Abstract
Of the three closo-carborane isomers (C2B10H12), closo-1,2-carborane has been used most widely in the synthesis of carboranyl amines. However, closo-1,2-carboranes are prone to deboronation to nido-7,8-carborane under various conditions including attack by basic amino groups. In order to overcome this problem, closo-1,7-carboranyl ethyl-, propyl-, and butylamine were synthesized, which should be more stable towards basic deboronation than their closo-1,2-carboranyl counterparts. These closo-1,7-carboranyl amines (5, 18 and 19) were synthesized using two different methods, both starting from the corresponding closo-1,7-carboranyl alkyl iodides (3, 14 and 15). One of the carboranyl alkyl amine (5) was conjugated with folic acid to form a closo-1,7-carborane-folic acid bioconjugate (20).
Keywords: m-closo-carborane; closo-1,7-carborane; carboranyl amine
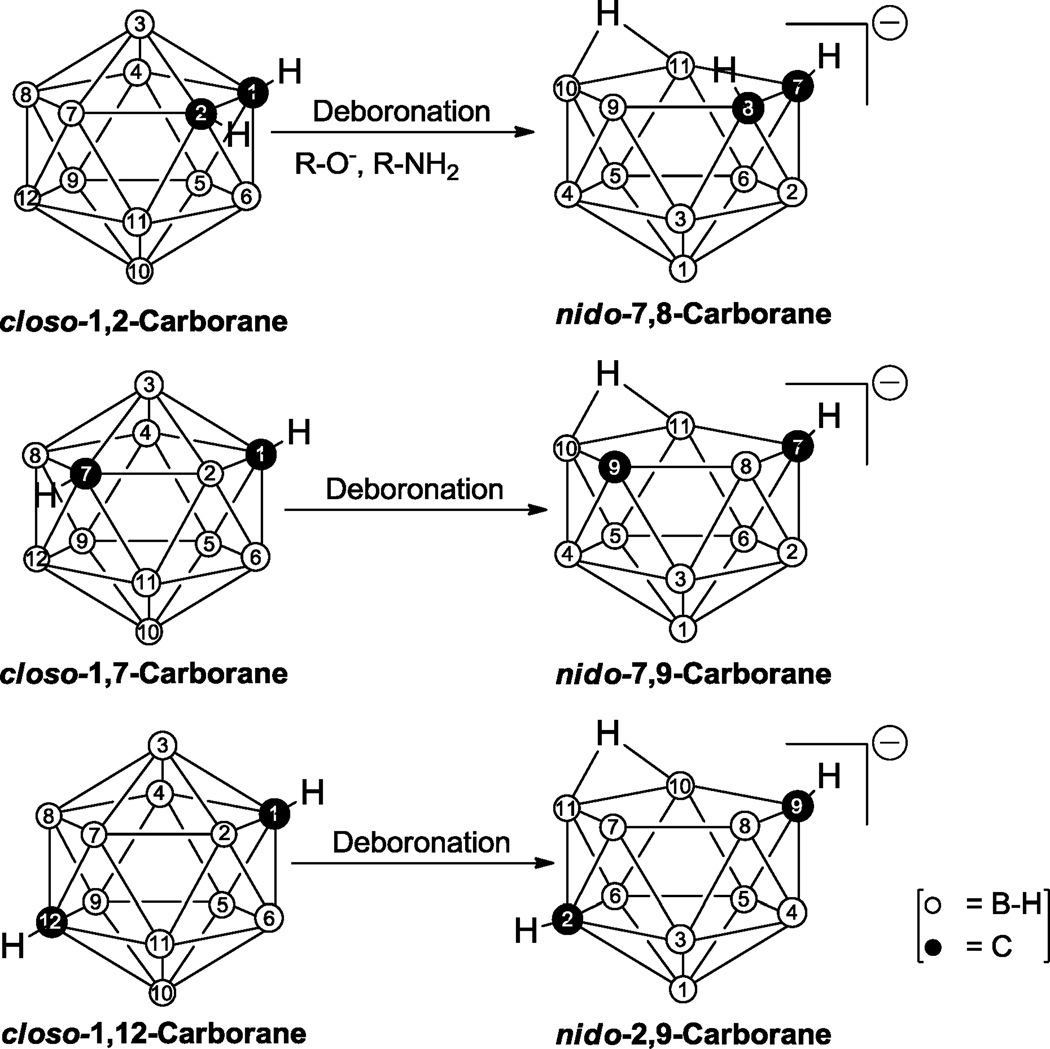
The hydrophobic dicarba-closo-dodecaboranes present a group of three isomers with the chemical formula C2B10H12. Depending on the position of the two carbons in the cage, the isomers are designated as closo-1,2 (ortho)-, closo-1,7 (meta)-, and closo-1,12 (para)-carborane. Nucleophilic deboronation of these closo-carboranes produces the corresponding negatively charged, hydrophilic dicarba-nido-undecaboranes 7,8-C2B9H12−, 7,9-C2B9H12−, and 2,9-C2B9H12− (Figure 1).1
Figure 1.
