Abstract
Background
Case control studies that randomly assign patients with diagnosis of acute appendicitis to either surgical or non-surgical treatment yield a relapse rate of approximately 14% at one year. It would be useful to know the relapse rate of patients who have, instead, been selected for a given treatment based on a thorough clinical evaluation, including physical examination and laboratory results (Alvarado Score) as well as radiological exams if needed or deemed helpful. If this clinical evaluation is useful, the investigators would expect patient selection to be better than chance, and relapse rate to be lower than 14%. Once the investigators have established the utility of this evaluation, the investigators can begin to identify those components that have predictive value (such as blood analysis, or US/CT findings). This is the first step toward developing an accurate diagnostic-therapeutic algorithm which will avoid risks and costs of needless surgery.
Methods/design
This will be a single-cohort prospective observational study. It will not interfere with the usual pathway, consisting of clinical examination in the Emergency Department (ED) and execution of the following exams at the physician's discretion: full blood count with differential, C reactive protein, abdominal ultrasound, abdominal CT. Patients admitted to an ED with lower abdominal pain and suspicion of acute appendicitis and not needing immediate surgery, are requested by informed consent to undergo observation and non operative treatment with antibiotic therapy (Amoxicillin and Clavulanic Acid). The patients by protocol should not have received any previous antibiotic treatment during the same clinical episode. Patients not undergoing surgery will be physically examined 5 days later. Further follow-up will be conducted at 7, 15 days, 6 months and 12 months. The study will conform to clinical practice guidelines and will follow the recommendations of the Declaration of Helsinki. The protocol was approved on November 2009 by Maggiore Hospital Ethical Review Board (ID CE09079).
Trial Registration
ClinicalTrials.gov identifier: NCT01096927.
Keywords: Lower abdominal Pain, right iliac fossa pain, acute appendicitis, antibiotic therapy, conservative Management, appendectomy, recurrence, length of hospital stay, sick leave time, short and long Term abdominal pain evaluation, study protocol, case control study
Article summary
Article focus
Acute appendicitis can have severe complications including perforation and generalised peritonitis.
The appendix is found to be free of disease in 15–30% of appendectomies.
As surgery carries various risks, conservative non-surgical treatment with antibiotics for suspected appendix inflammation may avoid needless surgery, in particular as the relapse rate is low and the rate of complications is similar.
Key messages
Case control studies that randomly assign patients with acute appendicitis to either surgical or non-surgical treatment show a relapse rate of approximately 14% at 1 year.
The relapse rate of patients who are treated based on a thorough clinical evaluation should be below 14%.
Once factors predictive of outcome and/or the need of surgery are identified, an accurate diagnostic-therapeutic algorithm which will help avoid the risks and costs of needless surgery can be developed.
Strengths and limitations of this study
This non-randomised controlled study will evaluate the effectiveness and short and long term outcomes of non-operative antibiotic treatment of acute appendicitis.
Amoxicillin and clavulanic acid are common and easily managed low cost drugs, available both for intravenous and oral use.
Better analysis of clinical data might lead to better decision-making in patients with right iliac fossa pain and suspected acute appendicitis.
The study also aims to evaluate the Alvarado score, which is used to diagnose acute appendicitis and discriminate patients needing immediate surgery from patients who may safely undergo observation and antibiotic treatment.
A large sample of patients undergoing non-operative antibiotic treatment will allow a statistically powerful evaluation of safety, efficacy and cost.
An additional objective is to identify clinical, laboratory and imaging findings that are predictive of failure of conservative treatment and/or relapse of appendicitis and need for appendectomy within 1 year.
As efficacy can not be reliably determined in the absence of a control group, a case series observation determining ‘efficacy’ has limited value.
The Alvarado score is used to separate those with acute appendicitis from those with similar symptoms but no appendicitis and there is no evidence that this score can identify those who would benefit from antibiotic treatment.
Background
Acute appendicitis is one of the most common urgent conditions seen in general surgery practice. Complications can be severe and include perforation and generalised peritonitis. Traditionally, surgical appendectomy has been the primary treatment, even in cases of unconfirmed diagnosis, given the low incidence of major complications. However, in 15–30% of cases the appendix is found to be free of disease upon resection.1 2 As appendectomy is associated with surgical wound infection, intestinal obstruction due to adhesions, pneumonia, and tubal infertility in females, the possibility of using conservative treatment merits investigation.
Non-operative treatment of a suspected appendicitis has safety implications. Delaying surgery may increase the risk of perforated appendicitis, intra-abdominal abscesses, and localised or diffuse peritonitis before surgery and wound infection, increased risk of adhesions and subsequent adhesive small bowel obstruction (ASBO) and infertility after surgery. Anaesthesia carries its own risks, and there can also be intraoperative (vascular lesions, enterotomies, urinary tract lesions, etc), early surgical postoperative (haematoma/bleeding, colonic fistula, surgical site infection (SSI), intra-abdominal abscess, adhesions and ileus/obstruction) with subsequent re-operation, late surgical postoperative (adhesions and subsequent ASBO and tubal infertility, incisional hernias) and general postoperative complications.
