Abstract
Background
Mild cognitive impairment (MCI) of an amnestic type is a common condition in older people and highly predictive of Alzheimer's disease (AD). To date, there is no clear consensus regarding the best antecedent biomarker to predict early conversion to AD.
Objective
The aim of the study is to demonstrate that 1H magnetic resonance spectroscopy (MRS) of the brain in MCI patients may predict early conversion to dementia within the 2-year period after baseline assessment.
Methods
A cohort of patients fulfilling the criteria of amnestic MCI were enrolled consecutively. At baseline the patients underwent neuropsychological examination, standard blood tests and APOE genotype. 1H-MRS (1.5 T) of the brain was carried out by exploring two areas: the posteromedial bilateral parietal lobe and left medial occipital lobe. The patients were followed up to detect conversion to probable AD according to the National Institute of Neurological and Communicative Disorders and Stroke and the Alzheimer's Disease and Related Disorders Association group criteria.
Results
After a 2-year follow-up, 27 (38%) patients converted to AD. The mean N-acetyl-aspartate/creatine (NAA/Cr) ratio in the posteromedial bilateral parietal cortex was 1.38 in converters versus 1.49 in non-converters (p<0.0001). An NAA/Cr ratio equal to or lower than 1.43 in this area predicted conversion to probable AD at 74.1% sensitivity and 83.7% specificity (area under the curve: 0.84; 95% CI 0.73 to 0.92). The cross-validated accuracy of classification was 82%, which reaches 85% when the APOE4 genotype and memory test are included in the analysis. In the left medial occipital lobe, the predictive value was somewhat lower with 85.2% sensitivity and 61.4% specificity (area under the curve: 0.8; 95% CI 0.69 to 0.89). Neither the APOE4 genotype nor leuco-araiosis was predictive of conversion to dementia.
Conclusion
MRS is a valuable biomarker to predict early conversion to dementia in patients with amnestic MCI.
Keywords: Mild cognitive impairment, magnetic resonance spectroscopy, adult neurology, dementia, magnetic resonance imaging
Article summary
Article focus
Amnestic mild cognitive impairment (MCI) is a common condition at increased risk of conversion to Alzheimer's disease (AD). There is no clear consensus regarding the ideal antecedent biomarker to predict early conversion.
There are a few longitudinal studies using brain magnetic resonance spectroscopy (MRS) as a predictor of conversion to dementia.
We hypothesise that MRS in MCI may identify patients at risk of early conversion to dementia.
Key messages
MRS is a reliable biomarker of AD and predicts early conversion to dementia in MCI.
Both posteromedial parietal and occipital regions were predictive of conversion, but the parietal region was better in terms of accuracy of classification.
Neither the APOE4 genotype nor leuco-araiosis was predictive of conversion.
Strengths and limitations
This is a longitudinal study, which is a non-invasive, reproducible and widely available tool. However, this technique is not free of artefacts limiting the accuracy of metabolite levels.
This study is based on early predictions (2 years from baseline). In the longer term, it is likely that all patients with objective memory impairment will convert to dementia.
Amnestic MCI is a common condition in older people mainly characterised by memory loss. Although there may be other subtle inefficiencies, the general cognitive function and daily living activities are preserved.1 The meaning of this concept varies across the scientific community: a transitional state between normality and dementia,1 an early phase of AD2 or an unstable condition that may evolve to dementia or may even revert to normality.3 Regardless of conceptualisation, most patients convert to AD over time, but some of them remain non-demented. A meta-analysis from clinical trials in MCI has revealed that treatment with cholinesterase inhibitors does not delay the onset of AD.4 5 However, in patients with mild to moderate AD, cholinesterase inhibitors can delay cognitive decline and deterioration in global health for at least 6 months.6 Given that it is not cost-effective to treat all MCI patients, we need a biomarker to predict conversion to dementia to start treatment as soon as possible in those at high risk.
Since brain pathology starts long before symptoms in AD, many studies have focused on antecedent biomarkers of AD to diagnose this disease in the early phase called MCI or even before. It is true that the atrophy of the medial temporal lobe structures, such as entorhinal cortex and hippocampus, has yielded encouraging results,7–16 and cerebrospinal fluid (CSF) biomarkers (τ and Aβ42 proteins) as well17–24 with large intra- and interindividual variations. So far, no standardised procedures have been established.
