Abstract
AIM: To examine whether administration of lentinan, purified β-1, 3-glucan, can prolong survival in advanced gastric cancer patients receiving S-1-based chemotherapy.
METHODS: Since 2004, 78 patients with metastatic or recurrent gastric cancer have received S-1-based chemotherapy as first-line treatment. Survival, side effects, and the ratio of granulocytes/lymphocytes (G/L ratio) were compared between 2 groups of patients who received chemo-immunotherapy using lentinan and chemotherapy alone.
RESULTS: Median overall survival was significantly longer in the former group than in the latter group [689 d (95% CI: 431-2339 d) vs 565 d (95% CI: 323-662 d), P = 0.0406]. In addition, the G/L ratio in patients who received lentinan was maintained around or below 2, which was significantly lower than that in patients who received chemotherapy alone (P < 0.001).
CONCLUSION: Chemo-immunotherapy with lentinan offers a significant advantage over S-1-based chemotherapy alone in terms of survival in patients with advanced gastric cancer.
Keywords: Gastric cancer, Lentinan, S-1-based chemotherapy
INTRODUCTION
The prognosis of unresectable gastric cancer remains poor; however, recent advances in chemotherapy for this type of cancer have considerably improved in terms of therapeutic effects[1,2]. Biological response modifiers (BRMs) have been used in combination with cytotoxic-chemotherapeutic agents[3], and the beneficial effects of BRMs in terms of the survival of patients with gastric cancer were previously described[4,5]. Lentinan is one of the BRMs whose effects are involved in host defense immune systems[6]. This agent is a purified polysaccharide of β-1, 3-glucan isolated from Shiitake mushrooms[7], which has been reported to have a life-prolonging effect in patients with unresectable or recurrent gastric cancer receiving an oral fluoropyrimidine (tegafur)[8].
A newly developed oral fluoropyrimidine, S-1, was designed to enhance the anticancer activity of tegafur by combining with 2 modulating substances: gimeracil to inhibit dihydropyrimidine dehydrogenase and potassium oxonate to reduce gastrointestinal toxicities[9]. The antitumor effects of fluoropyrimidines are known to be enhanced through the biochemical modulation of folate metabolism by cisplatin[10], and combination therapy using S-1 and cisplatin reportedly achieves high response rates[11,12]. Since the taxane derivatives, docetaxel and paclitaxel, have a unique mechanism of action that differs from those of fluoropyrimidines and platinum compounds[13,14], taxanes may be combined with either S-1 or S-1 plus cisplatin for the treatment of advanced gastric cancer[15-17]. However, disease progression is still observed in some patients receiving S-1-based chemotherapy[15,18] and further improvements in chemotherapy are necessary.
Recently, a meta-analysis conducted by Oba et al[19], showed that the addition of lentinan to fluoropyrimidine-based chemotherapy could prolong the survival of patients with advanced gastric cancer compared to chemotherapy alone. However, all 5 clinical studies included in this analysis were performed in the 1980s and S-1 was not prescribed at that time. Because S-1-based regimens are now widely used for the treatment of advanced gastric cancer in Japan, it is of great concern that lentinan may also have a synergistic effect with this new type of fluoropyrimidine. Therefore, with the aim of evaluating the effect of chemo-immunotherapy using S-1 and lentinan, we retrospectively examined all patients with unresectable/recurrent gastric cancer receiving S-1-based chemotherapy and then compared the overall survival (OS) and adverse effects between treatments with and without lentinan. We also compared the granulocyte/lymphocyte (G/L) ratio between groups of patients treated chemo-immunotherapy and chemotherapy alone in order to examine whether lentinan could have substantial immunological effects similar to other BRMs.
