Abstract
Background
The 2010 influenza vaccination program for children aged 6 months to 4 years in Western Australia (WA) was suspended following reports of severe febrile reactions, including febrile convulsions, following vaccination with trivalent inactivated influenza vaccine (TIV).
Methods
To investigate the association between severe febrile reactions and TIV, three studies were conducted: (i) rates of febrile convulsions within 72 h of receiving TIV in 2010 were estimated by vaccine formulation and batch; (ii) numbers of children presenting to hospital emergency departments with febrile convulsions from 2008 to 2010 were compared; and (iii) a retrospective cohort study of 360 children was conducted to compare the reactogenicity of available TIV formulations.
Findings
In 2010, an estimated maximum of 18 816 doses of TIV were administered and 63 febrile convulsions were recorded, giving an estimated rate of 3.3 (95% CI 2.6 to 4.2) per 1000 doses of TIV administered. The odds of a TIV-associated febrile convulsion was highly elevated in 2010 (p<0.001) and was associated with the vaccine formulations of one manufacturer—Fluvax and Fluvax Junior (CSL Biotherapies). The risk of both febrile convulsions (p<0.0001) and other febrile reactions (p<0.0001) was significantly greater for Fluvax formulations compared to the major alternate brand. The risk of febrile events was not associated with prior receipt of TIV or monovalent 2009 H1N1 pandemic vaccine. The biological cause of the febrile reactions is currently unknown.
Interpretation
One brand of influenza vaccine was responsible for the increase in febrile reactions, including febrile convulsions. Until the biological reason for this is determined and remediation undertaken, childhood influenza vaccination programs should not include Fluvax-type formulations and enhanced surveillance for febrile reactions in children receiving TIV should be undertaken.
Article summary
Article focus
Soon after commencement of the 2010 paediatric influenza vaccination program in Western Australia (WA), clinicians noted an apparent increase in children presenting with fever, vomiting and occasionally febrile convulsions, which led to a temporary suspension of the WA program for children under 5 years of age.
Three separate analytical studies were undertaken to determine the association between severe febrile adverse events and administration of the 2010 trivalent inactivated influenza vaccine (TIV) to children aged 6 months through 4 years in WA.
Key messages
The estimated rate of febrile convulsions in children aged under 5 years related to receipt of the 2010 TIV was found to be 3.3 per 1000 doses, more than 200 times higher than the only population-based published estimate.
Of the two major brands of TIV used in the program prior to its suspension, the elevated risk of febrile convulsions and other febrile reactions was found to be clearly associated with the Fluvax and Fluvax Junior formulations, manufactured by CSL Biotherapies; the reactions do not appear to be associated with the other TIV formulation.
As the biological mechanism has, to date, not been definitively identified, it seems reasonable that countries implement paediatric vaccination programs as usual using vaccine brands other than those of CSL Biotherapies, and undertake enhanced surveillance for febrile reactions and other adverse events in all children under 5 years of age receiving TIV.
Strengths and limitations
The major limitation of this study is the absence of reliable prospective data from an established, timely surveillance system designed to monitor adverse events resulting from vaccination. Accordingly, several data sources were used to gather numerator and denominator data, some already established and some created ad hoc and requiring retrospective data collection.
Its strengths are, first, the consistency of findings using different data sources and the strength of the statistical associations found, and second, the fact that this is the first report of an elevated rate of severe febrile reactions to TIV in children.
Introduction
Infants and young children are known to have an increased risk of severe disease from influenza A virus infection.1 2 Influenza-related hospitalisation rates for children less than 2 years of age are considerably higher than for older children and are comparable to rates in other groups considered to be at higher risk for complications from influenza, including the elderly.3 Some countries recommend universal annual vaccination for infants and children, including the USA, where vaccination is recommended for children aged 6 months and above.3 The Department of Health in the state of Western Australia (WA) has been offering trivalent inactivated influenza vaccine (TIV) to children aged 6 months to 4 years since 2008, following the deaths of three pre-school-aged children from influenza the previous year. Additionally, a new program providing TIV to children with underlying medical conditions was implemented throughout Australia in 2010.4
The medical literature supports the safety of TIV in young children.5–8 Fever has been found to be a relatively common adverse event in the few prospective clinical trials that aim to determine the safety of TIV in children, with rates ranging from 10% to 40%.9–11 Few studies large enough to accurately quantify the rate of uncommon potential adverse events, including febrile convulsions, following TIV administration have been published. A recent analysis from the US Vaccine Adverse Event Reporting System, which relies on passive reports, found that in children less than 2 years of age, seizures of any type, although rare, were the most commonly reported serious adverse events.6 A large population-based study from the US Vaccine Safety Datalink program found only one documented febrile convulsion (which occurred on day 3) in the 3 days following administration of 69 359 doses of TIV to children under 2 years of age (1.4 per 100 000).8
In 2010, distribution of TIV to immunisation providers in WA (general medical practitioners and government health services) began on 8 March and the childhood influenza vaccination program was publicly launched on 19 March. During the week beginning 12 April 2010, emergency department (ED) clinicians at a specialist paediatric referral hospital noted an apparent increase in children presenting with fever, vomiting and occasionally febrile convulsions, usually within 12 h of vaccination with TIV. Further reports led to a temporary suspension of the WA program for children under 5 years of age on 22 April 2010 to allow time to investigate whether this signal represented a true increase in adverse events, and whether any increase was common to all TIV formulations or specific to a particular formulation or batch(es). The following day, the federal government suspended the use of 2010 seasonal influenza vaccine for all children aged 5 years and under across Australia.