Studies with PET in MCI and AD show the areas involved in early phases. The posterior cingulate gyrus (PCG) is an area involved in memory and many times studied in MCI and AD. The patients with AD had a lower glucose metabolism than healthy controls in parietal, temporal, occipital, frontal and posterior cingulate cortices.25 FDDNP-PET (radiotracer binding to plaques and tangles)26 and FDG-PET27 findings can discriminate normality from MCI and AD, with the PCG being one of the most typically affected areas. In longitudinal studies, PET has yielded the highest accuracies to predict conversion to dementia, but most studies included small cohorts of MCI patients.28–31
Several cross-sectional studies with MRS found decreased N-acetyl-aspartate/creatine (NAA/Cr) ratios and increased myo-inositol/creatine (mI/Cr) ratios in the PCG of MCI and AD patients in comparison with controls,32–35 and in the occipital lobe of AD patients in comparison with controls36 37 and vascular dementia.38
Longitudinal studies with MRS are scarce. In a cohort with 53 MCI patients, the occipital NAA/Cr ratios, but not those of the hippocampus and mid-parietal lobe, were predictive of conversion to dementia with high accuracy.39 In another cohort of 119 MCI patients, the NAA/Cr ratios in the left occipital lobe were compared with those obtained in the posteromedial parietal cortex (PMPC), yielding similar predictive values, with the PMPC values being slightly more significant than those observed in the occipital lobe. This study included amnestic and multiple-domain MCI. At baseline, a significant correlation was observed between the ratios observed in these two locations.40 Another small cohort (25 MCI patients) study showed that the NAA/Cr ratios in the left paratrigonal area were also predictive of conversion to dementia.41 In a large cohort of 151 MCI patients (most being of the amnestic type) followed up for 3 years, MRS was individually predictive of conversion to dementia, but the accuracy of prediction improved when MRS was used in combination with hippocampal volumetry and the presence of cortical infarctions.42 In a small cohort of MCI (15) patients and controls (12), the ratios of NAA/Cr in the parietal lobe decreased longitudinally more in patients who converted to dementia than in non-converters.43
On the basis of all of the above and, given the paucity of longitudinal MRS studies in MCI, the purpose of this work is to investigate whether MRS measuring cerebral baseline NAA/Cr ratios in amnestic MCI are predictive of early conversion to dementia. We hypothesise that the occipital and parietal values are similarly predictive of conversion to dementia.
Patients and methods
A cohort of patients fulfilling the criteria of amnestic MCI according to the Petersen et al1 were recruited consecutively. The patients were referred by family physicians because of memory complaints corroborated by an informant. Those included in our cohort were first screened for memory impairment with the Memory Impairment Screen (MIS).44 In the MIS, four written words are presented to the patient, who must read aloud and memorise the words. After a period of 5 min, the patient is asked to recall these words; 2 points are given for every word recalled spontaneously, and 1 point is given for every word recalled with cues. At baseline, the patients underwent neuropsychological analysis encompassing the Mini-Mental test (Spanish version with a maximum possible score of 35 points),45 the Blessed Dementia Rating Scale, the clock drawing test, the Geriatric Depression Scale and the Rey Auditory Verbal Learning Test (RAVLT) delayed recall. The patients included in this study must score 5 or lower in the MIS, 0.5 in the CDR and a score in the Mini-Mental higher than 21 points. The cut-off points for the RAVLT 20 min delayed recall were as follows: ≤4 for patients aged up to 69 and ≤3 for patients aged 70 and older. Those who scored 11 points or higher in the Geriatric Depression Scale were re-evaluated after antidepressant treatment, so as to confirm that they had MCI.
The patients fulfilling the criteria mentioned above also underwent standard blood tests, including vitamin B12, serological test of syphilis and thyroid hormones. APOE genotype was also determined. Brain magnetic resonance techniques were also carried out as follows. All patients underwent brain T1- and T2-weighted MRI on a 1.5 T clinical scanner (Signa HD, GE, Milwaukee, Wisconsin). Single-voxel 1H-MRS was carried out by means of an echo time (TE) of 35 ms and a repetition time (TR) of 2000 ms with a spin echo technique that uses selective excitation with gradient spoiling for water suppression. The mode of spectral acquisition was probe-p (PRESS technique). The pure metabolite signal was spoiled, zero-filled and Fourier-transformed to produce a spectrum, scaled, drawn onto a 512×512 image, and stored as an image in the system database. Every spectrum was automatically fitted to four peaks corresponding to levels of N-acetyl-aspartate (NAA), 2.02 ppm; total creatine (Cr), 3.03 ppm; choline-containing compounds (Ch), 3.23 ppm; and myo-Inositol (mI), 3.56 ppm. We also obtained the peak amplitude of the metabolites relative to creatine. For this purpose, we used the algorithms provided by the GE software (Signa HD, GE software release 12.x), version 3.0, with the following steps: (1) setting a global frequency fit parameter; (2) performing line-width and line-shape enhancement by appropriate apodisation of the time-domain signal; (3) Fourier transformation of the signal to the appropriate frequency resolution and number of points; (4) calculation of a baseline correction from the frequency-domain signal; (5) and curve-fitting the desired regions of the frequency-domain signal. The volume voxel was 2×2×2 cm in each area explored. These areas of exploration were the left medial occipital lobe and the posteromedial parietal area bilaterally encompassing the posterior cingulated gyrus and the inferior precuneus (see figure 1). Spectra were rejected and repeated in the following cases: line width >10 Hz, line shape asymmetrical after eddy-current correction and the presence of artefacts. Data fits with %SD>20 from the Cramér–Rao inequality were eliminated.
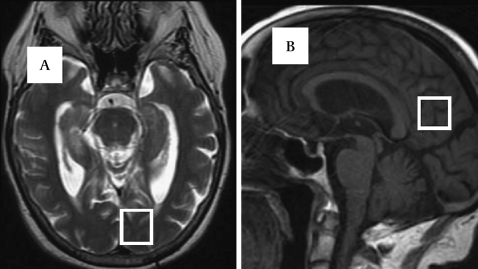
Figure 1.
(A) Axial T2-weighted MRI. Voxel placement in the left occipital lobe. (B) Sagittal T1-weighted MRI. Voxel placement in the posteromedial parietal cortex bilaterally.
Both areas we examined showed excellent reproducibility in two previous studies of test–retest reliability carried out with the same clinical scanner in AD patients.46 47 For the NAA/Cr ratios, the α value was 0.93 and 0.95 in the posteromedial bilateral parietal lobe respectively, and 0.89 and 0.87 in the left medial occipital lobe. In spite of these good α values seen previously, we also carried out a second immediate MRS in 22 patients without their being removed from the scanner to check reproducibility. The intraclass correlation coefficients were 0.92 and 0.9 for parietal and occipital lobes respectively.