MATERIALS AND METHODS
Patients
Lentinan has been used for the treatment of gastric cancer since August 2004 in our hospital in patients who gave informed consent. Patients receiving S-1-based chemotherapy between August 2004 and July 2010 with pathologically proven inoperable gastric cancer and at least 1 measurable lesion were enrolled in this analysis. They received either no prior chemotherapy or 1 regimen as postoperative adjuvant chemotherapy that was completed > 4 wk before entry. Patients over 85 years of age and those with severe impairment of liver [aspartate aminotransferase and alanine aminotransferase > 3 times the upper limit of normal (ULN)], kidney (serum creatinine more than ULN) and bone marrow function (white blood cell count < 4000/mm3, platelet count < 10 000/mm3, hemoglobin < 8 g/dL) were excluded. Patients with multiple cancers, those with an Eastern Cooperative Oncology Group performance status of 4, those with brain metastases, and those whose outcome were unclear were also excluded.
Treatment protocol
In this study, the S-1-based chemotherapy used included S-1 alone, S-1/paclitaxel, S-1/ cisplatin, and paclitaxel with S-1 plus cisplatin (PSC triple therapy). S-1 was given orally twice daily based on the patient’s body surface area (BSA), i.e. BSA < 1.25 m2, 40 mg; 1.25-1.50 m2, 50 mg; and BSA > 1.50 m2, 60 mg for 2 wk followed by either 1 wk rest for patients who received S-1 alone or 2 wk rest for those who received combination therapy. For the S-1/cisplatin therapy, cisplatin at a dose of 70 mg/m2 was administered by continuous infusion for 24 h on day 8[18,20]. For the S-1/paclitaxel therapy, paclitaxel at a dose of 50 mg/m2 was administered by a 2-h infusion on days 1 and 15[21,22]. For the PSC triple therapy, paclitaxel at a dose of 120 mg/m2 was administered by a 2-h infusion on day 1 and cisplatin at a dose of 60 mg/m2 by continuous infusion for 24 h on day 14[17]. Doses were adjusted at the initiation of subsequent cycles if severe toxicity (grade 3-4) was present; administration of S-1 was discontinued and then resumed at a reduced dose (10 mg/m2 per day) and the dose of paclitaxel was reduced by 25% in the next cycle if toxicity was resolved. Administration of cisplatin was postponed until toxicity resolved; the maximum duration of postponement was no more than 2 wk. In the lentinan-treated group, 2 mg/body of lentinan was intravenously administered for 30 min every 2 or 3 wk. OS was calculated from the start of S-1-based chemotherapy until death or the most recent follow-up day. The Kaplan-Meier method was used to plot OS curves and then OS rates were compared by means of the log-rank test between the lentinan group and the chemotherapy alone group. The National Cancer Institute common toxicity criteria version 4.0 was applied to evaluate adverse effects.
G/ L ratio
In order to assess the immunological effects of lentinan, the G/L ratio of each patient was determined at 3 points, i.e. before, at 3 mo, and at 1 year after initiation of the S-1-based chemotherapy or 1 mo prior to death. Then, the G/L ratios at the 3 time points were respectively compared between the groups of chemo-immunotherapy and chemotherapy alone using the χ2 test. P values of less than 0.05 were considered statistically significant. SAS version 9.1 (SAS Institute Inc., Cary, NC, United States) was used for all analyses.
RESULTS
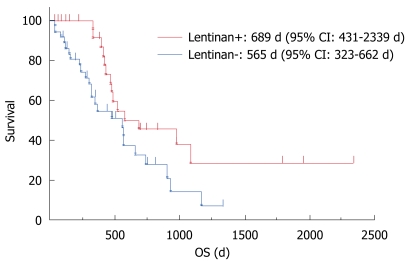
Among 78 patients with metastatic or recurrent gastric cancer receiving S-1-based chemotherapy as first-line treatment in our hospital during the 6 years between 2004 and 2010, 10 patients were excluded according to the criteria mentioned above. The chemotherapy alone group comprised 37 cases (29 men/8 women, median age; 68 years, range; 40-84 years), and the group that received chemo-immunotherapy with lentinan comprised 31 cases (19 men/12 women, median age; 67 years, range; 42-82 years). Patient characteristics are summarized in Table 1. S-1-based chemotherapy was continued as long as possible. The median OS was significantly longer in the group that received chemo-immunotherapy with lentinan than in the chemotherapy alone group [689 d (95% CI: 431-2339 d) vs 565 d (95% CI: 323-662 d), P = 0.0406] (Figure 1). One-, two-, and five-year survival rates were better in the group that received lentinan than in the group that received chemotherapy alone, (91.3% vs 59.4%, 45.7% vs 32.7%, 10.0% vs 0%, respectively). The most frequently observed severe (grades 3 and 4) toxicity was neutropenia (chemo-immunotherapy 50%, chemotherapy alone 45%). Grade 3 febrile neutropenia was observed in 1 case in each group. With regard to non-hematological toxicity, severe mucositis (grade 3) was observed in both groups with an incidence of 6.7% and 5.3%, respectively. Neither renal dysfunction nor hand-foot syndrome occurred in our series. There were essentially no differences in the incidence and degree of adverse effects between patients who did and those who did not receive lentinan.
