Abstract
To investigate the types of memory traces recovered by the medial temporal lobe (MTL), neural activity during veridical and illusory recognition was measured with the use of functional MRI (fMRI). Twelve healthy young adults watched a videotape segment in which two speakers alternatively presented lists of associated words, and then the subjects performed a recognition test including words presented in the study lists (True items), new words closely related to studied words (False items), and new unrelated words (New items). The main finding was a dissociation between two MTL regions: whereas the hippocampus was similarly activated for True and False items, suggesting the recovery of semantic information, the parahippocampal gyrus was more activated for True than for False items, suggesting the recovery of perceptual information. The study also yielded a dissociation between two prefrontal cortex (PFC) regions: whereas bilateral dorsolateral PFC was more activated for True and False items than for New items, possibly reflecting monitoring of retrieved information, left ventrolateral PFC was more activated for New than for True and False items, possibly reflecting semantic processing. Precuneus and lateral parietal regions were more activated for True and False than for New items. Orbitofrontal cortex and cerebellar regions were more activated for False than for True items. In conclusion, the results suggest that activity in anterior MTL regions does not distinguish True from False, whereas activity in posterior MTL regions does.
Our ability to remember past events, or episodic memory (1), critically depends on the function of the medial temporal lobe (MTL) region. Bilateral MTL damage can produce severe memory deficits in human patients and experimental animals (2). Functional neuroimaging studies have associated MTL both with the acquisition of new information (encoding) and with the recovery of stored information (retrieval) (3). During retrieval, neural activity in MTL was found to increase as a function of the amount of information recovered (4–10), suggesting that MTL is involved in the recovery of stored memory traces (11). However, the nature of the traces recovered by MTL is still a mystery.
What kinds of information does MTL recover? On one hand, there is evidence that MTL is sensitive to the sensory (perceptual) properties of recovered episodic information. For example, a positron emission tomography (PET) study found that MTL activity during object recognition was greater when study and test objects matched in size and orientation than when they mismatched (12). This pattern of results suggests that MTL contributes to the recovery of sensory traces. On the other hand, there is evidence that MTL recovers semantic (conceptual) but not sensory properties of episodic information. This evidence derives from PET and functional MRI (fMRI) studies of false recognition (13, 14). In these studies, subjects listened to a series of words (e.g., water, ice, wet, dark, freeze, etc.), which were all strongly related to a critical word that was not presented (e.g., cold), and later showed a strong tendency to falsely recognize the nonpresented critical word. Studied words (True items) and critical words (False items) elicited increased MTL activity relative to a fixation control condition, but MTL activity did not differentiate between True and False items (13, 14). This second pattern of results suggests that MTL is involved in the recovery of semantic traces (conceptual properties of episodic information), which presumably exist for both True and False items, rather than in the recovery of sensory traces (perceptual properties of episodic information), which presumably exist for True but not for False items (15).
A possible explanation of the lack of True–False differences in MTL activity in previous PET and fMRI studies of false recognition (13, 14) is that study conditions in these studies did not promote the encoding of sensory information. First, sensory information was not salient, because all words were presented in one format and one modality (one speaker's voice). Second, subjects were instructed to memorize the words, but memory for their sensory features was not required. To test this account, we conducted an fMRI study of false recognition with study conditions that promoted the encoding of sensory information. First, sensory information was made salient via an audiovisual presentation rich in sensory information: word lists were alternately presented by two different speakers (a Caucasian male and an Asian female). Second, subjects were instructed to remember not only the words but also the speaker who presented them. We expected that these study conditions would encourage the encoding of sensory information (e.g., speakers' voices, faces, locations, etc.) and lead to sensory-related differences in MTL activity during retrieval. Note that the old/new recognition task encourages but does not require the retrieval of sensory information. However, because previous neuroimaging studies of true vs. false recognition (13, 14) used old/new recognition tasks, use of the same task in the present study allowed us to compare our results directly with these previous findings.
A second goal of the study was to identify brain activity associated with recognizing old and new items. Activity elicited by old items can be attributed to the processing of recovered information and activity elicited by new items with additional recovery operations. There is evidence that recognizing old and new items differentially involves distinct subregions within the prefrontal cortex (PFC) (16, 17).
Methods
Subjects.