At baseline, we also carried out MRS in 35 healthy elderly controls with the voxels located in the same areas for comparison purposes. The subjects were healthy volunteers who agreed to participate to establish a normative group. The mean age was 70.3 (SD 7.8) years, and there were 23 women and 12 men.
The recruitment started in October 2007 and finished in July 2008. The patients were followed up and re-evaluated every 6 months or earlier to determine if they had converted to probable AD-type dementia according to the National Institute of Neurological and Communicative Disorders and Stroke and the Alzheimer's Disease and Related Disorders Association group criteria.48 Reassessment was based on the Mini-Mental, MIS, Blessed Dementia Rating Scale and clock drawing tests.
Statistical analysis
Quantitative variables such as metabolite values in converters and non-converters were compared using two-tailed t tests. Survival analysis was based on the Kaplan–Meier method and Cox proportional hazards model. According to the metabolite values found in MRS, we divided the patients into two groups: those with NAA/Cr ratios below the mean and those with mean values and higher. The proportions of patients free of conversion to dementia in each group were compared with the logrank test. The proportion of patients who did not convert to dementia was adjusted for potential confounders such as age, educational level, global cognitive function at baseline, memory and APOE genotype with the Cox regression model.
The predictive values of the different variables (APOE4, memory tests and brain metabolite values) were calculated with the analysis of receiver operating characteristic (ROC) curves. Parameters such as sensitivity, specificity, positive and negative predictive values, and accuracy of classifications are reported. The results were cross-validated using a discriminant analysis and leave-one-out technique. ROC curves were analysed using Med-Calc software, and the other statistical techniques using SPSS software, version 10.
We obtained informed consent from patients and relatives. This study was approved by our regional ethical committee.
Results
Initially, we recruited a cohort of 78 patients who scored 5 or lower in the MIS and fulfilled the criteria of amnestic MCI. However, MRS was not possible in six cases; three had claustrophobia, two wore a pacemaker, and one refused to participate. One patient had to be excluded because of an incidental brain-stem tumour. Therefore, 71 patients were finally included in the study. Table 1 lists the main baseline demographic variables and the results of memory tests and scales. There were no differences with regard to the female/male ratio between patients and controls, but the controls were somewhat younger than the patients (mean: 70.3 vs 74 years; p=0.01). The mean age of the seven excluded patients was 76.4 years.
Table 1.
Demographic variables and scales scores in the cohort of 71 patients with amnestic mild cognitive impairment
| Variables (N=71) | |
| Age, years | 74.6 (SD 6.4; range 58–88) |
| Sex | 43 female |
| MEC | 28.4 (SD 3.1; range 21–34) |
| Blessed Dementia Rating Scale | 2.8 (SD 0.9; range 1–4) |
| Memory Impairment Screen | 2.4 (SD 1.7; range 0–5) |
| Rey Auditory Verbal Learning Test | 3.1 (SD 2.5; range 0–6) in delayed recall |
| Educational level | |
| Elementary education, n patients | 65 |
| High school education, n patients | 6 |
| Higher education, n patients | 4 |
| Hypertension, n (%) | 24 (33.8) |
| APOE4 genotype | 21 had one or two alleles |
| Mean follow-up, months | 22 (range 6–34) |
| Mean no of visits | 4.4 (range 3–7) |
At baseline MRI (T1, T2 and fluid-attenuated inversion recovery sequences) we detected the following abnormalities: diffuse cortical atrophy in 40 patients, isolated hippocampal atrophy in nine patients, leuco-araiosis in 38 patients and microinfarctions in six patients. Atrophy was evaluated only visually.
After a mean follow-up of 22 (range: 6–34) months, 27 (38%) patients out of 71 converted to probable AD according to the National Institute of Neurological and Communicative Disorders and Stroke and the Alzheimer's Disease and Related Disorders Association criteria, and none of them reverted to normality. None of the converters showed any symptoms or signs of parkinsonism, hallucinations, cognitive fluctuations or focal symptoms. No differences were seen in the male/female ratio proportion, but converters were older than non-converters (mean age: 76 (SD 6.5) years for converters vs 73 (SD 5.8) years for non-converters; p=0.01).
Spectroscopic results
Controls versus MCI patients
Table 2 lists the values of the different variables for converters and non-converters, and also for controls. When we compared the NAA/Cr ratios in the occipital lobe of the 71 MCI patients with 35 controls, we found significant differences: 1.56 (SD 0.09) in patients in comparison with 1.65 (SD 0.08) in controls (t=5; p<0.0001). No significant differences were seen in the rest of metabolite ratios. In the PMPC, no global differences were observed in the mean NAA/Cr ratios: 1.46 for controls and 1.45 for MCI patients. The differences in the NAA levels were significant: 134.06 (SD 18.3) for controls versus 123.17 (SD 17.3) for MCI patients (t=2.99; p=0.003). We did not find any significant differences for the other metabolites and ratios.
Table 2.