Surgery may be associated with a longer hospital stay and higher costs compared with NOM with antibiotics, but delayed treatment and a perforated appendix may worsen morbidity, duration of sick leave and costs. However, NOM with antibiotics may be a cost-effective alternative to surgery in a large percentage of patients without increasing the risk, and may reduce hospital stay and costs in both developed and third world countries.
There is considerable debate regarding the utility of conservative treatment compared with surgical treatment in some cases of acute appendicitis, as few studies have addressed this issue to date.3–5 If conservative treatment is to be considered, it will be very importance to make an accurate diagnosis and assessment in every patient in order to select the most appropriate treatment.
The idea that appendicitis may resolve spontaneously is not new. In 1908 Alfred Stengel wrote: “Treated in a purely medical or tentative manner, the great majority of patients with appendicitis recover”.6
Restrained indications with few negative appendectomies are associated with a low incidence of diagnosed non-perforated appendicitis and a secondary high proportion of perforated appendicitis, but no increase in the incidence of perforations.7 This suggests that appendicitis in a significant number of patients may resolve undiagnosed. Resolving appendicitis may also be indicated by a history of recurrent appendicitis, which can be found in up to 6.5% of patients operated on for appendicitis.8
When perforation results from delayed treatment, the associated increase in morbidity and mortality must be weighed against the risks of a negative appendectomy. The excess mortality associated with non-perforated appendicitis and with negative appendectomy with a discharge diagnosis of non-specific abdominal pain, suggests that appendectomy itself carries risks. The decrease in mortality that may be achieved by one prevented perforation is therefore negated by each negative appendectomy.9 10
Other reports indicate that immediate appendectomy can be avoided for at least 24 h without increasing morbidity if antibiotics are administered.11 Other authors suggest that appendectomy may not be necessary for the majority of patients with acute uncomplicated appendicitis, as the condition resolves spontaneously in many patients and in others may be treatable with antibiotics alone.12 This approach has many advantages, including high success and low recurrence rates, reduced morbidity and mortality, less pain, shorter hospitalisation and sick leave, and reduced costs.13
In light of this, routine interval appendectomy after initial successful conservative treatment does not seem justified and should be abandoned. In fact, traditional interval appendectomy may prevent recurrent appendicitis in only 6.7% of patients after conservative treatment of acute appendicitis with an appendiceal mass. Thus 93.3% would have an unnecessary appendectomy.14
A recent meta-analysis comparing conservative treatment with acute appendectomy for complicated appendicitis (abscess or phlegmon) and including 1572 patients (847 patients received conservative treatment and 725 had acute appendectomy) showed that conservative treatment was associated with significantly fewer overall complications, wound infections, abdominal/pelvic abscesses, ileus/bowel obstructions and re-operations. Furthermore, there were significantly fewer overall complications in conservative treatment groups during sensitivity analysis of studies including only paediatric patients, high-quality studies, more recent studies, and studies with larger groups of patients.15
In particular, a randomised clinical trial (RCT) of antibiotic therapy versus appendicectomy as primary treatment of acute appendicitis in unselected patients showed that treatment efficacy was 90.8% for antibiotic therapy and 89.2% for surgery. In this trial minor complications were similar between the groups, while major complications were threefold higher in patients who had an appendicectomy (p<0.050). In total, 2.9% of the operated patients underwent a second operation, 3% of them developed abscesses, 2.4% postoperative small bowel obstruction (SBO), 1.8% wound rupture or wound hernia, 0.6% pulmonary embolism and 0.6% postoperative cardiac complications, and 1.2% underwent subsequent ileocaecal resection. Wound infection occurred in 7.6% and 1.2% of the patients had anaesthesia-related problems.16
In another RCT comparing appendectomy with antibiotic treatment in acute appendicitis, the complication rate in the surgery group was 14% (17/124) and was mainly due to wound infections. In the surgery group, time in hospital, sick leave taken and time lost from work were respectively 2.6, 6.0 and 10.1 days.5
The cumulated risk of surgically treated SBO after appendicectomy was 0.41% after 4 weeks, 0.63% after 1 year and 1.30% after 30 years of follow-up, compared with 0.003% at 1 year and 0.21% after 30 years of follow-up among non-operated controls, with perforated appendicitis, negative appendicectomy and high age risk factors for developing subsequent SBO.17
A further paper reviewing 1777 patients who underwent appendectomy for acute appendicitis, showed the overall SBO rate to be 2.8% over an average 4.1-year follow-up period or 0.0069 cases per person-year.18
The laparoscopic approach also carries risks, including intraoperative complications ranging from 3.1% to 0.7%, surgical postoperative complications ranging from 6.1% to 1.9%, general postoperative complications ranging from 4.9% to 1.5% and rates of re-operations ranging from 3.4% to 0.7%.19
