Abstract
Objectives
To determine whether polymorphisms at codon 487 (*1, GAA=Glu; *2, AAA=Lys) of mitochondrial aldehyde dehydrogenase 2 (ALDH2) influence nitroglycerine (glyceryl trinitrate (GTN))-induced vasodilation, and whether GTN or isosorbide dinitrate (ISDN) is a more effective antianginal agent in each ALDH2 genotype.
Design
A randomised, open-label, crossover trial with 117 healthy Japanese (20–39 years) whose genotypes were determined (*1/*1, n=47; *1/*2, n=48; *2/*2, n=22) was performed at Kyushu University Hospital, Fukuoka, Japan. Participants were randomly assigned to treatment: sublingual spray of GTN (0.3 mg) or ISDN (1.25 mg). After ≥1 week, measurements were repeated using the other drug. The main outcome measures were the maximal rate of increase in the brachial artery diameter determined by ultrasonography, the time required to attain maximal dilation (Tmax) and the time required to attain 90% maximal dilation (T0.9).
Results
The maximal artery diameter increase in response to GTN or ISDN did not differ among genotypes. However, GTN Tmax was significantly longer for *2/*2 (299.7 s, 269.0–330.4) than *1/*1 (254.7 s, 238.6–273.4; p=0.0190). GTN T0.9 was significantly longer in the *1/*2 (206.1 s, 191.7–219.3) and *2/*2 (231.4 s, 211.8–251.0) genotypes than *1/*1 (174.9 s, 161.5–188.3; p=0.0068, p<0.0001, respectively). In contrast, the time-course of ISDN-induced vasodilation did not differ among genotypes. GTN Tmax and T0.9 among *1 allele carriers (*1/*1 and *1/*2) were significantly shorter than those of ISDN, whereas the time course of GTN and ISDN vasodilation did not differ among participants carrying *2/*2.
Conclusions
The amplitude of GTN-induced vasodilation was not influenced by the ALDH2 genotype, but the response was significantly delayed in *2 allele carriers, especially *2/*2. GTN dilated the artery more quickly than ISDN in *1/*1 and *1/*2, but not in *2/*2.
Trial registration number
UMIN000001492 (UMIN-CTR database).
Keywords: Clinical pharmacology, therapeutics, hypertension, ischaemic heart disease, heart failure
Article summary
Article focus
There were few reliable human studies on the influence of aldehyde dehydrogenase 2 (ALDH2) polymorphisms at codon 487 on the response to nitroglycerine (GTN). In particular, there was no information about the response to GTN in *2/*2 homozygotes.
It was unclear whether GTN or isosorbide dinitrate (ISDN) is a more effective antianginal agent in each ALDH2 genotype.
We aimed at comparing vasodilation induced by GTN and ISDN in individuals with different ALDH2 genotypes.
Key messages
The maximal rate of increase in arterial diameter after GTN treatment did not differ among ALDH2 genotype groups.
The time required to attain 90% maximal vasodilation was longer in the *1/*2 and *2/*2 groups than in the *1/*1 group.
GTN (0.3 mg) induced vasodilation more rapidly than ISDN (1.25 mg) in the *1/*1 and *1/*2 groups, but there was no difference between GTN and ISDN in the *2/*2 group.
Strengths and limitations of this study
The choice of healthy young individuals as participants may have strengthened the validity of the study; the participants' backgrounds were likely to be more homogeneous by excluding the influence of multiple concomitant factors that could not be controlled when studying patients or older subjects. However, the results could have been different if the study had been performed with patients with angina, because haemodynamic changes in patients might alter the response to nitrates.
Sublingual spray formulations of nitrates were used instead of tablets to minimise the fluctuation in absorption rate. However, this may have made the dosage slightly unreliable.
A randomised crossover design was used, and arterial diameter was determined by a single measurer blinded to genotype. However, the participants and investigators were not blinded to treatment.
Arterial diameter was accurately measured using a semiautomatic ultrasonography system. However, the brachial artery diameter was measured instead of the coronary artery diameter, and moreover, we did not evaluate venous dilation.
Introduction
Nitroglycerine (glyceryl trinitrate (GTN)) has been the drug of choice to relieve angina pectoris since 1879 when William Murrell published his results on the clinical effect of GTN.1 However, the effectiveness of GTN is not consistent among individual patients.2 A sublingual 0.3 mg dose of GTN typically alleviates chest pain, but some patients require a larger dose or repeated administration. The reason for this differential effect may be complex, since several biological processes are involved in mediating the pharmacological effects of GTN. GTN is a prodrug that must be reduced to glyceryl dinitrate to generate nitrite, which is further reduced to nitric oxide (NO). NO is the active metabolite that relaxes vascular smooth muscle by stimulating soluble guanylate cyclase to produce cyclic guanosine monophosphate.3 4 Proposed mechanisms for GTN bioactivation include the non-enzymatic interaction with thiol compounds such as cysteine and N-acetylcysteine,5 and an enzymatic reaction such as glutathione S-transferase6–8 and cytochrome P4508 9; however, the relative importance of these mechanisms had not been understood until recently.