Deboronation of closo-1,2-, closo-1,7-, and closo-1,12-dicarba-dodecaboranes to corresponding nido-7,8-, nido-7,9- and nido-2,9-dicarba-undecaboranes, respectively.
Numerous types of carboranyl amines have been described.2–18 In most cases, closo-1,2- or nido-7,8-carborane were used for their synthesis. Many closo-1,2- and nido-7,8-carboranyl amines were synthesized for conjugation with pre-existing biomedical molecules, such as the epidermal growth factor (EGF),19 cyanocobalamin,6 and pyropheophorbide.17 These bioconjugates were prepared for use in Boron Neutron Capture Therapy (BNCT). Similarly, Tolmachev et al. prepared dextran conjugates of nido-7,8-carboranyl propyl amines for tumor imaging studies.20,21. Only very few reports are available describing mcloso-1,7-, nido-7,9-, and closo-1,12-carboranyl amines.14–16
Among the three closo-carboranyl isomers, closo-1,2-carborane is the most susceptible to deboronation by bases, such as alkoxides and amines.1 Deboronation of closo-1,2-carborane derivatives has even been reported under physiological and neutral aqueous conditions such as wet DMSO.22,23 In addition, Schinazi et al. observed intracellular nido-formation of 5-closo-1,2-carboranyl-2′-deoxyuridine in vitro.24 Therefore, nido-7,8-carboranes may not be suitable for biomedical applications which rely on the lipophilic character of a neutral closo-carborane cluster, as may the case for compounds that enter cells via passive diffusion.25 In addition, degradation of closo-1,2-carborane under physiological condition24 or during synthetic procedures involving carboranyl amines23 is generally not desirable when a closo-carboranyl compound is the desired target compound. To the best of our knowledge, deboronation of 1,7- and 1,12-carboranes under neutral aqueous conditions has not yet been reported and both clusters are in general far more stable than closo-1,2-carborane. In contrast to closo-1,7-carborane, closo-1,12-carborane is very expensive and has not been readily available in recent years in larger quantities. Therefore, closo-1,7-carboranes may be the best candidate for the synthesis of closo-carboranyl alkyl amines to be used in the preparation of closo-carboranyl bioconjugates that are stable under physiological conditions.
