Abstract
Immunotherapies, including vaccines, represent a potent tool to prevent or contain disease with high morbidity or mortality such as infections and cancer. However, despite their widespread use, we still have a limited understanding of the mechanisms underlying the induction of protective immune responses.
Immunity is made of a multifaceted set of integrated responses involving a dynamic interaction of thousands of molecules; among those is a growing appreciation for the role the innate immunity (i.e. pathogen recognition receptors - PRRs) plays in determining the nature and duration (immune memory) of adaptive T and B cell immunity. The complex network of interactions between immune manipulation of the host (immunotherapy) on one side and innate and adaptive responses on the other might be fully understood only employing the global level of investigation provided by systems biology.
In this framework, the advancement of high-throughput technologies, together with the extensive identification of new genes, proteins and other biomolecules in the "omics" era, facilitate large-scale biological measurements. Moreover, recent development of new computational tools enables the comprehensive and quantitative analysis of the interactions between all of the components of immunity over time.
Here, we review recent progress in using systems biology to study and evaluate immunotherapy and vaccine strategies for infectious and neoplastic diseases. Multi-parametric data provide novel and often unsuspected mechanistic insights while enabling the identification of common immune signatures relevant to human investigation such as the prediction of immune responsiveness that could lead to the improvement of the design of future immunotherapy trials. Thus, the paradigm switch from "empirical" to "knowledge-based" conduct of medicine and immunotherapy in particular, leading to patient-tailored treatment.
Review
Cross-talks between innate and adaptive immune systems
Pathogen recognition receptors (PRRs) detect foreign antigens in the form of living pathogen or vaccine [1,2] activating specific signaling pathways that drive biological and immunological responses. Among the PRRs, Toll-like receptors (TLRs) are widely present on innate immune cells (including DCs, macrophages, mast cells, neutrophils), endothelial cells and fibroblasts, and their expression is regulated by several factors, including foreign antigens, vaccines and cytokines [3-6].
Twelve members of the TLR family have been identified in mammals to date, and different TLRs are expressed extra- or intracellularly. In particular, TLRs 1, 2, 4, 5, and 6 are expressed on the cell surface, whereas TLR3, 7, 8, and 9 are found almost exclusively in intracellular compartments such as endosomes [1,2]. Each member of the TLR family recognizes a specific structural component of pathogens [1,7-19]. In addition to TLRs, other important families of PRRs are plasma-membrane and cytoplasmic receptors, including the C type lectins, which recognize a range of microbial stimuli from pathogens such as HIV, HCV, Helicobacter pylori, and Mycobacterium tuberculosis [20,21], and NOD proteins, which recognize components of intracellular bacteria [22].
The interaction between PRRs and foreign antigens expressed by the vaccine triggers a downstream signaling cascade leading to several cellular processes, including production of proinflammatory cytokines and chemokines. In particular, TLR activation induces a signal cascade via several intermediates, whose endpoint is the activation of transcription factors turning on the expression of inflammatory cytokine genes, such as TNF-β, IL-6, IL-1β, and IL-12 [1,23].
Subsequent to the TLR-ligand interaction, APCs (mainly DCs) uptake and process vaccine antigens to be exposed on cell membrane surface in association to major histocompatibility complex (MHC) molecules for efficient presentation to adaptive immune cells [24]. DCs undergo full activation and maturation, characterized by the increased expression of co-stimulatory molecules (CD40, CD80, CD86) and production of chemokines (TNFα, RANTES, MIP1α, MIP1β). Activated and matured DCs migrate to the regional lymph node, where they provide antigen-specific activation as well as co-stimulatory signals to naïve T cells and possibly B cells, bridging innate with adaptive immunity [25-27].
Engaged naïve T cells undergo clonal expansion and differentiation into effector CD4+ T helper cells or CD8+ cytotoxic T lymphocytes (CTL). CD4+ T-helper cells can be directed into a Th1, Th2 or T-Reg polarization upon direct contact with antigen-bearing APCs and induction by specific cytokines. The polarized T-helper cells can antagonize each other's actions and will ultimately lead the adaptive immune system toward either a cellular (Th1), a humoral (Th2) or a tolerance (T-Reg) response [28-33]. A subset of polarized activated effector T cells will further differentiate into long-lasting memory cells to readily induce an immune response at subsequent encounters with the same antigen [34].
In such framework, a successful vaccine must be effective in activating PRRs. However, the degree of TLRs engagement has been studied only for few licensed vaccines, including Bacillus Calmette-Guerin (BCG) [35,36], Haemophilus influenzae type b (HiB) [37] and live attenuated yellow fever vaccines [38]. For other licensed vaccines, although the engagement of TLRs has not been documented, it could be inferred that live attenuated vaccines activate innate immunity following the same pathway induced by the corresponding native fully replicating virus. On the contrary, inactivated or subunit vaccines may be not efficient in activating the innate system, requiring the addition of an adjuvant in the formulation to improve their immunogenicity. Among the very few adjuvants approved for human use, only the Monophosphoryl Lipid A (MPL) is known to engage a TLR (TLR-4), being a non-toxic derivative of the lipopolysaccharide (LPS) of Salmonella Minnesota [39,40], but many new vaccine adjuvants are under development and evaluated in clinical trials, whose mechanism of action is mediated by TLRs [41-46]. Recently, we reviewed the differential transcriptional activation of monocyte-derived DCs following distinct and frequently applied maturation strategies and their strong effect on Th-polarization. This review clearly demonstrated that the stimulus applied for DC maturation can affect dramatically the global expression pattern of these cells and, consequently, their in vitro and in vivo function [47].
Vaccine development
The goal of a successful vaccine is to induce long-term protective immunity based on the generation of an antigen-specific immunological memory. This is achieved via several levels of cross-talks between the innate (antigen-presenting cells - APCs) and adaptive (T and B lymphocytes) immune systems involving both cell-to-cell contact and/or soluble factors (i.e. cytokines and chemokines).
Most of the current successful vaccines are based on live attenuated or inactivated pathogens which show distinctive biological and immunological characteristics. The live attenuated vaccines are viruses with a limited replication in the vaccinated host, carrying the native pathogen-associated molecular signals - PAMS (i.e.: viral genetic material) which bind the pathogen recognition receptors - bind PRRs and trigger the activation of the innate immune system. Such attenuated viruses mimic a natural infection and spread to multiple host immune organs or tissues, eliciting immune responses similar to those induced by fully-replicative pathogens, which are often effective after a single administration [48]. The major drawback of such strategy is the mild-to-severe adverse effects as consequence of the limited replication in the vaccine recipients. The inactivated vaccines are viruses which cannot replicate due to irreversible damage of genetic material induced by heat or chemical treatment. Although safer than attenuated vaccines, they are generally less effective and require multiple administrations to boost the immune response documented for instance by enhancement of antibody titers over time. The inactivated vaccines are made of either whole virus or subunits (i.e. viral proteins) relevant to the conferring of protective immunity.
In the last years, also for safety reasons, alternative non-replicating vaccine strategy, including recombinant proteins, synthetic peptides, DNA, particulate structures (i.e.: Virus-Like Particles) have been developed [49]. However, despite relevant safety advantages, such vaccine strategies are not always effectively processed by antigen presenting cells and presented to the adaptive immune system, lacking "danger" signals necessary to trigger activation of the innate system and, downstream, of the adaptive immune response [50].