In 2002, Chen et al10 identified mitochondrial aldehyde dehydrogenase 2 (ALDH2) as an important enzyme for GTN bioactivation.11 They purified a protein fraction able to metabolise 14C-GTN and sequenced its amino acids.10 Subsequently, they showed that ALDH2 was essential for GTN metabolism using ALDH2 knockout mice.12 They performed additional in vivo experiments in dogs to demonstrate the role of ALDH2 in GTN bioactivation.13 These findings were supported by clinical studies. Mackenzie et al14 reported that the effect of GTN administered by intra-arterial injection was 33% lower in healthy volunteers if the ALDH2 inhibitor disulfiram was given. The loss-of-function single nucleotide polymorphism at codon 487 of ALDH2, in which Glu (GAA, *1) is replaced with Lys (AAA, *2), is common in East Asian populations including Japanese. In an in vitro assay, the GTN reductase activity of ALDH2 *2 was shown to be two orders of magnitude lower than that of the wild-type enzyme.10 15 Therefore, Mackenzie et al14 evaluated the effect of GTN in 11 Asian *2 allele carriers (*1/*2 and *2/*2) and found that it was 40% lower than its effect in carriers of *1/*1. Li et al16 reported that the effect of sublingual GTN was significantly lower in *2 allele carriers than in *1/*1 carriers among Chinese patients with angina; however, it is unclear whether this decrease in sensitivity to GTN should be considered in medical practice. Furthermore, the sensitivity to GTN of *2/*2 carriers should be distinguished from that of *1/*2 carriers, since the number of *2/*2 carriers included in previous studies was too small to be independently analysed. It is unknown whether another drug may be more effective for *2/*2 patients. In Japan, isosorbide dinitrate (ISDN) is the only alternative to GTN available for sublingual administration during anginal attacks. Since bioactivation of ISDN appears to be mediated by cytochrome P450 but not ALDH2,9 17 we wanted to compare the effectiveness of GTN and ISDN in individuals with different ALDH2 polymorphisms.
To answer these questions, we conducted a randomised crossover study to compare the vasodilation effect of GTN with that of ISDN in healthy young Japanese participants, including sufficient numbers of each ALDH2 genotype.
Methods
Study design
From September 2009 to May 2010, we performed this randomised, open-label, crossover trial at Kyushu University Hospital, Fukuoka, Japan with volunteers whose ALDH2 genotypes were determined. After an initial treatment with a standard therapeutic dose of either GTN (0.3 mg) or ISDN (1.25 mg), the brachial artery diameter was continuously measured by ultrasonography. After ≥1 week, the participants received the other drug, and the measurements were repeated. The main outcome measures were the maximal rate of diameter increase, the time required to attain maximal dilation (Tmax) and the time required to attain 90% of maximal dilation (T0.9).
Participants
From April 2009 to March 2010, we recruited Japanese men and women (20–39 years old) among the students and staff of Kyushu University for participation in the study, and selected the final participants based on ALDH2 genotype. Persons who met the following criteria were excluded: (1) requiring treatment for a disease, (2) under medical treatment, (3) history of cardiovascular disease, (4) serious liver damage, (5) serious renal damage, (6) pregnancy, (7) history of adverse reaction to an organic nitrate, (8) receiving phosphodiesterase-5 inhibitors (sildenafil, vardenafil or tadalafil), (9) systolic blood pressure <90 mm Hg, (10) body weight <40 kg or >90 kg or (11) judged by the principal investigator (TS) to be inappropriate for participation in the study.
Genotyping
Genomic DNA was extracted from peripheral blood with a DNA isolation kit (GenTLE; Takara Bio, Ohtsu, Japan); for the 11 participants who did not wish to have blood drawn, DNA was extracted from saliva with a DNA self-collection kit (Oragene; DNA Genotek, Ottawa, Canada). DNA extraction was carried out with the kits according to the manufacturers' instructions. ALDH2 genotypes at codon 487 were determined by PCR and restriction fragment length polymorphism analysis. The PCR primer sequences were 5′-TTGGTGGCTACAAGATGTCG-3′ (forward) and 5′-AAACACTGATGGCCTCAAGC-3′ (reverse). The 324-bp PCR products were digested with TspRI (New England BioLabs, Ipswich, Massachusetts) at 65°C for 20 min. The restriction digest cut the *1 genotype DNA into three fragments (68, 255 and 1 bp) and the *2 genotype DNA into two fragments (323 and 1 bp), which were separated on a 3% agarose gel and visualised with ethidium bromide. Genotypes were determined by the existence of 255 bp and/or 323 bp fragments.
