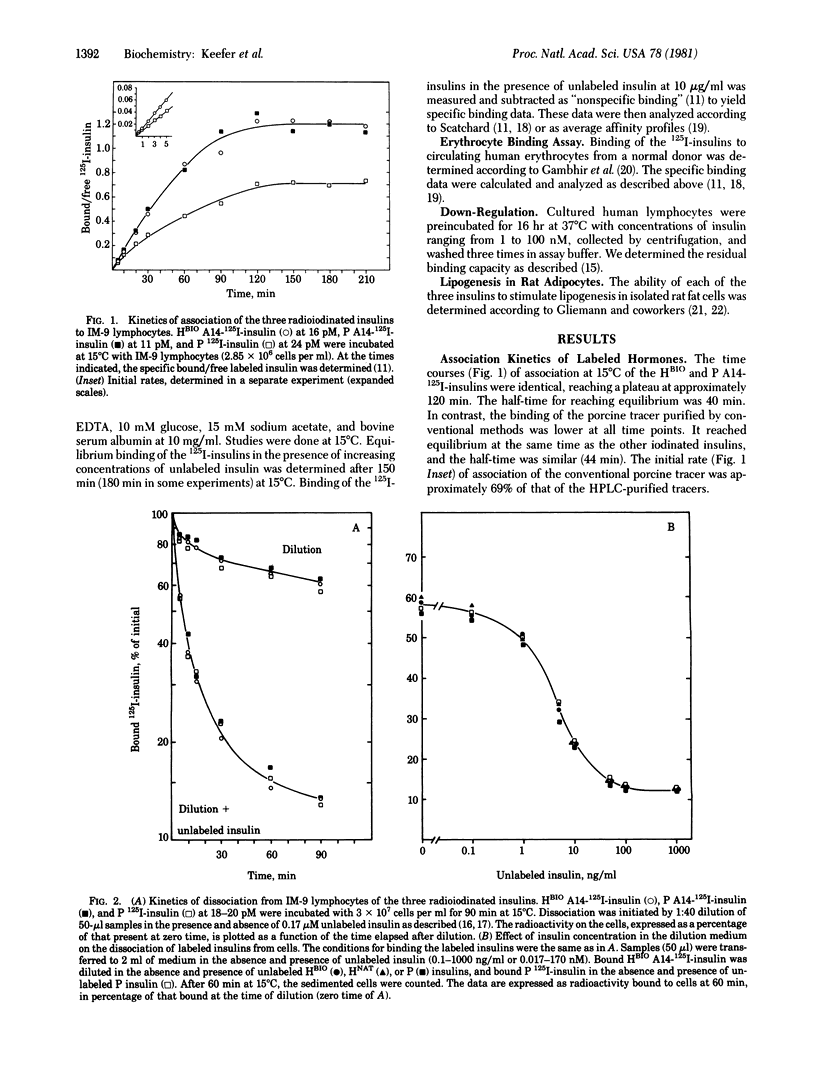
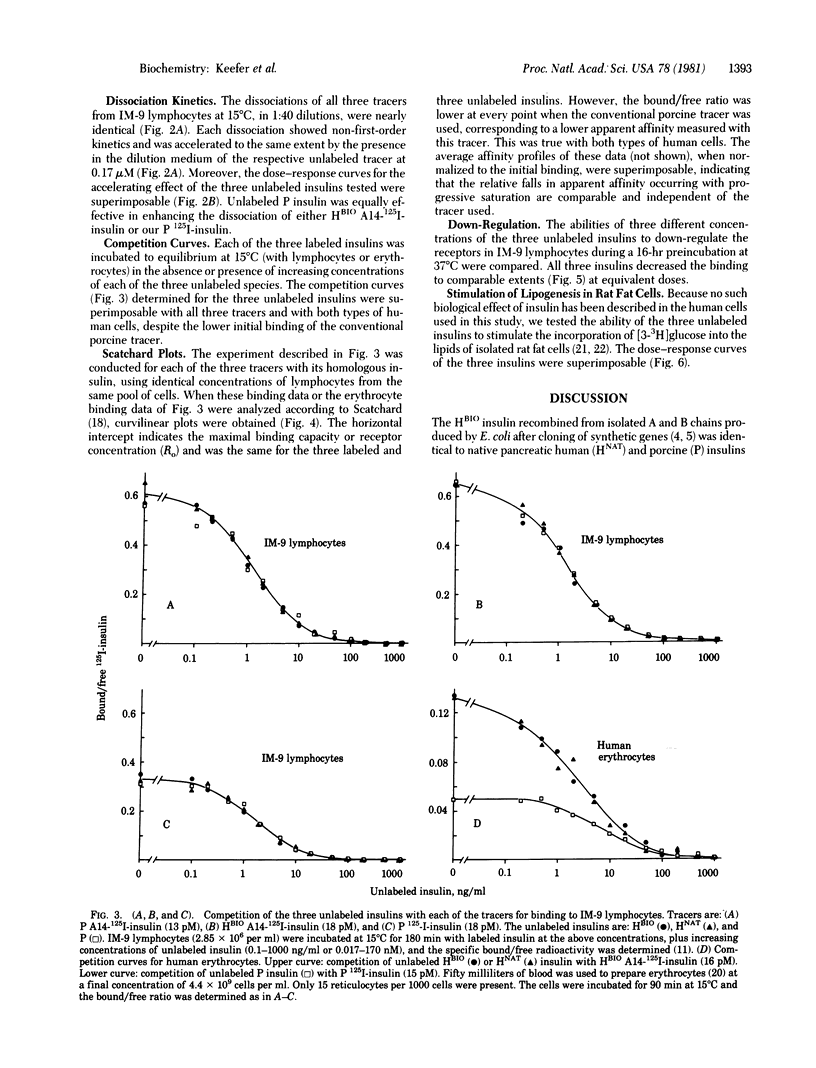
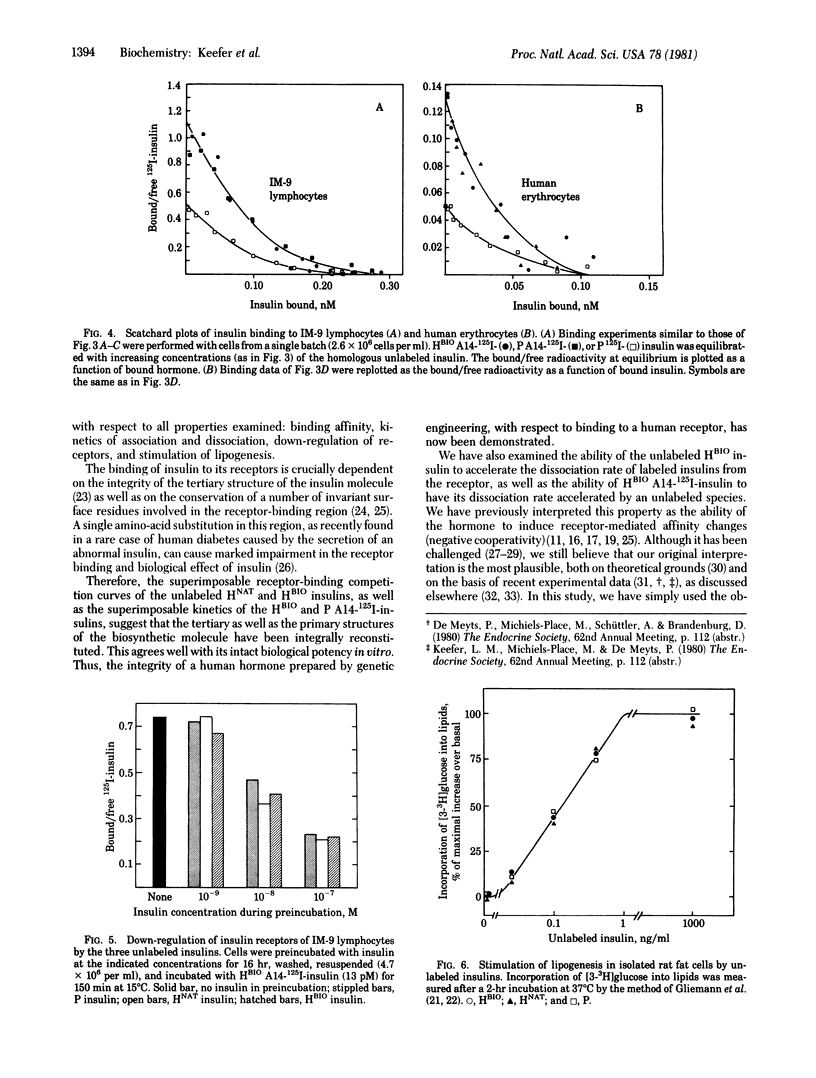
Abstract
Human insulin synthesized from A and B chains separately produced in Escherichia coli from cloned synthetic genes (prepared by the Eli Lilly Research Laboratories, Indianapolis, IN) was characterized by examining its interaction with human cultured lymphocytes, human circulating erythrocytes in vitro, and isolated rat fat cells. The binding behavior of the biosynthetic insulin with human cells was indistinguishable from that of native human or porcine insulins, with respect to affinity, association and dissociation kinetics, negative cooperativity, and the down-regulation of lymphocyte receptors. Similarly, the biosynthetic insulin was as potent as the native insulins in stimulating lipogenesis in isolated rat fat cells. We also examined the receptor binding characteristics of 125I-labeled human and porcine insulins monoiodinated solely at Tyr-A14, which were obtained by means of high-performance liquid chromatography of the iodination reaction mixture (this material was prepared by B. Frank, Eli Lilly Research Laboratories). In all aspects studied, the pure [TyrA14-125I]iodoinsulins were superior as tracers to the monoiodoinsulin purified by the more conventional method of gel filtration.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Crea R., Kraszewski A., Hirose T., Itakura K. Chemical synthesis of genes for human insulin. Proc Natl Acad Sci U S A. 1978 Dec;75(12):5765–5769. doi: 10.1073/pnas.75.12.5765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DU Y. C., ZHANG Y. S., LU Z. X., TSOU C. L. Resynthesis of insulin from its glycyl and phenylalanyl chains. Sci Sin. 1961 May;10:84–104. [PubMed] [Google Scholar]