In Australia, there is a dual system for reporting adverse events following immunisation (AEFI). In seven of eight Australian states and territories, AEFI are notifiable conditions, meaning there is a legal requirement to report them to state/territory health authorites.12 Vaccination providers and members of the public can also report AEFI directly to an established national drug adverse event reporting system.13 Reports received by either route are shared between national and state bodies. Because data from such passive surveillance systems for adverse events are limited due to lack of a denominator, under-reporting and biased reporting, we undertook a number of analytical studies to determine the association between severe febrile adverse events and administration of the 2010 TIV to children aged 6 months through 4 years in WA.
Methods
Setting
WA has a total population of 2.3 million people, with 78% residing in the capital, Perth. An estimated 134 000 children aged 6 months through 4 years were eligible for free TIV in 2010.
Vaccines from three manufacturers were used in the paediatric influenza vaccination program in 2010: CSL Biotherapies (Parkville, Australia) supplied Fluvax (0.5 ml) and the identically formulated Fluvax Junior (0.25 ml), Solvay Pharmaceuticals (Pymble, Australia) supplied Influvac, and Sanofi Pasteur (Lyon, France) supplied Vaxigrip. Fluvax Junior was indicated for use in children aged 6 months to 3 years and the other formulations for use in those aged 6 months and over. All contained the three influenza haemagglutinin antigens recommended by the WHO for the 2010 Southern hemisphere influenza season (A/California/7/2009 (H1N1)-like virus, A/Perth/16/2009 (H3N2)-like virus and B/Brisbane/60/2008-like virus).14 The recommended doses were 7.5 μg of each antigen for those aged 6–36 months (0.25 ml vaccine) and 15 μg of each antigen for 4-year-olds (0.5 ml vaccine). It is recommended that children aged less than 10 years receive two doses at least 1 month apart in the first year of vaccination, and one dose in subsequent years. As fewer than 100 doses of Vaxigrip were given to children prior to cessation of the program, this formulation was excluded from assessment.
Ethics approval was not required for this study as the data were gathered as part of a public health investigation.
Febrile convulsions following 2010 TIV vaccination
The period between the time TIV was made available to providers in 2010 and the suspension of the program was 46 days. We defined the investigation period as 49 days (the 46-day period of the TIV program plus 3 days to capture febrile convulsions occurring after the last TIV vaccination). Febrile convulsions occurring outside the investigation period were assumed to be unrelated to 2010 TIV.
Febrile convulsion cases during the investigation period were identified through passive notifications to the national AEFI reporting system and from ED diagnostic codes using the WA Emergency Department Information System (EDIS). EDIS collects discharge diagnosis data in near real-time from EDs at nine Perth hospitals that have a combined catchment of approximately 1.76 million people (77% of the total population of WA). Children aged 6 months through 4 years who presented to an EDIS-enrolled hospital during the investigation period and were assigned an ICD-10 code of R56.0 (febrile convulsion)15 were investigated.
To identify a possible temporal relationship between EDIS-reported febrile convulsions and prior receipt of TIV, we cross-checked the reported febrile convulsion episodes with vaccination data recorded with the Australian Childhood Immunisation Register (ACIR). Verification was also sought from the child's primary care givers and/or the child's vaccine provider to determine if the child had received TIV in the 72 h prior to the febrile convulsion. The influenza vaccination histories of cases were obtained from ACIR and the child's primary care giver or vaccine provider.
The case details for all 2010 R56.0-coded febrile convulsions were identified through review of the case records and details for any additional febrile convulsion cases identified through passive reporting were collected from interviews with the primary care giver or vaccination provider using a standardised survey instrument. All records were reviewed by one author (CB) to ensure diagnostic accuracy. A febrile convulsion was defined as any generalised convulsive seizure (level 1, 2 or 3 according to the Brighton Collaboration case definitions16) that occurred in a febrile child (temperature on presentation to a health practitioner >37.5°C). A vaccine associated febrile convulsion was defined as any febrile convulsion that occurred within 72 h of either the first or subsequent dose of TIV for which an alternative cause of fever could not be identified either clinically or microbiologically.
A number of data sources were analysed to ascertain the contribution of childhood infective causes to the febrile convulsion cases over this time period: results of microbiological work-up of the febrile convulsion cases, presentations of gastroenteritis or influenza-like illness to participants in the WA General Practice Sentinel Surveillance Network and to EDIS-enrolled hospitals, and review of microbiological results from the only metropolitan paediatric hospital laboratory in Perth.
Rates of TIV-associated febrile adverse events in 2010
We obtained denominator data by surveying WA vaccine providers following the suspension of the program using a standardised form that requested information on numbers of children vaccinated by age group (6–24 months, 3–4 years) and vaccine brand/formulation and batch number. To account for possible under-ascertainment of the number of vaccine doses administered due to non-response by vaccine providers, the final totals were adjusted upwards by a factor of 1.4. This figure was determined to provide the upper limit of the number of doses that might really have been given. To estimate rates of both convulsive and ‘other’ (non-convulsive) febrile reactions per 1000 TIV vaccinations, cases of febrile convulsions were determined from passive reports and R56.0-coded presentations to EDIS-enrolled hospitals (as described above), and cases of ‘other’ febrile reactions were derived from passive reports by doctors, other immunisation providers and parents to the AEFI reporting system.