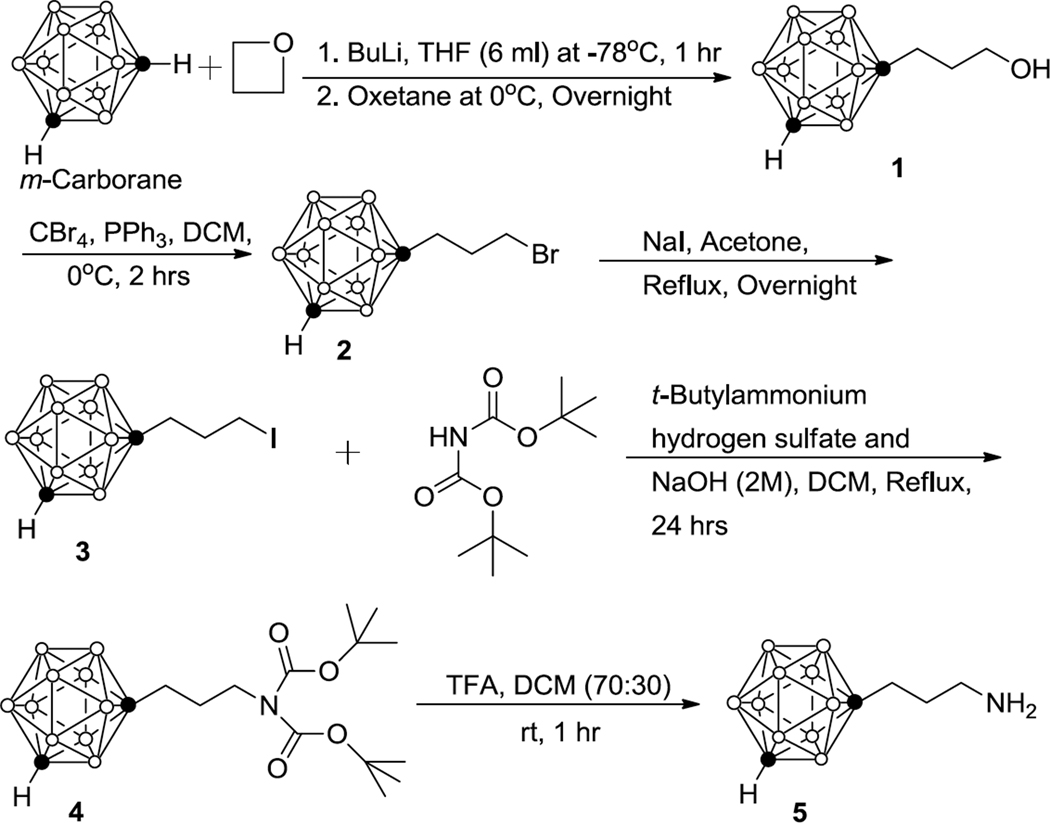
In this paper, we discuss the synthesis of closo-1,7-carboranyl ethyl-, propyl-, and butyl amine using two different synthetic strategies. To the best of our knowledge, the synthesis of 1,7-carborane-containing amines with spacers between amino group and cluster has not been described yet. In the first approach, closo-1,7-carboranyl propyl amine (5) was synthesized using a method that was previously reported for the preparation of the corresponding closo-1,2-carboranyl propyl amine (Scheme 1).21 The synthesis of 5 was initiated by reacting closo-1,7-carborane with butyl lithium in anhydrous tetrahydrofuran (THF) at −78°C for 2 hrs (Scheme 1). Subsequently, oxetane was added at 0 °C followed by stirring at room temperature overnight to produce 1-(3-hydroxypropyl)-closo-1,7-carborane (1) and 1,7-bis-(3-hydroxypropyl)-closo-1,7-carborane in 55% and 20% yields, respectively.21 Compound 1 was then reacted with carbon tetrabromide (CBr4) in presence of triphenyl phosphene (TPP) at 0 °C to produce 1-(3-bromopropyl)-closo-1,7-carborane (2) in 95% yield. Conversion to 1-(3-iodopropyl)-closo-1,7-carborane (3) in 95% yield was accomplished by refluxing 2 with sodium iodide (NaI) in anhydrous acetone. N-alkylation of 3 was achieved using di-tert-butylimminodicarboxylate (diBoc amine) in a phase transfer catalyzed reaction.26 Compound 3, diBoc amine, tetrabutylammonium hydrogen sulfate, and sodium hydroxide solution (2M) were refluxed (50°C) in dichloromethane to produce N-[3-closo-1,7-carboranyl propyl]-N,N-diBoc amine (4) in 70% yield.21 Formation of nido-carborane was not observed under these reaction conditions. Finally, removal of the Boc protective groups using 70% trifluoroacetic acid in dichloromethane at room temperature afforded a white precipitate of target compound 5 in 90% yield.
Scheme 1.
