Abstract
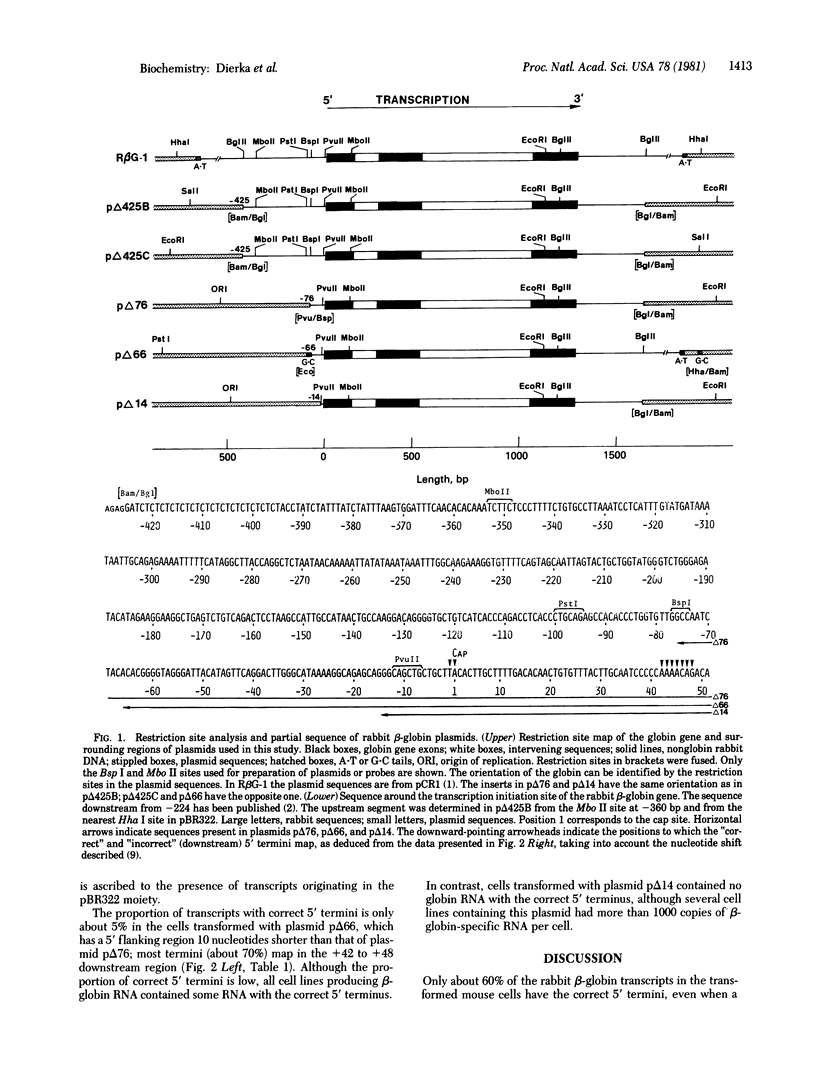
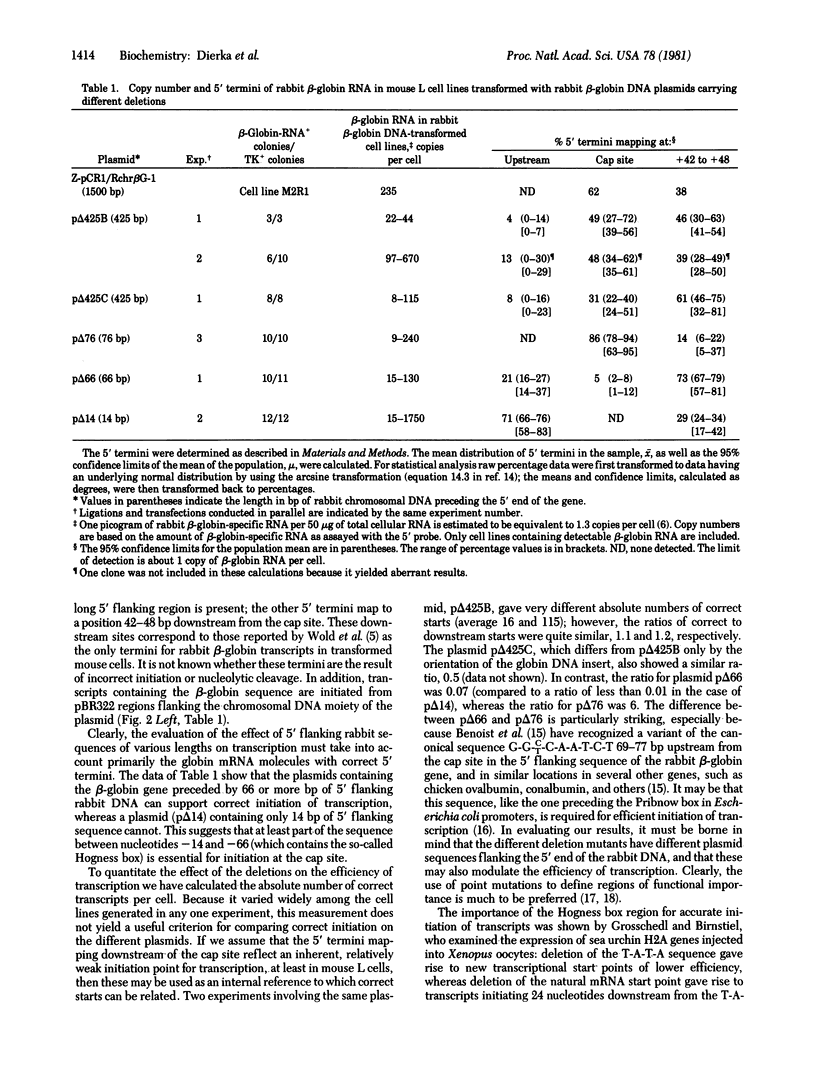
Mouse thymidine kinase-negative (TK-) L cells were transformed with concatenates of cloned herpes simplex virus 1 TK DNA and different rabbit beta-globin DNAs in which the globin genes were preceded by native flanking sequences of 14, 66, 76, 425, and 1500 nucleotides.l In all cases, selection for TK+ cell lines led to a high yield of lines producing 5-1500 mature rabbit beta-globin-specific RNA strands per cell. The 5' termini of the transcripts mapped to (i) the "cap" nucleotide, (ii) positions 42 to 48 nucleotides downstream from the cap site, or (iii) positions in the vector DNA preceding the gene. In the case of the gene with only 14 base pairs of 5' flanking sequence, a high level of rabbit beta-globin RNA was produced, but none of the transcripts had the correct 5' end; most of them originated in the vector moiety. With 66 base pairs of 5' flanking sequence, 5% of the 5' termini were correct, and with 76 or more base pairs, 30-85% were correct. The region between 14 and 66 base pairs preceding the cap site contains the Hogness box and appears to be essential for correct initiation of transcription. The region between 66 and 76 base pairs before the cap site contains a variant of the canonical sequence G-G-C-T-C-A-A-T-C-T found preceding many other genes at a similar location, and this region may modulate the efficiency of transcription. The sequence of 425 nucleotides preceding the rabbit beta-globin gene is reported.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Auffray C., Rougeon F. Purification of mouse immunoglobulin heavy-chain messenger RNAs from total myeloma tumor RNA. Eur J Biochem. 1980 Jun;107(2):303–314. doi: 10.1111/j.1432-1033.1980.tb06030.x. [DOI] [PubMed] [Google Scholar]
- Benoist C., O'Hare K., Breathnach R., Chambon P. The ovalbumin gene-sequence of putative control regions. Nucleic Acids Res. 1980 Jan 11;8(1):127–142. doi: 10.1093/nar/8.1.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berk A. J., Sharp P. A. Sizing and mapping of early adenovirus mRNAs by gel electrophoresis of S1 endonuclease-digested hybrids. Cell. 1977 Nov;12(3):721–732. doi: 10.1016/0092-8674(77)90272-0. [DOI] [PubMed] [Google Scholar]
- Corden J., Wasylyk B., Buchwalder A., Sassone-Corsi P., Kedinger C., Chambon P. Promoter sequences of eukaryotic protein-coding genes. Science. 1980 Sep 19;209(4463):1406–1414. doi: 10.1126/science.6251548. [DOI] [PubMed] [Google Scholar]