A comparison of 3025 open versus 14 174 laparoscopic appendectomies showed SSI occurred in 4% and 1.2%, deep incisional wound infection in 1.2% and 0.2%, wound disruption in 0.3% and 0.1%, organ space infection in 1.3% and 1.7%, pneumonia in 0.6% and 0.3%, renal failure in 0.2% and 0.3%, urinary tract infection (UTI) in 0.5% and 0.4%, deep vein thrombosis in 0.4% and 0.1%, sepsis in 1.4% and 1.0% and septic shock 0.3% and 0.1%, respectively. Appendectomy may also be associated with mortality (0.3% for open and 0.1% for laparoscopic).20
Another large population based appraisal including 32 683 patients reported morbidity and mortality for both open and laparoscopic appendectomy.21 Overall morbidity was 8.84% and 4.46%, respectively, serious morbidity 4.23% and 2.58%, SSI 6.65% and 3.26%, serious morbidity/mortality 4.26% and 2.60%, and mortality 0.13% and 0.07%. Superficial SSI incidence was, respectively, 3.89% and 1.26%, deep incisional SSI 0.99% and 0.24%, organ space SSI 1.72% and 1.79%, wound disruption 0.45% and 0.06%, pneumonia 0.43% and 0.24%, pulmonary embolism 0.08% in both open and lap groups, sepsis or septic shock 2.16% and 1.15%, bleeding 0.01 and 0.04% and UTIs 0.36% and 0.37%.
The long term follow-up of a RCT of open versus laparoscopic appendicectomy showed a 42.3% incidence of overall complications in the open surgery group versus 12.8% in the laparoscopic group, with wound-related complications as high as 30.77% versus 4.2%.22 After a mean follow-up of more than 9 years, 5.7% and 6.4% of patients, respectively, had adhesions or adhesion-related symptoms and 0.2% underwent another operation for adhesions. The risk of SBO after open appendectomy is between 0.33 and 1.51%.
Finally, it has been demonstrated that a history of perforated appendix in childhood does not seem to have long term negative consequences on female fertility. This may have important implications for the management of young women with suspected appendicitis as the liberal attitude to surgical exploration with a subsequently high rate of removal of a normal appendix is no longer justified by a perceived increased risk of infertility after perforation.23
In addition, a liberal attitude to exploration among patients with suspected appendicitis does not prevent perforations.24
Hansson et al16 conducted a RCT investigating the efficacy of conservative treatment compared to surgery for acute appendicitis. They reported that conservative treatment with antibiotics was efficacious in 91% of cases, with a 14% relapse rate at 12-month follow-up. One third of relapses occurred within the first 10 days of hospital discharge, while most of the remaining two thirds occurred between 3 and 16 months following discharge. The rates of minor complications such as diarrhoea, vomiting and nosocomial infections were similar among patients treated conservatively and those treated surgically. The incidence of major complications such as appendiceal abscess, paralytic ileus and pulmonary embolism, however, was significantly higher in those treated surgically (p<0.05).
A recent prospective randomised study conducted by Malik A and colleagues1 compared antibiotic therapy to appendectomy in acute appendicitis. The authors reported that conservative treatment was not only safe and efficacious, but also caused the patients less pain than surgery, reducing the need for analgesia (p<0.001). Ten per cent of conservatively treated patients relapsed within 12 months of discharge.
A multicentre randomised trial conducted in Sweden16 yielded similar results: the rate of relapse in antibiotic treated patients was 14% at 1 year after discharge. Interestingly, this was equal to the rate of post-operative complications in patients treated surgically.
Based on these reports, with the diagnostic accuracy of acute appendicitis being as high as 71%–87% with a combination of modern preoperative investigations,25 conservative treatment with antibiotics seems to valid for cases of suspected or probable/proven acute appendicitis. Relapse rate is low and complications are no higher than the rate of surgical complications.
Rationale
Case control studies that randomly assign patients with suspected acute appendicitis to either surgical or non-surgical treatment show a relapse rate of approximately 14% at 1 year. It would, therefore, be useful to determine the relapse rate of patients treated according to the results of a thorough clinical evaluation, including physical examination and laboratory results (all characteristics used to determine the Alvarado score26) as well as radiological evaluation. Imaging such as ultrasound may be helpful but is not usually necessary in diagnosing acute appendicitis and deciding its further treatment (operative or non-operative). As the Alvarado and AIR27 scores are based only on clinical and laboratory characteristics, only clinical signs and symptoms and laboratory values are routinely evaluated in patient with suspected acute appendicitis. If this clinical evaluation is effective, we would expect patient selection to be better than chance, and the relapse rate to be below 14%. Once we have established the utility of this evaluation, we can begin to identify those components that have predictive value (such as blood chemistry analysis or CT findings). This would be a first step towards developing an accurate diagnostic–therapeutic algorithm which could be used to avoid the risks and costs of needless surgery.