Comparing TIV-associated febrile convulsions in 2008–2010
To examine whether the number of febrile convulsions in children aged 6 months through 4 years had increased in 2010, we compared the R56.0-coded visits to the nine EDIS-enrolled hospitals for 2010 to those identified using the same data extraction process for corresponding 49-day periods following commencement of the paediatric TIV program in both 2008 (from 31 March) and 2009 (from 17 February). As for febrile convulsions reported in 2010, we cross-checked records of children with febrile convulsions identified in 2008 and 2009 with the ACIR and sought verification from the child's primary care givers and/or the child's vaccination provider.
Retrospective cohort study of paediatric TIV recipients
To assess the comparative reactogenicity of the three main TIV formulations, we conducted a retrospective cohort study to quantify the proportion of children experiencing a range of post-TIV vaccination symptoms. All children less than 5 years of age who had received the 2010 TIV at a large Perth public immunisation clinic were stratified by age and brand of vaccine administered. We then randomly selected 120 children from each of the three vaccine groups: Fluvax, Fluvax Junior and Influvac. The Fluvax and Influvac groups were stratified by age, with 60 children aged ≤2 years and 60 aged 3–4 years selected from each group. All children in the Fluvax Junior group were aged ≤2 years.
Primary care givers were interviewed by telephone using a standardised questionnaire, which sought information about symptoms in the 72 h following the 2010 TIV vaccination, body temperature measurement or self-reported fever, healthcare attendance, and prior history of vaccination with seasonal TIV or monovalent pandemic influenza vaccine. If the child received two doses of TIV, information was recorded about the first vaccination experience. Interviewers were blinded to the brand/formulation of vaccine the child had received.
Statistical analysis
Simple descriptive statistics were generated using Microsoft Excel or EpiInfo v 3.5.1 (CDC, Atlanta, Georgia, USA). For comparisons of adverse events by vaccine formulations, rates per 1000 vaccine doses and 95% CIs were determined. Relative risks (adjusted for age for the under-5 year category) and Mantel–Haenszel χ2 statistics were calculated for the combined Fluvax vaccines compared to Influvac. Fisher's exact test was used if expected cell frequencies were <5.
For analyses of adverse event data from the retrospective cohort study of TIV recipients from a large immunisation clinic, we computed odds ratios comparing events associated with combined Fluvax vaccines to Influvac, and controlled for age (continuous) using unconditional logistic regression. A multivariate logistic regression model was also computed to determine if vaccine brand was still associated with ‘fever’ or a ‘significant febrile adverse event’ (defined as fever ≥39°C recorded at home or during healthcare attendance and/or rigors and/or a febrile convulsion) while simultaneously controlling for three potential a priori confounders: age, prior vaccination with monovalent pandemic vaccine and prior vaccination with TIV, as dichotomous variables.
Results
Characteristics of febrile convulsion cases occurring in 2010
Based upon the uptake of influenza vaccine by children in 2008 and 2009, it is estimated that 85% of children vaccinated with the 2010 TIV resided in the catchment area of the EDIS-enrolled hospitals. During the investigation period, a total of 99 children aged 6 months–4 years presented to Perth EDIS-enrolled hospitals and were coded as R56.0 febrile convulsions. Of these, 38 (37.4%) occurred within 72 h of receipt of TIV. An additional 25 TIV-associated febrile convulsion cases that met diagnostic criteria were reported by ED clinicians, primary care givers or vaccine providers to the national AEFI reporting system, bringing the total number of TIV-associated febrile convulsions in 2010 to 63. The TIV brand/formulation could be determined for 62 of these 63 cases.
Of the 63 cases of febrile convulsions found to be associated with TIV, vaccination data for 41 (65%) were determined from cross-checking ACIR records (with corroboration by their primary care giver and/or immunisation provider), and the remaining 22 cases (35%) had their influenza vaccination status ascertained through interviews with their primary care giver and/or immunisation provider alone. The number of doses of vaccine cases had received in 2010 (either one or two) were known for 57 of 63 (90%), and all of these experienced their febrile convulsion after their first dose.
The demographic and clinical features of the 2010 cases derived from case record review and/or interviews with their primary care givers or vaccine providers are shown in table 1. The median time from receipt of TIV to onset of symptoms in the febrile convulsion cases was 7 h, with 93% having onset within 12 h. Vomiting was a conspicuous symptom, reported by 57.1% of cases, but local reactions were infrequent, with only 4.8% of cases reporting swelling at the injection site.
Table 1.