- Flavell R. A., Sabo D. L., Bandle E. F., Weissmann C. Site-directed mutagenesis: generation of an extracistronic mutation in bacteriophage Q beta RNA. J Mol Biol. 1974 Oct 25;89(2):255–272. doi: 10.1016/0022-2836(74)90517-8. [DOI] [PubMed] [Google Scholar]
- Graham F. L., van der Eb A. J. A new technique for the assay of infectivity of human adenovirus 5 DNA. Virology. 1973 Apr;52(2):456–467. doi: 10.1016/0042-6822(73)90341-3. [DOI] [PubMed] [Google Scholar]
- Grosschedl R., Birnstiel M. L. Identification of regulatory sequences in the prelude sequences of an H2A histone gene by the study of specific deletion mutants in vivo. Proc Natl Acad Sci U S A. 1980 Mar;77(3):1432–1436. doi: 10.1073/pnas.77.3.1432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grosschedl R., Birnstiel M. L. Spacer DNA sequences upstream of the T-A-T-A-A-A-T-A sequence are essential for promotion of H2A histone gene transcription in vivo. Proc Natl Acad Sci U S A. 1980 Dec;77(12):7102–7106. doi: 10.1073/pnas.77.12.7102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mantei N., Boll W., Weissmann C. Rabbit beta-globin mRNA production in mouse L cells transformed with cloned rabbit beta-globin chromosomal DNA. Nature. 1979 Sep 6;281(5726):40–46. doi: 10.1038/281040a0. [DOI] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. A new method for sequencing DNA. Proc Natl Acad Sci U S A. 1977 Feb;74(2):560–564. doi: 10.1073/pnas.74.2.560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stefano J. E., Ackerson J. W., Gralla J. D. Alterations in two conserved regions of promoter sequence lead to altered rates of polymerase binding and levels of gene expression. Nucleic Acids Res. 1980 Jun 25;8(12):2709–2723. doi: 10.1093/nar/8.12.2709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wasylyk B., Derbyshire R., Guy A., Molko D., Roget A., Téoule R., Chambon P. Specific in vitro transcription of conalbumin gene is drastically decreased by single-point mutation in T-A-T-A box homology sequence. Proc Natl Acad Sci U S A. 1980 Dec;77(12):7024–7028. doi: 10.1073/pnas.77.12.7024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wasylyk B., Kédinger C., Corden J., Brison O., Chambon P. Specific in vitro initiation of transcription on conalbumin and ovalbumin genes and comparison with adenovirus-2 early and late genes. Nature. 1980 Jun 5;285(5764):367–373. doi: 10.1038/285367a0. [DOI] [PubMed] [Google Scholar]