Much research into the cause of diseases relies on cohort, case–control or cross-sectional studies. Observational studies also have a role in research into the benefits and harms of medical interventions. Randomised trials cannot answer all important questions about a given intervention. For example, observational studies are more suitable for detecting rare or late adverse effects of treatments, and are more likely to provide an indication of what is achieved in daily medical practice.28
Study description
This will be a single-cohort prospective observational study. Patients presenting to the emergency department will undergo some or all of the following tests: complete blood count with differential, C reactive protein, abdominal ultrasound and abdominal CT scan. Patients with lower abdominal pain and suspicion of acute appendicitis not requiring immediate surgery, will be requested to undergo observation and non-operative treatment with antibiotic therapy (amoxicillin and clavulanic acid) and provide informed consent.
Suspected acute appendicitis is defined as patient presenting with right iliac fossa (RIF) pain AND absence of a definite alternative diagnosis, either of a gastrointestinal disease (such as inflammatory bowel disease (IBD), irritable bowel syndrome, colitis, etc) or urinary tract disease (such as UTI, renal colic, urinary tract stones, etc) or an obstetric-gynaecological cause (such as pregnancy, pelvic inflammatory disease, ovulation, etc).
Patients needing immediate surgery are defined as those with diffuse peritonitis and/or signs of sepsis, as well as patients with clinico-radiological (US or CT scan) evidence of an intra-abdominal collection/abscess.
The patients by protocol should not have received any previous antibiotic treatment during the same clinical episode. Patients not undergoing surgery will be physically examined 5 days later. If their condition has not improved or worsened, they will be admitted for surgical appendectomy. If they have improved, they will be given information about the study and invited to participate, and asked to sign an informed consent form for further follow-up. If the patient is under the age of 18 years, consent will be obtained from a parent or other legal guardian.
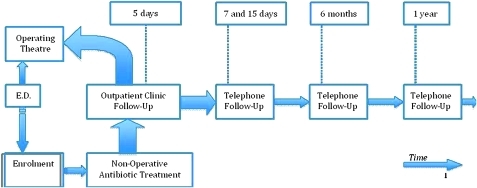
Telephone (or email) follow-up will be conducted at 7 and 15 days, 6 months and 1 year (see figure 1). In the case of patients under the age of 18 years, the phone interview will be conducted with a parent or legal guardian. The patient will be asked if he/she has undergone surgery since the first visit (5 days after presenting to the emergency department). If not, the patient will be asked:
Figure 1.
Non-operative treatment for acute appendicitis: patient outcomes and follow-up in a single-cohort prospective observational study. Flow diagram of the study according to CONSORT 2010.
Has your illness improved, stayed the same, or worsened since its onset?
Have you undergone any further tests or had additional doctor's visits for your illness?
After your initial emergency department visit, how long did it take to return to your normal activities (physical activity, work, etc)?
Statistical analysis
Data will be entered into a spreadsheet using Epi-Info (v 6.04d, Centers for Disease Control and Prevention) and analysed using SPSS software (v 15.0, SPSS). Descriptive statistics will be reported as mean and SD for normally distributed variables and median and IQR for variables not normally distributed. The χ2 analysis of variance will be used to compare differences for categorical variables. ORs with 95% CIs will be calculated. Student's paired sample t test will be used to compare mean differences between continuous variables. The Mann–Whitney U test will be used to compare non-parametric continuous variables (eg, age, white cell count). Statistical significance will be defined as p<0.05. Variables with clinically relevant cut-off points will be dichotomised.
In order to determine independent predictors of the short and long term efficacy of antibiotic treatment (in terms of failure rate of conservative treatment, recurrence rate of clinical episodes of acute appendicitis and definite improvement without need for surgery within 1 year of follow-up) in the general study population, numerous variables will be assessed including demographic characteristics (sex, age), clinical features (past medical history, gynaecological status, IBD history), clinical status (body temperature), laboratory studies (white cell count, neutrophils count, C reactive protein), whether or not empiric antibiotics were previously administered, and time to administration and duration of treatment with amoxicillin and clavulanic acid.
Univariate analyses will be used to identify which variables with a p value less than 0.20 should be included in the multivariate models. Stepwise backward logistic regression will be used to determine whether these covariates are independent predictors of treatment efficacy; covariates will be eliminated when p values are greater than or equal to 0.05.
The same methods will be used to assess predictors of abdominal pain after discharge, length of hospital stay, number of outpatient clinic follow-up appointments and sick leave.