Interventions and outcome measures
Participants were prohibited from drinking alcohol and smoking cigarettes the night before testing, but meals were not restricted. After spending at least 60 min without exercise, participants were randomly assigned 1:1 to a single sublingual spray administration of GTN (0.3 mg; Myocor spray, Toa Eiyo, Tokyo, Japan) or ISDN (1.25 mg; Nitorol spray, Eisai Co, Tokyo, Japan) as initial treatment. A random allocation sequence was made by HA using random digits generated by the Mersenne Twister method18 and conveyed to the investigators (SS, TY, HO and TS) by sealed numbered envelopes, one for each participant, with instructions to use the envelopes in numerical order. Participants rested at least 5 min in a supine position in a quiet examination room at 24°C, and then the right brachial artery was scanned in a longitudinal section 1 to 10 cm above the elbow in an arm maintained in the same position throughout the study, with a 10 MHz linear array transducer probe. To determine the maximal diameter of the artery, the end-diastolic diameter (length from one side of the media–adventitia interface to the other side) coincident with the R-wave on a monitoring electrocardiogram was continuously measured from 30 s before nitrate administration to 10 min after administration. This measurement was performed using a semiautomatic high-resolution B-mode ultrasound imaging system (UNEX EF 18G II, UNEX Corporation, Nagoya, Japan)19 20 by a single expert (SS) blinded to genotype and recorded on a hard disk. Pulse rate and blood pressure were measured every 5 min. More than 7 days later, each participant received the other drug, and the measurements were repeated. The prespecified primary outcome measures were the maximal rate of increase in brachial artery diameter and the time required to attain maximal dilation (Tmax). However, the time required to attain 90% maximal dilation (T0.9) was also determined, so as to minimise fluctuation of the outcome measure. In addition, changes in pulse rate and blood pressure were assigned as secondary outcome measures.
Statistical analysis
The minimum sample size for this clinical trial was based on results obtained in previous studies. The mean and SD for the increase in forearm blood flow induced by GTN in *1/*1 and *2 allele carriers were reported by Mackenzie et al.14 Because no data were available regarding the homozygous *2/*2 population, we estimated the mean and SD values based on an experiment by Chen et al12 using ALDH2 knockout mice. According to their results, we assumed that the increase in blood flow in *1/*1 and *2/*2 genotypes was 4.5±3.36 and 1.9±1.99 (ml/100 ml/min), respectively. Power calculation indicated that the minimum sample sizes of *1/*1 versus *2/*2 carriers were 24:24, 27:19, 30:15, 37:13 or 43:11 to detect the difference (2.6 ml/100 ml/min) between these two genotypes with a statistical power of 90% and a p value of 0.05. The resultant sample size (*1/*1, n=47; *2/*2, n=22) had an 80% power to detect a minimal difference of 3.7% in arterial diameter change in response to nitrates between these groups. Likewise, there was an 80% power to detect a minimal difference of 35 s in time required for arterial dilation between the two genotypes.
Differences in baseline characteristics of participants were compared according to genotype, using the χ2 test for the categorical variable and one-way analysis of variance (ANOVA) for continuous variables. Primary outcome measures were displayed according to genotype group using box-and-whisker plots. Overall group differences in the primary and secondary outcomes were determined by one-way ANOVA and analysis of covariance (ANCOVA) with sex, age, body mass index and systolic blood pressure as covariates. The Tukey–Kramer test was used for multiple comparisons. Differences in outcomes between GTN and ISDN were also determined by one-way ANOVA and ANCOVA with the same covariates in each genotype. All data were analysed with SAS version 9.2. A p value of <0.05 was considered significant.
Results
The frequency of the ALDH2 *2 allele is approximately 20% in the Japanese population, although regional differences may exist.21 The frequencies of each genotype are: *1/*1, 60% to 70%; *1/*2, 30% to 40%; and *2/*2, 3% to 5%. To overcome the difficulty of recruiting enough *2/*2 homozygotes by random screening only, we intentionally recruited participants who were unable to drink alcohol by advertisement at Kyushu University. We analysed genotypes of 305 individuals (*1/*1, n=156; *1/*2, n=111; *2/*2, n=38) and included 117 individuals in the trial to fulfil the required sample size (*1/*1, n=47; *1/*2, n=48; *2/*2, n=22). As shown in table 1, baseline characteristics did not differ among the genotype groups. We analysed the outcomes from all the participants without loss or exclusion.
Table 1.
Baseline characteristics of the participants
| Total | *1/*1 | *1/*2 | *2/*2 | p Value | |
| No | 117 | 47 | 48 | 22 | |
| Male (%) | 72.7 | 72.3 | 70.8 | 77.3 | 0.85 |
| Age (years) | 26.6±4.3 | 25.8±4.0 | 26.8±4.3 | 28.0±4.8 | 0.11 |
| Height (cm) | 167.5±8.4 | 168.0±8.5 | 168.1±8.1 | 165.0±8.9 | 0.41 |
| Weight (kg) | 59.6±9.3 | 59.9±8.5 | 59.9±10.4 | 57.7±8.4 | 0.62 |
| Body mass index | 21.2±2.3 | 21.3±2.1 | 21.1±2.7 | 21.1±1.9 | 0.91 |
| Pulse rate (beats/min) | 65.8±10.9 | 64.5±10.4 | 64.9±10.1 | 65.9±11.7 | 0.99 |
| Systolic blood pressure (mm Hg) | 119.5±11.8 | 118.4±13.5 | 117.0±12.5 | 118.3±10.9 | 0.60 |
| Diastolic blood pressure (mm Hg) | 67.1±8.4 | 66.7±8.7 | 65.9±8.1 | 64.3±6.8 | 0.42 |
Demographic and physiological parameters of the participants were evaluated by χ2 test for sex and by one-way ANOVA for body mass index, pulse rate, systolic blood pressure and diastolic blood pressure. Results are expressed as mean±SD.
Initially, we planned to assess the effectiveness of nitrates primarily with two parameters: (1) the maximal rate of increase in brachial artery diameter (Dmax−D0)/D0, where D0 is the diameter at time 0, and Dmax is the maximal diameter achieved after nitrate administration; and (2) the time required to attain maximal dilation (Tmax).