Demographic and clinical details of 63 children aged 6 months to 4 years presenting with febrile convulsions within 72 h of receiving trivalent inactivated influenza vaccine during the investigation period, 8 March to 25 April 2010
| Febrile convulsion presentations (n=63) | ||
| Demographics and past history | Mean age (years) | 1.85 |
| Median age (years) | 1.53 | |
| Male gender | 38 (60.3%) | |
| History of febrile convulsions | 8 (12.7%) | |
| History of seizures (other than febrile convulsions) | 3 (4.8%) | |
| History of AEFI | 4 (6.3%) | |
| Chronic medical condition* | 7 (11.1%) | |
| Symptoms on presentation | Fever (self-report) | 63 (100%) |
| Recorded temperature ≥38°C | 57 (90%) | |
| Rigors | 28 (44.4%) | |
| Respiratory | 12 (19.0%) | |
| Vomiting | 36 (57.1%) | |
| Fatigue | 22 (34.9%) | |
| Diarrhoea | 3 (4.8%) | |
| Headache | 3 (4.8%) | |
| Rash | 10 (15.9%) | |
| Local swelling | 3 (4.8%) | |
| Clinical | Admitted to hospital | 28 (44.4%)† |
| Admitted to intensive care unit | 2 (3.1%) | |
| Sequelae | 1 (1.6%)‡ | |
| Time from vaccination to symptom onset | Median time (h) | 7.0§ |
| Mean time (h) | 8.9§ | |
| Onset within 12 h | 93%§ | |
Chronic medical conditions included diabetes, chronic heart, respiratory, renal, neurological and blood disease, metabolic disease and impaired immunity.
Mean length of stay 3 days; range 1–50 days.
Hypoxic ischaemic encephalopathy.
Data available for 57 cases.
AEFI, adverse event following immunisation.
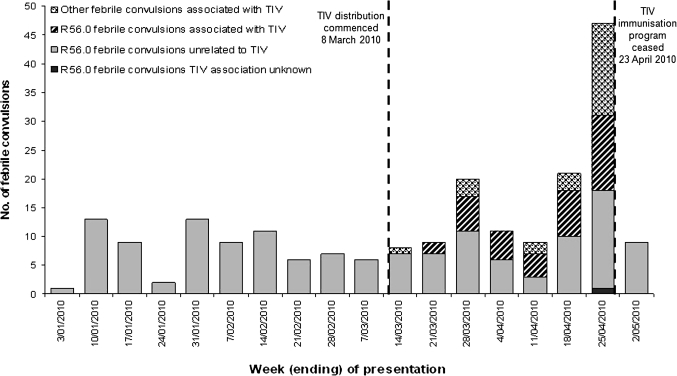
Figure 1 shows the temporal distribution of febrile convulsions from 1 January to 2 May 2010, and the timing of the vaccination program. Presentations of febrile convulsions increased following introduction of the TIV program and decreased to baseline soon after its suspension.
Figure 1.
Presentations of children under 5 years of age with febrile convulsions (ICD-10 code R56.0) to nine Perth hospital emergency departments, 1 January to 2 May 2010. TIV, trivalent inactivated influenza vaccine.
Presentations of gastroenteritis or influenza-like illness to participants in the WA General Practice Sentinel Surveillance Network and to EDIS-enrolled hospitals over the study period indicated low rates of gastrointestinal and respiratory viral illness in the community. Paediatric hospital laboratory data did not show an appreciable increase in childhood respiratory and gastrointestinal pathogens. Only a minority of children presenting to EDs with febrile convulsions in 2010 had a microbiological investigation conducted, as an infective cause of the fever was not often suspected. Of those who did have specimens taken, no pathogens were detected that could explain their illness.
Rates of adverse events by vaccine
From the vaccine provider survey, it was estimated that up to 18 816 TIV vaccinations had occurred in children aged 6 months–4 years in WA prior to the program's suspension, giving a conservatively estimated rate of 3.3 (95% CI 2.6 to 4.2) febrile convulsions per 1000 doses.
The rate of febrile convulsions and other febrile reactions, by vaccine brand and formulation, is shown in table 2. There was a significantly increased risk of both febrile convulsions and ‘other’ febrile reactions associated with Fluvax, Fluvax Junior or both Fluvax preparations combined, compared to Influvac. Adjusted for age, children receiving a Fluvax formulation were 14.8 times (95% CI 8.3 to 26.7) more likely to have a reported other febrile reaction than children receiving Influvac (p<0.0001). Relative risk could not be calculated for febrile convulsions, as no such events were reported for the estimated 4720 doses of Influvac given in children under 5 years of age, compared to an estimated rate of 4.4 per 1000 doses (95% CI 3.4 to 5.6) of Fluvax products (p<0.0001 for age adjusted comparison).
Table 2.
Incidence of trivalent inactivated influenza vaccine (TIV)-associated febrile convulsions and other febrile reactions in children aged 6 months to 4 years in Western Australia, by vaccine brand and formulation, for the period 8 March to 25 April 2010
| Fluvax |
Fluvax Junior |
Combined Fluvax and Fluvax Junior (CF) |
Influvac |
CF vs Influvac† |
|||||||
| n/N* | Rate/1000 TIV doses (95% CI) | n/N | Rate/1000 TIV doses (95% CI) | n/N | Rate/1000 TIV doses (95% CI) | n/N | Rate/1000 TIV doses (95% CI) | RR (95% CI) | p Value | ||
| Febrile convulsions‡ | ≤2 years | 28/5754 | 4.9 (3.4 to 7.1) | 23/2915 | 7.9 (5.3 to 11.8) | 51/8669 | 5.9 (4.5 to 7.7) | 0/2124 | 0 (0 to 1.8) | Undefined | <0.001 |
| 3–4 years | 7/5015 | 1.4 (0.7 to 2.9) | 4/413 | 9.7 (3.8 to 24.7) | 11/5428 | 2.0 (1.1 to 3.6) | 0/2596 | 0 (0 to 1.5) | Undefined | <0.05 | |
| All <5 years | 35/10769 | 3.3 (2.4 to 4.6) | 27/3327 | 8.1 (5.6 to 11.8) | 62/14096 | 4.4 (3.4 to 5.6) | 0/4720 | 0 (0 to 0.8) | Undefined | <0.0001 | |
| Other febrile reactions§ | ≤2 years | 282/5754 | 49.0 (43.7 to 54.9) | 138/2915 | 47.3 (40.2 to 55.6) | 420/8669 | 48.4 (44.1 to 53.1) | 9/2124 | 4.2 (2.2 to 8.0) | 11.4 (5.9 to 22.1) | <0.0001 |
| 3–4 years | 127/5015 | 25.3 (21.3 to 30.0) | 11/413 | 26.6 (14.9 to 47.0) | 138/5428 | 25.4 (21.5 to 29.9) | 2/2596 | 0.8 (0.2 to 2.9) | 33.0 (8.2 to 133.2) | <0.0001 | |
| All <5 years | 409/10769 | 38.0 (34.6 to 41.8) | 149/3327 | 44.8 (38.3 to 52.4) | 558/14096 | 39.6 (36.5 to 42.9) | 11/4720 | 2.3 (1.3 to 4.1) | 14.8 (8.3 to 26.7) | <0.0001 | |
n/N indicates number of febrile convulsions or other febrile reactions/adjusted number of doses given within age group and vaccine category.