Finally, cost analysis will be carried out on antibiotic course, length of hospital stay, outpatient clinic follow-up appointments and sick leave days.
Study objectives
Main objective
The main objective is to evaluate the outcome of patients treated conservatively and assess the reliability of the initial clinical evaluation in predicting which conservatively-treated patients should have been treated surgically.
The primary outcomes are:
Short term efficacy of antibiotic treatment: failure of conservative treatment with 7 days of amoxicillin and clavulanic acid therapy, defined as readmission due to lack of clinical improvement and/or worsening abdominal pain and/or localised/diffuse peritonitis.
Long term efficacy of antibiotic treatment: efficacy of antibiotic therapy for acute appendicitis defined as incidence of recurrences of clinical episodes of appendicitis up to follow-up at 1 year (at 7 days, 15 days, 6 months, 1 year).
Long term efficacy of antibiotic treatment (no need for surgery): efficacy of antibiotic therapy for acute appendicitis defined as definite improvement without the need for surgery up to follow-up at 1 year (at 7 days, 15 days, 6 months, 1 year).
Safety of antibiotic treatment: major side effects/drug- or treatment-related complications (ie, allergy or other treatment-related complications such as abscess formation).
The secondary outcomes are:
Minor complications: minor side effects/drug- or treatment-related complications (ie, bloating, diarrhoea, flatulence, headache, heartburn, nausea and vomiting) (at 7 days, 15 days).
Abdominal pain after discharge: assessment of abdominal pain/discomfort evaluated by means of a numerical rating scale (at 7 days, 15 days).
Length of hospital stay: length of clinical observation as an inpatient for non-operated patients.
Outpatient clinic follow-up: number of follow-up appointments scheduled in the outpatient clinic.
Sick leave: number of days of sick leave needed by the patient.
Cost analysis: analysis of the costs of antibiotics, length of hospital stay, outpatient clinic follow-up appointments and sick leave days.
Secondary objective
An additional objective is to identify clinical, laboratory and imaging findings that are predictive of failure of conservative treatment and/or relapse of appendicitis and need for appendectomy within 1 year.
Study design
This is a single cohort prospective non-randomised observational study. No experimental interventions or treatments will be employed beyond routine clinical care.
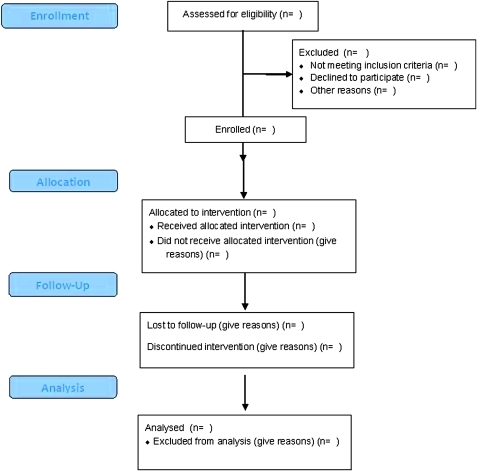
A flowchart of the study is given in figure 2 and a tempogram showing the study steps is given in figure 1.
Figure 2.
CONSORT 2010 flow diagram.
Estimated enrolment and study dates
The estimated sample size of the study population is 160 patients in 1 year, which is the average number of patients assessed annually by the emergency surgical team in our hospital for suspected acute appendicitis, conservatively treated with antibiotics and observed. The crude incidence of acute appendicitis is 86 per 100 000 inhabitants per year, varying between 74 and 96 per 100 000 during a 5-year period in Norway in an urban and rural catchment area with 265 000 inhabitants. A peak incidence of acute appendicitis was found in patients 13–40 years of age, with more males than females in this age group (ratio 1.34:1.00). Among young children and the elderly, significantly (p=0.002) more females had acute appendicitis.29 A further paper reported the incidence of appendicitis to be about 100 per 100 000 person-years in Europe/America.30
The appendectomy rate continues to decrease, although the incidence of appendicitis is now nearly stable and the incidence of perforated appendicitis has not changed (approximately 20 per 100 000 person-years) over the last 30 years. These data suggest the rate of surgery for acute appendicitis is decreasing in an attempt to avoid unnecessary appendectomies.
The catchment population of Bologna is half a million people. Maggiore Hospital is the largest hospital in the city and covers the northern area. The numbers of patients aged over 14 years admitted to the emergency department with right iliac fossa pain and/or suspected appendicitis have been monitored for the last 3 years and range from 328 to 443 cases/year.
The study start date is January 2010, the estimated study completion date is December 2010 and the estimated primary date of completion of follow-up is December 2011 (the final data collection date for all primary outcome measures and 1 year follow-up).
Inclusion and exclusion criteria
Any patient, male or female, above the age of 14 years (non-paediatric), who returns for a follow-up visit 5 days after an emergency department visit and consents to participation between 1 January 2010 and 31 December 2010 will be eligible for inclusion in the study.