Metabolite levels and ratios to creatine in the two areas explored with Magnetic Resonance Spectroscopy
| Variable | Controls (n=35) | Converters (n=27) | Non-converters (n=44) | p Value |
| Posteromedial parietal cortex | ||||
| NAA | 134 (18.3) | 120 (19.89) | 125.8 (15.59) | NS |
| NAA/Cr | 1.46 (0.08) | 1.38 (0.09) | 1.49 (0.08) | <0.0001 |
| Ch/Cr | 0.61 (0.07) | 0.62 (0.05) | 0.59 (0.1) | NS |
| mI/Cr | 0.66 (0.08) | 0.63 (0.08) | 0.6 (0.09) | NS |
| NAA/mI | 2.19 (0.35) | 2.4 (0.29) | 2.3 (0.29) | NS |
| Occipital lobe | ||||
| NAA | 133.3 (23.1) | 133.7 (25.1) | 146.9 (24.4) | 0.03 |
| NAA/Cr | 1.65 (0.08) | 1.49 (0.08) | 1.6 (0.08) | <0.0001 |
| Ch/Cr | 0.6 (0.07) | 0.55 (0.05) | 0.57 (0.07) | NS |
| mI/Cr | 0.65 (0.1) | 0.59 (0.06) | 0.6 (0.07) | NS |
| NAA/mI | 2.52 (0.36) | 2.63 (0.29) | 2.61 (0.4) | NS |
Statistical significance refers to the differences found between converters and non-converters. Statistical significance is represented by the p value on the right column
Ch, choline; Cr, creatine; mI, myo-inositol; NAA, N-acetyl-aspartate; NS, not significant.
Converters versus non-converters
The mean NAA/Cr ratio in the posteromedial cortex was 1.30 (SD 0.09) in converters versus 1.49 (SD 0.08) in non-converters (t=9.96; p<0.0001). In the occipital lobe it was 1.48 (SD 0.08) in converters versus 1.6 in non-converters (t=4.89; p=0.0001). The absolute occipital NAA level was 133.7 (SD 25.1) in converters versus 146.9 (SD 24.4) in non converters (p=0.03). Figure 2A,B presents an example in a non-converter and converter respectively. The differences were not significant for the other metabolite values (see table 2). It is worth mentioning that the baseline NAA/Cr values in the posteromedial and occipital cortices correlated significantly (r=0.56; p<0.0001) in the whole sample of 71 patients.
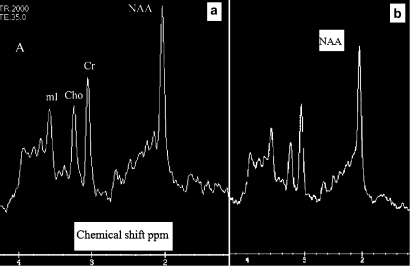
Figure 2.
(A) Example of a spectrum in the parietal lobe in a non-converter. (B) Example of a spectrum in a converter. The N-acetyl-aspartate (NAA) peak is lower than in the previous example in relation to creatine. Ch, choline compounds; Cr, creatine; mI, myo-inositol.
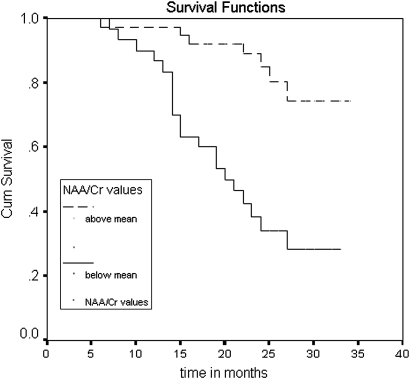
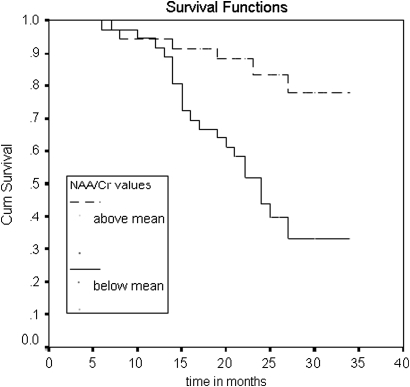
In the survival analysis, we saw significant differences in the proportion of dementia-free patients at follow-up (see figures 3, 4). The patients with NAA/Cr ratios below the mean were more likely to convert to dementia than those with values above the mean in both posteromedial parietal (logrank test: 17.83, p<0.0001) and occipital lobe (logrank test: 11.7; p=0.0007). The differences were adjusted for potential confounders (age, educational level, global cognitive function, memory scale and APOE genotype) with the Cox regression model. Only global cognition Mini Examen Cognoscitivo (MEC) and memory scale (RAVLT) at baseline were also predictive of conversion to dementia. The adjusted HR for the NAA/Cr ratios below the mean in the posteromedial parietal lobe was 7.03 (95% CI 2.6 to 18.9). In the occipital lobe, the HR was 5.06 (96% CI 1.73 to 14.8). The results were also predictive when NAA/Cr ratios were factored out as continuous variables.
Figure 3.
Comparison of survival curves for the variable N-acetyl-aspartate/Cr in the posteromedial parietal cortex. The curves represent the proportion of patients not converting to dementia across the time according to the NAA/Cr ratios. Upper curve: patients with ratios equal to or above mean. Lower curve: patients with ratios below mean.
Figure 4.
Comparison of survival curves for the variable N-acetyl-aspartate/Cr in the left occipital lobe. Proportion of patients free of dementia across the time in the patients with an N-acetyl-aspartate/Cr ratio equal to or higher than the mean (upper curve) and in those with ratios below the mean (lower curve).