The role of active specific immunization (vaccines) among immunotherapy strategies
Immunotherapy is a broad term that encompasses any manipulation of the immune response to elicit clearance of unwanted conditions such as infections, cancer, autoimmunity and rejection of heterologous organs. The purposes are somewhat opposite in some compared to other conditions as in some cases immune mediated, tissue-specific destruction is desired to clear the organism of cancer cells or cells infected with pathogens while in other cases efforts are made to dampen the same immune mechanisms that are actively causing unwanted destruction of tissues such is the case for autoimmunity, transplant rejection and graft versus host disease; we have recently shown that although the mechanisms leading to each of this pathological states or their resolution may be different, the final mechanism leading to tissue destruction is quite similar and involves the activation of a limited number of immune effector genes which we have called the immunologic constant of rejection (ICR) [51]. Thus, the role of immunotherapy is to enhance the chances of reaching full activation of ICR genes on one hand and reduce it on the other. It has become clear, thanks to transcriptional analyses that activation of an effector immune response is a multifactorial event that includes the activation of all arms of effector immunity including both innate and adaptive mechanisms and that neither alone is sufficient to induce tissue specific rejection [51,52]. Thus, it is likely that enhancement of one or another aspect of immunity by isolated immunotherapy strategies will be unlikely to achieve clearance of disease and combined strategies should be sought. Currently, immunotherapy strategies could be characterized by four major approaches: those aiming at the systemic and non-specific activation of immune cells (active immunotherapy), those aiming at the activation of specific antigen recognition pathways by T or B cells (active-specific immunotherapy), those in which cells deemed to bear important effector mechanisms (either antigen specific or non-specific) are expanded ex vivo and transfused in large number is patients (adoptive immunotherapy) and, finally, those in which effector molecules such as antibodies are transfused into patients (passive immunotherapy). While each one of these approaches has shown marginal benefit in the treatment of chronic infections and cancer, none of them alone has been dramatically effective in clearing disease and enhancing survival. In this context, active specific immunization with vaccines has the limited purpose of enhancing antigen-specific recognition by immune cells; interestingly, this has not been very successful in inducing dramatic clinical responses both in viral and in cancer systems. Recently, however, several clinical trials in which active specific immunization has been tested as a modality of treatment for cancer in large randomized trials have shown that survival is modestly but significantly enhanced compared to control groups treated with standard therapy. These studies provide a proof of principle that vaccination is probably enhancing the ability of the immune system to specifically recognize diseased tissues and control their growth. Yet, as we postulated in the ICR paper, the mechanisms leading to control of growth and presumably complete clearance of abnormal cells expand from the simple T or B cell interactions with their target to the activation in the diseased organ of an acute inflammatory process, triggered by the production of pro-inflammatory chemokines and lymphokines by antigen recognizing cells which can progressively recruit all arms of immune effector function [51].
Induction of adaptive immune response by vaccines
Most of the successful vaccines developed to date induce a long-lasting protective humoral adaptive immune response, eliciting the production of neutralizing or opsonizing antibodies [53]. However, such vaccines are effective mainly against pathogens characterized either by a limited and stable range of serotypes (i.e. smallpox, rubella, polio) or by seasonal serotypes (influenza). In the latter case, however, flu vaccine must be manufactured and administered each year according to the circulating seasonal variant.
However, in many cases the humoral antibody response by itself is not sufficient to protect against pathogens and, in the last years, the development of vaccines able to elicit also an effective cellular adaptive immune response has become a priority. In fact, if antibody-based vaccines provide prevention and protection from infection, T-cell-based vaccines may be relevant in controlling established chronic infections, such as HCV and HIV viruses [54-57], or a cancer [58-60]. This is particularly the case for chronic infections in which viruses are harbored within the cellular compartment. In that case, antibodies have no ability to recognize their target as they cannot cross the cell membrane and only immune cells with cytotoxic function can kill the infected cells and stop the perpetuation of the infectious process.
Regardless of the humoral or cellular immunity elicited by vaccines, relevant markers are needed to evaluate the vaccine efficacy and/or to optimize its development. Concerning the humoral immune response, a direct relationship between protective effect and serum titers of antibodies with high specificity and affinity, measured in ELISA or in neutralization has been widely accepted [61]. However, in few cases, other markers are considered better surrogates of protection, such as mucosal IgA titers for Rotavirus vaccine [62] or CD4+ T cell responses for Zoster vaccine [63], although in such cases no threshold levels for protection have been identified.
On the contrary, in situations where the humoral antibody response is not the protective arm of the immune response, specific parameters need to be validated to assess the relationship between protection and levels as well as type of cellular immunity. Indeed, a consensus has not been reached yet and different experimental models have been taken into account effector CD8+ cytotoxic T cell activity [64,65], perforin expression [66-68] or cytokine production [69,70].
Systems biology in vaccine development
The need for clear immunological markers to predict and evaluate the immunogenicity of vaccines and to optimize vaccine formulation critically exemplifies the usefulness of systems biology approaches. A great example was recently provided by a phase III randomized study in which a MAGE-A3 peptide was used to treat patients with advanced non-small-cell lung cancer or melanoma; this study not only demonstrated that the vaccine improved survival in either disease but also, by applying global transcriptional analysis to pre-treatment samples, the investigators identified signatures that clearly predict responsiveness to treatment both by immunological assessment and clinical benefit [71]. Indeed, the advancement of high-throughput technologies, together with the extensive identification of new genes, proteins and other biomolecules in the "omics" era, has facilitated large-scale biological measurements. The new experimental paradigm of systems biology aims to consider a biological system as not just a set of distinct elements, but rather as a complex product of the interactions among these elements and their relationship with the surrounding environment (Figure 1). Importantly, there are two major approaches to systems biology: a top down view in which potential permutations predicted by experimentally formed knowledge are predicted with a deductive approach; conversely, and inductive approach is often applied in clinical research following a bottom up approach; in this case, the global picture associated with a specific biological occurrence are "photographed" using high throughput technology and the reasons for their occurrences are then hypothesized using a reverse engineering approach [72]. We contend that this evidence based approach is a necessary preliminary step to focus the aim of future investigations because it identifies concept relevant to human physiopathology during the natural course of the disease or it response to treatment.
Figure 1.
Systems biology approaches for vaccine studies interactions and implications on translational research.
Despite the increasing use of such approaches in oncology [73-75], autoimmunity and infection [76,77] for the identification of prognostic or predictive biomarkers, systems biology has been only recently applied to vaccinology though the number of information are steadily increasing [78-80]. Each of the available high throughput methodologies have been employed to study the immune response induced by vaccines and a short description is reported here (Table 1).
Table 1.
Examples of Systems Biology applied to vaccinology
Transcriptomics
Transcriptomics enable the identification of set of genes and pathways differentially regulated in immune cells upon encounter with a foreign antigen or other non antigen-specific stimulation. DNA microarray technology has provided new insights into interactions between pathogens and innate immunity [81-85], which represent the background information for understanding and predicting the host response to vaccines. More recently, new powerful transcriptomics technologies have become widely available, including next-generation sequencing, exon and microRNA arrays [86,87].
Vaccines for infectious diseases
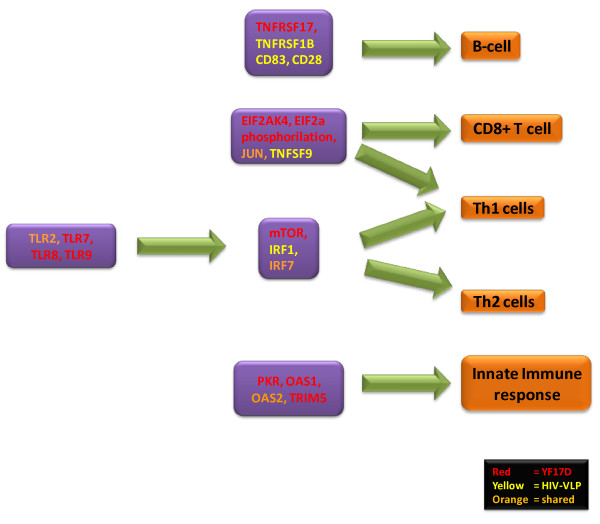
Gene 'signatures' in humans have been recently identified to predict immune responses to yellow fever vaccine (YF-17D) [88,89] and VLP-based HIV vaccine [90-93]. Transcriptional profile of early innate immune genes in PBMCs from vaccinated individuals showed that vaccination with YF-17D induced in most vaccine recipients a signature inclusive of genes involved in innate sensing of viruses and antiviral immunity. Among these, indeed, were observed genes encoding innate sensing receptors (i.e.: TLR7, RIG-I), transcription factors that regulate the expression of type I IFNs, IFN regulatory factor 7 (IRF7) and signal transducer and activator of transcription 1 (STAT1). A group of transcription factors, including IRF7, STAT1 and ETS2, were identified as key regulators of the early innate immune response to the YF-17D vaccine [88,89]. Furthermore, the enhanced transcription of several downstream genes that play critical roles in the maturation and differentiation of T cells, B cells, NK cells, and macrophages was observed [89].
Further bioinformatics approaches applied in a second YF-17D vaccine trial identified two genes - solute carrier family 2, member 6 (SLC2A6) and eukaryotic translation initiation factor 2 alpha kinase 4 (EIF2AK4) - that did correlate (with 90% accuracy) with the magnitude of antigen specific CD8+ T cell responses and antibody titers. In particular, EIF2AK4 regulates protein synthesis in response to environmental stresses by phosphorylating elongation initiation factor 2α (eIF2α) [94,95]. Indeed, YF-17D vaccination induced the phosphorylation of eIF2α as well as the formation of stress granules, and other genes involved in the stress response pathway correlated with the CD8+ T cell response [88].
