CH3), 2.04 (m, 4H, CH2), 1.66 (m, 4H, CH2), 1.35 (b, 6H, CH2); 13C NMR (D2O): d 48.05, 45.05, 39.46 (NCH2), 27.97, 25.73, 24.09 (CH2), 18.71 (CH3).
In vitro cultivation of P. falciparum
P. falciparum 3D7 (chloroquine sensitive), W2 (chloroquine resistant) and HB3 (antifolate resistant) strains were used to assess the in vitro antimalarial efficacy of the polyamine analogues. The parasites were maintained in O+ human red blood cells suspended at 5% hematocrit in RPMI-1640 culture medium containing 23.81 mM sodium bicarbonate; 0.024 mg/mL gentamycin; 25 mM HEPES; 0.2% glucose; 0.2 mM hypoxanthine and 5 g/L Albumax II. The parasites were incubated with moderate shaking at 60 rpm at 37°C in an atmosphere of 5% CO2, 5% O2 and 90% N2.24 Cultures were synchronized to >95% in the ring stage with 10% (w/v) D-sorbitol treatment.
In vitro assessment of antimalarial activity
In vitro activity against erythrocytic stages of P. falciparum (strains 3D7 and W2) was determined using the Malaria SYBR Green I–based fluorescence assay (MSF)25 based on the DNA binding properties of this dye. Compounds were dissolved in a non-lethal DMSO concentration (< 0.013%),26 serially diluted and added to ring stage P. falciparum (1% parasitemia, 2% hematocrit) and incubated at 37°C, static for 96 h. Subsequently, equal volumes (100 μl) of the parasite suspension were added to SYBR Green I lysis buffer [0.2 μL/mL of 10 000x SYBR Green I (Invitrogen), 20 mM Tris, pH 7.5; 5 mM EDTA; 0.008% (w/v) saponin; 0.08% (v/v) Triton X-100] and incubated in the dark for 1 hr at room temperature. Fluorescence was read with a Fluoroskan Ascent FL microplate reader at an excitation of 485 nm and emission of 538 nm. The data was represented as percentage of untreated control to determine cell proliferation. Non-linear regression curves were generated using Sigma Plot 11.0, from which the 50% inhibitory concentrations (IC50) could be determined. Each compound was tested in duplicate for at least three independent biological replicates.
Determination of parasite DNA replication
The effect of the compounds on P. falciparum DNA replication and nuclear division was determined using flow cytometry. Parasites (2% parasitemia, 2% hematocrit) were treated with test compounds (2xIC50) and 50 μL samples were isolated at set time intervals following drug exposure. Parasites were fixed with of 1 mL of 0.025% glutaraldehyde for 45 min and kept at 4°C until use. Fixed cells were washed twice with 1xPBS, resuspended in 20 μL PBS and stained with 20 μL 1:1000 SYBR Green I for 30 min in the dark at room temperature. DNA fluorescence was measured with a BD FACS Aria I flow cytometer (Becton Dickinson) analyzing 106 cells for each sample with fluorescence emission collected at an excitation wavelength of 488 nm with 502 nm long-band-pass and 530 nm band-pass emission filters. BD FACS Diva Software (6.1.1) and FlowJo v9.1 (Tree Star) were used to analyze the data.
Determination of polyamine reversal of inhibition
The ability of exogenous putrescine to rescue polyamine analogue treated parasites was determined using polyamine reversal studies that were set up in the format similar to that of the MSF assay. P. falciparum 3D7 cultures were treated in early ring stages with test compounds at 2xIC50. After 24 and 48 h, the cultures were supplemented with 1 mM putrescine and incubated for a further 24 h or 48 h, after which parasitemia was determined as described for the MSF assay. Statistical analysis was performed with GraphPad InStat 3.10, all data given are the mean of at least three independent biological repeats.
Cytotoxicity determinations in mammalian cells
Human hepatocellular liver carcinoma cells (HepG2, kind gift by Duncan Cromarty, University of Pretoria) were maintained in Dulbecco’s Modified Eagle’s Medium (DMEM) supplemented with 10% heat inactivated fetal bovine serum and 1% penicillin/Streptomycin at 37°C (5% CO2, 90% humidity). Cytotoxicity was measured using the lactate dehydrogenase assay (LDH). Cells (100 000) were seeded in 96-well plates and grown for 24 h at 37°C, after which cells were treated with various concentrations of the compounds. After 48 h exposure, cells were pelleted at 250xg for 10 min and LDH activity was measured in the supernatant (10 μL) by adding 100 μL LDH reaction mix (BioVision) and incubating for 30 min at room temperature. Colorimetric detection of NADH levels (as a measurement of LDH-mediated oxidation of lactate indicating LDH activity) occurred at 450 nm. Experiments were performed in duplicate for at least two independent biological repeats.