Table 1.
Patient characteristics
| Chemotherapy alone (n = 37) | Chemo-immunotherapy (n = 31) | |
| Gender | ||
| Male | 29 | 19 |
| Female | 8 | 12 |
| Age (yr) | 40-84 | 42-82 |
| Median | 68 | 67 |
| Performance status | ||
| 0 | 5 | 5 |
| 1 | 26 | 15 |
| 2 | 3 | 5 |
| 3 | 3 | 6 |
| Pathology | ||
| Intestinal | 13 | 10 |
| Diffuse | 24 | 21 |
| S-1-based chemotherapy | ||
| S-1 alone | 12 | 12 |
| S-1/paclitaxel | 4 | 7 |
| S-1/cisplatin | 18 | 6 |
| PSC | 3 | 6 |
PSC: Paclitaxel plus S-1/cisplatin combination therapy.
Figure 1.
Kaplan-Meier curves of overall survival. Lentinan+: The group that received chemo-immunotherapy with lentinan (n = 31); Lentinan-: The group that received S-1-based chemotherapy alone (n = 37). OS: Overall survival.
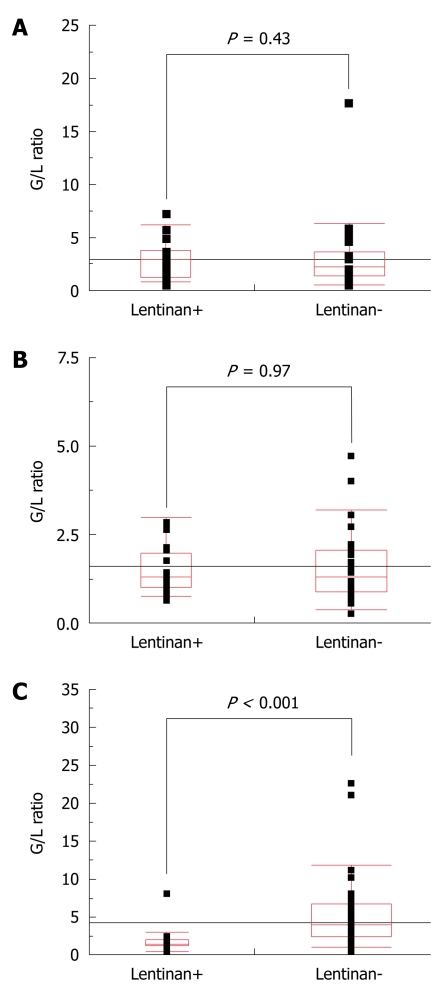
The G/L ratio was almost the same between the treatment with or without lentinan at the start of and 3 mo after chemotherapy (Figure 2A and B). In sharp contrast, the G/L ratio in patients receiving lentinan was maintained around or below 2, which was significantly lower than that in patients who received chemotherapy alone (P < 0.001, Figure 2C), at either 1 year after initiation of chemotherapy or 1 mo before death (cases in which the survival time was < 1 year after chemotherapy).
Figure 2.