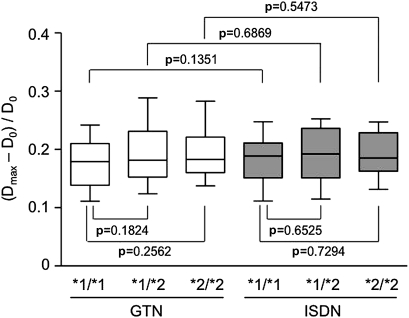
Unexpectedly, the first parameter (Dmax−D0)/D0 did not differ among the genotype groups after either treatment (figure 1), even after adjusting for sex, age, body mass index and systolic blood pressure. This finding suggests that GTN and ISDN eventually produce the same level of vasodilation regardless of genotype.
Figure 1.
Maximal rate of increase in the brachial artery diameter. After sublingual administration of glyceryl trinitrate (GTN) (0.3 mg) or isosorbide dinitrate (ISDN) (1.25 mg), the brachial artery diameter was measured continuously for 10 min. The maximal rate of increase was calculated by the formula (Dmax−D0)/D0, where D0 was the baseline diameter, and Dmax was the maximal diameter achieved. The values of (Dmax−D0)/D0 after GTN treatment were: total, 0.188 (0.178–0.199); *1/*1 genotype, 0.176 (0.163–0.196); *1/*2 genotype, 0.195 (0.177–0.210); and *2/*2 genotype, 0.196 (0.172–0.221). The values of (Dmax−D0)/D0 after ISDN treatment were: total, 0.187 (0.178–0.196); *1/*1 genotype, 0.183 (0.169–0.199); *1/*2 genotype, 0.189 (0.174–0.204); *2/*2 genotype, 0.189 (0.167–0.210). Results are shown as box-and-whisker plots indicating the 10th, 25th, 50th, 75th and 90th percentiles. Group results were compared by one-way ANOVA for crude data and by ANCOVA after adjusting for sex, age, body mass index and systolic blood pressure.
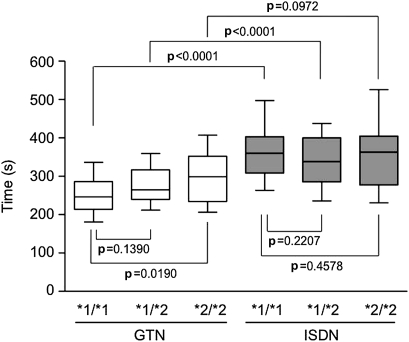
We then analysed the second parameter, Tmax. As shown in figure 2, the GTN Tmax was significantly longer in the *2/*2 genotype group than in the *1/*1 group (crude data, p=0.0190; adjusted data, p=0.0234), but no significant difference was observed between the *1/*1 and *1/*2 groups (crude, p=0.1390; adjusted, p=0.1834). The p trend among the three genotypes was significant for both crude (p=0.004) and adjusted (p=0.0002) data. In contrast, ISDN Tmax did not differ among the genotype groups.
Figure 2.
Time required to attain maximal dilation (Tmax). After sublingual administration of glyceryl trinitrate (GTN) (0.3 mg) or isosorbide dinitrate (ISDN) (1.25 mg), the brachial artery diameter was measured continuously for 10 min. The values of the time when Dmax was achieved (Tmax) after GTN treatment were: total, 272.6 s (261.4–284.2 s); *1/*1 genotype, 254.7 s (238.6–273.4 s); *1/*2 genotype, 277.4 s (259.4–294.3 s); *2/*2 genotype, 299.7 s (269.0–330.4 s). Tmax values after ISDN treatment were: total, 351.4 s (337.6–368.7 s); *1/*1 genotype, 365.0 s (340.5–389.9 s); *1/*2 genotype, 340.3 s (316.2–364.5 s); *2/*2 genotype, 347.0 s (320.7–391.3 s). Results for the different genotypes and nitrate treatments are shown as box-and-whisker plots indicating the 10th, 25th, 50th, 75th and 90th percentiles. Group results were compared by one-way ANOVA for crude data and by ANCOVA after adjusting for sex, age, body mass index and systolic blood pressure.
The brachial artery typically began to dilate about 1 min after nitrate administration. The diameter rapidly increased until 3 min, after which dilation slowed, and the diameter reached the maximum size at 4 to 6 min. However, it was often not easy to determine Tmax, because the artery diameter usually approached the maximum size slowly. Thus, an artefact in the trace could significantly affect the determination of Tmax. To avoid this problem, we introduced the parameter T0.9, which is the time point at which the arterial diameter reached 90% of the maximal level, to minimise fluctuation of the outcome measure.
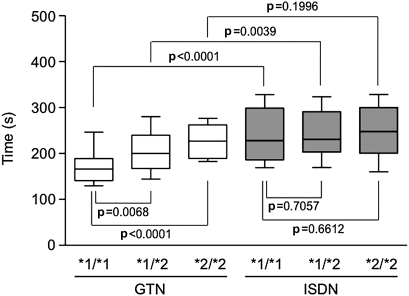
As shown in figure 3, GTN T0.9 was significantly longer in participants with *2/*2 (crude, p<0.0001; adjusted, p=0.0002) and *1/*2 (crude, p=0.0068; adjusted, p=0.0157) genotypes than those with *1/*1. The p trend among the three genotypes was also significant (crude, p<0.0001; adjusted, p<0.0001). On the other hand, there was no difference in the T0.9 of ISDN. A similar result was obtained when the time required for 50% maximal dilation was analysed (supplementary online figure).