Specifically, the inclusion criteria are:
Age >14 years
Lower/right iliac fossa abdominal pain
-
Clinical suspicion of acute appendicitis, that is:
–Alvarado score 5–6 (equivocal for acute appendicitis)
–Alvarado score 7–8 (probable appendicitis)
–Alvarado score 9–10 (highly probable appendicitis)
Informed consent (patient or legal representative).
Exclusion criteria are:
Diffuse peritonitis
Antibiotic (penicillin) documented allergy
Ongoing previously commenced antibiotic therapy
Previous appendectomy
Positive pregnancy test
IBD history or suspicion of IBD reoccurrence.
Follow-up assessment will be by telephone interview (or email). No interviewer training will be necessary as the surgeons conducting the study will also conduct the phone interviews. Informed consent will be obtained from the patient (or guardian) before enrolling him/her in the study. Confidentiality of personal and health information will be guaranteed and will only be accessible to the surgeons responsible for the study. Members of the project management team are as follows: study chair: Gregorio Tugnoli, MD; principal investigators: Salomone Di Saverio MD, Eleonora Giorgini MD, Nicola Antonacci, MD, Andrea Biscardi, MD, Nicola Clemente MD and Silvia Villani MD. The over-seeing authority is the Bologna Local Health District Ethics Committee (approval number CE 09079). The study follows the recommendations of the Declaration of Helsinki31 and conforms with the accepted best practice guidelines of the STROBE statement for observational studies (cohort, case–control or cross-sectional designs)32 (table 1).
Table 1.
STROBE 2007 (v4) Statement—Checklist of items that should be included in reports of case–control studies
| Item # | Recommendation | Reported on page # | |
| Title and abstract | 1 | (a) Indicate the study's design with a commonly used term in the title or the abstract | 2–3 |
| (b) Provide in the abstract an informative and balanced summary of what was done what was found | 2–3 | ||
| Introduction | |||
| Background/rationale | 2 | Explain the scientific background and rationale for the investigation being reported | 5–10 |
| Objectives | 3 | State specific objectives, including any prespecified hypotheses | 12–14 |
| Methods | |||
| Study design | 4 | Present key elements of study design early in the paper | 14 |
| Setting | 5 | Describe the setting, locations, and relevant dates, including periods of recruitment, exposure, follow-up, and data collection | 10–11/14–16 |
| Participants | 6 | (a) Give the eligibility criteria, and the sources and methods of case ascertainment and control selection. Give the rationale for the choice of cases and controls | 15 |
| (b) For matched studies, give matching criteria and the number of controls per case | |||
| Variables | 7 | Clearly define all outcomes, exposures, predictors, potential confounders, and effect modifiers. Give diagnostic criteria, if applicable | 10–11/12–14 |
| Data sources/measurement | 8* | For each variable of interest, give sources of data and details of methods of assessment (measurement). Describe comparability of assessment methods if there is more than one group | 11–12 |
| Bias | 9 | Describe any efforts to address potential sources of bias | 4 |
| Study size | 10 | Explain how the study size was arrived at | 14 |
| Quantitative variables | 11 | Explain how quantitative variables were handled in the analyses. If applicable, describe which groupings were chosen and why | 11–12 |
| Statistical methods | 12 | (a) Describe all statistical methods, including those used to control for confounding | 11–12 |
| (b) Describe any methods used to examine subgroups and interactions | 11–12 | ||
| (c) Explain how missing data were addressed | 11–12 | ||
| (d) If applicable, explain how matching of cases and controls was addressed | 11–12 | ||
| (e) Describe any sensitivity analyses | 11–12 | ||
| Results | |||
| Participants | 13* | (a) Report numbers of individuals at each stage of study—for example, numbers potentially eligible, examined for eligibility, confirmed eligible, included in the study, completing follow-up, and analysed | Not yet applicable |
| (b) Give reasons for non-participation at each stage | Not yet applicable | ||
| (c) Consider use of a flow diagram | Not yet applicable | ||
| Descriptive data | 14* | (a) Give characteristics of study participants (eg, demographic, clinical, social) and information on exposures and potential confounders | Not yet applicable |
| (b) Indicate number of participants with missing data for each variable of interest | Not yet applicable | ||
| Outcome data | 15* | Report numbers in each exposure category, or summary measures of exposure | Not yet applicable |
| Main results | 16 | (a) Give unadjusted estimates and, if applicable, confounder-adjusted estimates and their precision (eg, 95% CI). Make clear which confounders were adjusted for and why they were included | Not yet applicable |
| (b) Report category boundaries when continuous variables were categorized | Not yet applicable | ||
| (c) If relevant, consider translating estimates of RR into absolute risk for a meaningful time period | Not yet applicable | ||
| Other analyses | 17 | Report other analyses done—for example, analyses of subgroups and interactions, and sensitivity analyses | Not yet applicable |
| Discussion | Not yet applicable | ||
| Key results | 18 | Summarise key results with reference to study objectives | Not yet applicable |
| Limitations | 19 | Discuss limitations of the study, taking into account sources of potential bias or imprecision. Discuss both direction and magnitude of any potential bias | Not yet applicable |
| Interpretation | 20 | Give a cautious overall interpretation of results considering objectives, limitations, multiplicity of analyses, results from similar studies, and other relevant evidence | Not yet applicable |
| Generalisability | 21 | Discuss the generalisability (external validity) of the study results | Not yet applicable |
| Other information | Not yet applicable | ||
| Funding | 22 | Give the source of funding and the role of the funders for the present study and, if applicable, for the original study on which the present article is based | 16 |
Give information separately for cases and controls in case-control studies and, if applicable, for exposed and unexposed groups in cohort and cross-sectional studies.