RR, 95% CI and p value calculated for comparison of rates of adverse events for the combined Fluvax and Fluvax Junior group, versus Influvac. The RR and p value for the ‘All <5 years’ category is weighted for age.
Febrile convulsion presentations derived from notifications received via the AEFI reporting system and from emergency department ICD-10 diagnostic code R56.0 ‘febrile convulsion’.
‘Other febrile reactions’ derived from passive reports by doctors, other immunisation providers and parents to the AEFI reporting system.
AEFI, adverse event following immunisation; TIV, trivalent inactivated influenza vaccine.
The risk of other febrile reactions appeared to be consistent across batches of the adult Fluvax and Fluvax Junior products (data not shown). However, there was a significantly increased rate of febrile convulsions associated with Fluvax Junior (8.1 per 1000 doses (95% CI 5.6 to 11.8)) versus Fluvax (3.3 per 1000 doses (95% CI 2.4 to 4.6)): this was only partly explained by the fact that Fluvax Junior was used more frequently in children under 3 years of age, as the rate in children aged 3–4 years was also significantly elevated when compared to Fluvax. Although there was some variability in the risk of febrile convulsions across batches of Fluvax (but not for Fluvax Junior, as one batch dominated usage), this did not reach statistical significance (data not shown).
Historical comparison of febrile convulsions 2008–2010
There were 38 febrile convulsions temporally associated with TIV identified by R56.0 coding in the nine EDIS-enrolled hospital EDs in 2010, compared to only one in 2008 and none in 2009. The number of febrile convulsions of any cause during the investigation period was greatest for 2010 (table 3). The proportion of febrile convulsions temporally associated with TIV vaccination was significantly higher for 2010 compared to both 2008 and 2009 (p<0.001), and the odds of having a TIV-associated febrile convulsion was significantly elevated in 2010 (p<0.001).
Table 3.
Presentations of febrile convulsions in children aged 6 months to 4 years to nine Perth hospital emergency departments in the 49-day period following commencement of the trivalent inactivated influenza vaccination program, 2008–2010*
| Year |
Number of febrile convulsion† presentations within the investigation period‡ |
OR of temporally related§ febrile convulsions | p Value for difference | |||
| Total | Not temporally related§ to TIV | Temporally related§ to TIV | Unknown¶ | |||
| 2008 | 74 | 70 | 1 | 3 | Reference | Reference |
| 2009 | 68 | 65 | 0 | 3 | 0 (0–19) | 0.34 |
| 2010 | 99 | 60 | 38 | 1 | 44 (6–894) | <0.001 |
Unadjusted estimates of number of TIV doses given were 26 037 in 2008, 11 909 in 2009 (both derived from program data) and 11 243 in 2010 (derived from vaccine provider survey).
ICD-10 code R56.0 ‘febrile convulsion’. No febrile convulsions were recorded for children who had received monovalent H1N1 pandemic influenza vaccine.
The 49-day period allows for febrile convulsion to develop 72 h after vaccination up to day 46.
A temporal relationship is defined as febrile convulsion occurring within 72 h of receiving TIV.
‘Unknown’ if either not listed on the Australian Childhood Immunisation Register or uncontactable by telephone after four attempts, and immunisation provider not known. ‘Unknowns’ were excluded from statistical analysis.
TIV, trivalent inactivated influenza vaccine.
Pattern of clinical reactions by vaccine formulation
The results of the retrospective cohort study are shown in table 4. Of the 360 study subjects enrolled, 87.2% had primary care givers who were contactable; there was no significant difference in the response rate across the three vaccine formulation groups. The frequencies of reported symptoms following vaccination with either Fluvax vaccine formulation were similar and so were combined for comparisons with Influvac. Fever, fatigue, vomiting and rigors were each significantly more common in children who received a Fluvax vaccine compared to those who received Influvac, both in univariate analyses and in logistic regression analyses controlling for age. There was no difference in the frequency of other adverse events, including injection site reactions, and no significant differences in prior history of vaccination with TIV or monovalent pandemic influenza vaccine, between the Fluvax and Influvac groups. In a multivariate logistic regression model, ‘significant febrile adverse events’ were associated with receipt of either Fluvax vaccine formulation (OR 8.9; 95% CI 3.1 to 25.7; p<0.0005) and younger age (p=0.024), but no association was found between these events and receipt of seasonal influenza vaccine prior to 2010 or monovalent pandemic vaccine (p=0.4 and p=0.8, respectively).