Figure 3.
Time required to attain 90% maximal dilation (T0.9). After sublingual administration of glyceryl trinitrate (GTN) (0.3 mg) or isosorbide dinitrate (ISDN) (1.25 mg), the brachial artery diameter was measured continuously for 10 min. The values of the time at which the diameter achieved 90% Dmax (T0.9) after GTN treatment were: total, 198.3 s (189.0–207.6 s); *1/*1 genotype, 174.9 s (161.5–188.3 s); *1/*2 genotype, 206.1 s (191.7–219.3 s); *2/*2 genotype, 231.4 s (211.8–251.0 s). T0.9 values after ISDN treatment: total, 242.0 s (231.6–254.2 s); *1/*1 genotype, 236.8 s (220.8–257.0 s); *1/*2 genotype, 244.7 s (227.0–262.5 s); *2/*2 genotype, 247.4 s (221.5–273.3 s). Results for the different genotypes and nitrate treatments are shown as box-and-whisker plots indicating the 10th, 25th, 50th, 75th and 90th percentiles. Group results were compared by one-way ANOVA for crude data and by ANCOVA after adjusting for sex, age, body mass index and systolic blood pressure.
Next we investigated which organic nitrate was more effective in each ALDH2 genotype. As shown in figure 1, the effects of GTN and ISDN on (Dmax−D0)/D0 did not differ in any genotype group. In contrast, the Tmax of GTN was significantly smaller than that of ISDN in the *1/*1 group (crude, p<0.0001; adjusted, p<0.0001) and *1/*2 group (crude, p<0.0001; adjusted, p<0.0001), but no difference was observed between the effects of these nitrates in the *2/*2 group (crude, p=0.0972; adjusted, p=0.1023) (figure 2). The T0.9 of GTN was also significantly smaller than that of ISDN in the *1/*1 group (crude, p<0.0001; adjusted, p<0.0001) and *1/*2 group (crude, p=0.0039, adjusted, p=0.0013), but this value did not differ between the treatments in the *2/*2 group (crude, p=0.1996, adjusted, p=0.3122) (figure 3).
The results of secondary outcome measures are summarised in the supplementary online table. No significant differences were observed in pulse rate, diastolic blood pressure or systolic blood pressure measured 10 min after nitrate administration among the ALDH2 genotype groups. Further, no serious adverse events were observed throughout the study.
Discussion
Principal findings
First, the maximal rate of increase in diameter after GTN treatment did not differ among ALDH2 genotype groups, suggesting that GTN eventually induces maximal vasodilation regardless of genotype. Second, the GTN Tmax was significantly longer in the *2/*2 group than in the *1/*1 group, and GTN T0.9 was significantly longer in the *1/*2 and *2/*2 groups than in the *1/*1 group, suggesting that the *1 allele is associated with a more rapid response to GTN. However, this differential response was not observed after ISDN treatment. Third, GTN (0.3 mg) induced vasodilation more rapidly than ISDN (1.25 mg) in the *1/*1 and *1/*2 groups, but the Tmax and T0.9 values of GTN and ISDN did not differ in the *2/*2 group.
Strengths and limitations of the study
This study was carefully designed and conducted; however, our results require careful interpretation. First, the study participants were not patients with angina. Healthy, young volunteers were used because organic nitrates can also induce vasodilation in healthy people. The choice of participants may have strengthened the validity of the study; the participants' backgrounds were likely to be more homogeneous by excluding the influence of multiple concomitant factors that could not be controlled when studying patients or older subjects. In addition, using healthy, young participants made it easy to recruit a large enough sample of *2/*2 homozygotes. However, the results could have been different if the study had used patients with angina, because haemodynamic changes in patients might alter the response to organic nitrates. Second, we used sublingual spray formulations instead of tablets for both nitrates to minimise the fluctuation in absorption rate. However, this may have made the dosage slightly unreliable,22 although this would have affected all the participants similarly and therefore should not have biased the results. Third, to minimise bias, a randomised crossover design was used, and arterial diameter was determined by a single measurer blinded to genotype. However, the participants and investigators were not blinded to treatment. Fourth, arterial diameter was accurately measured using a semiautomatic ultrasonography system.19 20 However, the brachial artery diameter was measured instead of the coronary artery diameter. Moreover, we did not evaluate venous dilation, although venodilation is thought to be a mechanism that is as important as coronary artery dilation for the antianginal effects of nitrates.3 23 24
Comparison with other studies
Other than the present study, three previous clinical studies have investigated the influence of the ALDH2 polymorphism on GTN effects, but the study performed by Mackenzie et al14 was the only experimental study (clinical trial). In that study, GTN was injected into the brachial artery of healthy participants, and forearm blood flow measured by venous occlusion plethysmography was 33% lower if the ALDH2 inhibitor disulfiram was administered. Analysing 16 healthy East Asian participants (*1/*1, n=5; *1/*2, n=9; *2/*2, n=2), they also showed that the effect of GTN was 40% lower in carriers of the *2 allele than in participants with the *1/*1 genotype. However, the analysis did not differentiate between *1/*2 and *2/*2 genotypes, probably because the sample size was too small. In contrast, the present study included enough participants to allow analysis of the *2/*2 population. Moreover, we used ultrasonography to assess arterial dilation, which is a more direct method than plethysmography. The other two studies were both observational studies. Li et al16 divided 80 Chinese patients with stable angina according to whether pain relief, assessed using a pain judgement scale, was obtained within 10 min after sublingual administration of GTN. ALDH2 genotypes of each group were then analysed, revealing that the effect of GTN was significantly smaller in carriers of the *2 allele than in participants with the *1/*1 genotype. Zhang et al25 reported that in 142 Chinese patients with coronary heart disease, the rate of efficacious response to GTN was significantly higher in patients with the *1/*1 genotype than in those carrying the *2 allele. However, these studies did not distinguish between the *1/*2 and *2/*2 genotypes. Moreover, the efficacy of GTN was assessed according to the patients' subjective reporting of symptoms, potentially introducing bias. Therefore, few reliable human studies on the influence of the ALDH2 polymorphisms on the response to GTN were available until our study was conducted; in particular, there was no information about the response to GTN in *2/*2 homozygotes.