Synthesis of 1-(3-aminopropyl)-1,7-dicarba-closo-dodecaborane (5)
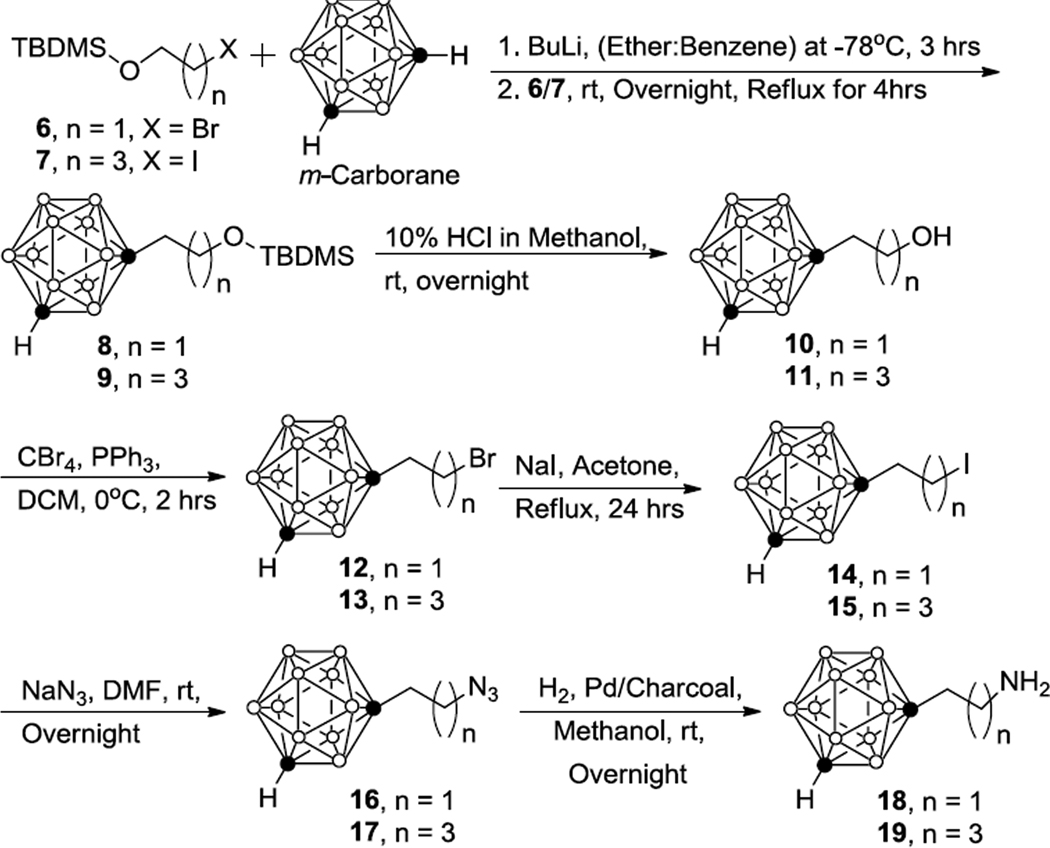
In the second approach, closo-1,7-carboranyl ethyl amine (18) and closo-1,7-carboranyl butyl amine (19) were synthesized adapting a procedure previously described by Soloway et al. for the preparation of closo-1,2-carboranylethyl amine (Scheme 2).3 Commercially available haloalcoxy-tert-butyl-dimethylsilane (6/7) was used for reaction with closo-1,7-carborane instead of oxetane. closo-1,7-Carborane was dissolved in anhydrous diethylether:benzene (2:1 mixture v/v), butyl lithium was added at 0 °C, and the reaction mixture was stirred for 2 hrs. Following this, 6/7 was added to produce 1,7-carboranyl ethoxy-tert-butyl-dimethylsilane (8) and 1,7-carboranyl ethoxy-tert-butyl-dimethylsilane (9) in 43% and 49% yield, respectively. Compound 8 and 1,7-carborane have similar Rf values (0.60 hexanes), and only partial purification of 8 was achieved to get 1,7-carborane-contaminated 8 (~30% m-carborane). The TBDMS protective groups of 8 and 9 were removed with a solution of 10% HCl in methanol to give 1,7-carboranyl ethanol (10) and 1,7-carboranyl butanol (11) in 79% and 84% yield, respectively.
Scheme 2.
Synthesis of 1-(2-aminoethyl)-1,7-dicarba-closo-dodecaborane (18) and 1-(4-aminobutyl)-1,7-dicarba-closo-dodecaborane (19).
Using the same procedure as described for the synthesis of closo-1,7-carboranyl propanol (1, Scheme 1), bromoethyl-closo-1,7-carborane (12) and bromobutyl-closo-1,7-carborane (13) were synthesized in 48% and 53% yield. A reaction time of exactly two hrs was crucial for an optimal yield of ~50%. Increased reaction times resulted in reduced yields and increased formation of impurities. Unreacted 10/11 were recovered by column chromatography and used again to further improve the yield. Longer reaction times resulted in increased formation of uncharacterized side products, which were difficult to separate from 12/13.