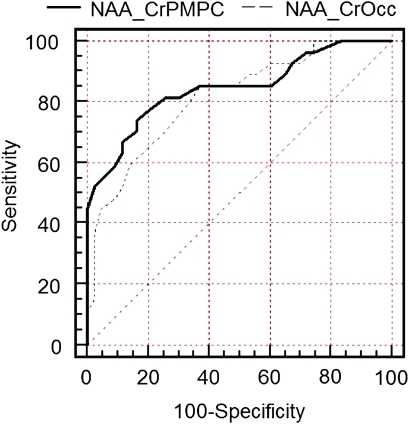
An NAA/Cr ratio equal to or lower than 1.43 in the posteromedial bilateral parietal cortex predicted conversion to probable AD at 74.1% sensitivity and 83.7% specificity, with a positive predictive value of 74.1% and a negative predictive value of 83.7%. The area under the curve was 0.84 (95% CI 0.73 to 0.92). The cross-validated accuracy of classification was 82%, reaching 85% when the APOE genotype and memory test were included in the analysis. In the left medial occipital lobe, the predictive value was somewhat lower with 85.2% sensitivity, 61.4% specificity, a positive predictive value of 57.5% and a negative predictive value of 87.1%. The area under the curve was 0.8 (95% CI 0.69 to 0.89). The ROC curves are presented in the figure 5.
Figure 5.
Comparison of the receiver operating characteristic curves for the N-acetyl-aspartate/Cr ratio in the posteromedial parietal cortex (continuous line) and the N-acetyl-aspartate/Cr ratio in the left occipital lobe (discontinuous line). Each curve represents the estimations of prediction of conversion to dementia for each of either variables. All predictive values are given in the text.
The APOE4 genotype alone yielded low predictive values in terms of sensitivity as 18 patients converted to dementia despite not having APOE4 alleles, and only eight of converters had one or two alleles (33% sensitivity and 72% specificity). The RAVLT yielded a low sensitivity but high specificity (55.6% and 84% respectively). The presence/absence of white-matter hyperintensities (leuco-araiosis) was not predictive of conversion to dementia.
Discussion
The diagnosis of AD in early phases is still challenging. Several tools, clinical and radiological, have been used so far, but there is no consensus on which is best. In addition, the techniques determined are not available in every medical centre, so each department should take advantage of those available.
MRS represents a valuable technique in AD on the basis of previous cross-sectional and longitudinal published works. Additionally it was found that MRS correlates well with histopathology. The NAA levels were lower in the brain of AD than in controls and that this decrease was correlated with the number of neuritic plaques and neurofibrillar tangles in tissue sections.49 The value of proton MRS as a biomarker has also been assessed ante mortem in a series of 54 patients ranging from a low to high likelihood of having AD and those who underwent an autopsy. Decreases in NAA/Cr and increases in mI/Cr ratios were correlated with higher Braak neuropathological stages in the posterior bilateral cingulate gyrus.50 Furthermore, the value of MRS as a biomarker has been confirmed by the fact that changes in metabolite ratios are detected years before the clinical onset of AD in subjects carrying mutations in presenilins51 or protein τ52 genes.
The predictive NAA/Cr ratios of the posteromedial parietal lobe agree with the early involvement of the PCG in AD but its occurrence in the occipital lobe may be surprising, as histopathological changes appear in more advanced stages of the disease. However, two cross-sectional studies with MRS showed lower NAA/Cr ratios in AD patients than in controls in the occipital cortex,36 37 and one study in MCI showed lower NAA/Cr ratios in converters than in converters.39 Two more studies point to an earlier involvement than previously thought for the occipital cortex in AD. A PET study in 13 patients with mild to moderate AD revealed that glucose metabolism was correlated to cognitive performance in the parietal lobes, but for the activation condition, the authors also found correlations within the primary and association visual areas.53 A neuropathological study revealed dense AD pathology in area 19 in some subjects with preclinical AD and in all patients with MCI, and noted that it was present even in the absence of hippocampal and entorhinal pathology.54
Apart from the intrinsic predictive value of a biomarker, this should be weighed in comparison with other biomarkers available. On the one hand, volumetry of the medial temporal lobe structures is widely used but not without its limitations (artefacts, lack of standardisation, complexity and long duration for the process). On the other hand, the CSF biomarkers need an invasive procedure and admission to hospital. Furthermore, these biomarkers do not differ greatly from MRS in terms of accuracy of prediction. At a fixed 80% specificity, the hippocampus plus entorhinal cortex volume predicted conversion to AD at 66.7% sensitivity in a cohort of 139 MCI patients followed up for 5 years.16 According to a large prospective multicentre cohort results with 750 MCI subjects followed up for a minimum of 2 years, the combination of Aβ42/P-τ ratio and T-τ in the CSF predicted conversion to AD with a sensitivity of 83%, a specificity of 72%, a positive predictive value of 62% and a negative predictive value of 88%, but the analytical techniques are pending standardisation.24 PET appears to be a robust predictor of conversion to dementia, but it is expensive and of limited availability. The excellent values found in the previous studies with PET28–31 have not been confirmed in the cohort of the AD Neuroimaging Initiative group where the positive predictive value was 41%, and the negative predictive value was 79%.55
PET with Pittsburg radiotracer (amyloid plaques binding) also seems promising in terms of specificity. In a study including 31 MCI patients, only 17 were PIB positive at baseline, and 14 (82%) of them converted to dementia over a 3 year follow-up. However, only 17 MCI patients (55%) were PIB-positive at baseline.56
In this context of expensive and sophisticated techniques, it should be borne in mind that neuropsychological tests can reveal good predictions of conversion to dementia in experienced hands.57
Of course, MRS also has some shortcomings. First, with the large voxels analysed, it is sensitive to artefacts in the magnetic field and partial volume effect in areas near osseous structures and cerebral ventricles.58 For this reason, it is likely that in our previous cohort, we did not find any predictive values in the hippocampus, although this area is theoretically involved very early in AD. It is expected that modern 3T scanners with smaller voxel analysed will overcome these limitations.59 60 Second, quantification of absolute metabolite values is complex, so the metabolite ratios to creatine are much more reliable than the absolute levels, as they can minimise systematic errors.61 For example, in table 2 the controls had lower NAA levels in the occipital lobe than did non-converters, whereas the NAA/Cr ratios were higher in controls as it is expected to occur. Third, we were not able to make any corrections for atrophy and CSF.