Based on the earlier studies, we initially hypothesised that the maximal response to GTN would be reduced in *2 allele carriers, but the results obtained were different from our expectation. Although the time required to obtain the response was different among the genotype groups, there was no difference in the size of response (the reason for this is discussed later). However, we cannot specify the reason for the difference in the results between earlier studies and ours. Probably multiple factors may be involved, such as study design, sample size, GTN dosage, studied vascular beds, measurement method or outcome measures.
Meaning of the study
The results of this study may have clinical implications for the daily use of organic nitrates. First, although there was no significant difference in the magnitude of response to GTN, the delay in attaining near maximal dilation was about 1 min for *2/*2 participants who took 0.3 mg of GTN. Given that the purpose of GTN is to provide rapid relief for chest pain, we believe that a 1 min delay cannot be ignored, although we observed only brachial artery dilation but not disappearance of the symptom. To practise personalised medicine for patients with angina, it may be useful to know the individual ALDH2 genotypes before treatment. If gene analysis is not available, a patient's genotype could be estimated by asking about drinking history or using the ethanol patch test.26 Second, this study suggests the first choice drug for treating angina in terms of ALDH2 polymorphisms. Assuming that a rapid response is better, GTN may still be the first choice for *1/*1 patients and perhaps also for *1/*2 patients, because GTN dilated the artery more rapidly than ISDN in participants with these genotypes. However, ISDN may be a better choice for *2/*2 patients, because it dilated the artery as fast as GTN; moreover, larger or repeated doses of GTN often induce tolerance and oxidative stress, although ISDN can also cause tolerance.27 In addition, this study may also be informative for basic research on organic nitrate pharmacology. The maximal rate of diameter increase was comparable among the genotypes. One explanation for this finding is that even the small amount of NO generated by ALDH2 *2 allele is sufficient for maximal response, because the GTN reductase activity of ALDH2 *2 is small but not nil.15 Previously, maximal relaxation of rat aortic rings was shown to occur at only 3.4% of the maximal levels of cyclic guanosine monophosphate obtained with NO.28 Another, perhaps more likely explanation is that enzymes other than ALDH2, such as glutathione S-transferase,6 7 29 cytochrome P4508 9 and xanthine oxidoreductase,30 are also involved in the bioactivation of GTN. It has been suggested that ALDH2 activates GTN at the low concentrations achieved by therapeutic doses, whereas cytochrome P450 activates GTN at higher concentrations.27 However, our results suggest that ALDH2 and other enzymes are concurrently involved in GTN bioactivation, even at low concentrations achieved by the standard therapeutic dose. The other enzymes involved may be able to generate a sufficient amount of NO without ALDH2, whereas ALDH2 specifically may be responsible for the rapid generation of NO.
Unanswered questions and future research
First, this study suggested that decreased ALDH2 activity delayed GTN-induced vasodilation in *2 allele carriers; however, direct evidence for delayed GTN bioactivation in *2 allele carriers has not been obtained. Therefore, pharmacokinetic studies evaluating serum concentrations of GTN and its metabolites are needed to specifically assess whether GTN metabolism is delayed in *2 allele carriers. Second, we should also evaluate GTN-induced venous dilation in *2 allele carriers, since an in vitro experiment showed that venodilation was attenuated by pharmacological inhibition of ALDH2.31 Third, the influence of the ALDH2 polymorphism on the action of GTN should be investigated with a clinical trial of patients with angina. No clinical trial has been conducted with patients so far; however, the study protocol should be carefully designed to enable precise determination of outcome measures. Fourth, basic studies are needed to identify the enzymes other than ALDH2 involved in GTN metabolism. Finally, it may be necessary to promote the development of a new medicine that is more beneficial than GTN.32 33 This is not simply because the response to GTN varies among ALDH2 genotypes, but because repeated or long-term GTN administration may cause nitrate tolerance inactivating ALDH2 by superoxide generation,4 27 34–36 and moreover, inactivated ALDH2 could disturb cardiovascular cell functions.37 38
Supplementary Material
Acknowledgments
We thank M Nagamatsu for administrative and technical assistance.
