Abstract
Objective
To examine if the beneficial effect of statin medication on mortality seen in randomised clinical trials of type 2 diabetes applies equally to observational studies in the general population of older people.
Design
A prospective, population-based cohort study.
Setting
Reykjavik, Iceland.
Participants
5152 men and women from the Age, Gene/Environment Susceptibility-Reykjavik Study, mean age 77 years, range of 66–96 years.
Main outcome measure
Cardiovascular and all-cause mortalities and the RR of dying according to statin use and history of coronary heart disease (CHD) in persons with type 2 diabetes and those without diabetes with a median follow-up time of 5.3 years, until end of 2009.
Results
The prevalence of type 2 diabetes was 12.4% of which 35% used statins. Statin use was associated with a 50% (95% CI 8% to 72%) lower cardiovascular mortality and 53% (29% to 68%) lower all-cause mortalities in persons with diabetes. For those without diabetes, statin use was associated with a 16% (−24% to 43%) lower cardiovascular and 30% (11% to 46%) lower all-cause mortalities. Persons with diabetes using statins had a comparable risk of cardiovascular and all-cause mortality to that of the general population without diabetes. The effect was independent of the level of glycaemic control.
Conclusion
This observational study lends important support to existing data from randomised clinical trials. These data suggest that in the general population of older people with diabetes, statin medication markedly reduces the excess cardiovascular and all-cause mortality risk, irrespective of the presence or absence of coronary heart disease or glucose-lowering medication.
Keywords: Cohort study, type 2 diabetes, statins, older persons, cardiovascular disease mortality, AGES-Reykjavik
Article summary
Article focus
Clinical trials have shown that statin medication is beneficial for persons with diabetes as regards cardiovascular morbidity and mortality.
This is not well established, except within the rigours of randomised clinical studies.
Key messages
This population-based observational study of older individuals demonstrates that treatment with statins in persons with diabetes reduces cardiovascular mortality to a level comparable with that observed in those without diabetes.
The effect observed is of a magnitude comparable with that reported in randomised clinical trials.
Strengths and limitations of this study
A major strength of the study is the proportionally large national representation in this population-based cohort, the high participation rate and the comprehensive information on morbidity and mortality. The effect observed is of comparable magnitude to the effect reported in randomised clinical trials.
A limitation is the non-attendance of frail individuals in the study that may cause a possible bias towards more healthy individuals at baseline of this study. Non-attendees in the study have been shown, however, at earlier visits to have comparable levels of conventional cardiovascular risk factors. A limitation is the unavailability of dietary information for this analysis. A weakness in our study is the relatively low number of events during the 5-year follow-up. A limitation is the lack of glucose tolerance test for diagnosis of diabetes.
Introduction
The excess risk of vascular disease in persons with diabetes is about twofold compared with those without diabetes and is independent of other conventional cardiovascular risk factors. This was clearly demonstrated in the meta-analysis from 102 prospective studies, recently published by the Emerging Risk Factors Collaboration.1 The beneficial effects of statins in reducing major vascular events in patients with diabetes, irrespective of their baseline lipid levels, have been demonstrated in a number of randomised clinical trials.2–5 Additionally, improved life expectancy in recent years of persons with type 2 diabetes, relative to those without diabetes, has been reported,6–8 although a difference still exists. This improvement is possibly a result of better adherence to published clinical guidelines, advocating aggressive multifactorial treatment.9 However, many patients with type 2 diabetes are still not receiving treatment with statin medication.10 11
Although randomised clinical trials have clearly demonstrated the benefit of statin use with regard to cardiovascular morbidity and mortality, every physician will daily face the question of whether these trial results apply to their patient. A general population of individuals, with a varied background of comorbidities which may or may not have excluded them from participation, is not fully represented in the aforementioned trials. This is why it is of key importance to gather confirmatory information from population-based observational studies. One recent prospective population-based study10 from the UK General Practice Database has indeed reported the beneficial effect of statin treatment in lowering all-cause mortality in type 2 diabetes. Older persons are under-represented in clinical trial data, and it is therefore particularly important to obtain information on the potential improvement in life expectancy with statin use in older persons with type 2 diabetes, as well as data on the relative impact of lipid-lowering treatment in that age group. This will help in clarifying at a population-based level whether current guidelines should be applied to older persons, who may have had diabetes for an extended period of time.