Twelve college students participated in the experiment. All subjects were right handed and had no history of neurological or psychiatric illness. They were not taking medications and did not have medical conditions that could affect cerebral blood flow. The study was approved by ethics review boards at the Medical College of Wisconsin and the University of Alberta.
Materials.
The materials were 18 thematic (18) and categorical 14-word lists. Each thematic list consisted of words (e.g., water, ice, wet, dark, freeze, etc.) associated with a theme word (e.g., cold), and each categorical list consisted of instances (e.g., cucumber, pea, potato, onion, corn, etc.) of a natural category (e.g., vegetables). In the case of thematic lists, the first and second strongest associates were used as True items, and the theme word (e.g., cold) and the third strongest associate, as False items. In the case of associative lists, the second and fourth strongest associates were used as True Items, and the first and third, as False items. These procedures approximately equated the average associative value of True and False items. For each study list, two test lists were created, one including one of the targets and one of the critical lures, and the other including the other target and critical lures. The assignment of targets and critical lures was counterbalanced across lists. Additionally, test lists also included new words, which were selected from a word norm with equivalent letter number, frequency, and concreteness as the targets.
Behavioral Methods.
The experiment consisted of six critical blocks with two phases each: study and test. During the study phase, subjects watched a videotape segment in which a male speaker and a female speaker alternatively read six word lists (three thematic, three categorical) of 14 words each. Words were presented in order of decreasing strength of association with the theme word or the category, except for the two words to be used as targets that were shifted to positions 4 and 6. Subjects were instructed to remember not only what words were presented but also who presented them. Each word was presented at a rate of one word every 2 s, and there was an interval of several seconds between lists.
During the test phase, subjects performed two consecutive tests: a recognition test and a source test. The behavioral and neuroimaging results of the source test are not reported or discussed in this article. In the recognition test, each word was presented for 3 s and followed by a fixation cross until the end of the 12.5-s trial. For each word, subjects had to indicate whether the word was read by either of the speakers during the preceding videotape segment (“old” word) or whether it was not presented during this segment (“new” word). Subjects made old/new decisions as quickly as possible by pressing keys in a response box. Subjects were instructed to rest during the 9.5-s fixation until the next word was presented. The test list of each block consisted of 18 words: one true target from each of the six lists, one false target from each of the six lists, and six new words (18 trials × 12.5 s = 3.75-min fMRI run). Thus, across the six blocks, subjects completed 36 True, 36 False, and 36 New trials.
fMRI Methods.
Whole-brain, event-related fMRI was conducted on a 1.5-Tesla Signa scanner (General Electric) equipped with a three-axis local gradient head coil and an elliptical endcapped quadrature radiofrequency coil (Medical Advances, Milwaukee). Echo-planar images were collected in a single-shot, blipped, gradient-echo echo-planar pulse sequence (echo time = 40 ms; field of view = 24 cm; matrix size = 64 × 64). For each functional time series, 17 contiguous sagittal 7-mm-thick slices were selected to provide coverage of the entire brain (voxel size: 3.75 × 3.75 × 7 mm). The interscan interval was 2.5 s. During each imaging series, 94 sequential echo-planar images were collected. At the beginning of the experiment 3D spoiled gradient-recalled at steady-state anatomic images were collected (echo time = 5 ms, repetition time = 24 ms, 40° flip angle, number of excitations = 1, slice thickness = 1.2 or 1.3 mm, field of view = 24 cm, resolution = 256 × 192).
Functional images were generated using analysis of functional neuroimages software (19). Time-series images were spatially registered in three-dimensional space to minimize effects of head motion. The analysis of functional neuroimages program 3ddeconvolve was used to extract hemodynamic response functions (HRFs) of the fMRI signal on a voxel-wise basis. This program uses a sum of scaled and time-delayed versions of the stimulus time series, with the data itself determining (within limits) the functional form of the estimated response. The 3ddeconvolve program yielded the best linear least-squares fit for the following model parameters: constant baseline, linear trend in time series, BOLD response deviation from baseline (for each condition: true, false, new) 2.5, 5, 7.5, and 10 s after stimulus. This fit of the parameters produced an estimate of the hemodynamic response for images 2–5 post-stimulus onset for each condition (True, False, and New) relative to a baseline state (rest). Image 1 in the HRF is not estimated, but defined as 0. The deconvolution method estimates the baseline from all of the points in the time series. The subsequent points in the HRF are estimated as deviations from the baseline, which defines the baseline as 0 in the HRF. In the figures, the HRF was smoothed with the use of the “smooth line” feature in the program excel (Microsoft). The HRF in the figures shows a slope down, which appears to be a consequence of using all of the points in the times series as baseline. True and False items classified as “old,” and New items classified as “new” were entered into the analyses. Anatomical and functional images were then interpolated to volumes with 1 mm3 voxels, coregistered, converted to stereotaxic coordinate space (20), and blurred with the use of a 4-mm Gaussian full-width half-maximum filter.
