Abstract
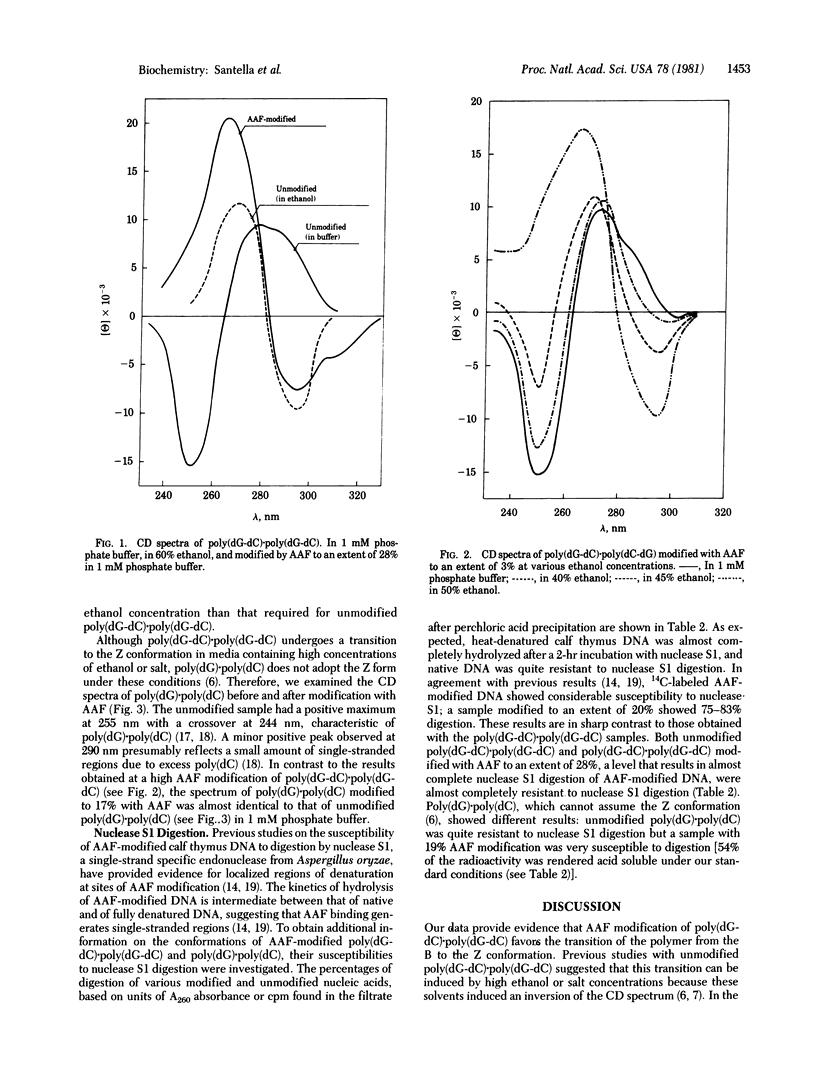
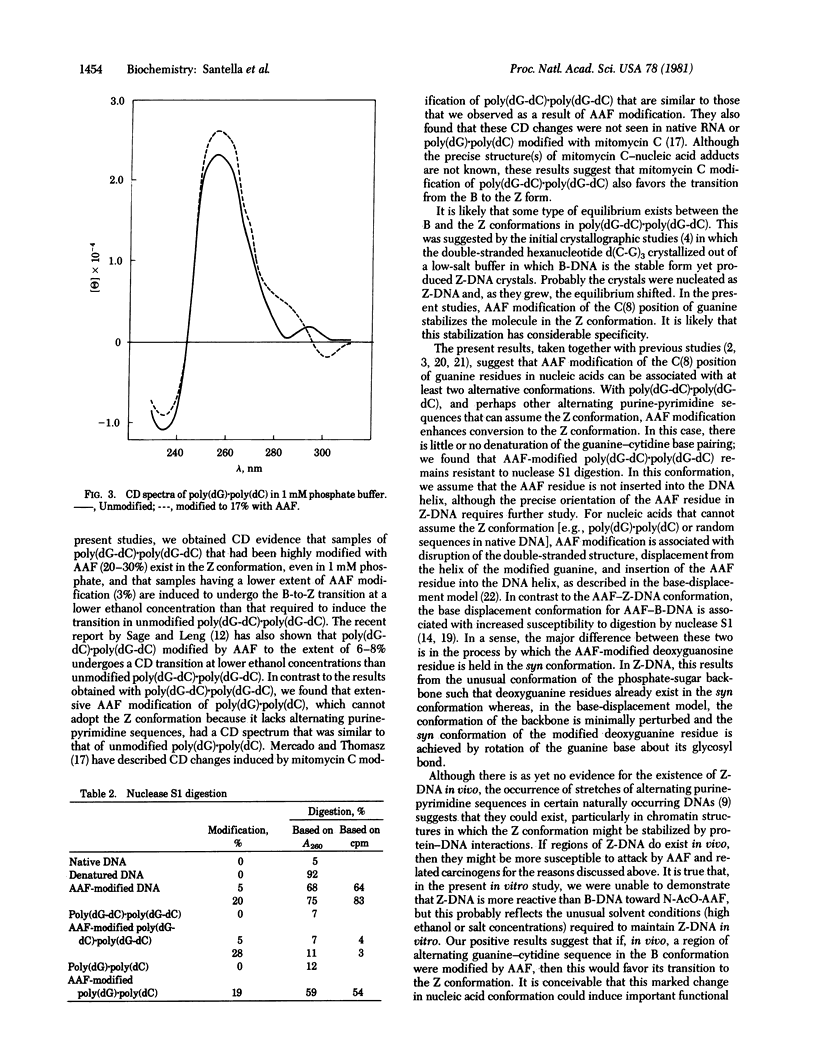
Poly(dG-dC).poly(dG-dC) and poly(dG).poly(dC) were modified by treatment with N-acetoxy-N-2-acetylaminofluorene, and their conformations were examined by circular dichroism and susceptibility to nuclease S1 digestion. A sample of poly(dG-dC).poly(dG-dC) modified to an extent of 28% with acetylaminofluorene (AAF) at the C(8) position of the deoxyguanosine residues showed a circular dichroism spectrum that had the characteristics of the Z conformation seen in unmodified poly(dG-dC).poly(dG-dC) at high ethanol or salt concentrations. A sample of poly(dG-dC).poly(dG-dC) modified only 3% by AAF showed a spectrum characteristic of the B form of DNA. However, it was converted to the Z form at ethanol concentrations lower than required to convert unmodified poly(dG-dC).poly(dG-dC) from the B to the Z form. Poly(dG).poly(dC), which does not undergo the B-to-Z transition at high ethanol concentrations, did not show any large conformational changes with high AAF modification. Susceptibility to digestion with nuclease S1 also suggested differences in the conformations of the two modified polynucleotides. Poly(dG-dC).poly(dG-dC) modified by AAF to an extent of 28% was almost completely resistant to nuclease S1 digestion. However, both poly(dG).poly(dC) and DNA modified to similar levels by AAF were highly susceptible to nuclease S1 digestion. Two different conformations for AAF-modified deoxyguanosine are proposed, depending on whether its position is in alternating purine-pyrimidine sequences or in random-sequence DNA.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arnott S., Chandrasekaran R., Birdsall D. L., Leslie A. G., Ratliff R. L. Left-handed DNA helices. Nature. 1980 Feb 21;283(5749):743–745. doi: 10.1038/283743a0. [DOI] [PubMed] [Google Scholar]
- Astell C. R., Smith M., Chow M. B., Ward D. C. Structure of the 3' hairpin termini of four rodent parvovirus genomes: nucleotide sequence homology at origins of DNA replication. Cell. 1979 Jul;17(3):691–703. doi: 10.1016/0092-8674(79)90276-9. [DOI] [PubMed] [Google Scholar]