Compounds 12 and 13 were converted in yields of 100% to the corresponding iodo-analogues 14 and 15, as described in Scheme 1 for compound 3. In contrast to iodopropyl-closo-1,7-carborane (3),21 the iodoalkyl-closo-1,7-carboranes 14 and 15 did not react with diBoc amine. To the best of our knowledge, the corresponding ortho-carboranyl analogues were never synthesized using this method. However, in the case of N-protected 4-(2-bromoethyl)- and 4-(4-bromobutyl)piperidines, for example, amination with diBoc amine proceeded without problems to the desired products.27 Therefore, compounds 14/15 were reacted with sodium azide in DMF at room temperature overnight to produce the closo-1,7-carboranyl ethyl azides 16 and 17 (both 100% yield).3 Finally, 16/17 were reduced with H2/PdC (10%) to the target compounds closo-1,7-carboranyl ethyl amine (18) and closo-1,7-carboranyl butyl amine (19) in 54% and 63% yield, respectively. All compounds were purified by silica gel column chromatography and analyzed by proton and carbon NMR spectroscopy as well as high-resolution mass spectrometry.
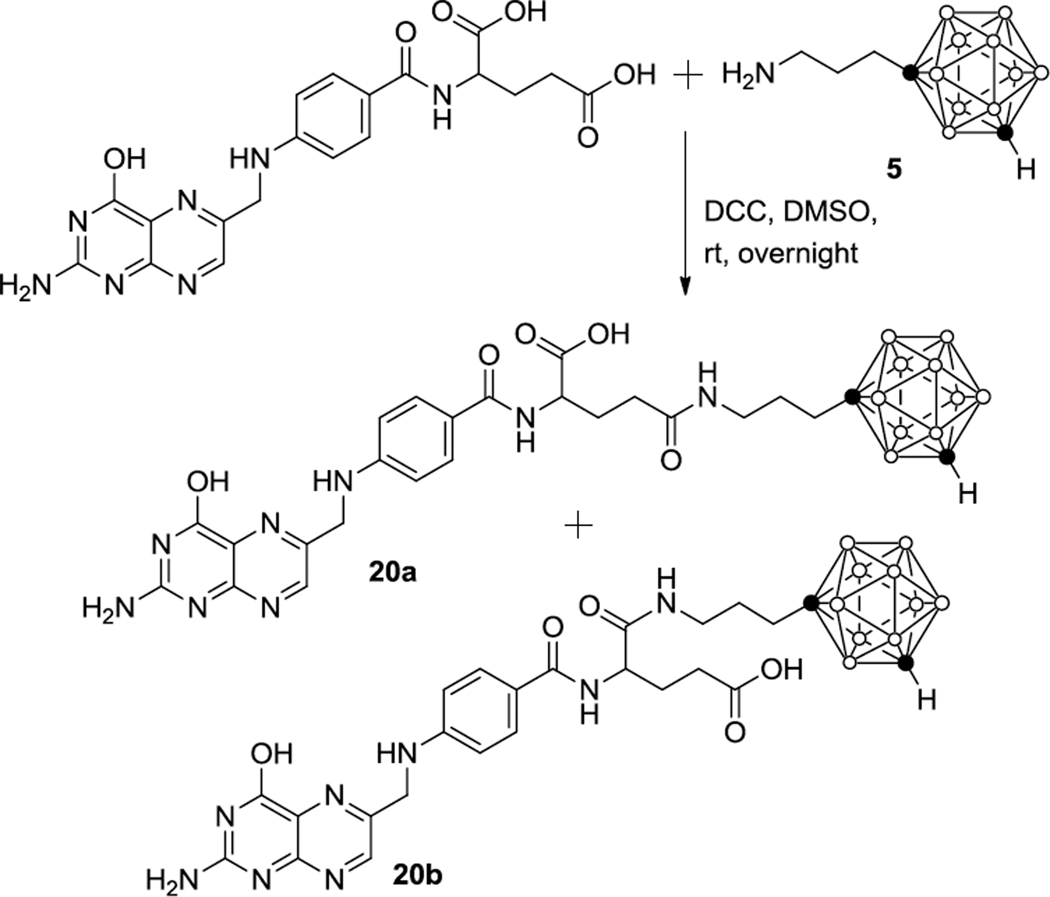
Finally, the closo-1,7-carboranyl propyl amine, 5, was conjugated with folic acid to yield the closo-1,7-carborane-folic acid bioconjugate, 20 (Scheme 3). Cellular uptake of folic acid and its bioconjugates is mediated by the folate receptor, which is overexpressed in various types of human cancer cells.28,29 In the past, boronated folic acid derivatives have been synthesized for BNCT studies.29 Cage-radiohalogenated forms of 20 could also be used for tumor imaging and the radiotherapy of cancer.28
Scheme 3.