The present study addresses this information gap by exploring treatment modalities and cardiovascular and total mortality in older individuals with diabetes in the population-based Age, Gene/Environment Susceptibility (AGES)-Reykjavik Study.
Methods
Study population
Between 2002 and 2006, the AGES-Reykjavik Study re-examined 5764 unselected survivors of the original cohort who had previously participated in the Reykjavik Study.12 In the present study, 5152 of these survivors are included, with a mean age of 77 years (range 66–96) and a median follow-up time of 5.3 years until the end of 2009. Informed consent was obtained from all study participants. The Reykjavik Study cohort originally comprised a random sample of 30 795 men and women born in 1907–1935, living in Reykjavik in 1967 that were invited to participate in a long-term prospective cardiovascular survey. A total of 19 381 attended, resulting in a 71% recruitment rate.12
As part of the baseline examination in the AGES-Reykjavik Study, a comprehensive questionnaire was administered. In order to eliminate any persons with type 1 diabetes in the study, participants reporting onset of diabetes before the age of 40 were not included; neither were participants not completing their questionnaire or having incomplete data for other study variables included: 66 had missing data about diabetes history on questionnaire; 21 were considered to have diabetes of type 1; 78 had missing data on risk factors (cholesterol, systolic blood pressure, body mass index, triglycerides); 447 had missing haemoglobin A1c (HbA1c). Participants were asked to bring all medications and supplements used in the previous 2 weeks to the clinic. All participants also had a fasting blood specimen drawn and analysed as documented below.
The criteria used for type 2 diabetes diagnosis were a fasting serum glucose of ≥7 mmol/l at the visit to the clinic, based on the WHO recommendations from 1999,13 self-reported diabetes in the questionnaire and/or use of diabetes medication.
Blood samples were drawn after overnight fasting. Total cholesterol, high-density lipoprotein (HDL) cholesterol, triglycerides, high-sensitivity C-reactive protein (CRP), glucose and HbA1c were analysed on a Hitachi 912, using reagents from Roche Diagnostics and following the manufacturer's instructions. Low-density lipoprotein (LDL) was calculated using the Friedewald equation.14
Blood pressure was measured with a mercury sphygmomanometer with a large cuff, and the mean value of two consecutive blood pressure measurements was used in the analysis. Height and weight were measured, and BMI was calculated as kg/m2.
Participants answered questions about frequency of moderate or vigorous physical activity, both current and in midlife. Answers were categorised into never, rarely, occasionally, moderate or high frequency of participation. In this study, a binary variable for physical activity was used as an indicator for occasional or a higher frequency of participation versus never or rarely participating.
Answers about education were categorised into a binary variable: higher than secondary education versus secondary education or less.
Information on the causes of death was based on data from a complete adjudicated registry of deaths available from the Icelandic National Roster (http://www.statice.is/Statistics/Population/Births-and-deaths). All-cause mortality was defined according to ICD 9-10. In this study, we calculated an individual's time at risk from the date of participation in the baseline survey until the date of death from cardiovascular disease (ICD-9 and ICD-10: defined as in the SCORE project15) or from all causes, or until the end of follow-up in the cohort. The information is collected from National Health System Records by the Icelandic Heart Association.
The study was approved by the National Bioethics Committee in Iceland (VSN 00-063) as well as the Institutional Review Board of the Intramural Research Program of the National Institute on Ageing and the Data Protection Authority in Iceland.
Statistical analyses
Baseline characteristics of participants by sex and diabetic status in the AGES-Reykjavik Study were compared using either linear or logistic regression with age adjustment. Skewed variables were log-transformed. The Cox proportional hazards regression model was used to estimate mortalities and HRs for the effect of risk factors and statin use. Time since entering the study was used as the timescale. For HR estimates, an adjustment was made for age and sex in a simple model, and additionally for the following cardiovascular risk factors: cholesterol, HDL cholesterol, systolic blood pressure, BMI, triglycerides, CRP, hypertensive medication and current smoking. An additional analysis of mortalities was done with adjustment for physical activity and education level. A separate term was used in the survival models to represent subgroups, formed by diabetes, history of CHD and statin use. The mortality was estimated from the average of the cumulative hazard function after a five-year follow-up and represented as the rate per 1000 person-years. The proportionality assumption for the HR associated with type 2 diabetes was inspected graphically and by testing the significance of the interaction of type 2 diabetes statuses with the logarithm of the follow-up of time analysed as a time-dependent covariate. Significance testing was two-sided and based on a 5% probability level. We analysed the data using SAS/STAT® software, version 9.2.