- De Meyts P., Roth J. Cooperativity in ligand binding: a new graphic analysis. Biochem Biophys Res Commun. 1975 Oct 27;66(4):1118–1126. doi: 10.1016/0006-291x(75)90473-8. [DOI] [PubMed] [Google Scholar]
- De Meyts P., Van Obberghen E., Roth J. Mapping of the residues responsible for the negative cooperativity of the receptor-binding region of insulin. Nature. 1978 Jun 15;273(5663):504–509. doi: 10.1038/273504a0. [DOI] [PubMed] [Google Scholar]
- DeMeyts P., Bainco A. R., Roth J. Site-site interactions among insulin receptors. Characterization of the negative cooperativity. J Biol Chem. 1976 Apr 10;251(7):1877–1888. [PubMed] [Google Scholar]
- Freychet P., Roth J., Neville D. M., Jr Insulin receptors in the liver: specific binding of ( 125 I)insulin to the plasma membrane and its relation to insulin bioactivity. Proc Natl Acad Sci U S A. 1971 Aug;68(8):1833–1837. doi: 10.1073/pnas.68.8.1833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gambhir K. K., Archer J. A., Carter L. Insulin radioreceptor assay for human erythrocytes. Clin Chem. 1977 Sep;23(9):1590–1595. [PubMed] [Google Scholar]
- Gavin J. R., 3rd, Roth J., Neville D. M., Jr, de Meyts P., Buell D. N. Insulin-dependent regulation of insulin receptor concentrations: a direct demonstration in cell culture. Proc Natl Acad Sci U S A. 1974 Jan;71(1):84–88. doi: 10.1073/pnas.71.1.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gliemann J. Assay of insulin-like activity by the isolated fat cell method. I. Factors influencing the response to crystalline insulin. Diabetologia. 1967 Aug;3(4):382–388. doi: 10.1007/BF02342631. [DOI] [PubMed] [Google Scholar]
- Gliemann J., Sonne O., Linde S., Hansen B. Biological potency and binding affinity of monoiodoinsulin with iodine in tyrosine A14 or tyrosine A19. Biochem Biophys Res Commun. 1979 Apr 27;87(4):1183–1190. doi: 10.1016/s0006-291x(79)80032-7. [DOI] [PubMed] [Google Scholar]