Footnotes
To cite: Sakata S, Yoshihara T, Arima H, et al. Differential effects of organic nitrates on arterial diameter among healthy Japanese participants with different mitochondrial aldehyde dehydrogenase 2 genotypes: randomised crossover trial. BMJ Open 2011;1:e000133. doi:10.1136/bmjopen-2011-000133
Funding: This work was supported by Grant-in-Aid for Scientific Research from the Ministry of Education, Culture, Sports, Science and Technology, Japan (grant number 20390160).
Competing interests: None.
Ethics approval: Ethics approval was provided by the Institutional Review Board for Clinical Trials, Kyushu University Hospital.
Contributors: TS, KM, HA and FT-Y conceived and designed the study and wrote the protocol. SS, TY, HO and TS recruited participants, obtained informed consent and acquired the data. FS determined participants' genotypes. SS, TY, KM, and TS analysed and interpreted the data. HA undertook sample size calculation, randomisation, and statistical analyses. TS obtained funding. All authors wrote and revised the manuscript, and all approved the publication of the final version. All authors had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. TS is guarantor.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data sharing statement: The full study protocol and data will not be publicly accessible. Interested individuals may contact the authors.
References
- 1.Smith E, Hart FD. William Murrell, physician and practical therapist. BMJ 1971;3:632–3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Eichler HG, Hiremath A, Katzir D, et al. Absence of age-related changes in venous responsiveness to nitroglycerin in vivo in humans. Clin Pharmacol Ther 1987;42:521–4 [DOI] [PubMed] [Google Scholar]
- 3.Torfgård KE, Ahlner J. Mechanisms of action of nitrates. Cardiovasc Drugs Ther 1994;8:701–17 [DOI] [PubMed] [Google Scholar]
- 4.Mayer B, Beretta M. The enigma of nitroglycerin bioactivation and nitrate tolerance: news, views and troubles. Br J Pharmacol 2008;155:170–84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Feelisch M, Noack E. Nitric oxide (NO) formation from nitrovasodilators occurs independently of hemoglobin or non-heme iron. Eur J Pharmacol 1987;142:465–9 [DOI] [PubMed] [Google Scholar]
- 6.Yeates RA, Schmid M, Leitold M. Antagonism of glycerol trinitrate activity by an inhibitor of glutathione S-transferase. Biochem Pharmacol 1989;38:1749–53 [DOI] [PubMed] [Google Scholar]
- 7.Tsuchida S, Maki T, Sato K. Purification and characterization of glutathione transferases with an activity toward nitroglycerin from human aorta and heart. Multiplicity of the human class Mu forms. J Biol Chem 1990;265:7150–7 [PubMed] [Google Scholar]
- 8.Bennett BM, McDonald BJ, Nigam R, et al. Biotransformation of organic nitrates and vascular smooth muscle cell function. Trends Pharmacol Sci 1994;15:245–9 [DOI] [PubMed] [Google Scholar]
- 9.Minamiyama Y, Takemura S, Akiyama T, et al. Isoforms of cytochrome P450 on organic nitrate-derived nitric oxide release in human heart vessels. FEBS Lett 1999;452:165–9 [DOI] [PubMed] [Google Scholar]
- 10.Chen Z, Zhang J, Stamler JS. Identification of the enzymatic mechanism of nitroglycerin bioactivation. Proc Natl Acad Sci U S A 2002;99:8306–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen Z, Stamler JS. Bioactivation of nitroglycerin by the mitochondrial aldehyde dehydrogenase. Trends Cardiovasc Med 2006;16:259–65 [DOI] [PubMed] [Google Scholar]
- 12.Chen Z, Foster MW, Zhang J, et al. An essential role for mitochondrial aldehyde dehydrogenase in nitroglycerin bioactivation. Proc Natl Acad Sci U S A 2005;102:12159–64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhang J, Chen Z, Cobb FR, et al. Role of mitochondrial aldehyde dehydrogenase in nitroglycerin-induced vasodilation of coronary and systemic vessels: an intact canine model. Circulation 2004;110:750–5 [DOI] [PubMed] [Google Scholar]
- 14.Mackenzie IS, Maki-Petaja KM, McEniery CM, et al. Aldehyde dehydrogenase 2 plays a role in the bioactivation of nitroglycerin in humans. Arterioscler Thromb Vasc Biol 2005;25:1891–5 [DOI] [PubMed] [Google Scholar]
- 15.Larson HN, Zhou J, Chen Z, et al. Structural and functional consequences of coenzyme binding to the inactive asian variant of mitochondrial aldehyde dehydrogenase: roles of residues 475 and 487. J Biol Chem 2007;282:12940–50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li Y, Zhang D, Jin W, et al. Mitochondrial aldehyde dehydrogenase-2 (ALDH2) Glu504Lys polymorphism contributes to the variation in efficacy of sublingual nitroglycerin. J Clin Invest 2006;116:506–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Daiber A, Oelze M, Coldewey M, et al. Oxidative stress and mitochondrial aldehyde dehydrogenase activity: a comparison of pentaerythritol tetranitrate with other organic nitrates. Mol Pharmacol 2004;66:1372–82 [DOI] [PubMed] [Google Scholar]