In conclusion, MRS is a useful technique as a biomarker in early AD, as it predicts early conversion to dementia. Although inferior to FDG-PET, it could yield a similar performance to structural neuroimaging and CSF biomarkers. We think MRS may play a role where no better instruments are available.
Supplementary Material
Footnotes
To cite: Modrego PJ, Fayed N, Sarasa M. Magnetic resonance spectroscopy in the prediction of early conversion from amnestic mild cognitive impairment to dementia: a prospective cohort study. BMJ Open 2011;1:e000007. doi:10.1136/bmjopen-2010-000007
Funding: This work was supported by the Spanish Ministry of Education and Science (Grant: SAF 2006-13332).
Competing interests: None.
Patient consent: Obtained.
Ethics approval: Ethics approval was provided by Comité de Etica del Instituto Aragones de Ciencias de la Salud.
Contributors: PJM: design, clinical data acquisition; statistical analysis; drafting of the manuscript. NF: MRI acquisition; design; critical review. MS: acquisition of funding. APOE4 genotype determination; critical review.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Petersen RC, Smith GE, Waring SC, et al. Mild cognitive impairment: clinical characterization and outcome. Arch Neurol 1999;56:303–8 [DOI] [PubMed] [Google Scholar]
- 2.Morris JC, Storandt M, Miller JP, et al. Mild cognitive impairment represents early-stage Alzheimer's disease. Arch Neurol 2001;58:397–405 [DOI] [PubMed] [Google Scholar]
- 3.Larrieu S, Letenneur L, Orgogozo JM, et al. Incidence and outcome of mild cognitive impairment in a population based cohort. Neurology 2002;59:1594–9 [DOI] [PubMed] [Google Scholar]
- 4.Birks J, Flicker L. Donepezil for mild cognitive impairment. Cochrane Database Syst Rev 2006;(3):CD006104. [DOI] [PubMed] [Google Scholar]
- 5.Raschetti R, Albanese E, Vanacore N, et al. Cholinesterase inhibitors in mild cognitive impairment: a systematic review of randomised trials. PLoS Med 2007;4:e338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wolfson C, Oremus M, Shukla V, et al. Donepezil and rivastigmine in the treatment of Alzheimer's disease: a best evidence synthesis of the published data on their efficacy and cost-effectiveness. Clin Ther 2002;24:862–86 [DOI] [PubMed] [Google Scholar]
- 7.De León MJ, Golomb J, George AE, et al. The radiologic prediction of Alzheimer's disease: the atrophic hippocampal formation. Am J Neuroradiol 1993;14:897–906 [PMC free article] [PubMed] [Google Scholar]
- 8.Jack CR, Petersen RC, Xu YC, et al. Prediction of AD with MRI based hippocampal volume in mild cognitive impairment. Neurology 1999;52:1397–403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Killiany RJ, Gómez-Isla T, Moss M, et al. Use of structural magnetic resonance imaging to predict who will get Alzheimer's disease. Ann Neurol 2000;47:430–9 [PubMed] [Google Scholar]
- 10.Dickerson BC, Goncharova I, Sullivan MP, et al. MRI-derived entorhinal and hippocampal atrophy in incipient and very mild AD. Neurobiol Aging 2001;22:747–54 [DOI] [PubMed] [Google Scholar]
- 11.Visser PJ, Verhey FR, Hofman PA, et al. Medial temporal lobe atrophy predicts Alzheimer's disease in mild cognitive impairment. J Neurol Neurosurg Psychiatry 2002;72:491–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Korf ES, Wahlund LO, Visser PJ, et al. Medial temporal lobe on MRI predicts dementia in patients with mild cognitive impairment. Neurology 2004;63:94–100 [DOI] [PubMed] [Google Scholar]
- 13.De Toledo-Morrell L, Stoub TR, Bulgakova M, et al. MRI-derived entorhinal volume is a good predictor of conversion from MCI to AD. Neurobiol Aging 2004;25:1197–203 [DOI] [PubMed] [Google Scholar]
- 14.Jack CR, Shiung MM, Weigand SD, et al. Brain atrophy rates predict subsequent clinical conversion in normal elderly with amnestic MCI. Neurology 2005;65:1227–31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stoub TR, Bulgakova M, Leurgans S, et al. MRI predictors of risk of incident Alzheimer's disease: a longitudinal study. Neurology 2005;64:1520–4 [DOI] [PubMed] [Google Scholar]
- 16.Devanand DP, Pradhaban G, Liu X, et al. Hippocampal and entorhinal atrophy in mild cognitive impairment: prediction of Alzheimer disease. Neurology 2007;68:828–36 [DOI] [PubMed] [Google Scholar]
- 17.Riemenschneider M, Lautenschlager N, Wagenpfeil S, et al. Cerebrospinal fluid tau and beta-amyloid 42 proteins identify Alzheimer's disease in subjects with mild cognitive impairment. Arch Neurol 2002;59:1729–34 [DOI] [PubMed] [Google Scholar]
- 18.Zatterberg H, Wahlung LO, Blennow K. Cerebrospinal fluid markers for prediction of Alzheimer's disease. Neurosci Lett 2003;352:67–9 [DOI] [PubMed] [Google Scholar]