Voxel-wise two-factor ANOVAs, with a fixed condition factor (True vs. False vs. New) and a random subject factor, were performed. The ANOVAs were conducted separately for each of images 2–5 to avoid the confounding influence of temporal autocorrelation. Each ANOVA was followed by posthoc pooled variance t tests for pairwise comparison of the three conditions. The significance threshold for activation peaks was set at a t value of 3.8 for analyses of MTL activity and at a t value of 4.5 for whole-brain exploratory analyses. To minimize false positives, only clusters with at least 0.2 ml above t = 2.8 were considered (21). Under these conditions, 10,000 Monte Carlo simulations on an average brain volume of 1,404 ml with 4-mm full-width half-maximum of spatial blurring yielded a final per-voxel probability of P < 0.0003.
Virtually all significant activation foci derived from the voxel-wise two-factor ANOVAs and posthoc t tests occurred in the analyses applied to images 2 and 3, the likely peak of the HRF. For all significant clusters, an average HRF was calculated for each subject and each condition (True, False, and New). This calculation was accomplished by defining a mask of all activated regions defined by the group analysis. By then applying this mask to brain volumes of individual subjects, the average HRF was calculated for each condition (True, False, and New) from all voxels and time points within the masked region. The average HRF was then subjected to pairwise t tests to identify specific patterns of activation differentiating the three conditions (these analyses were confined to a single image in the HRF rather than across time points to avoid statistical problems associated with temporal autocorrelation).
Results
Behavioral Results.
Subjects were generally accurate at accepting True items and rejecting New (unrelated) items, but at the same time they showed a strong tendency to accept False items. The mean proportion of items classified as “old” was 0.88 (SD = 0.12) for True items, 0.80 (SD = 0.13) for False items, and 0.12 (SD = 0.07) for New items. An ANOVA yielded a significant effect of item type [F(2, 22) = 246.56, P < 0.0001]. Fischer's probable least-squares differences were significant between True and New items (P < 0.0001) and between False and New items (P < 0.0001), and there was a trend for a difference between True and False items (P = 0.06). Responses were faster for True than for False items, and for False items than for New items. Mean reaction times were 1,419 ms for True items, 1,576 ms for False items, and 1,709 ms for New items. An ANOVA yielded a significant effect of item type [F(2, 22) = 12.36, P < 0.0003], and probable least-squares differences were all significant (P < 0.03). Thus, although recognition responses for True and False items were not significantly different, subjects were faster for True than for False items.
fMRI Results.
First, we identified activation differences between True, False, and New conditions in MTL regions by performing voxel-wise ANOVAs separately for each of images 2–5, followed by posthoc t tests. Results of this voxel-wise analysis identified significant areas of activation in the hippocampus bilaterally (left: xyz = −25, −22, −11, t = 4.6; right: xyz = 27, −29, −11, t = 5.1) and the left parahippocampal gyrus (xyz = −15, −36, 3; t = 4.1). A subsequent analysis of the average hemodynamic response function (average HRF; see fMRI Methods) yielded a dissociation between these posterior and anterior MTL regions (Fig. 1). In anterior MTL, bilateral hippocampal regions were more activated for True and False items than for New items, with no difference between True and False items (see Fig. 1A). This difference was observed primarily at image 3. Pairwise contrasts of the average HRF yielded significant differences between True and New [t (11) = 6.2, P < 0.0001] and between False and New [t (11) = 5.8, P < 0.0001], but not between True and False (P = 0.48). This activation pattern suggests that this anterior MTL region is involved in the recovery of semantic rather then sensory information. A different pattern across True, False, and New trials was observed in posterior MTL. Specifically, the left parahippocampal gyrus was more activated for True than for False and New items, with no difference between False and New items (see Fig. 1B). This difference was observed at image 2. Pairwise contrasts of the average HRF yielded significant differences between True and False [t (11) = 4.3, P < 0.0005] and between True and New [t (11) = 3.8, P < 0.001], but not between False and New (P = 0.29). This activation pattern suggests that this posterior MTL region is involved in the recovery of sensory rather than semantic information.