Table 4.
Number of children enrolled in the retrospective cohort study who were reported by primary care givers as having symptoms, or prior vaccination, and receiving trivalent inactivated influenza vaccine (TIV) in 2010, by brand and formulation
| Percentage of children with symptom |
CF versus Influvac | CF versus Influvac (controlling for age) |
||||||
| Fluvax (n=109) | Fluvax Junior (n=100) | Combined Fluvax and Fluvax Junior (CF) (n=209) | Influvac (n=110) | Univariate χ2 p value | OR (95% CI) | p Value | ||
| Reported symptom | Fever* | 52.3 | 61.0 | 56.5 | 17.3 | 0.00001 | 5.1 (2.9 to 9.2) | 0.0001 |
| Fatigue | 29.4 | 37.0 | 33.0 | 10.9 | 0.0001 | 3.5 (1.8 to 7.0) | 0.0003 | |
| Vomiting | 14.7 | 20.0 | 17.2 | 2.7 | 0.0001 | 6.0 (1.8 to 20.3) | 0.0037 | |
| Rigours | 11.9 | 19.0 | 15.3 | 0.9 | 0.0006 | 16.0 (2.1 to 119.5) | 0.0070 | |
| Swelling | 12.8 | 13.0 | 12.9 | 10.9 | 0.6 | 1.1 (0.5 to 2.3) | 0.7914 | |
| Diarrhoea | 6.4 | 4.0 | 5.3 | 0.9 | 0.052 | 5.3 (0.7 to 43.0) | 0.1160 | |
| Rash | 5.5 | 2.0 | 3.8 | 0.9 | 0.14 | 3.8 (0.5 to 31.2) | 0.2193 | |
| Headache | 3.7 | 3.0 | 3.4 | 0.9 | 0.18 | 4.3 (0.5 to 37.5) | 0.1843 | |
| Convulsions | 2.8 | 0.0 | 1.4 | 0.0 | 0.2 | NA | ||
| Significant SFAE | 22.9 | 36.0 | 29.2 | 4.5 | 0.0001 | 7.0 (2.7 to 18.3) | 0.0001 | |
| Percentage of children answering ‘Yes’ (n) |
CF versus Influvac | CF versus Influvac (controlling for age) |
||||||
| Fluvax (n=109) | Fluvax Junior (n=100) | Combined Fluvax and Fluvax Junior (CF) (n=209) | Influvac (n=110) | Univariate χ2 p value | OR (95% CI) | p Value | ||
| Prior influenza vaccination history | Had monovalent 2009 H1N1 pandemic influenza vaccine? (n=104) | 15.4 (104) | 15.6 (96) | 15.5 (200) | 19.6 (102) | 0.37 | 0.9 (0.4 to 1.6) | 0.64 |
| Had seasonal influenza vaccine before 2010? (n=107) | 50.5 (107) | 49 (100) | 49.8 (207) | 57.5 (108) | 0.19 | 1.5 (0.9 to 2.6) | 0.16 | |
SFAE, significant febrile adverse event (defined as fever ≥39°C and/or rigours and/or a febrile convulsion).
Self-reported fever, whether measured or not.
Discussion
The suspension of the WA paediatric influenza vaccination program is the first Australian vaccination program of any type to be suspended in recent memory. This analysis of available epidemiological data supports that decision and provides four important observations. First, the estimated rate of febrile convulsions related to receipt of the 2010 TIV is far in excess of published rates from post-marketing surveillance studies. The only population-based published study that allows a rate of TIV-associated febrile convulsions to be calculated, indicates a rate of 1.4 per 100 000 doses in children under 2 years of age within 72 h of receipt of TIV,8 which compares to the estimated rate in our study of 330 per 100 000 doses in children under 5 years of age. Another report estimated the rate of febrile convulsions in 2010 in WA children under 5 years of age within 24 h of receipt of TIV to be 900 per 100 000 doses, and approximately 500 per 100 000 doses for children from other Australian states.17
Second, there was a marked increase in the number of TIV-attributable febrile convulsions reported from Perth metropolitan EDs in 2010 compared to similar periods in both 2008 and 2009, years for which the available data indicate 32% and 34% of the 6-month through 4-year-old cohort in the metropolitan area received at least one dose of TIV, respectively.18
Third, of the three brands of TIV used in the program, the elevated risk of febrile convulsions and other febrile reactions was found to be clearly associated with the Fluvax and Fluvax Junior formulations, but not with Influvac, for which no febrile convulsions were identified in WA against an estimate of over 4000 doses given. The excess of febrile reactions was not due to a particular batch of the Fluvax product, as several batches were implicated. The reason for the higher rate of febrile convulsions associated with Fluvax Junior compared to Fluvax is unclear, but it did not appear to be explained by the younger age of Fluvax Junior recipients. As the rates of non-convulsive febrile reactions were almost identical for the two Fluvax formulations, it seems unlikely that the apparent difference in the rate of febrile convulsions represents a real difference between the formulations related to some variation in concentration of whatever factor is responsible for the excess in serious adverse events. However, this possibility cannot be discounted.