The TnF receptor superfamily, receptor 17 (TnFRSF17), which is a receptor for B cell-activating factor (BAFF), was shown to be a key gene in the predictive signatures. BAFF, indeed, is thought to optimize B cell responses to B cell receptor- and TLR-dependent signaling [96,97].
Similar studies have been performed using a baculovirus-expressed HIV-VLPs developed in our laboratory [98]. Such HIV-VLPs, indeed, induced specific transcriptional profiles of genes involved in the morphological and functional changes characterizing innate and early adaptive immune response. This immune signature was observed in MDDCs [90] as well as in PBMCs from HIV-1 seronegative and seropositive subjects [93,99].
In particular, as described for the yellow fever live attenuated YF-17D vaccine, HIV-VLPs induced a molecular signature including several genes involved in innate sensing of viruses and antiviral immunity. Expression of proinflammatory mediators CXC-chemokine ligand 10 (CXCL-10) and interleukin-1α (IL-1α) genes were found upregulated. Similarly several genes were identified encoding innate sensing receptors (i.e.: TLR2), transcription factors that regulate the expression of type I IFNs, IFN regulatory factor 1 (IRF1) and signal transducer and activator of transcription 2 (STAT2). The gene signature predictive of both humoral and cellular adaptive immune response included several genes. The CD83 and CD28 genes indicate a strong activation of the Th2 development and B lymphocytes [100-102]. The TNF receptor superfamily, receptor 1B and 6B (TnFRSF1B and TnFRSF6B) are a marker for T and B cell activation (TnFRSF1B) [103] and resistance to the pro-apoptotic activity of the FAS-ligand (TnFRSF6B) [104]. The TNFSF9 is a T-cell activation marker [105,106] and the CD40 is one of the key players in activation of both humoral and cell-mediated immune responses [107,108].
These studies provide a global description of the innate and adaptive immune responses induced by two vaccines based on two different strategies, live attenuated (YF-17D) and non-replicating Virus-Like Particles (HIV-VLPs), identifying commonalities between the signatures induced by the two vaccines. Such results suggest the possible identification of specific shared predictive gene expression meta-signatures with a broad application in vaccinology (Figure 2).
Figure 2.
Genes modulated by YF-17D and HIV-VLP vaccines identified by microarray analyses.
In such studies, predictive signatures of response to vaccine have been evaluated using two independent classification methods, called classification to nearest centroid (ClaNC) and discriminant analysis via mixed integer programming (DAMIP) [88]. In particular, ClaNC has been previously shown to successfully develop predictive transcriptional cancer models [109], while the DAMIP classification model has proven to be a powerful supervised-learning classification approach in predicting various biomedical and bio-behavioral phenomena [110] and to produce superior classification accuracy when compared to traditional quadratic or linear discriminant analysis [111]. The DAMIP method was found to useful in determining correlates of vaccine efficacy for both T cell and B cell responses [88].
Vaccines for cancer
A selected subset of genes differentially expressed in melanoma metastases lesions have been identified, which are associated with better response to vaccines [112]. In particular, patients with a clinical response showed a gene transcriptional profile characteristic of an inflammatory tumor microenvironment, including chemokines and T-cell markers, that existed before the initiation of vaccination. A transcriptional profile involving similar set of genes was associated with better clinical outcome upon administration of MAGE-A3 protein vaccine [113]. Indeed, upregulation of CCL5, CXCL9 and CXCL10 chemokine genes within the melanoma metastases has been identified in patients with improved clinical outcome following vaccination, suggesting an effective intra-lesion recruitment of effector T cells [112].
Very interestingly, the same set of genes has been correlated to better outcome in subjects affected by non-small-cell lung carcinoma (NSCLC) and vaccinated with the same MAGE-A3 strategy [114].
Such results suggest that the pre-existent inflamed tumor microenvironment able to recruit activated T cells may represent a favorable prognostic factor for clinical response to cancer vaccines.
Genetic polymorphisms in innate immunity genes
Genetic polymorphisms can adversely affect expression of genes as well as proteins of the innate immune system and, consequently, host-pathogen interactions and molecular signaling. This represents an additional level of analysis to be included in the global evaluation of factors involved in the host response to foreign antigens, including vaccines [115-119].
Measle Vaccine
Associations between SNPs in TLRs 3, 4, 5 and 6 and the downstream intracellular signaling molecules, MyD88 and MD2, with variations in both antibody and cellular responses following measles vaccination have been recently described [120]. A SNP in the 3'UTR of TLR3 (rs5743305 at -976 bp of TLR promoter) has been identified demonstrating an association between heterozygous variant AT and low antibody as well as low lymphoproliferative responses in vaccinees. Similarly, the GA variant of a non-synonymous SNP also in the TLR3 gene was associated with lower antibody production.
Moreover, heterozygous variants for two non-synonymous SNPs (Gly299Asp and Ile399Thr) have been identified in the TLR4 gene and associated with higher IL-4 secretion to the measles vaccine strain [120]. Of note, the same two SNPs have been studied extensively in association with septic shock after infection with gram negative bacteria, premature birth, myocardial infarction and allograft rejection [121]. More recently, the major alleles of coding SNPs in the TLR2 (rs3804100) and TLR4 (rs5030710) genes have been associated with a dose-related increase or decrease in measles-specific antibodies, respectively [122]. Similarly, associations between SNPs in TLR5 and TLR6 genes and variations in IFN-γ secretion in response to measles virus stimulation have been identified, whose significance is still unclear [120].
Associations between SNPs in genes of intracellular signaling molecules associated with TLRs and the immune response to measles have been also investigated and a minor allele variant for a SNP in the 3'UTR of MyD88, the intracellular adaptor molecule that signals for most of the TLRs, was found to be associated with a lower antibody response to measles vaccine. Furthermore, several intronic SNPs in TLR and their associated intracellular molecule genes were significantly associated with variations in cellular immune responses to measles vaccine [120].
Rubella Vaccine
Also for rubella vaccination, associations between SNPs in TLR and the downstream intracellular signaling molecules with variations in both antibody and cellular responses have been recently described [123]. Polymorphisms in promoter and intronic regions of TLR3 and TLR4 genes have been found associated with rubella virus specific cytokine immune responses, such as IFN-γ, IL-2, TNF-α, and GM-CSF. In particular, two SNPs in the TLR3 gene appear to be significantly associated with lower rubella IFN-γ secretion in an allele dose-related manner. Of note, the promoter polymorphism (rs5743305, -8441 A > T) in the TLR3 gene, associated with rubella virus induced GM-CSF secretion, is the same SNP suggested to be a risk factor for lower antibody and low lymphoproliferative responses to measles vaccine [120]. This finding strongly suggests that rs5743305 in the TLR3 gene may play a role in viral immunity and may be a key control point for humoral and cellular immune responses to both measles and rubella vaccines.
The same study identified 22 associations between polymorphisms in promoter and intronic regions of vitamin A and vitamin D receptor genes and their downstream mediators of signaling with different immune response to rubella-specific cytokine. Since SNPs in the vitamin D receptor (VDR) genes have been associated also with protection from HIV-1 infection [124], it can be postulated that pro-inflammatory immune responses to viral infection or live viral vaccination are influenced by functional polymorphisms in the VDR gene.
Finally, associations of polymorphisms in the TRIM5 gene with variations in rubella virus-specific immune responses (TNF-α, GM-CSF and IL-2) have been observed, in concordance with recent findings on the role of the same TRIM5-gene SNPs in the immune response to retroviral (HIV-1) infection [125].
Pertussis Vaccine
The involvement of TLR4 in immunity to B. pertussis vaccine has been extensively shown and specific SNPs in the promoter region of the TLR4 gene influencing the antibody response to the pertussis (PT) vaccine have been identified [126,127]. The evidence of association was most consistent and strong for the SNPs in the TOLLIP gene, which showed association in three independent analyses. TOLLIP is a small protein that binds the activated IL-1 receptor type I (IL-1RI) complex, as well as TLR2 and TLR4 complexes, coordinating optimal signaling through IL-1RI and TLR4 [128,129].
Furthermore, associations of SNPs in TIRAP and TICAM1 genes and immune response to PT vaccine can be explained by the knowledge that these two factors belong to the Toll/Interleukin-1 receptor (TIR) domain-containing adaptors, also including MyD88, that modulate TLR signaling pathways. Furthermore, the signal transduction mediators of the Toll and IL-1 receptor (IL-1R) families, namely IRAK3 and IRAK4, showed evidence for association with immune response to PT vaccine.