Figure 1.
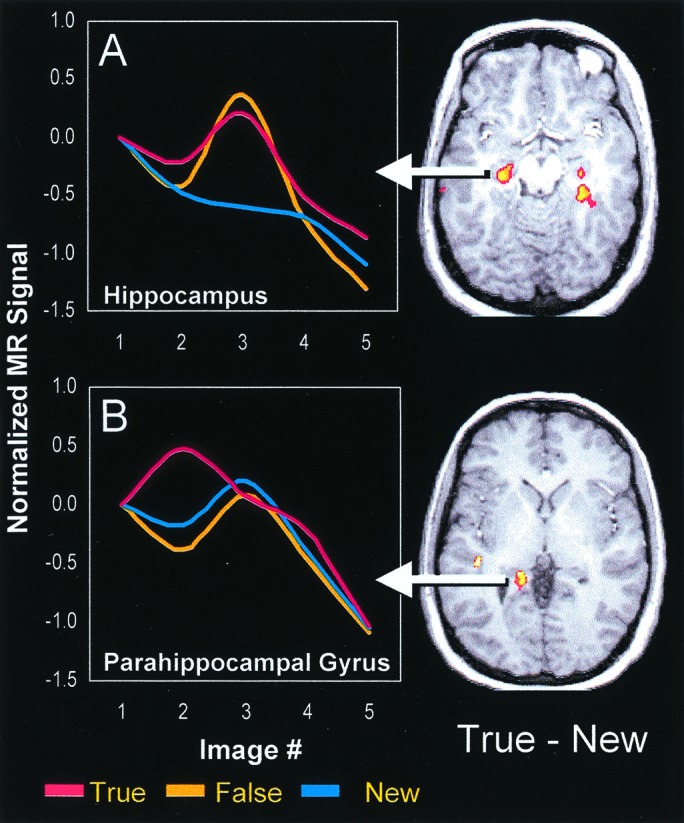
Significant activations in the MTL and their corresponding hemodynamic response functions. (A) Bilateral hippocampal regions were more activated for True and False than for New items, with no difference between True and False. (B) A left posterior parahippocampal region that was more activated for True than for False and New items, with no difference between False and New.
To investigate the dissociation between posterior and anterior MTL, we conducted a 2 (MTL region: left anterior vs. left posterior) × 3 (item: True vs. False vs. New) ANOVA on the average HRFs from images showing maximal differences (image 2 for posterior MTL, image 3 for anterior MTL). This analysis yielded a nonsignificant main effect of region (F < 1), a significant effect of item [F(2, 22) = 17.8, P < 0.0001], and a significant region x item interaction [F(2, 22) = 12.01, P < 0.0005]. This reliable interaction confirms the dissociation between activity patterns in anterior and posterior MTL.
Second, we conducted a whole-brain exploratory analysis to identify regions outside MTL showing differences between True, False, and New conditions (see Table 1). Because the True vs. New contrast and the False vs. New contrast yielded a very similar set of activations, we simplified the results by collapsing over these conditions (True/False vs. New). Every activation identified by this combined analysis was also significant in the separate analyses (True vs. New and False vs. New contrasts).
Table 1.