Results
The mean age of the 5152 AGES-Reykjavik Study participants was 77.0 (±5.8) years, with an age range of 66–96 years. The baseline characteristics in men and women with and without type 2 diabetes according to statin use are shown in table 1. A higher percentage of individuals with type 2 diabetes were hypertensive than those without diabetes; they also had lower HDL cholesterol but higher triglycerides and BMI, irrespective of statin use. Statin medication reduced the mean level of total and LDL cholesterol similarly in those with and without diabetes, or by about 1.2 mmol/l in both men and women. In statin users, CRP was lower by 0.30 mg/l in men and 0.50 mg/l in women without diabetes and 0.80 and 1.05 mg/l respectively in those with diabetes. The prevalence of coronary heart disease estimated from hospital records in persons without diabetes using statins was 71.1% in men and 34.4% in women compared with 7.9% and 2.2% respectively in those not using statins. In individuals with diabetes, the coronary heart disease prevalence was 59.4% in men and 24.2% in women using statins, compared with 14.8% and 8.6% respectively in those not on statins. Over 93% of all statin users were hypertensive, compared with 78% of non-statin users.
Table 1.
Baseline characteristics according to statin use in men and women with and without type 2 diabetes (T2D) in the AGES-Reykjavik Study, 2002–2006
| Variables | Men |
Women |
||||||
| Statins: no |
Statins: yes |
Statins: no |
Statins: yes |
|||||
| Mean±SD, IQR or % | Without T2D | With T2D | Without T2D | With T2D | Without T2D | With T2D | Without T2D | With T2D |
| No | 1357 | 230 | 477 | 128 | 2237 | 186 | 442 | 95 |
| Age, years | 77.4 (±5.8) | 77.9 (±6.1) | 76.0 (±4.8) | 75.5 (±4.4) | 77.0 (±6.1) | 78.7 (±6.0)*** | 76.1 (±4.8) | 76.3 (±4.5) |
| Cholesterol, mmol/l | 5.57 (±0.93) | 5.38 (±0.98)** | 4.32 (±0.82) | 4.14 (±0.84)* | 6.19 (±1.05) | 5.97 (±1.01)** | 4.96 (±0.83) | 4.67 (±0.94)** |
| High-density-lipoprotein cholesterol, mmol/l | 1.46 (±0.40) | 1.26 (±0.34)*** | 1.40 (±0.37) | 1.26 (±0.33)*** | 1.75 (±0.45) | 1.53 (±0.41)*** | 1.70 (±0.40) | 1.48 (±0.41)*** |
| Low-density-lipoprotein cholesterol, mmol/l | 3.61 (±0.85) | 3.40 (±0.89)** | 2.38 (±0.67) | 2.21 (±0.68)** | 3.90 (±0.98) | 3.73 (±0.91)* | 2.71 (±0.72) | 2.41 (±0.70)*** |
| Triglycerides, mmol/l, median (IQR) | 0.97 (0.56) | 1.33 (0.83)*** | 1.01 (0.65) | 1.29 (0.98)*** | 1.04 (0.63) | 1.38 (0.85)*** | 1.11 (0.67) | 1.57 (0.85)*** |
| C-reactive protein, mg/l, median (IQR) | 1.90 (2.70) | 2.20 (3.53)** | 1.60 (2.20) | 1.40 (2.10) | 2.00 (3.20) | 3.15 (5.65)*** | 1.50 (2.10) | 2.10 (3.30)* |
| BMI, kg/m2 | 26.4 (±3.7) | 28.3 (±4.2)*** | 27.0 (±3.7) | 28.4 (±3.7)*** | 27.0 (±4.9) | 29.0 (±5.5)*** | 26.8 (±4.0) | 30.2 (±5.1)*** |
| Systolic BP, mm Hg | 142.9 (±20.6) | 141.1 (±20.7) | 144.1 (±20.3) | 148.2 (±19.6)*** | 142.2 (±21.0) | 143.3 (±21.8) | 143.3 (±20.2) | 143.6 (±19.7) |
| Diastolic BP, mm Hg | 77.0 (±9.4) | 74.7 (±10.8)** | 74.4 (±9.7) | 74.6 (±10.3) | 72.6 (9.3) | 70.5 (±10.5)* | 71.6 (±10.2) | 69.7 (±8.9) |
| Hypertension (%)* | 73.5 | 88.7*** | 91.6 | 95.3 | 78.9 | 88.7** | 92.5 | 95.8 |
| Hypertensive medication (%) | 50.0 | 75.2*** | 85.5 | 87.5 | 58.7 | 79.6*** | 84.6 | 90.5 |
| Glucose-lowering medication | – | 46.1 | – | 64.1 | – | 36.6 | – | 64.2 |
| Coronary heart disease prevalence (%)† | 7.9 | 14.8** | 71.1 | 59.4* | 2.2 | 8.6*** | 34.4 | 24.2* |
| Family history of myocardial infarction (%) | 30.1 | 39.1** | 45.2 | 41.4 | 40.2 | 51.6** | 56.7 | 53.7 |