An Explanation and Elaboration article discusses each checklist item and gives methodological background and published examples of transparent reporting. The STROBE checklist is best used in conjunction with this article (freely available on the Web sites of PLoS Medicine at http://www.plosmedicine.org/, Annals of Internal Medicine at http://www.annals.org/, and Epidemiology at http://www.epidem.com/). Information on the STROBE Initiative is available at http://www.strobe-statement.org/.
Supplementary Material
Acknowledgments
The authors would like to acknowledge the head of the Department of Emergency of the Bologna Local Health District, Dr Giovanni Gordini MD, MPH for his support for the study and advice on public health policy. The authors would also like to acknowledge SIPAD (Società Italiana Patologia Apparato Digerente - Italian Society for Digestive Diseases) for acting as patron of the study.
Footnotes
To cite: Tugnoli G, Giorgini E, Biscardi A, et al. The NOTA study: non-operative treatment for acute appendicitis: prospective study on the efficacy and safety of antibiotic treatment (amoxicillin and clavulanic acid) in patients with right sided lower abdominal pain. BMJ Open 2011;1:e000006. doi:10.1136/bmjopen-2010-000006
Competing interests: None.
Contributors: GT, EG, SDS contributed to the conception and design of the study, acquisition of data, and analysis and interpretation of data. SDS drafted the manuscript and revised it critically for important intellectual content. GT, NA, EG and SDS conceived the study, participated in its design and coordination and helped draft the manuscript.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Malik AA, Bari SU. Conservative management of acute appendicitis. J Gastrointest Surg 2009;13:966–70 [DOI] [PubMed] [Google Scholar]
- 2.Deutsch AA, Shani N, Reiss R. Are some appendicectomies unnecessary? An analysis of 319 white appendices. J R Coll Surg Edinb 1983;28:35–40 [PubMed] [Google Scholar]
- 3.Pieper R, Kager L, Nasman P. Acute appendicitis: a clinical study of 1018 cases of emergency appendectomy. Acta Chir Scand 1982;148:51–62 [PubMed] [Google Scholar]
- 4.Eriksson S, Granstrom L. Randomized controlled trial of appendectomy versus antibiotic therapy for acute appendicitus. Br J Surg 1995;82:166–9 [DOI] [PubMed] [Google Scholar]
- 5.Styrud J, Eriksson S, Nilsson I, et al. Appendectomy versus antibiotic treatment in acute appendicitis. A prospective multicenter randomized controlled trial. World J Surg 2006;30:1033–7 [DOI] [PubMed] [Google Scholar]
- 6.Stengel A. Appendicitis. In: Osler W, McCrae T, eds. Modern Medicine, Vol V. Diseases of the Alimentary Tract. Philadelphia: Lea & Febiger; 1908 [Google Scholar]
- 7.Andersson RE. The natural history and traditional management of appendicitis revisited: spontaneous resolution and predominance of prehospital perforations imply that a correct diagnosis is more important than an early diagnosis. World J Surg 2007;31:86–92 [DOI] [PubMed] [Google Scholar]
- 8.Barber MD, McLaren J, Rainey JB. Recurrent appendicitis. Br J Surg 1997;84:110–12 [PubMed] [Google Scholar]
- 9.Blomqvist PG, Andersson RE, Granath F, et al. Mortality after appendectomy in Sweden, 1987–1996. Ann Surg 2001;233:455–60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Flum DR, Koepsell T. The clinical and economic correlates of misdiagnosed appendicitis: nationwide analysis. Arch Surg 2002;137:799–804; discussion 804. [DOI] [PubMed] [Google Scholar]
- 11.Shindoh J, Niwa H, Kawai K, et al. Predictive factors for negative outcomes in initial non-operative management of suspected appendicitis. J Gastrointest Surg 2010;14:309–14 [DOI] [PubMed] [Google Scholar]
- 12.Mason RJ. Surgery for appendicitis: is it necessary? Surg Infect (Larchmt) 2008;9:481–8 [DOI] [PubMed] [Google Scholar]
- 13.Sakorafas GH, Mastoraki A, Lappas C, et al. Conservative treatment of acute appendicitis: heresy or an effective and acceptable alternative to surgery? Eur J Gastroenterol Hepatol 2011;23:121–7 [DOI] [PubMed] [Google Scholar]