Fourth, the febrile convulsions resulting from vaccination with the Fluvax vaccines are unlikely to represent a new, distinct clinical syndrome associated with the vaccine. Rather, the data indicate that the febrile convulsions were occurring against a background of increased rates of febrile reactions following vaccination. This conclusion is supported by the results of the vaccine provider survey and the retrospective cohort study, which both showed a clearly higher rate of febrile reactions in recipients of Fluvax and Fluvax Junior compared to those receiving Influvac. There was no evidence that the elevated rate of febrile convulsions in 2010 resulted from an increased incidence of childhood infections.
The major limitation of this study results from the absence of reliable prospective data from an established, timely surveillance system designed to monitor adverse events resulting from vaccination. Accordingly, several data sources were used to gather numerator and denominator data, some already established and some created ad hoc and requiring retrospective data collection. However, the strength of the statistical associations found, together with the consistency of findings using different data sources and the tight temporal association of the febrile convulsions with TIV (93% of cases having symptom onset within 12 h of vaccination), suggest the conclusions are robust. Reporting bias relating to the number of febrile convulsion cases being reported in 2010, or to Fluvax-related events in particular, cannot explain this finding as there was no communication to the public or the clinicians prior to the recognition of the association and the program's cessation.
This is the first report of an elevated rate of severe febrile reactions to TIV in children, and the biological mechanism has, to date, not been definitively identified despite an extensive laboratory investigation of Fluvax and Fluvax Junior undertaken by the Therapeutic Goods Administration, the Australian regulator of prescription medicines including vaccines. These studies are ongoing, but endotoxins or abnormal levels of whole virus particles have not been identified. Pyrogenicity studies in different animal models have shown conflicting results. The Therapeutic Goods Administration has hypothesised that high content and enzyme activity of neuraminidase in the H1N1 component of the 2010 TIV compared to previous years may be responsible for different cytokine responses and the increased pyrogenicity of the CSL vaccine.17 In the meantime, the Australian government recommended use of vaccines other than the CSL products in children under 5 years of age for the remainder of 201019 and in 2011,20 and the US Advisory Committee on Immunization Practices has recommended that the CSL Biotherapies TIV marketed in the USA (Afluria) not be used in children aged from 6 months to 8 years.21
The data from WA indicate that the elevated risk of febrile adverse events was associated with TIV from a single manufacturer. Experience in New Zealand, the only other Southern hemisphere country to use Fluvax and Fluvax Junior and vaccine from another manufacturer in 2010, corroborates this finding.19 Furthermore, and reassuringly, the low rate of febrile adverse events and symptoms associated with Influvac in our studies, and Vaxigrip in New Zealand,19 indicates that TIV containing pandemic (H1N1) 2009 antigen are not inherently highly pyrogenic. Therefore, it seems reasonable that countries implement paediatric vaccination programs as usual using vaccine brands other than those of CSL Biotherapies. However, until a cause can be determined for the Australian findings, and further national and international data are available, it is recommended that enhanced surveillance be undertaken for febrile reactions and other adverse events in all children under 5 years of age receiving TIV.
What this paper adds.
Although the use of trivalent inactivated influenza vaccine (TIV) in young children is considered safe, few population-based studies large enough to accurately quantify the rate of uncommon potential adverse events, including febrile convulsions, following TIV administration have been published.
This study shows that children aged 4 years and under who had received TIV from one vaccine manufacturer (CSL Biotherapies) had a 200-fold higher rate of febrile convulsions than that of the only reliable published estimate.
This is the first peer-reviewed epidemiological report of an elevated rate of severe febrile adverse events following TIV administration in young children.
Until the biological cause is determined, alternative brands of TIV should be used in children aged 4 years and under, and enhanced surveillance for such adverse events should be undertaken.
Supplementary Material
Acknowledgments
We thank the staff of the Western Australian Communicable Disease Control Directorate, the Central Immunisation Clinic, the Vaccine Trials Group at the Telethon Institute for Child Health Research and Princess Margaret Hospital for Children, who contributed to these studies. We also gratefully acknowledge the many immunisation providers and parents who reported cases and assisted with the investigation.
Footnotes
To cite: Armstrong PK, Dowse GK, Effler PV, et al. Epidemiological study of severe febrile reactions in young children in Western Australia caused by a 2010 trivalent inactivated influenza vaccine. BMJ Open 2011;1:e000016. doi:10.1136/bmjopen-2010-000016
Funding: The investigations were funded from the internal resources of the authors' organisations.
Competing interest: None.
Contributors: PA, GD and PE designed the studies. FM, MS, CB, GG and DC were responsible for acquisition of the data. PE and GD were responsible for the statistical analysis. PA, GD, PE, CB, TW, DC and PR analysed and interpreted the data. All authors contributed to the writing of the report. All authors saw and approved the final report. All authors had full access to all of the data (including statistical reports and tables) in the study and can take full responsibility for the integrity of the data and the accuracy of the data analysis.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data sharing statement: Datasets are available from the corresponding author at paul.armstrong@health.wa.gov.au.