- 19.Andreasen N, Vanmechelen E, Vanderstichele H, et al. Cerebrospinal fluid levels of total-tau, phospho-tau and A Beta42 predicts development of Alzheimer's disease in patients with mild cognitive impairment. Acta Neurol Scand 2003;179:47–51 [DOI] [PubMed] [Google Scholar]
- 20.Hampel H, Teipel SJ, Fuchrberger T, et al. Value of CSF-Beta-amyloid1-42 and tau as predictors of Alzheimer's disease in mild cognitive impairment. Mol Psychiatry 2004;9:705–10 [DOI] [PubMed] [Google Scholar]
- 21.Ivanoiu A, Sindic CJ. Cerebrospinal fluid Tau protein and Amyloid-B42 in mild cognitive impairment: prediction of progression to Alzheimer's disease and correlation with the neurological examination. Neurocase 2005;11:32–9 [DOI] [PubMed] [Google Scholar]
- 22.de Leon MJ, Segal S, Tarshish CY, et al. Longitudinal cerebrospinal fluid tau load increases in mild cognitive impairment. Neurosci Lett 2002;333:183–6 [DOI] [PubMed] [Google Scholar]
- 23.Vanderstichele H, De Meyer G, Andreasen N, et al. Amino-truncated {beta}-Amyloid42 peptides in cerebrospinal fluid and prediction of progression of mild cognitive impairment. Clin Chem 2005;51:1650–60 [DOI] [PubMed] [Google Scholar]
- 24.Mattsson N, Zetterberg H, Hansson O, et al. CSF biomarkers and incipient Alzheimer disease in patients with mild cognitive impairment. JAMA 2009;302:385–93 [DOI] [PubMed] [Google Scholar]
- 25.Alexander GE, Chen K, Pietrini P, et al. Longitudinal PET evaluation of cerebral metabolic decline in dementia: A potential outcome measure in Alzheimer's disease treatment studies. Am J Psychiatry 2002;159:738–45 [DOI] [PubMed] [Google Scholar]
- 26.Small GW, Kepe V, Ercoli LM, et al. PET of brain amyloid and tau in mild cognitive impairment. N Engl J Med 2006;355:2652–63 [DOI] [PubMed] [Google Scholar]
- 27.Hunt A, Schonknecht P, Henze M, et al. Reduced cerebral glucose metabolism in patients at risk for Alzheimer's disease. Psychiatry Res 2007;155:147–54 [DOI] [PubMed] [Google Scholar]
- 28.de Leon MJ, Convit A, Wolf OT, et al. Prediction of cognitive decline in normal elderly subjects with 2-[(18)F]fluoro-2-deoxy-d-glucose/positron-emission tomography (FDG/PET). Proc Natl Acad Sci U S A 2001;98:10966–71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mosconi L, Perani D, Sorbi S, et al. MCI conversion to dementia and the APOE genotype: a prediction study with FDG-PET. Neurology 2004;63:2332–40 [DOI] [PubMed] [Google Scholar]
- 30.Drzezga A, Grimmer T, Riemenschneider M, et al. Prediction of individual clinical outcome in MCI by means of genetic assessment and (18)F-FDG PET. J Nucl Med 2005;46:1625–32 [PubMed] [Google Scholar]
- 31.Anchisi D, Borroni B, Franceschi M, et al. Heterogeneity of brain glucose metabolism in mild cognitive impairment and clinical progression to Alzheimer's disease. Arch Neurol 2005;62:1728–33 [DOI] [PubMed] [Google Scholar]
- 32.Kantarci K, Petersen RC, Boeve BF, et al. 1H MR spectroscopy in common dementias. Neurology 2004;63:1393–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kantarci K, Jack CR, Xu YC, et al. Regional metabolic patterns in mild cognitive impairment and Alzheimer's disease. Neurology 2000;55:210–17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hancu I, Zimmerman EA, Sailasuta N, et al. 1H MR spectroscopy using TE averaged PRESS: A more sensitive technique to detect neurodegeneration associated with Alzheimer's disease. Magn Reson Med 2005;53:777–82 [DOI] [PubMed] [Google Scholar]
- 35.Kantarci K, Xu YC, Shiung MM, et al. Comparative diagnostic utility of different MR modalities in mild cognitive impairment and Alzheimer's disease. Dement Geriatr Cogn Disord 2002;14:198–207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Miller BL, Moats RA, Shonk T, et al. Alzheimer's disease: depiction of increased cerebral myoinositol with proton MR spectroscopy. Radiology 1993;187:433–7 [DOI] [PubMed] [Google Scholar]
- 37.Shonk TK, Moats RA, Gifford P, et al. Probable Alzheimer disease: diagnosis with proton MR spectroscopy. Radiology 1995;195:65–72 [DOI] [PubMed] [Google Scholar]
- 38.Rai GS, McConnel JR, Waldman A, et al. Brain proton spectroscopy in dementia: an aid to clinical diagnosis. Lancet 1999;353:1063–4 [DOI] [PubMed] [Google Scholar]
- 39.Modrego PJ, Fayed N, Pina MA. Conversion from mild cognitive impairment to probable Alzheimer's disease predicted by brain magnetic resonance spectroscopy. Am J Psychiatry 2005;162:667–75 [DOI] [PubMed] [Google Scholar]
- 40.Fayed N, Davila J, Oliveros A, et al. Utility of different MR modalities in mild cognitive impairment and its use as a predictor of conversión to probable dementia. Acad Radiol 2008;15:1089–98 [DOI] [PubMed] [Google Scholar]