- 14.Tekin A, Kurtoğlu HC, Can I, et al. Routine interval appendectomy is unnecessary after conservative treatment of appendiceal mass. Colorectal Dis 2008;10:465–8 [DOI] [PubMed] [Google Scholar]
- 15.Simillis C, Symeonides P, Shorthouse AJ, et al. A meta-analysis comparing conservative treatment versus acute appendectomy for complicated appendicitis (abscess or phlegmon). Surgery 2010;147:818–29 [DOI] [PubMed] [Google Scholar]
- 16.Hansson J, Korner U, Khorram-Manesh A, et al. Randomized clinical trial of antibiotic therapy versus appendicectomy as primary treatment of acute appendicitis in unselected patients. Br J Surg 2009;96:473–81 [DOI] [PubMed] [Google Scholar]
- 17.Andersson RE. Small bowel obstruction after appendicectomy. Br J Surg 2001;88:1387–91 [DOI] [PubMed] [Google Scholar]
- 18.Leung TT, Dixon E, Gill M, et al. Bowel obstruction following appendectomy: what is the true incidence? Ann Surg 2009;250:51–3 [DOI] [PubMed] [Google Scholar]
- 19.Brügger L, Rosella L, Candinas D, et al. Improving outcomes after laparoscopic appendectomy: a population-based, 12-year trend analysis of 7446 patients. Ann Surg. Published Online First: 17 December 2010. doi: 10.1097/SLA.0b013e3181fc9d53 [DOI] [PubMed] [Google Scholar]
- 20.Page AJ, Pollock JD, Perez S, et al. Laparoscopic versus open appendectomy: an analysis of outcomes in 17,199 patients using ACS/NSQIP. J Gastrointest Surg 2010;14:1955–62 [DOI] [PubMed] [Google Scholar]
- 21.Ingraham AM, Cohen ME, Bilimoria KY, et al. Comparison of outcomes after laparoscopic versus open appendectomy for acute appendicitis at 222 ACS NSQIP hospitals. Surgery 2010;148:625–35; discussion 635–7. [DOI] [PubMed] [Google Scholar]
- 22.Kouhia ST, Heiskanen JT, Huttunen R, et al. Long-term follow-up of a randomized clinical trial of open versus laparoscopic appendicectomy. Br J Surg 2010;97:1395–400 [DOI] [PubMed] [Google Scholar]
- 23.Andersson R, Lambe M, Bergström R. Fertility patterns after appendicectomy: historical cohort study. BMJ 1999;318:963–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Andersson R, Hugander A, Thulin A, et al. Indications for operation in suspected appendicitis and incidence of perforation. BMJ 1994;308:107–10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Styrud J, Eriksson S, Segelman J, et al. Diagnostic accuracy in 2,351 patients undergoing appendicectomy for suspected acute appendicitis: a retrospective study 1986–1993. Dig Surg 1999;16:39–44 [DOI] [PubMed] [Google Scholar]
- 26.Alvarado A. A practical score for the early diagnosis of acute appendicitis. Ann Emerg Med 1986;15:557–64 [DOI] [PubMed] [Google Scholar]
- 27.Andersson M, Andersson RE. The appendicitis inflammatory response score: a tool for the diagnosis of acute appendicitis that outperforms the Alvarado score. World J Surg 2008;32:1843–9 [DOI] [PubMed] [Google Scholar]
- 28.Vandenbroucke JP, von Elm E, Altman DG, et al. Strengthening the Reporting of Observational Studies in Epidemiology (STROBE): explanation and elaboration. PLoS Med 2007;4:e297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Körner H, Söndenaa K, Söreide JA, et al. Incidence of acute nonperforated and perforated appendicitis: age-specific and sex-specific analysis. World J Surg 1997;21:313–17 [DOI] [PubMed] [Google Scholar]
- 30.Ohmann C, Franke C, Kraemer M, et al. [Status report on epidemiology of acute appendicitis] (In German). Chirurg 2002;73:769–76 [DOI] [PubMed] [Google Scholar]
- 31.World Medical Association Declaration Of Helsinki Ethical Principles for Medical Research Involving Human Subjects. 59th WMA General Assembly, Seoul, October 2008. World Medical Association DoH, 2008 [Google Scholar]
- 32.von Elm E, Altman DG, Egger M, et al. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. BMJ 2007;335:806–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.