| Physical activity in midlife (%) | 51.0 | 43.9 | 54.3 | 45.3 | 47.1 | 40.9 | 50.7 | 36.8* |
| Physical activity current (%) | 39.6 | 27.4*** | 45.3 | 35.2* | 31.3 | 26.3 | 34.2 | 16.8* |
| Education, more than secondary (%) | 30.0 | 27.4 | 29.8 | 28.9 | 21.9 | 20.4 | 17.2 | 16.8 |
| Smoking (%) | 11.9 | 12.2 | 9.0 | 9.4 | 12.4 | 10.2 | 12.9 | 9.5 |
| Haemoglobin A1c, % | 5.54 (±0.33) | 6.38 (±0.88)*** | 5.58 (±0.32) | 6.55 (±0.79)*** | 5.61 (±0.33) | 6.32 (±0.95)*** | 5.64 (±0.34) | 6.58 (±0.88)*** |
| Glucose, mmol/l | 5.57 (±0.50) | 7.95 (±2.09)*** | 5.58 (±0.53) | 7.92 (±2.19)*** | 5.44 (±0.51) | 7.60 (±2.00)*** | 5.41 (±0.52) | 7.97 (±2.38)*** |
Significance estimates for age-adjusted comparison between those with and without T2D and not using statins (no) and statin users (yes): *p<0.05; **p<0.01; ***p<0.001.
Hypertensive includes those with systolic blood pressure (BP) of >140 mm Hg or diastolic BP of >90 mm Hg, or on hypertensive medication.
Prevalence from history of myocardial infarction, percutaneous coronary intervention, and coronary-artery bypass grafting in hospital records.
The prevalence of type 2 diabetes and use of glucose-lowering treatment in men and women is shown in table 2. About 16% of men and 9.5% of women in the cohort had type 2 diabetes, and the proportion of persons with diabetes undiagnosed at baseline was 31%. In the group with previously diagnosed type 2 diabetes, 23% of the men and 35% of the women controlled their blood sugar level with diet only. As shown in table 2, less than 7% of persons with diagnosed diabetes were simultaneously taking three or four drugs for lowering blood glucose. For participants with a prior diagnosis of diabetes, the average time from diagnosis at baseline assessment was just over 10 years.
Table 2.
Prevalence of type 2 diabetes (T2D) and glucose-lowering treatment in the Age, Gene/Environment Susceptibility-Reykjavik Study, 2002–2006
| AGES-Reykjavik Study | Men | Women | Men and women | Percentage of total T2D |
| Total T2D at baseline, % (n) | 16.3 (358) | 9.5 (281) | 12.4 (639) | 100 |
| Diagnosed at study entry, % (n) | 5.1 (113) | 2.8 (84) | 3.8 (197) | 31 |
| With prevalent T2D at study entry, % (n) | 11.2 (245) | 6.7 (197) | 8.6 (442) | 69 |
| Mean T2D duration, years (±SD) | 10.7 (±10.0) | |||
| Glucose-lowering treatment in prevalent T2D | ||||
| On special diet only, % (n) | 23.3 (57) | 34.5 (68) | ||
| On diabetic medication, % (n) | 76.7 (188) | 65.5 (129) | ||
| Using one drug, % (n) | 43.7 (107) | 40.1 (79) | ||
| Using two drugs, % (n) | 25.7 (63) | 19.8 (39) | ||
| Using three drugs, % (n) | 6.9 (17) | 4.6 (9) | ||
| Using four drugs, % (n) | 0.4 (1) | 1.0 (2) | ||
Effect of statins on cardiovascular and all-cause mortality
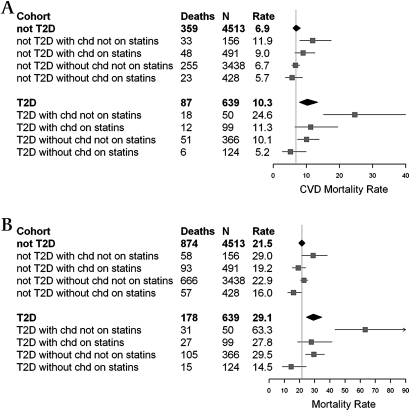
The 5-year average cardiovascular disease mortality and all-cause mortalities for those with and without diabetes are shown in figure 1. Mortality is estimated according to statin use and prevalence of coronary heart disease, adjusted to age 75, sex and the mean levels of cardiovascular risk factors (cholesterol, HDL cholesterol, systolic blood pressure, BMI, triglycerides, hypertensive medication and current smoking) within each cohort. About 26% of men and 16% of women without diabetes and about 35% in both sexes with diabetes were statin users. Statin medication was administered to 69.2% of persons with prevalent coronary heart disease and known diabetes compared with 31.6% of those with known diabetes but without coronary heart disease (supplementary table 1).