Comparison of the granulocyte/lymphocyte ratio between patients who did (Lentinan+) and those who did not receive lentinan (Lentinan-) using the χ2 test. A: Before therapy; B: At 3 mo after initiation of the S-1-based chemothrapy; C: At either 1 year after initiation of the S-1-based chemotherapy or 1 mo prior to death (cases in which the survival time was < 1 year after chemotherapy). P values of less than 0.05 were considered statistically significant. G/L: Granulocyte/lymphocyte.
DISCUSSION
We retrospectively investigated whether the administration of lentinan could prolong survival in patients with highly advanced gastric cancer receiving S-1-based chemotherapy. A previous study demonstrated that the addition of lentinan to the older fluoropyrimidines such as 5-FU, tegafur, and UFT significantly prolonged survival, compared to chemotherapy alone, in patients with advanced gastric cancer[19]. Recently, S-1 or S-1-based combination therapy has been widely used for the treatment of advanced gastric cancer especially in Eastern countries. If S-1-based chemotherapy becomes a standard treatment in advanced settings, it is very important to confirm the synergistic effects of lentinan with S-1. Therefore, we examined advanced gastric cancer patients receiving S-1-based chemotherapy and determined whether the addition of lentinan extended their survival time and enhanced immunological activity.
Our results showed that chemo-immunotherapy using lentinan was superior to chemotherapy alone in terms of survival, although the therapeutic regimens were not completely matched in the 2 treatment groups. The survival time was long in 3 patients in the chemo-immunotherapy group (lung metastasis, 5 years; peritoneal metastases, 5 years and 5 mo; and peritoneal metastases, 6 years and 6 mo after initiation of chemotherapy), while none of the patients in the chemotherapy alone group survived for more than 5 years. The survival rates at 1-, 2- and 5-year were also higher in the group that received lentinan. In this preliminary study, there were essentially no differences in the incidence and degree of adverse effects between patients who did and those who did not receive lentinan, which suggested that adverse effects are strongly associated with the duration of chemotherapy, and not with the additional administration of this agent. Neutropenia was the most common toxicity, and febrile neutropenia occurred in only 1 case in each group. All toxicities were manageable and no patient died due to adverse effects.
In order to assess the immunological effects of lentinan, the G/L ratio as well as the Th1/Th2 balance and the binding ratio of lentinan to monocytes were reported as parameters[23-25]. The G/L ratio is easily determined, even in a retrospective analysis, and was previously reported to be correlated with the prognosis of gastric cancer patients[23]. Accordingly, we chronologically determined the G/L ratio in each patient from the initiation of S-1-based chemotherapy until 1 year later or death. The administration of lentinan appeared to suppress the G/L ratio and to maintain it around or below 2. β-1, 3-glucan is considered a stimulator of cellular immunity[7]. Binding of β-1, 3-glucan to a specific receptor activates macrophages[26,27], increases the expression of adhesion molecules and adhesion to the endothelium, induces the secretion of cytokines, and triggers intracellular processes such as respiratory burst[28]. Non-specific macrophage activation by this immunomodulator has been reported to be involved in skewing of the Th1/Th2 balance to Th1[29], which may eventually decrease the G/L ratio[23]. A Th1-mediated attack on cancer cells may be correlated with prolongation of cancer patients’ survival.
Our data on S-1-based chemotherapy showed that the addition of lentinan to chemotherapy prolonged the survival of advanced gastric cancer patients along with immunological activation, compared to chemotherapy alone. Lentinan should be widely accepted for the treatment of unresectable or recurrent advanced gastric cancer. Chemo-immunotherapy combined with lentinan and an S-1-based regimen might be a candidate for the standard treatment of advanced gastric cancer. In Japan, a phase III study comparing therapy with S-1/lentinan with S-1 alone is now underway. The results of this control study are eagerly awaited.
COMMENTS
Background
Lentinan is one of the BRMs whose effects are involved in host defense immune systems [6]. This agent is a purified polysaccharide of beta-1, 3-glucan isolated from Shiitake mushrooms, which has been reported to have a life-prolonging effect in patients with unresectable or recurrent gastric cancer receiving an oral fluoropyrimidineS-1 or S-1-based combination therapy has been widely used for the treatment of advanced gastric cancer especially in Eastern countries. If S-1-based chemotherapy becomes a standard treatment in advanced settings, it is very important to confirm the synergistic effects of lentinan with S-1.