Acknowledgments
We thank Wayne Barnes at the Flow Cytometry and Cell Sorting Unit in the Department of Biochemistry, University of Pretoria for technical assistance and Bridgette Cummings for proofreading the manuscript. This work was supported by the South African Malaria Initiative (www.sami.org.za), the South African National Research Foundation (NRF Grant FA2007050300003) and the University of Pretoria (LMB) and National Institutes of Health grant 7RO1-CA149095 (PMW). BV was supported by grants from TATA Africa and the South African Malaria Initiative. Any opinion, findings and conclusions or recommendations expressed in this paper are those of the author(s) and therefore the NRF does not accept any liability in regard hereto.
ABBREVIATIONS
- BW-1
bis(phenylbenzyl) 3-7-3 analogue
- MDL 27695
N, N′-bis(benzyl)-substituted polyamine analogue
- MSF
Malaria SYBR Green I – based fluorescence assay
References
- 1.Dondorp A, Yeung S, White L, Nguon C, Day N, Socheat D, von Seidlein l. Artemisinin resistance: current status and scenarios for containment. Nat Rev Microbiol. 2010;8:272–280. doi: 10.1038/nrmicro2331. [DOI] [PubMed] [Google Scholar]
- 2.Heby O, Persson L, Rentala M. Targeting the polyamine biosynthetic enzymes: a promising approach to therapy of african sleeping sickness, chagas’ disease, and leishmaniasis. Amino Acids. 2007;33(2):359–366. doi: 10.1007/s00726-007-0537-9. [DOI] [PubMed] [Google Scholar]
- 3.Casero RA, Marton LJ. Targeting polyamine metabolism and function in cancer and other hyperproliferative diseases. Nat Rev Drug Discov. 2007;6(5):373–390. doi: 10.1038/nrd2243. [DOI] [PubMed] [Google Scholar]
- 4.Wallace HM, Fraser AV, Hughes A. A perspective of polyamine metabolism. Biochem J. 2003;15(376):1–14. doi: 10.1042/BJ20031327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Clark K, Niemand J, Reeksting S, Smit S, Van Brummelen A, Williams M, Louw AI, Birkholtz L. Functional consequences of perturbing polyamine metabolism in the malaria parasite. Amino Acids. 2010;38(2):633–644. doi: 10.1007/s00726-009-0424-7. [DOI] [PubMed] [Google Scholar]
- 6.Wallace HM, Niiranen K. Polyamine analogues - an update. Amino Acids. 2007;33(2):261–265. doi: 10.1007/s00726-007-0534-z. [DOI] [PubMed] [Google Scholar]
- 7.Casero RA, Woster PM. Recent advances in the development of polyamine analogues as antitumor agents. J Med Chem. 2009;52(15):4551–4573. doi: 10.1021/jm900187v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bi X, Lopez C, Bacchi C, Rattendib D, Woster P. Novel alkylpolyaminoguanidines and alkylpolyaminobiguanides with potent antitrypanosomal activity. Bioorg Med Chem Lett. 2006;16(12):3229–3232. doi: 10.1016/j.bmcl.2006.03.048. [DOI] [PubMed] [Google Scholar]
- 9.Zou Y, Wu Z, Sirisoma N, Woster PM, Casero RA, Weiss LM, Rattendi D, Lane S, Bacchi CJ. Novel alkylpolyamine analogues that possess both antitrypanosomal and antimicrosporidial activity. Bioorg Med Chem Lett. 2001;11(12):1613–1617. doi: 10.1016/s0960-894x(01)00315-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bitonti A, Dumont J, Bush T, Edwards M, Stemerick D, McCann P, Sjoerdsma A. Bis(benzyl)polyamine analogs inhibit the growth of chloroquineresistant human malaria parasites (Plasmodium falciparum) in vitro and in combination with α-difluoromethylornithine cure murine malaria. Proc Natl Acad Sci USA. 1989;86(2):651–655. doi: 10.1073/pnas.86.2.651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bacchi CJ, Yarlett N, Faciane E, Bi X, Rattendi D, Weiss LM, Woster PM. Metabolism of an alkyl polyamine analog by a polyamine oxidase from the microsporidian Encephalitozoon cuniculi. Antimicrob Agents Chemother. 2009;53(6):25992604. doi: 10.1128/AAC.00267-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bacchi CJ, Rattendi D, Faciane E, Yarlett N, Weiss LM, Frydman B, Woster PM, Wei B, Marton LJ, Wittner M. Polyamine metabolism in a member of the phylum Microspora (Encephalitozoon cuniculi): effects of polyamine analogues. Microbiology. 2004;150(5):1215–1224. doi: 10.1099/mic.0.26889-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sharma S, Wu Y, Steinbergs N, Crowley M, Hanson A, Casero RAJ, Woster P. (Bis)urea and (bis)thiourea inhibitors of lysine-specific demethylase 1 as epigenetic modulators. J Med Chem. 2010;53(14):5197–5212. doi: 10.1021/jm100217a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shearer BG, Oplinger JA, Lee S. S-2-Naphthylmethyl thioacetimidate hydrobromide: A new odorless reagent for the mild synthesis of substituted acetamidines. Tetrahedron Lett. 1997;38(2):179–182. [Google Scholar]