- 18.Matsumoto M, Nishimura T. Mersenne Twister: a 623-dimensionally equidistributed uniform pseudorandom number generator. ACM Trans Model Comput Simul 1998;8:3–30 [Google Scholar]
- 19.Tomiyama H, Matsumoto C, Yamada J, et al. The relationships of cardiovascular disease risk factors to flow-mediated dilatation in Japanese subjects free of cardiovascular disease. Hypertens Res 2008;31:2019–25 [DOI] [PubMed] [Google Scholar]
- 20.Inoue T, Matsuoka H, Higashi Y, et al. Flow-mediated vasodilation as a diagnostic modality for vascular failure. Hypertens Res 2008;31:2105–13 [DOI] [PubMed] [Google Scholar]
- 21.Li H, Borinskaya S, Yoshimura K, et al. Refined geographic distribution of the oriental ALDH2*504Lys (nee 487Lys) variant. Ann Hum Genet 2009;73:335–45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Price JW, Price JR. Accuracy and precision of metered doses of nitroglycerin lingual spray. Am J Health Syst Pharm 2008;65:1556–9 [DOI] [PubMed] [Google Scholar]
- 23.Tadjkarimi S, O'Neil GS, Luu TN, et al. Comparison of cyclic GMP in human internal mammary artery and saphenous vein: implications for coronary artery bypass graft patency. Cardiovasc Res 1992;26:297–300 [DOI] [PubMed] [Google Scholar]
- 24.Abrams J. Beneficial actions of nitrates in cardiovascular disease. Am J Cardiol 1996;77:31C–7C [DOI] [PubMed] [Google Scholar]
- 25.Zhang H, Chen YG, Xu F, et al. The relationship between aldehyde dehydrogenase-2 gene polymorphisms and efficacy of nitroglycerin. Zhonghua Nei Ke Za Zhi 2007;46:629–32 [PubMed] [Google Scholar]
- 26.Muramatsu T, Higuchi S, Shigemori K, et al. Ethanol patch test: a simple and sensitive method for identifying ALDH phenotype. Alcohol Clin Exp Res 1989;13:229–31 [DOI] [PubMed] [Google Scholar]
- 27.Münzel T, Daiber A, Mülsch A. Explaining the phenomenon of nitrate tolerance. Circ Res 2005;97:618–28 [DOI] [PubMed] [Google Scholar]
- 28.Kollau A, Hofer A, Russwurm M, et al. Contribution of aldehyde dehydrogenase to mitochondrial bioactivation of nitroglycerin: evidence for the activation of purified soluble guanylate cyclase through direct formation of nitric oxide. Biochem J 2005;385:769–77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhang XJ, Chang L, Zhang YM, et al. Comparing the role of glutathione-S-transferase and mitochondrial aldehyde dehydrogenase in nitroglycerin biotransformation and the correlation with calcitonin gene-related peptide. Eur J Pharmacol 2009;61:97–101 [DOI] [PubMed] [Google Scholar]
- 30.Golwala NH, Hodenette C, Murthy SN, et al. Vascular responses to nitrite are mediated by xanthine oxidoreductase and mitochondrial aldehyde dehydrogenase in the rat. Can J Physiol Pharmacol 2009;87:1095–101 [DOI] [PubMed] [Google Scholar]
- 31.Huellner MW, Schrepfer S, Weyand M, et al. Inhibition of aldehyde dehydrogenase type 2 attenuates vasodilatory action of nitroglycerin in human veins. FASEB J 2008;22:2561–8 [DOI] [PubMed] [Google Scholar]
- 32.Schuhmacher S, Schulz E, Oelze M, et al. A new class of organic nitrates: investigations on bioactivation, tolerance and cross-tolerance phenomena. Br J Pharmacol 2009;158:510–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schnorbus B, Schiewe R, Ostad MA, et al. Effects of pentaerythritol tetranitrate on endothelial function in coronary artery disease: results of the PENTA study. Clin Res Cardiol 2010;99:115–24 [DOI] [PubMed] [Google Scholar]
- 34.DiFabio J, Ji Y, Vasiliou V, et al. Role of mitochondrial aldehyde dehydrogenase in nitrate tolerance. Mol Pharmacol 2003;64:1109–16 [DOI] [PubMed] [Google Scholar]
- 35.Szöcs K, Lassègue B, Wenzel P, et al. Increased superoxide production in nitrate tolerance is associated with NAD(P)H oxidase and aldehyde dehydrogenase 2 downregulation. J Mol Cell Cardiol 2007;42:1111–18 [DOI] [PubMed] [Google Scholar]
- 36.Hink U, Daiber A, Kayhan N, et al. Oxidative inhibition of the mitochondrial aldehyde dehydrogenase promotes nitroglycerin tolerance in human blood vessels. J Am Coll Cardiol 2007;50:2226–32 [DOI] [PubMed] [Google Scholar]
- 37.Daiber A, Oelze M, Wenzel P, et al. Nitrate tolerance as a model of vascular dysfunction: roles for mitochondrial aldehyde dehydrogenase and mitochondrial oxidative stress. Pharmacol Rep 2009;61:33–48 [DOI] [PubMed] [Google Scholar]
- 38.Budas GR, Disatnik MH, Mochly-Rosen D. Aldehyde dehydrogenase 2 in cardiac protection: a new therapeutic target? Trends Cardiovasc Med 2009;19:158–64 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.