Differences in activity outside MTL
| Contrast | |||||||
|---|---|---|---|---|---|---|---|
| Region | Lat. | BA | X | Y | Z | Max. t | Image |
| True/False–New | |||||||
| Dorsolateral PFC | R | 46 | 38 | 38 | 6 | 5.4 | 3 |
| R | 8/6 | 37 | 8 | 52 | 4.6 | 3 | |
| L | 46 | −39 | 49 | 8 | 4.8 | 3 | |
| L | 8/9 | −32 | 17 | 40 | 5.2 | 3 | |
| Parietal ctx. | R | 40/39 | 40 | −51 | 22 | 6.6 | 3 |
| L | 40 | −47 | −50 | 38 | 10.0 | 3 | |
| Cuneus/precuneus | R | 31 | 12 | −48 | 37 | 6.4 | 3 |
| L | 7/19 | −9 | −68 | 32 | 5.1 | 3 | |
| Temporal ctx. | R | 21/37 | 57 | −48 | −3 | 5.2 | 3 |
| Thalamus | L | −20 | −34 | 13 | 5.1 | 3 | |
| New–True/False | |||||||
| Ventrolateral PFC | L | 45 | −42 | 24 | 6 | 4.6 | 4 |
| Central sulcus | L | 1/2 | −39 | −24 | 48 | 4.6 | 3 |
| True–False | |||||||
| Anterior cingulate | M | 24 | 16 | 32 | 8 | 5.2 | 3 |
| Posterior parietal | L | 40/39 | −53 | −55 | 32 | 4.7 | 2 |
| False–True | |||||||
| Temporal pole | R | 38 | 35 | 3 | −45 | 5.2 | 4 |
| Cerebellum | M | 3 | −78 | −26 | 4.8 | 4 | |
| Ventromedial PFC | R | 11 | 16 | 53 | −19 | 5.4 | 5 |
Lat, lateralization; L, left; R, right; M, midline; ctx., cortex; XYZ, coordinates in Talairach and Tournoux atlas (20); t, t value (df = 11). Image, number of the image showing the maximum difference in activation.
Compared with New items, True and False items elicited activations in bilateral dorsolateral PFC, as well as in parietal, cuneus/precuneus, temporal, and thalamic regions. Conversely, left ventrolateral PFC and central sulcus regions were more active for New than for True and False items. Thus, there was a dissociation between two PFC areas (see Fig. 2): whereas bilateral dorsolateral regions (e.g., Area 46) were more active for True and False than for New items (see Fig. 2 A and B), the left ventrolateral region (area 45) was more active for New than for True and False items (see Fig. 2C).
Figure 2.
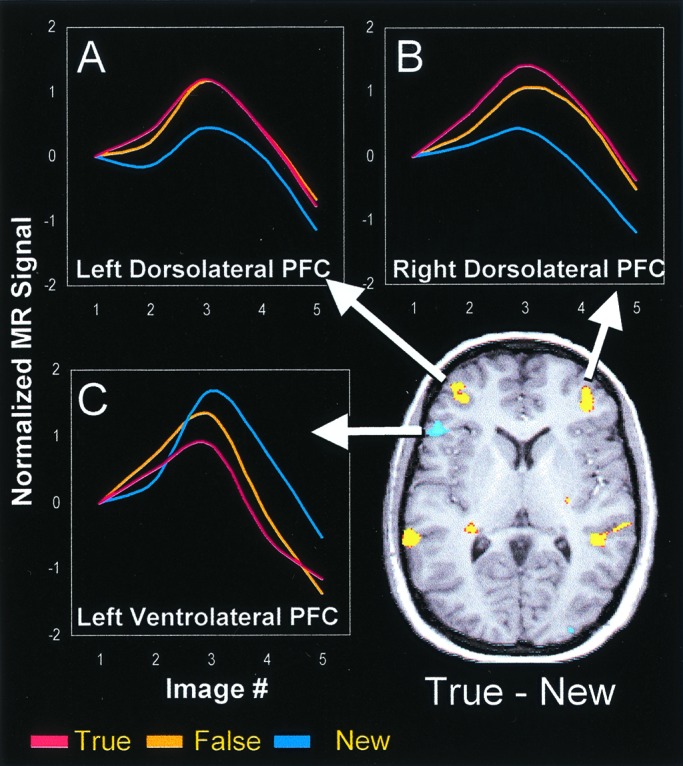
Significant activations in PFC and their corresponding hemodynamic response functions. (A and B) Bilateral dorsolateral PFC regions were more activated for True and False than for New items. (C) A left ventrolateral PFC region was more activated for New than for True and False items.
To investigate the dissociation between dorsolateral and ventrolateral left PFC regions, we conducted a 2 (left dorsolateral PFC vs. left ventrolateral PFC) × 3 (item: True vs. False vs. New) ANOVA on the average HRFs from images showing maximal differences (image 3 for left dorsolateral PFC, image 4 for left ventrolateral PFC). This analysis yielded a significant main effect of region [F(1, 11) = 10.7, P < 0.01], a nonsignificant effect of item [F(2, 22) = 1.3, P = 0.28], and a significant region × item interaction [F(2, 22) = 24.5, P < 0.0001]. This reliable interaction confirms the dissociation between activity patterns in dorsolateral and ventrolateral left PFC regions.