Influenza vaccine
The role of HLA gene polymorphisms in the response to influenza vaccine has been evaluated and HLAA* 1101 (p = 0.0001) as well as A*6801 (p = 0.09) alleles were associated with higher median levels of antibody titers to influenza vaccine [130]. In the same study, also polymorphisms of cytokine and cytokine receptor genes were associated with humoral response to seasonal influenza vaccination. Previously, the increased frequency of HLADRB1* 0701 and the decreased frequency of HLA-DQB1*0603-9/14 was identified in individuals non-responders to the influenza subunit vaccine [131].
The correlation between specific gene polymorphisms and responsiveness to the different vaccines has been evaluated by different strong statistical analyses. However, in order to validate such findings, it would be extremely relevant to perform a meta-analysis using the same algorithms.
Data integration
The most challenging aspect of systems biology approaches is the integration of different data types to unveil relationships between genes, transcripts, proteins, metabolites, and epigenetic regulators. To this aim, appropriate analysis and modeling tools are in a continuous progress of development.
Algorithms and software packages designed to integrate heterogeneous data types have been released [132-134] and several publicly available databases of immunology-related transcriptomic datasets have been created in the recent years [135-138]. Furthermore, to improve integration of immunology datasets of these different databases, the Immunological Genome Project initiative has been established recently with the ambitious goal to combine immunology and computational biology laboratories in a systems-level approach [139].
The exploitation of computational methods for data mining and machine learning as well as bioinformatics methods for incorporating prior biological knowledge into data analysis algorithms has been proved to be an effective strategy. Recently, it has been shown the efficacy of applying statistical strategies (i.e. Random Forests method) for integrating genetic (SNPs) and proteomic (cytokine serum concentrations) data collected to elucidate the mechanisms underlying the development of adverse events (AEs) in patients after smallpox vaccination [140]. Combining information from previous studies on AEs related to smallpox vaccination with the genetic and proteomic attributes identified by RF, the Authors were able to build a comprehensive model of AE development that included specific cytokines (i.e. ICAM-1, IL-10, and G-CSF) and a genetic polymorphism in the cytokine gene interleukin-4 (IL4).
Such examples show the efficacy of data integration analysis and it is an easy prediction that in the next future more applications will be reported in the literature.
Conclusions
The comprehensive analysis at system level of immune response to vaccines and immunotherapies (vaccinomics or systems vaccinology) will provide invaluable knowledge in immunology and genetics which will lead to optimized vaccine development - including the identification of optimal antigens, and antigen formulations (i.e.: adjuvant antigens), inducing the sought cluster of genes and immune pathways leading to the required adaptive immune response. Moreover, it will greatly facilitate screening for responsiveness to vaccines or immunotherapies and understanding eventual failures in individuals enrolled in clinical trials. Indeed, the identification of gene transcriptional profiles or gene polymorphisms closely associated with immune response to such immunological strategies shows the relevance of such comprehensive analysis in the personalization of treatment to obtain the best clinical outcome.
However, the systems biology in vaccinology will go through several steps before becoming a widely used approach. The science is still developing and the complexity and extensive polymorphic nature of immune response genes needs enhanced powerful bio-informatics approaches in order to inexpensively manage the vast mass of genetic information. Moreover, validation studies in larger settings of different genetic background will be required to distinguish between natural and immune-response related genetic - gene expression or polymorphism - modifications. Indeed, only few examples show that genes are modulated in response to vaccination with a cause-effective relationship [141,142]. One way to overcome such problem is to evaluate the results within the context of known pathways or to combine multiple data types. Moreover, according to the vaccine or immunotherapy evaluated, the predictive target cell population should be identified which is not always represented by peripheral blood mononuclear cells.
Nevertheless, regardless the technical, cultural and financial challenges, systems biology applied to vaccinology represents a primary way to go in order to develop novel vaccines and to re-develop established vaccines, switching from the "empirical" to the "knowledge-based" age of vaccinology. This should enable the development of even more successful vaccines for preventive as well as therapeutic intervention strategies for human diseases according to individual- or group-personalized strategy.
Abbreviations
PRRs: Pathogen recognition receptors; TLRs: Toll-like receptors; HIV: Human Immunodeficiency virus; HCV: Hepatitis C virus; TNFβ: Tumor necrosis factor beta; IL-6: interleukin 6; IL-1β: interleukin 1 beta; IL-12: interleukin 12; APCs: antigen-presenting cells; DCs: dendritic cells; TNFα: Tumor necrosis factor alpha; MIP1α: Macrophage inflammatory protein 1 alpha; MIP1β: Macrophage inflammatory protein 1 beta; PAMS: pathogen-associated molecular signals; MAGE-A3: Melanoma-associated antigen 3; IFN: Interferon; MDDCs: monocyte-derived dendritic cells; PMBCs: peripheral blood mononuclear cells; CCL5: Chemokine (C-C motif) ligand 5; CXCL9: Chemokine (C-X-C motif) ligand9; CXCL10: Chemokine (C-X-C motif) ligand10; SNP: single-nucleotide polymorphism; MyD88: Myeloid differentiation primary response 88; GM-CSF: Granulocyte-macrophage colony-stimulating factor; TOLLIP: Toll interacting protein; TIRAP: toll-interleukin 1 receptor (TIR) domain containing adaptor protein; TICAM1: Toll-like receptor adapter molecule 1
Competing interests
The authors declare that they have no competing interests.
Authors' contributions
LB was responsible for the overall planning and coordination of the review as well as writing the paper; EW, MLT, FMB and FM equally contributed to the elaboration of the paper. All authors read and approved the final manuscript.
Contributor Information
Luigi Buonaguro, Email: lbuonaguro@tin.it.
Ena Wang, Email: EWang@cc.nih.gov.
Maria Lina Tornesello, Email: mtornesello@istitutotumori.na.it.
Franco M Buonaguro, Email: irccsvir@unina.it.
Francesco M Marincola, Email: FMarincola@cc.nih.gov.
References
- Akira S, Uematsu S, Takeuchi O. Pathogen recognition and innate immunity. Cell. 2006;124(4):783–801. doi: 10.1016/j.cell.2006.02.015. [DOI] [PubMed] [Google Scholar]
- Pulendran B, Ahmed R. Translating innate immunity into immunological memory: implications for vaccine development. Cell. 2006;124(4):849–63. doi: 10.1016/j.cell.2006.02.019. [DOI] [PubMed] [Google Scholar]
- Takeda K, Kaisho T, Akira S. Toll-like receptors. Annu Rev Immunol. 2003;21:335–76. doi: 10.1146/annurev.immunol.21.120601.141126. [DOI] [PubMed] [Google Scholar]
- Germain RN. An innately interesting decade of research in immunology. Nat Med. 2004;10(12):1307–20. doi: 10.1038/nm1159. [DOI] [PubMed] [Google Scholar]
- Beutler B. Inferences, questions and possibilities in Toll-like receptor signalling. Nature. 2004;430(6996):257–63. doi: 10.1038/nature02761. [DOI] [PubMed] [Google Scholar]
- Janeway CA, Medzhitov R. Innate immune recognition. Annu Rev Immunol. 2002;20:197–216. doi: 10.1146/annurev.immunol.20.083001.084359. [DOI] [PubMed] [Google Scholar]