Synthesis of a closo-1,7-carborane-folic acid bioconjugate, (20).
Compound 20 was synthesized by reacting folic acid with 5 in the presence of N,N′-dicyclohexylcarbodiimide (DCC) as the coupling reagent and pyridine as the base in DMSO at room temperature overnight (Scheme 3). The reaction mixture was added to a mixture of methanol and ether (1/4, v/v). The resulting precipitate was collected and purified by preparative RP18 HPLC to yield 20 in 53% yield. NMR-spectroscopic analysis indicated that 20 was a mixture of ~ 60% γ-amide (20a) and ~ 40 % α-(20b). The formation of 20 demonstrates the feasibility of closo-1,7-carboranyl amines to react with biomolecules.
In summary, three closo-1,7-carboranyl alkyl amines (5, 18 and 19) were successfully synthesized using two different methods without observing nido-formation during preparation. The overall yield for closo-1,7-carboranyl propyl amine (5) was the highest with 45%. On the other hand, the overall yields for closo-1,7-carboranyl ethyl amine (18) and 1,7-carboranyl butyl amine (19) were 9 and 14%, respectively, due to comparatively lower yields in the first two reaction steps. As an example, 5 was conjugated with folic acid to form the closo-1,7-carborane-folic acid bioconjugate, 20. The described carboranyl amines could also be used for the conjugation to other biomedically active molecules, such as nucleosides to be used in BNCT.25 Preliminary biological studies in our laboratory with several closo-1,7-carboranyl amine bioconjugates indicated no cage degradation under physiological conditions. Additionally, the intermediate closo-1,7-carboranyl alcohols (1, 10 and 11) and iodides (3, 14 and 15) could also be used in the synthesis of biologically-active compounds, as has been described previously for the corresponding closo-1,2-carboranyl derivatives.13,17
Supplementary Material
Acknowledgments
This research work was supported by The Ohio State University College of Pharmacy and, in part, by NIH grant R01 CA127935.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Supplementary Material
Supporting Information include the following: (S3) General Information, (S5) Experimental section, (S16) Spectral characterization data.
References and notes
- 1.Grimes RN. Amsterdam: Academic Press (an imprint of Elsevier); 2011. [Google Scholar]
- 2.Wu Y, Quintana W. Inorg. Chem. 1999;38:2025–2029. doi: 10.1021/ic981223h. [DOI] [PubMed] [Google Scholar]
- 3.Wilson JG, Anisuzzaman AKM, Alam F, Soloway AH. Inorg. Chem. 1992;31:1955–1958. [Google Scholar]
- 4.Lee J-D, Lee Y-J, Jeong H-J, Lee JS, Lee C-H, Ko J, Kang SO. Organometallics. 2003;22:445–449. [Google Scholar]
- 5.Sevryugina Y, Julius RL, Hawthorne MF. Inorg. Chem. 2010;49:10627–10634. doi: 10.1021/ic101620h. [DOI] [PubMed] [Google Scholar]
- 6.Hogenkamp HPC, Collins DA, Live D, Benson LM, Naylor S. Nuc. Med. Bio. 2000;27:89–92. doi: 10.1016/s0969-8051(99)00081-5. [DOI] [PubMed] [Google Scholar]
- 7.Terrasson V, Planas JG, Prim D, Teixidor F, Viñas C, Light ME, Hursthouse MB. Chem. Eur. J. 2009;15:12030–12042. doi: 10.1002/chem.200901332. [DOI] [PubMed] [Google Scholar]