Finally, a few regions outside MTL showed significant differences between True and False conditions. The anterior cingulate and the left posterior parietal cortex (area 40/39) were more activated for True than for False items, whereas the right temporal pole (area 38), the cerebellum, and ventromedial PFC (area 11) were more activated for False than for True items.
Discussion
The main finding of the present study was a dissociation between two MTL regions as a function of the type of information recovered. Whereas a posterior MTL region in the parahippocampal gyrus was associated with the recovery of sensory information, an anterior MTL region in the hippocampus was associated with the recovery of semantic information. The parahippocampal activation suggests that MTL can be sensitive to sensory properties of recovered information (12), and it provides evidence that MTL activity can differentiate between True from False items. The fact that this region did not differentiate between False and New items suggests that it is involved in the recovery of sensory rather than semantic information. The hippocampal activation is consistent with previous functional neuroimaging studies of false recognition, which detected similar hippocampal activity for veridical and illusory recognition (13, 14). The fact that this region did not differentiate between True and False items implies that this more anterior part of MTL is involved in the recovery of semantic rather than sensory information.
The finding that posterior MTL is sensitive to the recovery of sensory information whereas the anterior hippocampal region is sensitive only to the recovery of semantic information provides an explanation for puzzling findings in false memory experiments (22, 23). On one hand, when participants in these experiments recall or recognize events that never happened, they are usually very confident (24). On the other hand, when they are asked to rate retrieved items in terms of sensory detail, they give greater ratings to True than to False items (25, 26). How can human participants believe in their illusory recollections and at the same time be able to differentiate them from veridical recollections? The present MTL dissociation provides a possible answer to this conundrum: the MTL memory system can generate two different messages. As illustrated by Fig. 1, whereas anterior hippocampal activity suggests that False items are like True items, posterior parahippocampal activity suggests that False items are like New items. These two messages are not contradictory: False items are like True items in terms of their semantic properties, but they are like New items in terms of their sensory properties.
The dissociation observed here is broadly consistent with the proposal that anterior and posterior MTLs contribute differently to memory processes (27, 28). The present MTL dissociation is also consistent with a hierarchical model of visual processing along the occipito-temporal ventral pathway (29). According to this model, information processing progresses from low-level sensory analyses in more posterior temporal regions to higher-order semantic processing in more anterior temporal regions. Consistent with this idea, in the present study, the posterior MTL was sensitive to sensory features of stimuli, whereas the anterior MTL was sensitive to semantic features of stimuli. The fact the posterior MTL activation was maximal in image 2 whereas the anterior MTL activation was maximal in image 3 is also consistent with a posterior-anterior progression of sensory-semantic analyses, but this timing difference should be interpreted with caution because of the interleaved acquisition of MRI slices.
In particular, sensory recovery was linked to the parahippocampal gyrus, and semantic recovery, to the hippocampus. The anatomy of these two MTL regions is consistent with the idea of a perceptual–conceptual continuum. The parahippocampal region (perirhinal and parahippocampal cortex) receives direct inputs from unimodal and polymodal cortical regions and provides about two-thirds of the inputs to the hippocampal region (30). The hippocampal region has intimate connection with diencephalic and prefrontal regions (31), but is farther from sensory input than the parahippocampal region. Thus, the anatomy of the MTL is consistent with the dissociation between sensory vs. semantic recovery implied by the present findings.
The association of True items with sensory-related activity is consistent with the results of a PET study of false recognition. In that study, the retrieval of auditorily encoded words was associated with activity in auditory cortex (13), whereas in the present study, the retrieval of audio-visually encoded words was associated with activity in the parahippocampal gyrus. The notion that the recovery of sensory information during episodic retrieval involves the reactivation of sensory brain regions (32, 33) has recently been bolstered by the results of functional neuroimaging studies (34, 35). In these studies, the recovery of sensory traces was associated with activity in visual or auditory cortices, whereas in the present study, it involved an association area with rich sensory input, the parahippocampal gyrus. The reason for this discrepancy is unclear, but it could be related to differences in presentation format (e.g., words paired with sounds vs. words paired with faces and a spatial context). An alternative explanation is that the parahippocampal activation did not reflect the recovery of sensory traces, but other differences between True and False items.