- Shimazu R, Akashi S, Ogata H. et al. MD-2, a molecule that confers lipopolysaccharide responsiveness on Toll-like receptor 4. J Exp Med. 1999;189(11):1777–82. doi: 10.1084/jem.189.11.1777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poltorak A, He X, Smirnova I. et al. Defective LPS signaling in C3H/HeJ and C57BL/10ScCr mice: mutations in Tlr4 gene. Science. 1998;282(5396):2085–8. doi: 10.1126/science.282.5396.2085. [DOI] [PubMed] [Google Scholar]
- Alexopoulou L, Thomas V, Schnare M. et al. Hyporesponsiveness to vaccination with Borrelia burgdorferi OspA in humans and in TLR1- and TLR2-deficient mice. Nat Med. 2002;8(8):878–84. doi: 10.1038/nm732. [DOI] [PubMed] [Google Scholar]
- Ozinsky A, Underhill DM, Fontenot JD. et al. The repertoire for pattern recognition of pathogens by the innate immune system is defined by cooperation between toll-like receptors. Proc Natl Acad Sci USA. 2000;97(25):13766–71. doi: 10.1073/pnas.250476497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeuchi O, Kawai T, Muhlradt PF. et al. Discrimination of bacterial lipoproteins by Toll-like receptor 6. Int Immunol. 2001;13(7):933–40. doi: 10.1093/intimm/13.7.933. [DOI] [PubMed] [Google Scholar]
- Takeuchi O, Sato S, Horiuchi T. et al. Cutting edge: role of Toll-like receptor 1 in mediating immune response to microbial lipoproteins. J Immunol. 2002;169(1):10–4. doi: 10.4049/jimmunol.169.1.10. [DOI] [PubMed] [Google Scholar]
- Alexopoulou L, Holt AC, Medzhitov R, Flavell RA. Recognition of double-stranded RNA and activation of NF-kappaB by Toll-like receptor 3. Nature. 2001;413(6857):732–8. doi: 10.1038/35099560. [DOI] [PubMed] [Google Scholar]
- Hemmi H, Kaisho T, Takeuchi O. et al. Small anti-viral compounds activate immune cells via the TLR7 MyD88-dependent signaling pathway. Nat Immunol. 2002;3(2):196–200. doi: 10.1038/ni758. [DOI] [PubMed] [Google Scholar]
- Heil F, Hemmi H, Hochrein H. et al. Species-specific recognition of single-stranded RNA via toll- like receptor 7 and 8. Science. 2004;303:1526–9. doi: 10.1126/science.1093620. [DOI] [PubMed] [Google Scholar]
- Diebold SS, Kaisho T, Hemmi H, Akira S, Reis eSC. Innate antiviral responses by means of TLR7-mediated recognition of single-stranded RNA. Science. 2004;303:1529–31. doi: 10.1126/science.1093616. [DOI] [PubMed] [Google Scholar]
- Ahmad-Nejad P, Hacker H, Rutz M, Bauer S, Vabulas RM, Wagner H. Bacterial CpG-DNA and lipopolysaccharides activate Toll-like receptors at distinct cellular compartments. Eur J Immunol. 2002;32(7):1958–68. doi: 10.1002/1521-4141(200207)32:7<1958::AID-IMMU1958>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- Latz E, Schoenemeyer A, Visintin A. et al. TLR9 signals after translocating from the ER to CpG DNA in the lysosome. Nat Immunol. 2004;5(2):190–8. doi: 10.1038/ni1028. [DOI] [PubMed] [Google Scholar]
- Hayashi F, Smith KD, Ozinsky A. et al. The innate immune response to bacterial flagellin is mediated by Toll-like receptor 5. Nature. 2001;410(6832):1099–103. doi: 10.1038/35074106. [DOI] [PubMed] [Google Scholar]
- den Dunnen J, Gringhuis SI, Geijtenbeek TB. Innate signaling by the C-type lectin DC-SIGN dictates immune responses. Cancer Immunol Immunother. 2009;58(7):1149–57. doi: 10.1007/s00262-008-0615-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geijtenbeek TB, van Vliet SJ, Engering A, 't Hart BA, van Kooyk Y. Self- and nonself-recognition by C-type lectins on dendritic cells. Annu Rev Immunol. 2004;22:33–54. doi: 10.1146/annurev.immunol.22.012703.104558. [DOI] [PubMed] [Google Scholar]
- McDonald C, Nunez G. NOD-LRR proteins: role in host-microbial interactions and inflammatory disease. Annu Rev Biochem. 2005;74:355–83. doi: 10.1146/annurev.biochem.74.082803.133347. [DOI] [PubMed] [Google Scholar]
- Takeda K, Akira S. Toll-like receptors. Curr Protoc Immunol. 2007;Chapter 14 doi: 10.1002/0471142735.im1412s77. [DOI] [PubMed] [Google Scholar]
- Villadangos JA, Young L. Antigen-presentation properties of plasmacytoid dendritic cells. Immunity. 2008;29(3):352–61. doi: 10.1016/j.immuni.2008.09.002. [DOI] [PubMed] [Google Scholar]
- Alvarez D, Vollmann EH, von Andrian UH. Mechanisms and consequences of dendritic cell migration. Immunity. 2008;29(3):325–42. doi: 10.1016/j.immuni.2008.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasare C, Medzhitov R. Toll-like receptors: linking innate and adaptive immunity. Adv Exp Med Biol. 2005;560:11–8. doi: 10.1007/0-387-24180-9_2. [DOI] [PubMed] [Google Scholar]
- von Andrian UH, Mempel TR. Homing and cellular traffic in lymph nodes. Nat Rev Immunol. 2003;3(11):867–78. doi: 10.1038/nri1222. [DOI] [PubMed] [Google Scholar]
- McGuirk P, Mills KH. Pathogen-specific regulatory T cells provoke a shift in the Th1/Th2 paradigm in immunity to infectious diseases. Trends Immunol. 2002;23(9):450–5. doi: 10.1016/S1471-4906(02)02288-3. [DOI] [PubMed] [Google Scholar]
- Moser M, Murphy KM. Dendritic cell regulation of TH1-TH2 development. Nat Immunol. 2000;1(3):199–205. doi: 10.1038/79734. [DOI] [PubMed] [Google Scholar]
- O'Garra A, Vieira P. Regulatory T cells and mechanisms of immune system control. Nat Med. 2004;10(8):801–5. doi: 10.1038/nm0804-801. [DOI] [PubMed] [Google Scholar]
- O'Garra A, Robinson D. Development and function of T helper 1 cells. Adv Immunol. 2004;83:133–62. doi: 10.1016/S0065-2776(04)83004-9. [DOI] [PubMed] [Google Scholar]
- Mowen KA, Glimcher LH. Signaling pathways in Th2 development. Immunol Rev. 2004;202:203–22. doi: 10.1111/j.0105-2896.2004.00209.x. [DOI] [PubMed] [Google Scholar]
- Groux H, O'Garra A, Bigler M. et al. A CD4+ T-cell subset inhibits antigen-specific T-cell responses and prevents colitis. Nature. 1997;389(6652):737–42. doi: 10.1038/39614. [DOI] [PubMed] [Google Scholar]
- Kaech SM, Wherry EJ, Ahmed R. Effector and memory T-cell differentiation: implications for vaccine development. Nat Rev Immunol. 2002;2(4):251–62. doi: 10.1038/nri778. [DOI] [PubMed] [Google Scholar]
- Tsuji S, Matsumoto M, Takeuchi O. et al. Maturation of human dendritic cells by cell wall skeleton of Mycobacterium bovis bacillus Calmette-Guerin: involvement of toll-like receptors. Infect Immun. 2000;68(12):6883–90. doi: 10.1128/IAI.68.12.6883-6890.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uehori J, Matsumoto M, Tsuji S. et al. Simultaneous blocking of human Toll-like receptors 2 and 4 suppresses myeloid dendritic cell activation induced by Mycobacterium bovis bacillus Calmette-Guerin peptidoglycan. Infect Immun. 2003;71(8):4238–49. doi: 10.1128/IAI.71.8.4238-4249.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Latz E, Franko J, Golenbock DT, Schreiber JR. Haemophilus influenzae type b-outer membrane protein complex glycoconjugate vaccine induces cytokine production by engaging human toll-like receptor 2 (TLR2) and requires the presence of TLR2 for optimal immunogenicity. J Immunol. 2004;172(4):2431–8. doi: 10.4049/jimmunol.172.4.2431. [DOI] [PubMed] [Google Scholar]
- Querec T, Bennouna S, Alkan S. et al. Yellow fever vaccine YF-17D activates multiple dendritic cell subsets via TLR2, 7, 8, and 9 to stimulate polyvalent immunity. J Exp Med. 2006;203(2):413–24. doi: 10.1084/jem.20051720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casella CR, Mitchell TC. Putting endotoxin to work for us: monophosphoryl lipid A as a safe and effective vaccine adjuvant. Cell Mol Life Sci. 2008;65(20):3231–40. doi: 10.1007/s00018-008-8228-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mata-Haro V, Cekic C, Martin M, Chilton PM, Casella CR, Mitchell TC. The vaccine adjuvant monophosphoryl lipid A as a TRIF-biased agonist of TLR4. Science. 2007;316(5831):1628–32. doi: 10.1126/science.1138963. [DOI] [PubMed] [Google Scholar]
- Lahiri A, Das P, Chakravortty D. Engagement of TLR signaling as adjuvant: towards smarter vaccine and beyond. Vaccine. 2008;26(52):6777–83. doi: 10.1016/j.vaccine.2008.09.045. [DOI] [PubMed] [Google Scholar]