- 15.Keller O, Keller WE, van Look G, Wersin G. tert-Butoxycarbonylation of amino acids and their derivatives: N-tert-Butoxycarbonyl-l-Phenylalanine. Org Synth. 1985;63:160–171. [Google Scholar]
- 16.Bellevue FH, Baohbedason M, Wu R, Woster PM, Casero RAJ, Rattendi D, Lane S, Bacchi CJ. Structural comparison of alkylpolyamine analogues with potent in vitro antitumor or antiparasitic activity. Bioorg Med Chem Lett. 1996;6(22):2765–2770. [Google Scholar]
- 17.Reilly HB, Want H, Steuter JA, Marx AM, Ferdig MT. Quantitative dissection of clone-specific growth rates in cultures malaria parasites. Int J Parasitol. 2007;37(14):1599–1607. doi: 10.1016/j.ijpara.2007.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Izumiyama S, Omura M, Takasaki T, Ohmae H, Asahi H. Plasmodium falciparum: development and validation of a measure of intraerythrocytic growth using SYBR Green I in a flow cytometer. Exp Parasitol. 2009;121(2):144–150. doi: 10.1016/j.exppara.2008.10.008. [DOI] [PubMed] [Google Scholar]
- 19.Assaraf YG, Abu-Elheiga L, Spira DT, Desser H, Bachrach U. Effect of polyamine depletion on macromolecular synthesis of the malarial parasite, Plasmodium falciparum, cultured in human erythrocytes. Biochem J. 1987;242(1):221–226. doi: 10.1042/bj2420221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Das Gupta R, Krause-Ihle T, Bergmann B, Müller I, Khomutov A, Müller S, Walter R, Lüersen K. 3-Aminooxy-1-aminopropane and derivatives have an antiproliferative effect on cultured Plasmodium falciparum by decreasing Intracellular polyamine concentrations. Antimicrob Agents Chemother. 2005;49(7):2857–2864. doi: 10.1128/AAC.49.7.2857-2864.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Assaraf YG, Golenser J, Spira DT, Messer G, Bachrach U. Cytostatic effect of DL-alpha-difluoromethylornithine against Plasmodium falciparum and its reversal by diamines and spermidine. Parasitol Res. 1987;73(4):313–318. doi: 10.1007/BF00531084. [DOI] [PubMed] [Google Scholar]
- 22.Li Z, Fennie MW, Ganem B, Hancock MT, Kosbaslija M, Rattendi D, Bacchi CJ, O’Sullivan MC. Polyamines with N-(3-phenylpropyl) substituents are effective competitive inhibitors of trypanothione reductase and trypanocidal agents. Bioorg Med Chem Lett. 2001;11(2):251–254. doi: 10.1016/s0960-894x(00)00643-0. [DOI] [PubMed] [Google Scholar]
- 23.Duraisingh MT, Voss TS, Marty AJ, Duffy MF, Good RT, Thompson JK, Freitas-Junior LH, Scherf A, Crabb BS, Cowman AF. Heterochromatin silencing and locus repositioning linked to regulation of virulence genes in Plasmodium falciparum. Cell. 2005;121(1):13–24. doi: 10.1016/j.cell.2005.01.036. [DOI] [PubMed] [Google Scholar]
- 24.Trager W, Jensen JD. Human malaria parasites in continuous culture. Science. 1976;193(4254):637. doi: 10.1126/science.781840. [DOI] [PubMed] [Google Scholar]
- 25.Smilkstein M, Sriwilaijaroen N, Kelly J, Wilairat P, Riscoe M. Simple and inexpensive fluorescence-based technique for high-throughput antimalarial drug screening. Antimicrob Agents Chemother. 2004;48(5):1803–1806. doi: 10.1128/AAC.48.5.1803-1806.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Grobusch MP, Hanscheid T, Gobels K, Slevogt H, Zoller T, Rogler G, Teichmann D. Comparison of three antigen detection tests for diagnosis and follow-up of falciparum malaria in travellers returning to Berlin, Germany. Parasitol Res. 1998;89(5):354–357. doi: 10.1007/s00436-002-0764-7. [DOI] [PubMed] [Google Scholar]