- Fuchs R. P. In vitro recognition of carcinogen-induced local denaturation sites native DNA by S1 endonuclease from Aspergillus oryzae. Nature. 1975 Sep 11;257(5522):151–152. doi: 10.1038/257151a0. [DOI] [PubMed] [Google Scholar]
- Fuchs R. P., Lefevre J. F., Pouyet J., Daune M. P. Comparative orientation of the fluorene residue in native DNA modified by N-acetoxy-N-2-acetylaminofluorene and two 7-halogeno derivatives. Biochemistry. 1976 Jul 27;15(15):3347–3351. doi: 10.1021/bi00660a027. [DOI] [PubMed] [Google Scholar]
- Fuchs R., Daune M. Physical studies on deoxyribonucleic acid after covalent binding of a carcinogen. Biochemistry. 1972 Jul 4;11(14):2659–2666. doi: 10.1021/bi00764a017. [DOI] [PubMed] [Google Scholar]
- Gray D. M. A circular dichroism study of poly dG, poly dC, and poly dG:dC. Biopolymers. 1974;13(10):2087–2102. doi: 10.1002/bip.1974.360131011. [DOI] [PubMed] [Google Scholar]
- Grunberger D., Blobstein S. H., Weinstein I. B. Modification of ribonucleic acid by chemical carcinogens. VI. Effect of N-2-acetylaminofluorene modification of guanosine on the codon function of adjacent nucleosides in oligonucleotides. J Mol Biol. 1974 Feb 5;82(4):459–468. doi: 10.1016/0022-2836(74)90241-1. [DOI] [PubMed] [Google Scholar]
- Grunberger D., Nelson J. H., Cantor C. R., Weinstein I. B. Coding and conformational properties of oligonucleotides modified with the carcinogen N-2-acetylaminofluorene. Proc Natl Acad Sci U S A. 1970 Jun;66(2):488–494. doi: 10.1073/pnas.66.2.488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isono K., Yourno J. Chemical carcinogens as frameshift mutagens: Salmonella DNA sequence sensitive to mutagenesis by polycyclic carcinogens. Proc Natl Acad Sci U S A. 1974 May;71(5):1612–1617. doi: 10.1073/pnas.71.5.1612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivanov V. I., Minchenkova L. E., Schyolkina A. K., Poletayev A. I. Different conformations of double-stranded nucleic acid in solution as revealed by circular dichroism. Biopolymers. 1973;12(1):89–110. doi: 10.1002/bip.1973.360120109. [DOI] [PubMed] [Google Scholar]