References
- 1.Poehling KA, Edwards KM, Weinberg GA, et al. The underrecognized burden of influenza in young children. N Engl J Med 2006;355:31–40 [DOI] [PubMed] [Google Scholar]
- 2.Izurieta HS, Thompson WW, Kramarz P, et al. Influenza and the rates of hospitalization for respiratory disease among infants and young children. N Engl J Med 2000;342:232–9 [DOI] [PubMed] [Google Scholar]
- 3.Fiore AE, Uyeki TM, Broder K, et al. Centers for Disease Control and Prevention. Prevention and control of seasonal influenza with vaccines: recommendations of the Advisory Committee on Immunization Practices (ACIP), 2010. MMWR Recomm Rep 2010;59:1–62 [PubMed] [Google Scholar]
- 4.Australia Government Department of Health and Ageing, Immunise Australia Program. Influenza Vaccination for Individuals aged 6 months and over with medical conditions predisposing to severe influenza. http://www.immunise.health.gov.au/internet/immunise/publishing.nsf/Content/immunise-influenza#infants (accessed 20 Jun 2010).
- 5.France EK, Glanz JM, Xu S, et al. Safety of the trivalent inactivated influenza vaccine among children: a population-based study. Arch Pediatr Adolesc Med 2004;158:1031–6 [DOI] [PubMed] [Google Scholar]
- 6.Rosenberg M, Sparks R, McMahon A, et al. Serious adverse events rarely reported after trivalent inactivated influenza vaccine (TIV) in children 6–23 months of age. Vaccine 2009;27:4278–83 [DOI] [PubMed] [Google Scholar]
- 7.McMahon AW, Iskander J, Haber P, et al. Adverse events after inactivated influenza vaccination among children less than 2 years of age: analysis of reports from the vaccine adverse event reporting system, 1990–2003. Pediatrics 2005;115:453–60 [DOI] [PubMed] [Google Scholar]
- 8.Hambidge SJ, Glanz JM, France EK, et al. Vaccine Safety Datalink Team Safety of trivalent inactivated influenza vaccine in children 6 to 23 months old. JAMA 2006;296:1990–7 [DOI] [PubMed] [Google Scholar]
- 9.Neuzil KM, Dupont WD, Wright PF, et al. Efficacy of inactivated and cold-adapted vaccines against influenza A infection, 1985 to 1990: the pediatric experience. Pediatr Infect Dis J 2001;20:733–40 [DOI] [PubMed] [Google Scholar]
- 10.Nolan T, Richmond PC, McVernon J, et al. Safety and immunogenicity of an inactivated thimerosal-free influenza vaccine in infants and children. Influenza Other Respi Viruses 2009;3:315–25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Baxter R, Jeanfreau R, Block SL, et al. A Phase III evaluation of immunogenicity and safety of two trivalent inactivated seasonal influenza vaccines in US children. Pediatr Infect Dis J 2010;29:924–30 [DOI] [PubMed] [Google Scholar]
- 12.Australian Government Department of Health and Ageing The Australian Immunisation Handbook. 9th edn Australian Government Department of Health and Ageing, 2008 [Google Scholar]
- 13.Menzies R, Mahajan D, Gold MS, et al. Annual report: surveillance of adverse events following immunisation in Australia, 2008. Commun Dis Intell 2009;33:365–81 [PubMed] [Google Scholar]
- 14.World Health Organization Global Alert and Response. Recommended Composition of Influenza Virus Vaccines for use in the 2010 Southern Hemisphere Influenza Season. http://www.who.int/csr/disease/influenza/recommendations2010south/en/index.html (accessed 20 Jun 2010).
- 15.World Health Organization International Statistical Classification of Diseases and Related Health Problems: 10th Revision Version for 2007. http://apps.who.int/classifications/apps/icd/icd10online (accessed 20 Jun 2010).
- 16.Bonhoeffer J, Menkes J, Gold MS; Brighton Collaboration Seizure Working Group. Generalized convulsive seizure as an adverse event following immunization: case definition and guidelines for data collection, analysis, and presentation. Vaccine 2004;22:557–62 [DOI] [PubMed] [Google Scholar]
- 17.Australian Government Department of Health and Ageing Therapeutic Goods Adminstration Overview of Vaccine Regulation and Safety Monitoring and Investigation into Adverse Events Following 2010 Seasonal Influenza Vaccination in Young Children. 2010. http://www.tga.gov.au/alerts/medicines/vaccine-overview.htm (accessed 10 Jan 2011). [Google Scholar]
- 18.Dixon GA, Moore HC, Kelly H, et al. Lessons from the first year of the WAIVE study investigating the protective effect of influenza vaccine against laboratory-confirmed influenza in hospitalised children aged 6–59 months. Influenza Other Respi Viruses 2010;4:231–4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Australia Government Department of Health and Ageing, Immunise Australia Program ATAGI Statement: Clinical advice for Immunisation Providers on Resumption of the Use of 2010 Trivalent Seasonal Vaccines in Children Less Than 5 years of Age: 30 July 2010. http://www.immunise.health.gov.au/internet/immunise/Publishing.nsf/content/immunise-atagi-statement-tiv (accessed 15 Aug 2010).
- 20.Australia Government Department of Health and Ageing, Immunise Australia Program Chief Medical Officer Advice. 7 March 2011: Seasonal Influenza Vaccination. http://www.health.gov.au/internet/immunise/publishing.nsf/Content/immunise-cmo (accessed 20 Apr 2011).
- 21.Centers for Disease Control and Prevention (CDC) Update: Recommendations of the Advisory Committee on Immunization Practices (ACIP) Regarding Use of CSL Seasonal Influenza Vaccine (Afluria) in the United States During 2010–11. MMWR Morb Mortal Wkly Rep 2010;59;989–92 [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.