- Goeddel D. V., Kleid D. G., Bolivar F., Heyneker H. L., Yansura D. G., Crea R., Hirose T., Kraszewski A., Itakura K., Riggs A. D. Expression in Escherichia coli of chemically synthesized genes for human insulin. Proc Natl Acad Sci U S A. 1979 Jan;76(1):106–110. doi: 10.1073/pnas.76.1.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keen H., Glynne A., Pickup J. C., Viberti G. C., Bilous R. W., Jarrett R. J., Marsden R. Human insulin produced by recombinant DNA technology: safety and hypoglycaemic potency in healthy men. Lancet. 1980 Aug 23;2(8191):398–401. doi: 10.1016/s0140-6736(80)90443-2. [DOI] [PubMed] [Google Scholar]
- MEIENHOFER J., SCHNABEL E., BREMER H., BRINKHOFF O., ZABEL R., SROKA W., KLOSTERMAYER H., BRANDENBURG D., OKUDA T., ZAHN H. SYNTHESE DER INSULINKETTEN UND IHRE KOMBINATION ZU INSULINAKTIVEN PRAEPARATEN. Z Naturforsch B. 1963 Dec;18:1120–1121. [PubMed] [Google Scholar]
- Moody A. J., Stan M. A., Stan M., Gliemann J. A simple free fat cell bioassay for insulin. Horm Metab Res. 1974 Jan;6(1):12–16. doi: 10.1055/s-0028-1093895. [DOI] [PubMed] [Google Scholar]
- Olefsky J. M., Chang H. Insulin binding to adipocytes. Evidence for functionally distinct receptors. Diabetes. 1978 Sep;27(9):946–958. doi: 10.2337/diab.27.9.946. [DOI] [PubMed] [Google Scholar]
- Pollet R. J., Standaert M. L., Haase B. A. Hormone-receptor interactions are noncooperative: application to the beta-adrenergic receptor. Proc Natl Acad Sci U S A. 1980 Jul;77(7):4340–4344. doi: 10.1073/pnas.77.7.4340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollet R. J., Standaert M. L., Haase B. A. Insulin binding to the human lymphocyte receptor. Evaluation of the negative cooperativity model. J Biol Chem. 1977 Aug 25;252(16):5828–5834. [PubMed] [Google Scholar]