Figure 1.
(A) Cardiovascular disease (CVD) mortality and (B) all-cause mortality per 1000 person years for subjects without type 2 diabetes (not T2D) and with type 2 diabetes (T2D) according to statin use and prevalent coronary heart disease (CHD). Rates have been adjusted to age 75, sex and the mean levels of cardiovascular risk factors (cholesterol, high-density-lipoprotein cholesterol, systolic blood pressure, BMI, triglycerides, hypertensive medication and current smoking) within each cohort. Follow-up was through 2009 (a median period of 5.3 years) for the Age, Gene/Environment Susceptibility-Reykjavik Study. The vertical lines represent the mortality of all without diabetes (not T2D, N=4513).
For individuals with diabetes and prevalent coronary heart disease, statin use was associated with a significantly lower rate of cardiovascular disease mortality (figure 1A) compared with those not using statins, or 11.3 vs 24.6 per 1000 person years. This amounts to a 54% (95% CI 14% to 75%) lower mortality in statin users. Similarly, statin use was associated with an all-cause mortality of 27.8 vs 63.3 per 1000 person years when comparing the same two groups (figure 1B), amounting to 55% (31% to 71%) lower mortality in statin users. In individuals with diabetes but without coronary heart disease, the rate was 48% (1% to 73%) lower for cardiovascular disease mortality and 52% (26% to 69%) lower for all-cause mortality in the group using statins compared with non-statin users. Combining the groups, the hazard of cardiovascular disease mortality was 50% lower in statin users compared with non-statin users, and the all-cause mortality was 53% lower (supplementary table 2).
Statin use was associated with 16% (−24% to 43%) lower cardiovascular mortality in individuals without diabetes, as shown in figure 1, albeit not statistically significant. For all-cause mortality, statin users had a 30% (11% to 46%) lower mortality than non-statin users.
The effect of statins was not modified by the level of HbA1c, the use of oral hypoglycaemic or hypertensive medication, or whether the diabetes was prevalent or newly diagnosed (supplementary figures 1, 2).
An additional analysis of mortalities with adjustment for current physical activity and education level did not have any material effect on the results or the conclusions drawn from the data. The additionally adjusted mortalities are shown in supplementary figure 3.
The dramatic effect of statin medication use on mortality in persons with diabetes is also reflected in the HRs for the RR of cardiovascular and all-cause mortality in individuals with diabetes compared with those without, as shown in table 3. In persons with diabetes and prevalent coronary heart disease not on statins, the RR of dying from cardiovascular disease was 3.33 (2.05–5.50) when compared with those without diabetes, after adjusting for age, sex and cardiovascular risk factors. The individuals treated with statins, however, had an RR of 1.51 (0.83–2.75). In persons with diabetes but without coronary heart disease not on statins, the RR was 1.40 (1.03–1.89) compared with 0.71 (0.31–1.62) for those on statins. Adding CRP to the risk factor adjustment showed minimal attenuation of the HRs (table 3). This implies that the protective effect of statins is not solely attributable to the effect on these cardiovascular risk factors.
Table 3.