- 8.Zhuo J-C, Cai J, Soloway AH, Barth RF, Adams DM, Ji W, Tjarks W. J. Med. Chem. 1999;42:1282–1292. doi: 10.1021/jm980703f. [DOI] [PubMed] [Google Scholar]
- 9.Ol'shevskaya VA, Dutikova YV, Tyutyunov AA, Kononova EG, Petrovskii PV, Sung DD, Kalinin VN. Synlett. 2010;8:1265–1267. [Google Scholar]
- 10.Ma L, Hamdi J, Huang J, Hawthorne MF. Inorg. Chem. 2005;44:7249–7258. doi: 10.1021/ic050895m. [DOI] [PubMed] [Google Scholar]
- 11.Zakharkin LI, Ol'shevskaya VA, Vinogradova LE. Russ. J. Gen. Chem. 1999;69:917–919. [Google Scholar]
- 12.Tsuji M. J. Org. Chem. 2003;68:9589–9597. doi: 10.1021/jo035090f. [DOI] [PubMed] [Google Scholar]
- 13.Cai J, Soloway AH. Tetrahedron Lett. 1996;37:9283–9286. [Google Scholar]
- 14.Byun Y, Tjarks W. Tetrahedron Lett. 2006;47:5649–5652. [Google Scholar]
- 15.Yoneyama H, Shimoda A, Araki L, Hatano K, Sakamoto Y, Kurihara T, Yamatodani A, Harusawa S. J. Org. Chem. 2008;73:2096–2104. doi: 10.1021/jo702181x. [DOI] [PubMed] [Google Scholar]
- 16.Kasar RA, Knudsen GM, Kahl SB. Inorg. Chem. 1999;38:2936–2940. doi: 10.1021/ic990037o. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Luzgina VN, Ol'shevskaya VA, Sekridova AV, Mironov AF, Kalinin VN, Pashchenko VZ, Gorokhov VV, Tusov VB, Shtil AA. Russ. J. Org. Chem. 2007;43:1243–1251. [Google Scholar]
- 18.Gruzdev DA, Levit GL, Bazhov IV, Sadretdinova LS, Ol'shevskaya VA, Kalinin VN, Krasnov VP, Chupakhin ON. Russ. Chem. Bull. 2010;59:110–115. [Google Scholar]
- 19.Carlsson J, Gedda L, Grönvik C, Hartman T, Lindstroem P, Lundquist H, Lövqvist A, Malmqvist J, Olsson P, Essand M, Ponten J, Sjöberg S, Westermark B. Int. J. Radiation. Oncology Bio. Phys. 1994;30:105–115. doi: 10.1016/0360-3016(94)90525-8. [DOI] [PubMed] [Google Scholar]
- 20.Tolmachev V, Bruskin A, Sjöberg S, Carlsson J, Lundquist HJ. Radioanal. Nucl. Chem. 2004;261:107–112. [Google Scholar]
- 21.Ghirmai S, Malmquist J, Lundquist H, Tolmachev V, Sjöberg S. J. Labelled Comp. Rad. 2004;47:557–569. [Google Scholar]
- 22.Powell CL, Schulze M, Black SJ, Thompson AS, Threadgill MD. Tetrahedron Lett. 2007;48:1251–1254. [Google Scholar]
- 23.Kononov LO, Orlova AV, Zinin AI, Kimel BG, Sivaev IB, Bregadze VI. J. Organomet. Chem. 2005;690:2769–2774. [Google Scholar]
- 24.Moore C, Hernandez-Santiago BI, Hurwitz SJ, Tan C, Wang C, Schinazi RF. J. Neurooncol. 2005;74:275–280. doi: 10.1007/s11060-004-8323-y. [DOI] [PubMed] [Google Scholar]
- 25.Al-Madhoun AS, Johnsamuel J, Barth Rolf F, Tjarks W, Eriksson S. Can. Res. 2004;64:6280–6286. doi: 10.1158/0008-5472.CAN-04-0197. [DOI] [PubMed] [Google Scholar]
- 26.Hashimoto T, Keiji M. In: Maruoka K, editor. Weinheim, Germany: Wiley-VCH Verlag GmbH & Co. KGaA; 2008. [Google Scholar]
- 27.Berque-Bestel I, Soulier JL, Giner M, Rivail L, Langlois M, Sicsic S. J. Med. Chem. 2003;46:2606–2620. doi: 10.1021/jm0307887. [DOI] [PubMed] [Google Scholar]
- 28.Müller C, Schibli R. J Nucl Med. 2011;52:1–4. doi: 10.2967/jnumed.110.076018. [DOI] [PubMed] [Google Scholar]
- 29.Shukla S, Wu G, Chatterjee M, Yang W, Sekido M, Diop LA, Müller R, Sudimack JJ, Lee RJ, Barth RF, Tjarks W. Bioconjug Chem. 2003;14:158–167. doi: 10.1021/bc025586o. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.