- Kang SM, Compans RW. Host responses from innate to adaptive immunity after vaccination: molecular and cellular events. Mol Cells. 2009;27(1):5–14. doi: 10.1007/s10059-009-0015-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed SG, Bertholet S, Coler RN, Friede M. New horizons in adjuvants for vaccine development. Trends Immunol. 2009;30(1):23–32. doi: 10.1016/j.it.2008.09.006. [DOI] [PubMed] [Google Scholar]
- Kwissa M, Kasturi SP, Pulendran B. The science of adjuvants. Expert Rev Vaccines. 2007;6(5):673–84. doi: 10.1586/14760584.6.5.673. [DOI] [PubMed] [Google Scholar]
- Fraser CK, Diener KR, Brown MP, Hayball JD. Improving vaccines by incorporating immunological coadjuvants. Expert Rev Vaccines. 2007;6(4):559–78. doi: 10.1586/14760584.6.4.559. [DOI] [PubMed] [Google Scholar]
- Coffman RL, Sher A, Seder RA. Vaccine adjuvants: putting innate immunity to work. Immunity. 2010;33(4):492–503. doi: 10.1016/j.immuni.2010.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castiello L, Sabatino M, Jin P. et al. Monocyte-derived DC maturation strategies and related pathways: a transcriptional view. Cancer Immunol Immunother. 2011;60(4):457–66. doi: 10.1007/s00262-010-0954-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bachmann MF, Zinkernagel RM, Oxenius A. Immune responses in the absence of costimulation: viruses know the trick. J Immunol. 1998;161(11):5791–4. [PubMed] [Google Scholar]
- Zepp F. Principles of vaccine design-Lessons from nature. Vaccine. 2010;28(Suppl 3):C14–C24. doi: 10.1016/j.vaccine.2010.07.020. [DOI] [PubMed] [Google Scholar]
- Pashine A, Valiante NM, Ulmer JB. Targeting the innate immune response with improved vaccine adjuvants. Nat Med. 2005;11(4 Suppl):S63–S68. doi: 10.1038/nm1210. [DOI] [PubMed] [Google Scholar]
- Wang E, Worschech A, Marincola FM. The immunologic constant of rejection. Trends Immunol. 2008;29(6):256–62. doi: 10.1016/j.it.2008.03.002. [DOI] [PubMed] [Google Scholar]
- Wang E, Marincola FM. In: Immunological signatures of rejection. Marincola FM, Wang E, editor. Springer; 2011. Immune-mediated tumor rejection; pp. 281–304. [Google Scholar]
- Plotkin SA. Vaccines: the fourth century. Clin Vaccine Immunol. 2009;16(12):1709–19. doi: 10.1128/CVI.00290-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houghton M, Abrignani S. Prospects for a vaccine against the hepatitis C virus. Nature. 2005;436(7053):961–6. doi: 10.1038/nature04081. [DOI] [PubMed] [Google Scholar]
- Sallberg M, Frelin L, Weiland O. DNA vaccine therapy for chronic hepatitis C virus (HCV) infection: immune control of a moving target. Expert Opin Biol Ther. 2009;9(7):805–15. doi: 10.1517/14712590902988444. [DOI] [PubMed] [Google Scholar]
- Korber BT, Letvin NL, Haynes BF. T-cell vaccine strategies for human immunodeficiency virus, the virus with a thousand faces. J Virol. 2009;83(17):8300–14. doi: 10.1128/JVI.00114-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rerks-Ngarm S, Pitisuttithum P, Nitayaphan S. et al. Vaccination with ALVAC and AIDSVAX to Prevent HIV-1 Infection in Thailand. N Engl J Med. 2009 Dec 3;361(23):2209–20. doi: 10.1056/NEJMoa0908492. [DOI] [PubMed] [Google Scholar]
- Draper SJ, Heeney JL. Viruses as vaccine vectors for infectious diseases and cancer. Nat Rev Microbiol. 2010;8(1):62–73. doi: 10.1038/nrmicro2240. [DOI] [PubMed] [Google Scholar]
- Banchereau J, Klechevsky E, Schmitt N, Morita R, Palucka K, Ueno H. Harnessing human dendritic cell subsets to design novel vaccines. Ann N Y Acad Sci. 2009;1174:24–32. doi: 10.1111/j.1749-6632.2009.04999.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sioud M. Does our current understanding of immune tolerance, autoimmunity, and immunosuppressive mechanisms facilitate the design of efficient cancer vaccines? Scand J Immunol. 2009;70(6):516–25. doi: 10.1111/j.1365-3083.2009.02326.x. [DOI] [PubMed] [Google Scholar]
- Plotkin SA. Correlates of protection induced by vaccination. Clin Vaccine Immunol. 2010;17(7):1055–65. doi: 10.1128/CVI.00131-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franco MA, Angel J, Greenberg HB. Immunity and correlates of protection for rotavirus vaccines. Vaccine. 2006;24(15):2718–31. doi: 10.1016/j.vaccine.2005.12.048. [DOI] [PubMed] [Google Scholar]
- Weinberg A, Zhang JH, Oxman MN. et al. Varicella-zoster virus-specific immune responses to herpes zoster in elderly participants in a trial of a clinically effective zoster vaccine. J Infect Dis. 2009;200(7):1068–77. doi: 10.1086/605611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akondy RS, Monson ND, Miller JD. et al. The yellow fever virus vaccine induces a broad and polyfunctional human memory CD8+ T cell response. J Immunol. 2009;183(12):7919–30. doi: 10.4049/jimmunol.0803903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller JD, van der Most RG, Akondy RS. et al. Human effector and memory CD8+ T cell responses to smallpox and yellow fever vaccines. Immunity. 2008;28(5):710–22. doi: 10.1016/j.immuni.2008.02.020. [DOI] [PubMed] [Google Scholar]
- Migueles SA, Osborne CM, Royce C. et al. Lytic granule loading of CD8+ T cells is required for HIV-infected cell elimination associated with immune control. Immunity. 2008;29(6):1009–21. doi: 10.1016/j.immuni.2008.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hersperger AR, Pereyra F, Nason M. et al. Perforin expression directly ex vivo by HIV-specific CD8 T-cells is a correlate of HIV elite control. PLoS Pathog. 2010;6(5):e1000917. doi: 10.1371/journal.ppat.1000917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makedonas G, Hutnick N, Haney D. et al. Perforin and IL-2 upregulation define qualitative differences among highly functional virus-specific human CD8 T cells. PLoS Pathog. 2010;6(3):e1000798. doi: 10.1371/journal.ppat.1000798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khader SA, Bell GK, Pearl JE. et al. IL-23 and IL-17 in the establishment of protective pulmonary CD4+ T cell responses after vaccination and during Mycobacterium tuberculosis challenge. Nat Immunol. 2007;8(4):369–77. doi: 10.1038/ni1449. [DOI] [PubMed] [Google Scholar]
- Yuan L, Wen K, Azevedo MS, Gonzalez AM, Zhang W, Saif LJ. Virus-specific intestinal IFN-gamma producing T cell responses induced by human rotavirus infection and vaccines are correlated with protection against rotavirus diarrhea in gnotobiotic pigs. Vaccine. 2008;26(26):3322–31. doi: 10.1016/j.vaccine.2008.03.085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dieu-Nosjean MC, Antoine M, Danel C. et al. Long-term survival for patients with non-small-cell lung cancer with intratumoral lymphoid structures. J Clin Oncol. 2008;26(27):4410–7. doi: 10.1200/JCO.2007.15.0284. [DOI] [PubMed] [Google Scholar]
- Wang E, Marincola FM. Bottom up: a modular view of immunology. Immunity. 2008;29(1):9–11. doi: 10.1016/j.immuni.2008.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potti A, Dressman HK, Bild A. et al. Genomic signatures to guide the use of chemotherapeutics. Nat Med. 2006;12(11):1294–300. doi: 10.1038/nm1491. [DOI] [PubMed] [Google Scholar]
- Alizadeh AA, Staudt LM. Genomic-scale gene expression profiling of normal and malignant immune cells. Curr Opin Immunol. 2000;12(2):219–25. doi: 10.1016/S0952-7915(99)00078-3. [DOI] [PubMed] [Google Scholar]
- Sorlie T, Perou CM, Tibshirani R. et al. Gene expression patterns of breast carcinomas distinguish tumor subclasses with clinical implications. Proc Natl Acad Sci USA. 2001;98(19):10869–74. doi: 10.1073/pnas.191367098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramilo O, Allman W, Chung W. et al. Gene expression patterns in blood leukocytes discriminate patients with acute infections. Blood. 2007;109(5):2066–77. doi: 10.1182/blood-2006-02-002477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaussabel D, Quinn C, Shen J. et al. A modular analysis framework for blood genomics studies: application to systemic lupus erythematosus. Immunity. 2008;29(1):150–64. doi: 10.1016/j.immuni.2008.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pulendran B, Li S, Nakaya HI. Systems vaccinology. Immunity. 2010;33(4):516–29. doi: 10.1016/j.immuni.2010.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buonaguro L, Pulendran B. Immunogenomics and systems biology of vaccines. Immunol Rev. 2011;239(1):197–208. doi: 10.1111/j.1600-065X.2010.00971.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buonaguro FM, Tornesello ML, Buonaguro L. In: Immunological signatures of rejection. Marincola FM, Wang E, editor. Springer; 2011. Immune signatures and systems biology of vaccines; pp. 141–67. [Google Scholar]