HRs for the RR of cardiovascular disease (CVD) mortality and all-cause mortality in people with type 2 diabetes (T2D) compared with all non-diabetics according to prevalent coronary heart disease (chd) and statin use* in the AGES-Reykjavik Study, 2002–2006
| Adjusted for age and sex | Adjusted for age, sex, and CVD risk factors† | Adjusted for age, sex, CVD risk factors† and C-reactive protein | |
| HR (95% CI) | HR (95% CI) | HR (95% CI) | |
| Death from CVD | |||
| All T2D | 1.71 (1.35 to 2.17) | 1.48 (1.15 to 1.90) | 1.45 (1.13 to 1.86) |
| According to CHD and statin use | |||
| T2D with CHD not on statins | 4.71 (2.93 to 7.58) | 3.33 (2.02 to 5.50) | 3.20 (1.94 to 5.29) |
| T2D with CHD on statins | 1.92 (1.08 to 3.43) | 1.51 (0.83 to 2.75) | 1.55 (0.85 to 2.82) |
| T2D without CHD and not on statins | 1.53 (1.14 to 2.05) | 1.40 (1.03 to 1.89) | 1.34 (0.99 to 1.82) |
| T2D without CHD and on statins | 0.83 (0.37 to 1.85) | 0.71 (0.31 to 1.62) | 0.75 (0.33 to 1.69) |
| Death from all causes | |||
| All T2D | 1.44 (1.22 to 1.69) | 1.35 (1.14 to 1.61) | 1.32 (1.11 to 1.57) |
| According to CHD and statin use | |||
| T2D with CHD not on statins | 3.48 (2.43 to 4.99) | 2.88 (1.98 to 4.18) | 2.72 (1.87 to 3.95) |
| T2D with CHD on statins | 1.61 (1.10 to 2.37) | 1.34 (0.90 to 1.99) | 1.37 (0.92 to 2.04) |
| T2D without CHD not on statins | 1.34 (1.09 to 1.64) | 1.34 (1.08 to 1.64) | 1.27 (1.03 to 1.57) |
| T2D without CHD on statins | 0.77 (0.46 to 1.28) | 0.70 (0.42 to 1.17) | 0.73 (0.44 to 1.23) |
Individuals on statin medication are identified as on statins; those not on statin medication as not on statins.
CVD risk factors: cholesterol, high-density-lipoprotein cholesterol, systolic blood pressure, BMI, triglycerides, hypertensive medication and current smoking.
Discussion
The major finding in our population-based AGES-Reykjavik Study of older individuals with diabetes is the marked improvement in survival associated with statin medication when compared with those not receiving statins, with respect to cardiovascular and all-cause mortality. This is independent of prevalent coronary heart disease or glucose-lowering treatment, reducing the mortality in individuals with diabetes to a level comparable with those without diabetes.
Statin medication in diabetes has increased gradually during the last decade. Data from the UK General Practice Research Database showed an increase in statin use from about 5% in 1996 to 63.5% in women and 71.0% in men in 2005,10 which was associated with HRs of mortality of 0.29 in women and 0.34 in men in the first 2 years following diagnosis of diabetes. Similarly, in Denmark, only 7% of patients receiving glucose-lowering medication also received statins in 1997, but this had increased to 62% in 200711; information on mortality in that study is however lacking. In our cohort of older persons with diabetes, the prevalence of statin medication use was 35% overall. In those with known diabetes but without coronary heart disease, the prevalence of statin medication use was 31.6%, but 11.6% in those newly diagnosed without coronary heart disease. One may speculate if this low prevalence of statin use is due to Icelandic physicians' unawareness of results from recent clinical trials or simply their belief that the evidence does not apply to older patients in general.
It is of course well recognised that statin treatment favourably impacts vascular event rates in both those with and without diabetes. In the Heart Protection Study,2 for example, it was concluded that 40 mg of simvastatin daily would probably reduce the rate of first major vascular event by about a third. Similarly a 2-year treatment period with atorvastatin in type 2 diabetes in the Collaborative Atorvastatin Diabetes Study reduced the death rate by 27%, prompting an early termination of the trial.16 The authors concluded that lipid-lowering treatment should at least receive the same attention as glycaemic and blood-pressure control in patients with type 2 diabetes without a history of cardiovascular disease, which is in harmony with current clinical guidelines. Aggressive multifactorial treatment of high-risk individuals with type 2 diabetes has been shown to be important,9 and may also be appropriate for persons diagnosed above the age of 70. Recent results from several large studies17 18 have, however, indicated that caution may be needed as regards aggressive glucose-lowering treatment in those with a long-established coronary heart disease and diabetes of long duration. In our study, statin use at baseline, but not use of glucose-lowering medication, is associated with the marked reduction in cardiovascular and total mortality, reducing mortality in those with diabetes to a level similar to those without diabetes.
