Abstract
In the United States, there are significant racial disparities in the incidence and prevalence of end-stage renal disease. The disparities are greatest for the Blacks and the magnitude of disparity is significantly greater than is evident from the incidence and prevalence data of end-stage renal disease – early stage chronic kidney disease is less common in Blacks and during that stage, mortality rate is significantly higher for that racial group. Recent studies have identified a genetic predisposition for non-diabetic renal disease among Blacks. However, genetic factors explain only part of the higher risk and the racial disparities are a result of a complex interplay of biology and sociology. Herein we focus on two factors and their role in explaining the higher risk for progression of chronic kidney disease among Blacks – one biologic (vitamin D deficiency) and one sociologic (neighborhood poverty). A greater Understanding of these factors is important in order to reduce the racial disparities in the United States.
Keywords: renal disparities, genetic predisposition, vitamin D deficiency, neighbourhood poverty, diabetes, hypertension
Epidemiologic studies have shown that socially and economically disadvantaged populations bear a disproportionate burden of end-stage renal disease (ESRD). In the United States, the incidence of treated ESRD is 2.6-, 1.1-, and 1.2-fold higher in the Black, Asian, and Native Americans, compared to Whites [1]. The prevalence of treated ESRD is 4.2-, 1.5-, and 2.3-fold higher respectively in these disadvantaged populations [1]. Two recent observations suggest that the incidence and prevalence data of treated ESRD under-estimate the magnitude of racial disparity in the progression of chronic kidney disease (CKD) among Blacks. First, the prevalence of early stage CKD in Blacks is actually lower than in Whites [2]. Second, the relative risk for death in Blacks with early stage CKD is significantly higher than Whites – a risk that is modified by age: While among individuals with CKD < 65 years of age, the risk for death among Blacks is 78% higher than Whites, no significant differences are seen in older individuals [3]. Thus, lower prevalence of, and a higher probability of death during early stage CKD, and a higher incidence and prevalence of treated ESRD provide a better measure of disparity in the progression of CKD among Blacks.
Numerous investigators have tried to understand the reasons underlying this disparity. Studies have focused on risk factors present at birth (genetic factors, gene-environment interactions, low birth weight and nephron number), higher prevalence and greater severity of risk factors for CKD(diabetes mellitus, hypertension, systemic lupus erythematosus, human immunodeficiency virus infection, obesity), and behavioral factors (smoking, adherence). In this paper, we will focus on two issues – one biologic factor (hypovitaminosis D), and the other sociologic (neighborhood, and individual socio-economic deprivation).
Hypovitaminosis D and racial disparities in progression of chronic kidney disease
Accumulating evidence supports the notion that vitamin D exerts a wide variety of pleiotropic effects, beyond its important role in bone and mineral metabolism [4]. Since there are few dietary sources rich in vitamin D, humans obtain most of the vitamin D through the conversion of 7-dehydrotachysterol in the skin via ultraviolet light. Vitamin D formed in the skin is 25-hydroxylated in the liver and circulating 25-hydroxyvitamin D levels is a sensitive measure of body stores of vitamin D [4]. Large proportions of populations living in temperate climates have vitamin D levels that are insufficient to prevent secondary hyperparathyroidism. Recent studies have also demonstrated that vitamin D deficiency is an independent risk factor for fatal and non-fatal cardiovascular events (reviewed in [5]). Hypovitaminosis D is much more prevalent in dark-skinned individuals, and thus, is more prevalent in the two largest disadvantaged populations in the United States (Blacks and Mexican-Americans) [6]. Similarly, despite abundant sunlight and low use of sunscreens, hypovitaminosis D is highly prevalent in India [7].