Research frontiers
Recent advances in chemotherapy for this type of cancer have considerably improved in terms of therapeutic effects. A newly developed oral fluoropyrimidine, S-1, was designed to enhance the anticancer activity of tegafur by combining with 2 modulating substances: gimeracil to inhibit dihydropyrimidine dehydrogenase and potassium oxonate to reduce gastrointestinal toxicities
Innovations and breakthroughs
The data on S-1-based chemotherapy showed that the addition of lentinan to chemotherapy prolonged the survival of advanced gastric cancer patients along with immunological activation, compared to chemotherapy alone.
Applications
Lentinan should be widely accepted for the treatment of unresectable or recurrent advanced gastric cancer. Chemo-immunotherapy combined with lentinan and an S-1-based regimen might be a candidate for the standard treatment of advanced gastric cancer. In Japan, a phase III study comparing therapy with S-1/lentinan with S-1 alone is now underway.
Peer review
The paper by Ina and Furuta is well written. The results about the improvement of the survival of patients with unresectable or recurrent gastric cancer treated with chemo-immunotherapy consisting of S-1-based chemotherapy plus lentinan as compared to the S-1-based chemotherapy alone are important and of interest to readers.
Footnotes
Peer reviewers: Murielle Mimeault, PhD, Department of Biochemistry and Molecular Biology, College of Medicine, Eppley Cancer Institute, 7052 DRC, University of Nebraska Medical Center, 985870 Nebraska Medical Center, Omaha, NE 68198-5870, United States; Vaclav Vetvicka, Professor, Department of Pathology and Laboratory Medicine, University of Louisville, 511 S. Floyd St., MDR Bldg., Rm. 224, Louisville, KY 40202,
United States
S- Editor Tian L L- Editor Webster JR E- Editor Zheng XM
References
- 1.Hohenberger P, Gretschel S. Gastric cancer. Lancet. 2003;362:305–315. doi: 10.1016/s0140-6736(03)13975-x. [DOI] [PubMed] [Google Scholar]
- 2.Sastre J, Garcia-Saenz JA, Diaz-Rubio E. Chemotherapy for gastric cancer. World J Gastroenterol. 2006;12:204–213. doi: 10.3748/wjg.v12.i2.204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vetvicka V. Glucan-immunostimulant, adjuvant, potential drug. World J Clin Oncol. 2011;2:115–119. doi: 10.5306/wjco.v2.i2.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nakazato H, Koike A, Saji S, Ogawa N, Sakamoto J. Efficacy of immunochemotherapy as adjuvant treatment after curative resection of gastric cancer. Study Group of Immunochemotherapy with PSK for Gastric Cancer. Lancet. 1994;343:1122–1126. doi: 10.1016/s0140-6736(94)90233-x. [DOI] [PubMed] [Google Scholar]
- 5.Sakamoto J, Teramukai S, Nakazato H, Sato Y, Uchino J, Taguchi T, Ryoma Y, Ohashi Y. Efficacy of adjuvant immunochemotherapy with OK-432 for patients with curatively resected gastric cancer: a meta-analysis of centrally randomized controlled clinical trials. J Immunother. 2002;25:405–412. doi: 10.1097/00002371-200209000-00004. [DOI] [PubMed] [Google Scholar]
- 6.Hamuro J, Röllinghoff M, Wagner H. beta(1 leads to 3) Glucan-mediated augmentation of alloreactive murine cytotoxic T-lymphocytes in vivo. Cancer Res. 1978;38:3080–3085. [PubMed] [Google Scholar]