- Ricciardi-Castagnoli P, Granucci F. Opinion: Interpretation of the complexity of innate immune responses by functional genomics. Nat Rev Immunol. 2002;2(11):881–9. doi: 10.1038/nri936. [DOI] [PubMed] [Google Scholar]
- Elkon R, Linhart C, Halperin Y, Shiloh Y, Shamir R. Functional genomic delineation of TLR-induced transcriptional networks. BMC Genomics. 2007;8:394. doi: 10.1186/1471-2164-8-394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenner RG, Young RA. Insights into host responses against pathogens from transcriptional profiling. Nat Rev Microbiol. 2005;3(4):281–94. doi: 10.1038/nrmicro1126. [DOI] [PubMed] [Google Scholar]
- McCaffrey RL, Fawcett P, O'Riordan M. et al. A specific gene expression program triggered by Gram-positive bacteria in the cytosol. Proc Natl Acad Sci USA. 2004;101(31):11386–91. doi: 10.1073/pnas.0403215101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leber JH, Crimmins GT, Raghavan S, Meyer-Morse NP, Cox JS, Portnoy DA. Distinct TLR- and NLR-mediated transcriptional responses to an intracellular pathogen. PLoS Pathog. 2008;4(1):e6. doi: 10.1371/journal.ppat.0040006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z, Gerstein M, Snyder M. RNA-Seq: a revolutionary tool for transcriptomics. Nat Rev Genet. 2009;10(1):57–63. doi: 10.1038/nrg2484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa V, Angelini C, De F, Ciccodicola A. Uncovering the complexity of transcriptomes with RNA-Seq. J Biomed Biotechnol. 2010;2010:853916. doi: 10.1155/2010/853916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Querec TD, Akondy RS, Lee EK. et al. Systems biology approach predicts immunogenicity of the yellow fever vaccine in humans. Nat Immunol. 2009;10(1):116–25. doi: 10.1038/ni.1688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaucher D, Therrien R, Kettaf N. et al. Yellow fever vaccine induces integrated multilineage and polyfunctional immune responses. J Exp Med. 2008;205(13):3119–31. doi: 10.1084/jem.20082292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aricò E, Wang E, Tornesello ML. et al. Immature monocyte derived dendritic cells gene expression profile in response to Virus-Like Particles stimulation. J Transl Med. 2005;3:45. doi: 10.1186/1479-5876-3-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buonaguro L, Tornesello ML, Tagliamonte M. et al. Baculovirus-derived human immunodeficiency virus type 1 virus-like particles activate dendritic cells and induce ex vivo T-cell responses. J Virol. 2006;80(18):9134–43. doi: 10.1128/JVI.00050-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buonaguro L, Tornesello ML, Gallo RC, Marincola FM, Lewis GK, Buonaguro FM. Th2 Polarization in Peripheral Blood Mononuclear Cells from Human Immunodeficiency Virus (HIV)-Infected Subjects, as Activated by HIV Virus-Like Particles. J Virol. 2009;83(1):304–13. doi: 10.1128/JVI.01606-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monaco A, Marincola FM, Sabatino M. et al. Molecular immune signatures of HIV-1 vaccines in human PBMCs. FEBS Lett. 2009;583(18):3004–8. doi: 10.1016/j.febslet.2009.07.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richter JD, Sonenberg N. Regulation of cap-dependent translation by eIF4E inhibitory proteins. Nature. 2005;433(7025):477–80. doi: 10.1038/nature03205. [DOI] [PubMed] [Google Scholar]
- Ron D, Walter P. Signal integration in the endoplasmic reticulum unfolded protein response. Nat Rev Mol Cell Biol. 2007;8(7):519–29. doi: 10.1038/nrm2199. [DOI] [PubMed] [Google Scholar]
- Hoek KL, Carlesso G, Clark ES, Khan WN. Absence of mature peripheral B cell populations in mice with concomitant defects in B cell receptor and BAFF-R signaling. J Immunol. 2009;183(9):5630–43. doi: 10.4049/jimmunol.0901100. [DOI] [PubMed] [Google Scholar]
- Khan WN. B cell receptor and BAFF receptor signaling regulation of B cell homeostasis. J Immunol. 2009;183(6):3561–7. doi: 10.4049/jimmunol.0800933. [DOI] [PubMed] [Google Scholar]
- Buonaguro L, Buonaguro FM, Tornesello ML. et al. High efficient production of Pr55gag Virus-like Particles expressing multiple HIV-1 epitopes, including a gp120 protein derived from an Ugandan HIV-1 isolate of subtype A. Antiviral Research. 2001;49(1):35–47. doi: 10.1016/S0166-3542(00)00136-4. [DOI] [PubMed] [Google Scholar]
- Buonaguro L, Monaco A, Arico E. et al. Gene expression profile of peripheral blood mononuclear cells in response to HIV-VLPs stimulation. BMC Bioinformatics. 2008;9(Suppl 2):S5. doi: 10.1186/1471-2105-9-S2-S5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magistrelli G, Jeannin P, Elson G. et al. Identification of three alternatively spliced variants of human CD28 mRNA. Biochem Biophys Res Commun. 1999;259(1):34–7. doi: 10.1006/bbrc.1999.0725. [DOI] [PubMed] [Google Scholar]
- Andres PG, Howland KC, Nirula A. et al. Distinct regions in the CD28 cytoplasmic domain are required for T helper type 2 differentiation. Nat Immunol. 2004;5(4):435–42. doi: 10.1038/ni1044. [DOI] [PubMed] [Google Scholar]
- Kozlow EJ, Wilson GL, Fox CH, Kehrl JH. Subtractive cDNA cloning of a novel member of the Ig gene superfamily expressed at high levels in activated B lymphocytes. Blood. 1993;81(2):454–61. [PubMed] [Google Scholar]
- Beltinger CP, White PS, Maris JM. et al. Physical mapping and genomic structure of the human TNFR2 gene. Genomics. 1996;35(1):94–100. doi: 10.1006/geno.1996.0327. [DOI] [PubMed] [Google Scholar]
- Pitti RM, Marsters SA, Lawrence DA. et al. Genomic amplification of a decoy receptor for Fas ligand in lung and colon cancer. Nature. 1998;396(6712):699–703. doi: 10.1038/25387. [DOI] [PubMed] [Google Scholar]
- Alderson MR, Smith CA, Tough TW. et al. Molecular and biological characterization of human 4-1BB and its ligand. Eur J Immunol. 1994;24(9):2219–27. doi: 10.1002/eji.1830240943. [DOI] [PubMed] [Google Scholar]
- Stephan MT, Ponomarev V, Brentjens RJ. et al. T cell-encoded CD80 and 4-1BBL induce auto- and transcostimulation, resulting in potent tumor rejection. Nat Med. 2007;13(12):1440–9. doi: 10.1038/nm1676. [DOI] [PubMed] [Google Scholar]
- Kawabe T, Naka T, Yoshida K. et al. The immune responses in CD40-deficient mice: impaired immunoglobulin class switching and germinal center formation. Immunity. 1994;1(3):167–78. doi: 10.1016/1074-7613(94)90095-7. [DOI] [PubMed] [Google Scholar]
- Schonbeck U, Libby P. The CD40/CD154 receptor/ligand dyad. Cell Mol Life Sci. 2001;58(1):4–43. doi: 10.1007/PL00000776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dabney AR. Classification of microarrays to nearest centroids. Bioinformatics. 2005;21(22):4148–54. doi: 10.1093/bioinformatics/bti681. [DOI] [PubMed] [Google Scholar]
- Lee EK. Large-scale optimization-based classification models in medicine and biology. Ann Biomed Eng. 2007;35(6):1095–109. doi: 10.1007/s10439-007-9317-7. [DOI] [PubMed] [Google Scholar]
- Gallagher RJ, Lee EK. Mixed integer programming optimization models for brachytherapy treatment planning. Proc AMIA Annu Fall Symp. 1997. pp. 278–82. [PMC free article] [PubMed]
- Harlin H, Meng Y, Peterson AC. et al. Chemokine expression in melanoma metastases associated with CD8+ T-cell recruitment. Cancer Res. 2009;69(7):3077–85. doi: 10.1158/0008-5472.CAN-08-2281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Louahed J, Gruselle O, Gaulis S, Expression of defined genes identified by pre-treatment tumor profiling: association with clinical responses to the GSK MAGE-A3 immunotherapeutic in metastatic melanoma patients. J Clin Oncol 26S. 2008.