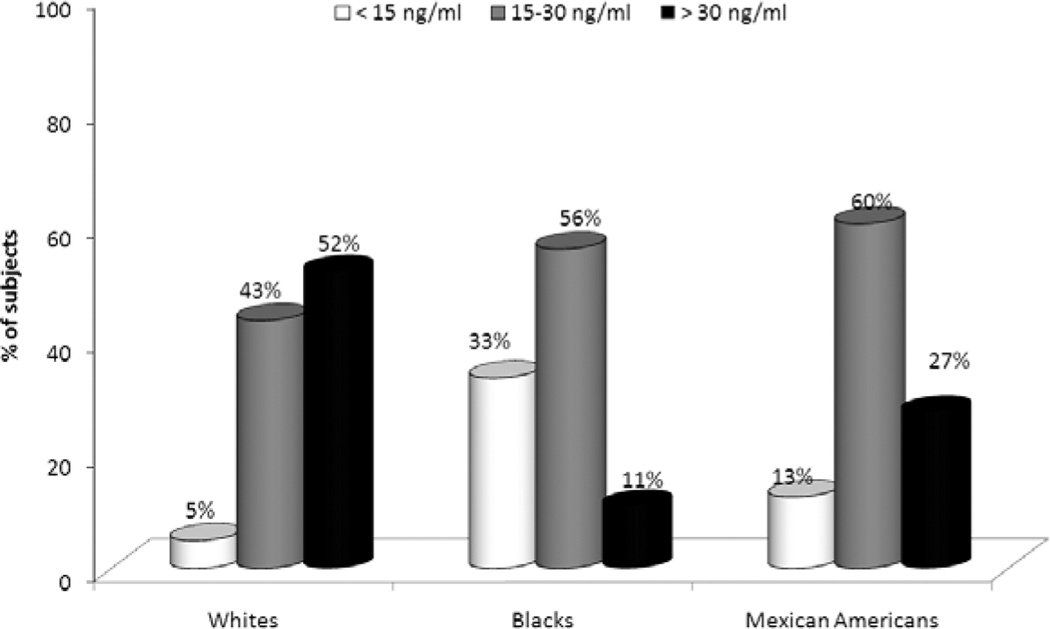
The odds of vitamin D deficiency (serum 25-hydroxyvitamin D < 15 ng/ml) are 32% higher in individuals with CKD, compared to the general population [8]. Most Blacks have serum 25-hydroxyvitamin D levels < 30 ng/ml; the prevalence of vitamin D deficiency and insufficiency is also more common in Mexican-Americans, when compared to Whites (Figure 1) [8, 9]. As in the general population, there is a graded, inverse relationship between vitamin D levels and all-cause mortality in chronic kidney disease [9]. This raises the possibility that the higher risk for death in racial and ethnic minorities with CKD in the United States may be, at least in part, secondary to vitamin D deficiency.
Figure 1.
Prevalence of vitamin D deficiency (< 15 ng/ml), insufficiency (15 – 30 ng/ml), and sufficiency (> 30 ng/ml) in Whites, Blacks, and Mexican-Americans with chronic kidney disease. Data taken from the Third National Health and Nutrition Examination Survey.
Emerging evidence also links hypovitaminosis D to progression of CKD. The biologic data linking hypovitaminosis D to progression of CKD is elegantly summarized in a review by Tian et al. [10]. Thus, vitamin D deficiency has been associated with 1) increased inflammation, 2) mesangial and epithelial proliferation, 3) activation of the renin-angiotensin-aldosterone system, 4) glomerular hyperfiltration and hypertrophy, 5) proteinuria, 6) increased renal expression of pro-fibrotic transforming growth factor-β, and 7) decreased expression of E-cadherin, a key first step in epithelial-mesenchymal transformation [10]. All of these processes are expected to lead to increased glomerulosclerosis, and tubulointerstitial fibrosis.
There are at least three interventional studies suggesting that treatment with active vitamin D compounds may have an antiproteinuric effect. Pooling data from three placebo-controlled, randomized controlled trials (28% non-White, 59% diabetic), Agarwal et al. [11] showed that twice as many Stage 3 or 4 CKD subjects treated with paricalcitol had regression of proteinuria, when compared to those treated with placebo (51% vs. 24%, p = 0.004). In a follow-up randomized controlled trial of 24 CKD subjects, either 1 µg, or 2 µg of parcialcitol was associated with an almost 50% reduction in urine albumin excretion over 1 month; subjects treated with placebo had a 35% increase in albuminuria over the same time-period [12]. The reduction in albuminuria was also associated with reduction in serum high-sensitivity C-reactive protein levels that achieved statistical significance in subjects treated with 2 µg parcialcitol [12]. Finally, in an open-label, uncontrolled study, 0.5 µg calcitriol twice weekly was associated with a significant reduction in proteinuria in 10 subjects with biopsy-proven IgA nephropathy [13].
Each of these three studies has significant limitations – small sample size, the decision to treat with active vitamin D compounds was not based on measurement of serum 25-hydroxyvitamin D levels, and none of the studies measured change in glomerular filtration, or racial differences in response to therapy. Despite their limitations, they raise the possibility that hypovitaminosis D may underlie at least some of the increased risk for progression of CKD in racial/ethnic minorities in the United States. Given the potential for significant benefits, randomized, controlled trials are needed to determine if correction of hypovitaminosis D will reduce the racial disparities in CKD – to reduce the risk for both the progression to ESRD, and cardiovascular risk. It is also important to determine if hypovitaminosis D is an operative mechanism for progression of CKD in disadvantaged populations in other parts of the world.