- Roth J. Methods for assessing immunologic and biologic properties of iodinated peptide hormones. Methods Enzymol. 1975;37:223–233. doi: 10.1016/s0076-6879(75)37018-3. [DOI] [PubMed] [Google Scholar]
- Schlichtkrull J., Brange J., Christiansen A. H., Hallund O., Heding L. G., Jorgensen K. H. Clinical aspects of insulin--antigenicity. Diabetes. 1972;21(2 Suppl):649–656. doi: 10.2337/diab.21.2.s649. [DOI] [PubMed] [Google Scholar]
- Sieber P., Kamber B., Hartmann A., Jöhl A., Riniker B., Rittel W. Totalsynthese von Humaninsulin. IV. Beschreibung der Endstufen. Helv Chim Acta. 1977 Jan 26;60(1):27–37. doi: 10.1002/hlca.19770600105. [DOI] [PubMed] [Google Scholar]
- Sodoyez J. C., Sodoyez-Goffaux F., Goff M. M., Zimmerman A. E., Arquilla E. R. [127-I]- or carrier-free [125-I]monoiodoinsulin. J Biol Chem. 1975 Jun 10;250(11):4268–4277. [PubMed] [Google Scholar]
- Tager H., Given B., Baldwin D., Mako M., Markese J., Rubenstein A., Olefsky J., Kobayashi M., Kolterman O., Poucher R. A structurally abnormal insulin causing human diabetes. Nature. 1979 Sep 13;281(5727):122–125. doi: 10.1038/281122a0. [DOI] [PubMed] [Google Scholar]
- Wood S. P., Blundell T. L., Wollmer A., Lazarus N. R., Neville R. W. The relation of conformation and association of insulin to receptor binding; x-ray and circular-dichroism studies on bovine and hystricomorph insulins. Eur J Biochem. 1975 Jul 15;55(3):531–542. doi: 10.1111/j.1432-1033.1975.tb02190.x. [DOI] [PubMed] [Google Scholar]
- de Meyts P., Roth J., Neville D. M., Jr, Gavin J. R., 3rd, Lesniak M. A. Insulin interactions with its receptors: experimental evidence for negative cooperativity. Biochem Biophys Res Commun. 1973 Nov 1;55(1):154–161. doi: 10.1016/s0006-291x(73)80072-5. [DOI] [PubMed] [Google Scholar]