- 41.Metastasio A, Rinaldi P, Tarducci R, et al. Conversión of MCI to dementia: role of proton magnetic resonance spectroscopy. Neurobiol Aging 2006;27:926–32 [DOI] [PubMed] [Google Scholar]
- 42.Kantarci K, Weigand SD, Przybelski SA, et al. Risk of dementia in MCI. Combined effect of cerebrovascular disease, volumetric MRI, and 1H MRS. Neurology 2009;72:1519–25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pilatus U, Lais C, Rochmont Adu M, et al. Conversion to dementia in mild cognitive impairment is associated with decline of N-acetylaspartate and creatine as revealed by magnetic resonance spectroscopy. Psychiatry Res 2009;173:1–7 [DOI] [PubMed] [Google Scholar]
- 44.Buschke H, Kuslansky G, Katz M, et al. Screening for dementia with the memory impairment screen. Neurology 1999;52:231–8 [DOI] [PubMed] [Google Scholar]
- 45.Lobo A, Ezquerra J, Gomez Burgada F, et al. El Mini-Examen cognoscitivo: un test sencillo y practico para detectar alteraciones intelectuales en pacientes médicos (in Spanish). Actas Luso Esp Neurol Psiquiatr Cienc Afines 1979;7:189–202 [PubMed] [Google Scholar]
- 46.Fayed N, Modrego PJ, Medrano J. Comparative test–retest reliability of metabolite values assessed with magnetic resonance spectroscopy of the brain. The LCModel versus the manufacturer software. Neurol Res 2009;31:472–7 [DOI] [PubMed] [Google Scholar]
- 47.Modrego PJ, Fayed N, Errea JM, et al. Memantine versus donepezil in Alzheimer's disease. A randomized trial with magnetic resonance spectroscopy. Eur J Neurol 2010;17:405–12 [DOI] [PubMed] [Google Scholar]
- 48.McKhan G, Drachman D, Folstein M, et al. Clinical diagnosis of Alzheimer's disease: report of the NINCDS-ADRDA work group. Neurology 1984;34:939–44 [DOI] [PubMed] [Google Scholar]
- 49.Klunk WE, Panchalingam K, Moossy J, et al. N-acetyl-l-aspartate and other amino acid metabolites in Alzheimer's disease brain: a preliminary proton nuclear magnetic resonance study. Neurology 1992;42:1578–85 [DOI] [PubMed] [Google Scholar]
- 50.Kantarci K, Knopman DS, Dickson DW, et al. Alzheimer disease: postmortem neuropathologic correlates of antemortem 1H MR spectroscopy metabolite measurements. Radiology 2008;248:210–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Godbolt AK, Waldman AD, MacManus DG, et al. MRS shows abnormalities before symptoms in familial Alzheimer disease. Neurology 2006;66:718–22 [DOI] [PubMed] [Google Scholar]
- 52.Kantarci K, Boeve BF, Wszolek Z, et al. MRS in presymptomatic MAPT mutation carriers. Neurology 2010;75:771–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bokde AL, Teipel SJ, Drzezga A, et al. Association between cognitive performance and cortical glucose metabolism in patients with mild Alzheimer's disease. Dement Geriatr Cogn Disord 2005;20:352–7 [DOI] [PubMed] [Google Scholar]
- 54.McKee AC, Cabral HJ, Kowall NW, et al. Visual association pathology in preclinical Alzheimer disease. J Neuropathol Exp Neurol 2006;65:621–30 [DOI] [PubMed] [Google Scholar]
- 55.Landau SM, Harvey D, Madison CM, et al. Comparing predictors of conversion and decline in mild cognitive impairment. Neurology 2010;75:230–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Okello A, Koivunen J, Edison P, et al. Conversion of amyloid positive and negative MCI to AD over 3 years: an 11C-PIB PET study. Neurology 2009;73:754–60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Modrego PJ. Predictors of conversion to dementia of probable Alzheimer type in patients with mild cognitive impairment. Curr Alzheimer Res 2006;3:161–70 [DOI] [PubMed] [Google Scholar]
- 58.Fayed N, Olmos S, Morales H, et al. Physical basis of magnetic resonance spectroscopy and its application to central nervous system diseases. Am J Appl Sci 2006;3:1836–45 [Google Scholar]
- 59.Wang Z, Zhao C, Yu L, et al. Regional metabolic changes in the hippocampus and posterior cingulated area detected with 3-Tesla magnetic resonance spectroscopy in patients with mild cognitive impairment and Alzheimer's disease. Acta Radiol 2009;50:312–19 [DOI] [PubMed] [Google Scholar]
- 60.Caserta MT, Ragin A, Hermida AP, et al. Single voxel MR spectroscopy at 3T in a memory disorders clinic: early right hippocampal NAA/Cr loss in mildly impaired subjects. Psychiatry Res 2008;164:154–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Danielsen ER, Ross B. Basic physics of MRS. In: Danielsen ER, Ross B, eds. Magnetic Resonance Spectroscopy Diagnosis of Neurological Diseases. New York: Marcel Dekker, 1999:5–22 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.