Neighborhood, poverty, and kidney disease
Socio-cultural and economic factors increase vulnerability to several diseases, including CKD, by increasing the stressors both for the community in which an individual resides, and for the individual her/himself [14]. Several studies from different parts of the world have demonstrated the complex interplay between socio-economic status and risk for CKD, as well as the complex interplay of race and poverty on the incidence of ESRD.
In an analysis of 34,767 new dialysis patients in three southern states in the United States over a 5-year period, there was a progressively higher incidence of ESRD in census tracts with increasing proportions of individuals with income below the poverty level [15]. A significant interaction was noted between race, and poverty such that neighborhood was associated with a greater increase in the incidence of ESRD in Blacks than in Whites [15]. The relationship between neighborhood economic deprivation and incidence of ESRD has also been shown in other parts of the world, such as Australia and the United Kingdom [16, 17]. The effect of socio-economic status also appears to be greater on ESRD secondary to diseases like diabetes and hypertension that are amenable to individual, community, and healthcare intervention, when compared to diseases such as autosomal dominant polycystic kidney disease [18].
Individual socio-economic deprivation is also associated with a higher prevalence of lower levels of glomerular filtration in both Sweden, and United Kingdom – two societies that offer universal health insurance [19, 20]. Finally, life-time social position may be more important than socio-economic status at a given point in time in determining an individual’s risk for CKD [21].
Conclusions
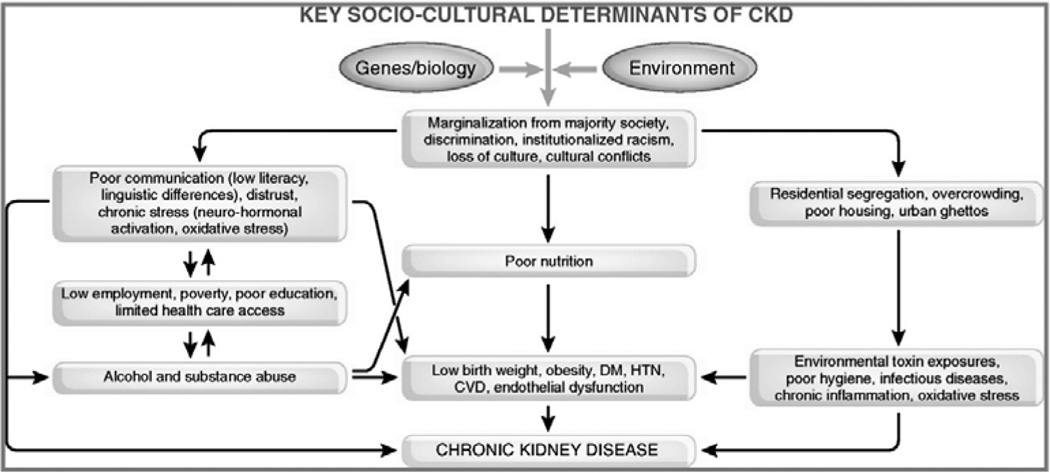
Disadvantaged populations have a higher risk for the development and progression of CKD, and have a higher risk for death in early stages of CKD. This disparity in health outcomes represents a complex interplay of biology and sociology (Figure 2) [22]. Understanding these factors will allow us to develop interventions that could lead to a reduction in the societal burden of CKD.
Figure 2.
Effect of sociology on the biology of chronic kidney disease. Reproduced, with permission from reference [22].