- 7.Chihara G, Maeda Y, Hamuro J, Sasaki T, Fukuoka F. Inhibition of mouse sarcoma 180 by polysaccharides from Lentinus edodes (Berk.) sing. Nature. 1969;222:687–688. doi: 10.1038/222687a0. [DOI] [PubMed] [Google Scholar]
- 8.Nakano H, Namatame K, Nemoto H, Motohashi H, Nishiyama K, Kumada K. A multi-institutional prospective study of lentinan in advanced gastric cancer patients with unresectable and recurrent diseases: effect on prolongation of survival and improvement of quality of life. Kanagawa Lentinan Research Group. Hepatogastroenterology. 1999;46:2662–2668. [PubMed] [Google Scholar]
- 9.Shirasaka T, Shimamato Y, Ohshimo H, Yamaguchi M, Kato T, Yonekura K, Fukushima M. Development of a novel form of an oral 5-fluorouracil derivative (S-1) directed to the potentiation of the tumor selective cytotoxicity of 5-fluorouracil by two biochemical modulators. Anticancer Drugs. 1996;7:548–557. doi: 10.1097/00001813-199607000-00010. [DOI] [PubMed] [Google Scholar]
- 10.Scanlon KJ, Newman EM, Lu Y, Priest DG. Biochemical basis for cisplatin and 5-fluorouracil synergism in human ovarian carcinoma cells. Proc Natl Acad Sci USA. 1986;83:8923–8925. doi: 10.1073/pnas.83.23.8923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lenz HJ, Lee FC, Haller DG, Singh D, Benson AB, Strumberg D, Yanagihara R, Yao JC, Phan AT, Ajani JA. Extended safety and efficacy data on S-1 plus cisplatin in patients with untreated, advanced gastric carcinoma in a multicenter phase II study. Cancer. 2007;109:33–40. doi: 10.1002/cncr.22329. [DOI] [PubMed] [Google Scholar]
- 12.Koizumi W, Narahara H, Hara T, Takagane A, Akiya T, Takagi M, Miyashita K, Nishizaki T, Kobayashi O, Takiyama W, et al. S-1 plus cisplatin versus S-1 alone for first-line treatment of advanced gastric cancer (SPIRITS trial): a phase III trial. Lancet Oncol. 2008;9:215–221. doi: 10.1016/S1470-2045(08)70035-4. [DOI] [PubMed] [Google Scholar]
- 13.Ohtsu A, Boku N, Tamura F, Muro K, Shimada Y, Saigenji K, Akazawa S, Kitajima M, Kanamaru R, Taguchi T. An early phase II study of a 3-hour infusion of paclitaxel for advanced gastric cancer. Am J Clin Oncol. 1998;21:416–419. doi: 10.1097/00000421-199808000-00021. [DOI] [PubMed] [Google Scholar]
- 14.Sulkes A, Smyth J, Sessa C, Dirix LY, Vermorken JB, Kaye S, Wanders J, Franklin H, LeBail N, Verweij J. Docetaxel (Taxotere) in advanced gastric cancer: results of a phase II clinical trial. EORTC Early Clinical Trials Group. Br J Cancer. 1994;70:380–383. doi: 10.1038/bjc.1994.310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yamaguchi K, Shimamura T, Hyodo I, Koizumi W, Doi T, Narahara H, Komatsu Y, Kato T, Saitoh S, Akiya T, et al. Phase I/II study of docetaxel and S-1 in patients with advanced gastric cancer. Br J Cancer. 2006;94:1803–1808. doi: 10.1038/sj.bjc.6603196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sato Y, Takayama T, Sagawa T, Takahashi Y, Ohnuma H, Okubo S, Shintani N, Tanaka S, Kida M, Sato Y, et al. Phase II study of S-1, docetaxel and cisplatin combination chemotherapy in patients with unresectable metastatic gastric cancer. Cancer Chemother Pharmacol. 2010;66:721–728. doi: 10.1007/s00280-009-1215-2. [DOI] [PubMed] [Google Scholar]