- Levine A. F., Fink L. M., Weinstein I. B., Grunberger D. Effect of N-2-acetylaminofluorene modification on the conformation of nucleic acids. Cancer Res. 1974 Feb;34(2):319–327. [PubMed] [Google Scholar]
- Mercado C. M., Tomasz M. Circular dichroism of mitomycin-DNA complexes. Evidence for a conformational change in DNA. Biochemistry. 1977 May 3;16(9):2040–2046. doi: 10.1021/bi00628a044. [DOI] [PubMed] [Google Scholar]
- Miller E. C. Some current perspectives on chemical carcinogenesis in humans and experimental animals: Presidential Address. Cancer Res. 1978 Jun;38(6):1479–1496. [PubMed] [Google Scholar]
- Patel D. J., Canuel L. L., Pohl F. M. "Alternating B-DNA" conformation for the oligo(dG-dC) duplex in high-salt solution. Proc Natl Acad Sci U S A. 1979 Jun;76(6):2508–2511. doi: 10.1073/pnas.76.6.2508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pohl F. M. Polymorphism of a synthetic DNA in solution. Nature. 1976 Mar 25;260(5549):365–366. doi: 10.1038/260365a0. [DOI] [PubMed] [Google Scholar]
- Sage E., Leng M. Conformation of poly(dG-dC) . poly(dG-dC) modified by the carcinogens N-acetoxy-N-acetyl-2-aminofluorene and N-hydroxy-N-2-aminofluorene. Proc Natl Acad Sci U S A. 1980 Aug;77(8):4597–4601. doi: 10.1073/pnas.77.8.4597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogt V. M. Purification and further properties of single-strand-specific nuclease from Aspergillus oryzae. Eur J Biochem. 1973 Feb 15;33(1):192–200. doi: 10.1111/j.1432-1033.1973.tb02669.x. [DOI] [PubMed] [Google Scholar]
- Wang A. H., Quigley G. J., Kolpak F. J., Crawford J. L., van Boom J. H., van der Marel G., Rich A. Molecular structure of a left-handed double helical DNA fragment at atomic resolution. Nature. 1979 Dec 13;282(5740):680–686. doi: 10.1038/282680a0. [DOI] [PubMed] [Google Scholar]
- Yamasaki H., Pulkrabek P., Grunberger D., Weinstein I. B. Differential excision from DNA of the C-8 and N2 guanosine adducts of N-acetyl-2-aminofluorene by single strand-specific endonucleases. Cancer Res. 1977 Oct;37(10):3756–3760. [PubMed] [Google Scholar]