References
- 1.United States Renal Data System. US Department of Public Health and Human Services, National Institutes of Health. Bethesda: Public Health Service; 2008. [Google Scholar]
- 2.Coresh J, Astor BC, Greene T, et al. Prevalence of chronic kidney disease and decreased kidney function in the adult US population: Third National Health and Nutrition Examination Survey. Am J Kidney Dis. 2003;41:1–12. doi: 10.1053/ajkd.2003.50007. [DOI] [PubMed] [Google Scholar]
- 3.Mehrotra R, Kermah D, Fried LF, et al. Racial differences in mortality among those with CKD. J Am Soc Nephrol. 2008;19:1403–1410. doi: 10.1681/ASN.2007070747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Holick MF. Vitamin D deficiency. N Engl J Med. 2007;357:266–281. doi: 10.1056/NEJMra070553. [DOI] [PubMed] [Google Scholar]
- 5.Artaza JN, Mehrotra R, Norris KC. Vitamin D and the cardiovascular system. Clin JAmSoc Nephrol. 2009 doi: 10.2215/CJN.02260409. (in press) [DOI] [PubMed] [Google Scholar]
- 6.Zadshir A, Tareen N, Pan D, et al. The prevalence of hypovitaminosis D among US adults: data from the NHANES III. Ethn Dis. 2005;15 5-97-101. [PubMed] [Google Scholar]
- 7.Harinarayan CV, Ramalakshmi T, Prasad UV, et al. Vitamin D status in Andhra Pradesh: a population based study. Indian J Med Res. 2008;127:211–218. [PubMed] [Google Scholar]
- 8.Mehrotra R, Kermah D, Budoff M, et al. Hypovitaminosis D and chronic kidney disease. Clin J Am Soc Nephrol. 2008;3:1144–1151. doi: 10.2215/CJN.05781207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mehrotra R, Kermah D, Salusky I, Wolf M, Thadhani R, Chiu YW, Martins D, Adler SA, Norris K. Chronic kidney disease, hypovitaminosis D, and mortality in the United States. Kidney Int. 2009;76:977–983. doi: 10.1038/ki.2009.288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tian J, Liu Y, Williams LA, et al. Potential role of active vitamin D in retarding the progression of chronic kidney disease. Nephrol Dial Transplant. 2007;22:321–328. doi: 10.1093/ndt/gfl595. [DOI] [PubMed] [Google Scholar]
- 11.Agarwal R, Acharya M, Tian J, et al. Antiproteinuric effect of oral paricalcitol in chronic kidney disease. Kidney Int. 2005;68:2823–2828. doi: 10.1111/j.1523-1755.2005.00755.x. [DOI] [PubMed] [Google Scholar]
- 12.Alborzi P, Patel NA, Peterson C, et al. Paricalcitol reduces albuminuria and inflammation in chronic kidney disease: a randomized double-blind pilot trial. Hypertension. 2008;52:249–255. doi: 10.1161/HYPERTENSIONAHA.108.113159. [DOI] [PubMed] [Google Scholar]
- 13.Szeto CC, Chow KM, Kwan BC, et al. Oral calcitriol for the treatment of persistent proteinuria in immunoglobulin A nephropathy: an uncontrolled trial. Am J Kidney Dis. 2008;51:724–731. doi: 10.1053/j.ajkd.2007.12.038. [DOI] [PubMed] [Google Scholar]
- 14.Gee GC, Payne-Sturges DC. Environmental health disparities: a framework integrating psychosocial and environmental concepts. Environ Health Perspect. 2004;112:1645–1653. doi: 10.1289/ehp.7074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Volkova N, McClellan W, Klein M, et al. Neighborhood poverty and racial differences in ESRD incidence. J Am Soc Nephrol. 2008;19:356–364. doi: 10.1681/ASN.2006080934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cass A, Cunningham J, Wang Z, et al. Social disadvantage and variation in the incidence of end-stage renal disease in Australian capital cities. Aust N Z J Public Health. 2001;25:322–326. doi: 10.1111/j.1467-842x.2001.tb00587.x. [DOI] [PubMed] [Google Scholar]
- 17.Maheswaran R, Pearson T, Jordan H, et al. Socioeconomic deprivation, travel distance, location of service, and uptake of breast cancer screening in North Derbyshire, UK. J Epidemiol Community Health. 2006;60:208–212. doi: 10.1136/jech.200X.038398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ward MM. Socioeconomic status and the incidence of ESRD. Am J Kidney Dis. 2008;51:563–572. doi: 10.1053/j.ajkd.2007.11.023. [DOI] [PubMed] [Google Scholar]
- 19.Fored CM, Ejerblad E, Fryzek JP, et al. Socio-economic status and chronic renal failure: a population-based case-control study in Sweden. Nephrol Dial Transplant. 2003;18:82–88. doi: 10.1093/ndt/18.1.82. [DOI] [PubMed] [Google Scholar]
- 20.Bello AK, Peters J, Rigby J, et al. Socioeconomic status and chronic kidney disease at presentation to a renal service in the United Kingdom. Clin J Am Soc Nephrol. 2008;3:1316–1323. doi: 10.2215/CJN.00680208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shoham DA, Vupputuri S, Kaufman JS, et al. Kidney disease and the cumulative burden of life course socioeconomic conditions: the Atherosclerosis Risk in Communities (ARIC) study. Soc Sci Med. 2008;67:1311–1320. doi: 10.1016/j.socscimed.2008.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Norris KC, Agodoa LY. Unraveling the racial disparities associated with kidney disease. Kidney Int. 2005;68:914–924. doi: 10.1111/j.1523-1755.2005.00485.x. [DOI] [PubMed] [Google Scholar]