Statin medication under the rigours of controlled study conditions in selected individuals has thus been shown to be helpful.2 4 5 The novelty of our study is that statin medication also is very likely to be important in older persons in the community, persons who may or may not fit the stringent criteria of normality imposed for inclusion into randomised clinical trials.
Recently, a large meta-analysis1 demonstrated that the increased cardiovascular mortality seen in type 2 diabetes is not explained by conventional cardiovascular risk factors. This suggests that the effect of the statins on cardiovascular mortality seen in our population-based cohort may reach beyond the statins' effect on cholesterol levels, possibly by reducing the damaging effect of inflammatory agents.19 20 Inflammation as measured by CRP, however, does not appear to be the explanation in our study, as adjusting for CRP in the risk models did not attenuate the RR, although those on statins had a markedly lower CRP. This warrants further study.
The prevalence of diabetes in Iceland is changing in the same fashion as in other western societies.21 Furthermore, the Icelandic population is similar to other western populations with respect to cardiovascular morbidity and mortality,22 and our results are therefore applicable to other Caucasian populations. Thus, considerable additional benefit can be expected by adhering to the current guidelines on statin use in treating individuals with diabetes, also in the older age-groups.
In summary, we have estimated the risk of death from cardiovascular disease and from all causes in a population-based cohort of older persons with type 2 diabetes. The main finding is that statin use, irrespective of glucose-lowering and antihypertensive medication, eliminates the difference in the mortality of older persons with type 2 diabetes, compared with those without diabetes. Our study suggests that treatment with statins is paramount in the multifaceted management of type 2 diabetes. Strict adherence to the current guidelines is therefore of key importance for older persons with diabetes, regardless of the presence or absence of cardiovascular disease or the level of glucose or blood pressure control.
Our results urgently call for other population-based studies with comparable information to confirm the effect of statin use on mortality in individuals with type 2 diabetes.
Supplementary Material
Footnotes
To cite: Olafsdottir E, Aspelund T, Sigurdsson G, et al. Effects of statin medication on mortality risk associated with type 2 diabetes in older persons: the population-based AGES-Reykjavik Study. BMJ Open 2011;1:e000132. doi:10.1136/bmjopen-2011-000132
Funding: This study has been funded by NIH contract N01-AG-1-2100, the NIA Intramural Research Program, Hjartavernd (the Icelandic Heart Association), and Althingi (the Icelandic parliament). The study is approved by the Icelandic National Bioethics Committee, VSN: 00-063. The researchers are indebted to the participants for their willingness to participate in the study.
Competing interests: None.
Patient consent: Obtained.
Contributors: VG had access to all the data and takes full responsibility for the content of this paper. Drafting of the manuscript: EO, TA, RB and VG. Statistical analysis: EO and TA. Data collection and preparation: EO, TA, VG, BT, GE, GS, LJL and TBH. All authors contributed to the interpretation of the results, read and commented on the manuscript, and approved the final version.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data sharing statement: No additional data available.