- Vansteenkiste JF, Zielinski M, Dahabreh IJ, Association of gene expression signature and clinical efficacy of MAGE-A3 antigen-specific cancer immunotherapeutic (ASCI) as adjuvant therapy in resected stage IB/II non-small cell lung cancer (NSCLC) J Clin Oncol 15S. 2008.
- Georgel P, Macquin C, Bahram S. The heterogeneous allelic repertoire of human toll-like receptor (TLR) genes. PLoS One. 2009;4(11):e7803. doi: 10.1371/journal.pone.0007803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bochud PY, Bochud M, Telenti A, Calandra T. Innate immunogenetics: a tool for exploring new frontiers of host defence. Lancet Infect Dis. 2007;7(8):531–42. doi: 10.1016/S1473-3099(07)70185-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Misch EA, Hawn TR. Toll-like receptor polymorphisms and susceptibility to human disease. Clin Sci (Lond) 2008;114(5):347–60. doi: 10.1042/CS20070214. [DOI] [PubMed] [Google Scholar]
- Dickinson AM, Holler E. Polymorphisms of cytokine and innate immunity genes and GVHD. Best Pract Res Clin Haematol. 2008;21(2):149–64. doi: 10.1016/j.beha.2008.03.004. [DOI] [PubMed] [Google Scholar]
- Poland GA, Ovsyannikova IG, Jacobson RM. Application of pharmacogenomics to vaccines. Pharmacogenomics. 2009;10(5):837–52. doi: 10.2217/pgs.09.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhiman N, Ovsyannikova IG, Vierkant RA. et al. Associations between SNPs in toll-like receptors and related intracellular signaling molecules and immune responses to measles vaccine: preliminary results. Vaccine. 2008;26(14):1731–6. doi: 10.1016/j.vaccine.2008.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroder NW, Schumann RR. Single nucleotide polymorphisms of Toll-like receptors and susceptibility to infectious disease. Lancet Infect Dis. 2005;5(3):156–64. doi: 10.1016/S1473-3099(05)01308-3. [DOI] [PubMed] [Google Scholar]
- Ovsyannikova IG, Haralambieva IH, Vierkant RA, Pankratz VS, Jacobson RM, Poland GA. The role of polymorphisms in Toll-like receptors and their associated intracellular signaling genes in measles vaccine immunity. Hum Genet. 2011. [DOI] [PMC free article] [PubMed]
- Ovsyannikova IG, Dhiman N, Haralambieva IH. et al. Rubella vaccine-induced cellular immunity: evidence of associations with polymorphisms in the Toll-like, vitamin A and D receptors, and innate immune response genes. Hum Genet. 2010;127(2):207–21. doi: 10.1007/s00439-009-0763-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de la Torre MS, Torres C, Nieto G. et al. Vitamin D receptor gene haplotypes and susceptibility to HIV-1 infection in injection drug users. J Infect Dis. 2008;197(3):405–10. doi: 10.1086/525043. [DOI] [PubMed] [Google Scholar]
- van Manen D, Rits MA, Beugeling C, van DK, Schuitemaker H, Kootstra NA. The effect of Trim5 polymorphisms on the clinical course of HIV-1 infection. PLoS Pathog. 2008;4(2):e18. doi: 10.1371/journal.ppat.0040018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banus S, Bottema RW, Siezen CL. et al. Toll-like receptor 4 polymorphism associated with the response to whole-cell pertussis vaccination in children from the KOALA study. Clin Vaccine Immunol. 2007;14(10):1377–80. doi: 10.1128/CVI.00175-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimman TG, Banus S, Reijmerink N. et al. Association of interacting genes in the toll-like receptor signaling pathway and the antibody response to pertussis vaccination. PLoS One. 2008;3(11):e3665. doi: 10.1371/journal.pone.0003665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Didierlaurent A, Brissoni B, Velin D. et al. Tollip regulates proinflammatory responses to interleukin-1 and lipopolysaccharide. Mol Cell Biol. 2006;26(3):735–42. doi: 10.1128/MCB.26.3.735-742.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bulut Y, Faure E, Thomas L, Equils O, Arditi M. Cooperation of Toll-like receptor 2 and 6 for cellular activation by soluble tuberculosis factor and Borrelia burgdorferi outer surface protein A lipoprotein: role of Toll-interacting protein and IL-1 receptor signaling molecules in Toll-like receptor 2 signaling. J Immunol. 2001;167(2):987–94. doi: 10.4049/jimmunol.167.2.987. [DOI] [PubMed] [Google Scholar]
- Poland GA, Ovsyannikova IG, Jacobson RM. Immunogenetics of seasonal influenza vaccine response. Vaccine. 2008;26(Suppl 4):D35–D40. doi: 10.1016/j.vaccine.2008.07.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gelder CM, Lambkin R, Hart KW. et al. Associations between human leukocyte antigens and nonresponsiveness to influenza vaccine. J Infect Dis. 2002;185(1):114–7. doi: 10.1086/338014. [DOI] [PubMed] [Google Scholar]
- Myers CL, Chiriac C, Troyanskaya OG. Discovering biological networks from diverse functional genomic data. Methods Mol Biol. 2009;563:157–75. doi: 10.1007/978-1-60761-175-2_9. [DOI] [PubMed] [Google Scholar]
- Joyce AR, Palsson BO. The model organism as a system: integrating 'omics' data sets. Nat Rev Mol Cell Biol. 2006;7(3):198–210. doi: 10.1038/nrm1857. [DOI] [PubMed] [Google Scholar]
- Moore JH, Asselbergs FW, Williams SM. Bioinformatics challenges for genome-wide association studies. Bioinformatics. 2010;26(4):445–55. doi: 10.1093/bioinformatics/btp713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hijikata A, Kitamura H, Kimura Y. et al. Construction of an open-access database that integrates cross-reference information from the transcriptome and proteome of immune cells. Bioinformatics. 2007;23(21):2934–41. doi: 10.1093/bioinformatics/btm430. [DOI] [PubMed] [Google Scholar]
- Abbas AR, Baldwin D, Ma Y. et al. Immune response in silico (IRIS): immune-specific genes identified from a compendium of microarray expression data. Genes Immun. 2005;6(4):319–31. doi: 10.1038/sj.gene.6364173. [DOI] [PubMed] [Google Scholar]
- Korb M, Rust AG, Thorsson V. et al. The Innate Immune Database (IIDB) BMC Immunol. 2008;9:7. doi: 10.1186/1471-2172-9-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardy JL, Lynn DJ, Brinkman FS, Hancock RE. Enabling a systems biology approach to immunology: focus on innate immunity. Trends Immunol. 2009;30(6):249–62. doi: 10.1016/j.it.2009.03.009. [DOI] [PubMed] [Google Scholar]
- Heng TS, Painter MW. The Immunological Genome Project: networks of gene expression in immune cells. Nat Immunol. 2008;9(10):1091–4. doi: 10.1038/ni1008-1091. [DOI] [PubMed] [Google Scholar]
- Reif DM, Motsinger-Reif AA, McKinney BA, Rock MT, Crowe JE, Moore JH. Integrated analysis of genetic and proteomic data identifies biomarkers associated with adverse events following smallpox vaccination. Genes Immun. 2009;10(2):112–9. doi: 10.1038/gene.2008.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bansal M, Belcastro V, mbesi-Impiombato A, di BD. How to infer gene networks from expression profiles. Mol Syst Biol. 2007;3:78. doi: 10.1038/msb4100120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu W, Li D, Liu Q, Zhu Y, He F. A novel parametric approach to mine gene regulatory relationship from microarray datasets. BMC Bioinformatics. 2010;11(Suppl 11):S15. doi: 10.1186/1471-2105-11-S11-S15. [DOI] [PMC free article] [PubMed] [Google Scholar]