- Weaver R. F., Weissmann C. Mapping of RNA by a modification of the Berk-Sharp procedure: the 5' termini of 15 S beta-globin mRNA precursor and mature 10 s beta-globin mRNA have identical map coordinates. Nucleic Acids Res. 1979 Nov 10;7(5):1175–1193. doi: 10.1093/nar/7.5.1175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber H., Taniguchi T., Müller W., Meyer F., Weissmann C. Application of site-directed mutagenesis to RNA and DNA genomes. Cold Spring Harb Symp Quant Biol. 1979;43(Pt 2):669–677. doi: 10.1101/sqb.1979.043.01.075. [DOI] [PubMed] [Google Scholar]
- Wigler M., Pellicer A., Silverstein S., Axel R. Biochemical transfer of single-copy eucaryotic genes using total cellular DNA as donor. Cell. 1978 Jul;14(3):725–731. doi: 10.1016/0092-8674(78)90254-4. [DOI] [PubMed] [Google Scholar]
- Wigler M., Sweet R., Sim G. K., Wold B., Pellicer A., Lacy E., Maniatis T., Silverstein S., Axel R. Transformation of mammalian cells with genes from procaryotes and eucaryotes. Cell. 1979 Apr;16(4):777–785. doi: 10.1016/0092-8674(79)90093-x. [DOI] [PubMed] [Google Scholar]
- Wilkie N. M., Clements J. B., Boll W., Mantei N., Lonsdale D., Weissmann C. Hybrid plasmids containing an active thymidine kinase gene of Herpes simplex virus 1. Nucleic Acids Res. 1979 Oct 25;7(4):859–877. doi: 10.1093/nar/7.4.859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wold B., Wigler M., Lacy E., Maniatis T., Silverstein S., Axel R. Introduction and expression of a rabbit beta-globin gene in mouse fibroblasts. Proc Natl Acad Sci U S A. 1979 Nov;76(11):5684–5688. doi: 10.1073/pnas.76.11.5684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Ooyen A., van den Berg J., Mantei N., Weissmann C. Comparison of total sequence of a cloned rabbit beta-globin gene and its flanking regions with a homologous mouse sequence. Science. 1979 Oct 19;206(4416):337–344. doi: 10.1126/science.482942. [DOI] [PubMed] [Google Scholar]
- van den Berg J., van Ooyen A., Mantei N., Schamböck A., Grosveld G., Flavell R. A., Weissmann C. Comparison of cloned rabbit and mouse beta-globin genes showing strong evolutionary divergence of two homologous pairs of introns. Nature. 1978 Nov 2;276(5683):37–44. doi: 10.1038/276037a0. [DOI] [PubMed] [Google Scholar]