References
- 1.Sarwar N, Gao P, Seshasai SR, et al. Diabetes mellitus, fasting blood glucose concentration, and risk of vascular disease: a collaborative meta-analysis of 102 prospective studies. Lancet 2010;375:2215–22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Collins R, Armitage J, Parish S, et al. MRC/BHF Heart Protection Study of cholesterol-lowering with simvastatin in 5963 people with diabetes: a randomised placebo-controlled trial. Lancet 2003;361:2005–16 [DOI] [PubMed] [Google Scholar]
- 3.Shepherd J, Barter P, Carmena R, et al. Effect of lowering LDL cholesterol substantially below currently recommended levels in patients with coronary heart disease and diabetes: the Treating to New Targets (TNT) study. Diabetes Care 2006;29:1220–6 [DOI] [PubMed] [Google Scholar]
- 4.Kearney PM, Blackwell L, Collins R, et al. Efficacy of cholesterol-lowering therapy in 18,686 people with diabetes in 14 randomised trials of statins: a meta-analysis. Lancet 2008;371:117–25 [DOI] [PubMed] [Google Scholar]
- 5.Brugts JJ, Yetgin T, Hoeks SE, et al. The benefits of statins in people without established cardiovascular disease but with cardiovascular risk factors: meta-analysis of randomised controlled trials. BMJ 2009;338:b2376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gulliford MC, Charlton J. Is relative mortality of type 2 diabetes mellitus decreasing? Am J Epidemiol 2009;169:455–61 [DOI] [PubMed] [Google Scholar]
- 7.Dale AC, Vatten LJ, Nilsen TI, et al. Secular decline in mortality from coronary heart disease in adults with diabetes mellitus: cohort study. BMJ 2008;337:a236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Barnett KN, McMurdo ME, Ogston SA, et al. Mortality in people diagnosed with type 2 diabetes at an older age: a systematic review. Age Ageing 2006;35:463–8 [DOI] [PubMed] [Google Scholar]
- 9.Gaede P, Lund-Andersen H, Parving HH, et al. Effect of a multifactorial intervention on mortality in type 2 diabetes. N Engl J Med 2008;358:580–91 [DOI] [PubMed] [Google Scholar]
- 10.Charlton J, Latinovic R, Gulliford MC. Explaining the decline in early mortality in men and women with type 2 diabetes: a population-based cohort study. Diabetes Care 2008;31:1761–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dominguez H, Schramm TK, Norgaard ML, et al. Initiation and persistence to statin treatment in patients with diabetes receiving glucose-lowering medications 1997–2006. Open Cardiovasc Med J 2009;3:152–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Harris TB, Launer LJ, Eiriksdottir G, et al. Age, Gene/Environment Susceptibility-Reykjavik Study: multidisciplinary applied phenomics. Am J Epidemiol 2007;165:1076–87 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.World Health Organization Expert Committee Definition dacodmaic. Report of a WHO consultation, part 1: diagnosis and classification of diabetes mellitus. Geneva: WHO Press, 1999 [Google Scholar]
- 14.Warnick GR, Knopp RH, Fitzpatrick V, et al. Estimating low-density lipoprotein cholesterol by the Friedewald equation is adequate for classifying patients on the basis of nationally recommended cutpoints. Clin Chem 1990;36:15–19 [PubMed] [Google Scholar]
- 15.Conroy RM, Pyorala K, Fitzgerald AP, et al. Estimation of ten-year risk of fatal cardiovascular disease in Europe: the SCORE project. Eur Heart J 2003;24:987–1003 [DOI] [PubMed] [Google Scholar]
- 16.Colhoun HM, Betteridge DJ, Durrington PN, et al. Primary prevention of cardiovascular disease with atorvastatin in type 2 diabetes in the Collaborative Atorvastatin Diabetes Study (CARDS): multicentre randomised placebo-controlled trial. Lancet 2004;364:685–96 [DOI] [PubMed] [Google Scholar]
- 17.Gerstein HC, Miller ME, Byington RP, et al. Effects of intensive glucose lowering in type 2 diabetes. N Engl J Med 2008;358:2545–59 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Duckworth W, Abraira C, Moritz T, et al. Glucose control and vascular complications in veterans with type 2 diabetes. N Engl J Med 2009;360:129–39 [DOI] [PubMed] [Google Scholar]
- 19.Sever PS, Poulter NR, Dahlof B, et al. Reduction in cardiovascular events with atorvastatin in 2,532 patients with type 2 diabetes: Anglo-Scandinavian Cardiac Outcomes Trial–lipid-lowering arm (ASCOT-LLA). Diabetes Care 2005;28:1151–7 [DOI] [PubMed] [Google Scholar]
- 20.Ridker PM, Danielson E, Fonseca FA, et al. Reduction in C-reactive protein and LDL cholesterol and cardiovascular event rates after initiation of rosuvastatin: a prospective study of the JUPITER trial. Lancet 2009;373:1175–82 [DOI] [PubMed] [Google Scholar]
- 21.Bergsveinsson J, Aspelund T, Gudnason V, et al. [Prevalence of type 2 diabetes mellitus in Iceland 1967–2002]. Laeknabladid 2007;93:397–402 [PubMed] [Google Scholar]
- 22.Aspelund T, Thorgeirsson G, Sigurdsson G, et al. Estimation of 10-year risk of fatal cardiovascular disease and coronary heart disease in Iceland with results comparable with those of the Systematic Coronary Risk Evaluation project. Eur J Cardiovasc Prev Rehabil 2007;14:761–8 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.