- 17.Iwase H, Shimada M, Tsuzuki T, Ina K, Sugihara M, Haruta J, Shinoda M, Kumada T, Goto H. A Phase II Multi-Center Study of Triple Therapy with Paclitaxel, S-1 and Cisplatin in Patients with Advanced Gastric Cancer. Oncology. 2011;80:76–83. doi: 10.1159/000328746. [DOI] [PubMed] [Google Scholar]
- 18.Ina K, Kataoka T, Takeuchi Y, Fukuoka T, Miwa T, Nishio T, Furuta R, Masaki A, Mori F, Kayukawa S, et al. Pathological complete response induced by the combination therapy of S-1 and 24-h infusion of cisplatin in two cases initially diagnosed as inoperable advanced gastric cancer. Oncol Rep. 2008;20:259–264. [PubMed] [Google Scholar]
- 19.Oba K, Kobayashi M, Matsui T, Kodera Y, Sakamoto J. Individual patient based meta-analysis of lentinan for unresectable/recurrent gastric cancer. Anticancer Res. 2009;29:2739–2745. [PubMed] [Google Scholar]
- 20.Iwase H, Shimada M, Tsuzuki T, Horiuchi Y, Kumada S, Haruta J, Yamaguchi T, Sugihara M, Ina K, Kusugami K, et al. A phase II multicentric trial of S-1 combined with 24 h-infusion of cisplatin in patients with advanced gastric cancer. Anticancer Res. 2005;25:1297–1301. [PubMed] [Google Scholar]
- 21.Narahara H, Fujitani K, Takiuchi H, Sugimoto N, Inoue K, Uedo N, Tsukuma H, Tsujinaka T, Furukawa H, Taguchi T. Phase II study of a combination of S-1 and paclitaxel in patients with unresectable or metastatic gastric cancer. Oncology. 2008;74:37–41. doi: 10.1159/000138978. [DOI] [PubMed] [Google Scholar]
- 22.Ina K, Furuta R, Kataoka T, Nishio T, Nagao S, Kayukawa S, Masaki A, Ando T, Goto H. [Two advanced gastric cancer cases with peritoneal metastases successfully treated by s-1/paclitaxel combination therapy] Gan To Kagaku Ryoho. 2009;36:979–981. [PubMed] [Google Scholar]
- 23.Nimura H, Mitsumori N, Takahashi N, Kashimura H, Takayama S, Kashiwagi H, Yanaga K. [S-1 combined with lentinan in patients with unresectable or recurrent gastric cancer] Gan To Kagaku Ryoho. 2006;33 Suppl 1:106–109. [PubMed] [Google Scholar]
- 24.Yoshino S, Tabata T, Hazama S, Iizuka N, Yamamoto K, Hirayama M, Tangoku A, Oka M. Immunoregulatory effects of the antitumor polysaccharide lentinan on Th1/Th2 balance in patients with digestive cancers. Anticancer Res. 2000;20:4707–4711. [PubMed] [Google Scholar]
- 25.Kubota E, Kataoka H, Hayashi K, Kamiya T, Sasaki M, Ogasawara N, Yamada T, Wada T, Mori Y, Mizoshita T, et al. Advanced stomach and pancreas cancer successfully treated with combination chemotherapy with S-1/paclitaxel/lentinan. Hepatogastroenterology. 2009;56:106–110. [PubMed] [Google Scholar]
- 26.Takeda K, Kaisho T, Akira S. Toll-like receptors. Annu Rev Immunol. 2003;21:335–376. doi: 10.1146/annurev.immunol.21.120601.141126. [DOI] [PubMed] [Google Scholar]
- 27.Brown GD, Gordon S. Immune recognition. A new receptor for beta-glucans. Nature. 2001;413:36–37. doi: 10.1038/35092620. [DOI] [PubMed] [Google Scholar]
- 28.Schepetkin IA, Quinn MT. Botanical polysaccharides: macrophage immunomodulation and therapeutic potential. Int Immunopharmacol. 2006;6:317–333. doi: 10.1016/j.intimp.2005.10.005. [DOI] [PubMed] [Google Scholar]
- 29.Murata Y, Shimamura T, Tagami T, Takatsuki F, Hamuro J. The skewing to Th1 induced by lentinan is directed through the distinctive cytokine production by macrophages with elevated intracellular glutathione content. Int Immunopharmacol. 2002;2:673–689. doi: 10.1016/s1567-5769(01)00212-0. [DOI] [PubMed] [Google Scholar]