The hippocampal activation is also consistent with functional neuroimaging evidence. Hippocampal activity during episodic retrieval has been found in several blocked PET and fMRI studies (27, 28) and to be associated with successful recovery of episodic information (4, 6–10). Hippocampal activity was also found in two recent event-related fMRI studies. In one study (36), recognition of old words was associated with activity in the left posterior parahippocampal gyrus, and recognition of new words, with activity in the left anterior hippocampus. This latter finding is inconsistent with the present results but is difficult to interpret because of marked differences in the probability of old and new items during the scan (37). In the other study (5), the hippocampus was activated when word recognition was based on recollection (Remember response) but not when it was based on familiarity (Know response). This finding is consistent with the present results, because in the false memory paradigm we used (24), Remember responses are as common for True as for False items (23). The present results suggest that hippocampal-based recollection reflects the recovery of semantic information, rather than the recovery of sensory information, which in the present study seemed to be mediated by posterior parahippocampal regions.
The study also yielded a dissociation between bilateral dorsolateral PFC, which was more activated for True and False items than for New items, and left ventrolateral PFC, which was more activated for New than for True and False items. Dorsolateral PFC has been found to be more active for old than for new items (16, 36, 38–40), possibly reflecting the monitoring of retrieved information (16). Consistent with this idea, decisions that involve post-retrieval demands in addition to those required by simple recognition, such as choosing between Remember and Know responses (5, 41) or rejecting lures made of old items (42), tend to engage dorsolateral PFC (37). Left ventrolateral PFC activity during episodic retrieval has been associated with the contributions of semantic processing to episodic memory retrieval (43–45). This interpretation is plausible in the present study, given that study materials were semantically organized. Thus, the present results suggest that in left PFC, the ventrolateral cortex is associated with semantic processing, whereas the dorsolateral cortex is associated with monitoring. This dissociation is consistent with the idea that ventrolateral PFC is involved in simple working memory operations, such as semantic processing, whereas dorsolateral PFC is involved in higher-order working memory operations, such as monitoring or selection within working memory (46–48).
However, other interpretations are also possible, particularly regarding the difference in left ventrolateral PFC. First, this difference could reflect conceptual priming for True and False items, as several studies have associated conceptual priming with deactivations in left ventrolateral PFC (49–54). This interpretation is not incompatible with the semantic processing interpretation because conceptual priming could involve a reduction in the semantic processing demands of True and False items, resulting in greater semantic processing for New items. Second, the left ventrolateral PFC difference could reflect incidental episodic memory encoding of verbal material, which has been associated with left ventrolateral PFC (3) and was probably greater for New than for True and False items. Left ventrolateral PFC activity during processing of a word has been found to predict subsequent memory for the word (55), even when the word is encountered as a cue during a recognition test (56). Again, this interpretation is not irreconcilable with the semantic processing hypothesis, as episodic encoding and semantic retrieval can be seen as two aspects of the same process (57).
Other regions that were more activated for True and False than for New items included the precuneus area and lateral parietal regions, particularly in the left hemisphere. Activations in these areas are frequently observed in functional neuroimaging studies of episodic retrieval (3). The precuneus activation is consistent with evidence that this region is involved in successful recovery operations (3, 37, 58). The left parietal cortex (area 40/39) showed a graded response to recovery, being more activated for True than for False items, and for False than for New items. This finding extends the results of recent event-related fMRI studies (40, 42, 59) and supports the idea that left parietal activity could underlie the left parietal event-related potential effect (59), which also shows a graded a response as a function of recovery (60).
Finally, right ventromedial PFC (orbitofrontal cortex) and cerebellar regions were more activated for False than for True items. These regions also showed a significant False–True difference in a previous PET study of false recognition (13) and may reflect verification processes during retrieval. Verification processes are particularly demanding in the case of False items, because they elicit a vivid recollective experience but less sensory recovery than True items. It is interesting to note that orbitofrontal lesions may lead to high levels of false recognition (61) and confabulation (62), suggesting a deficit with verification processes (22).
In summary, the main finding of the present study was a dissociation between two MTL regions. Compared with New items, a hippocampal region was similarly activated for True and False items, suggesting that it is involved in the recovery of semantic information. In contrast, a parahippocampal region was activated for True but not for False items, suggesting that it is involved in the recovery of sensory information. This dissociation could account for inconsistent findings in the false memory literature and is consistent with the anatomy of MTL. Thus, the answer to the question in the title appears to be that, whereas anterior MTL regions cannot distinguish True from False, posterior MTL regions can.
Acknowledgments
We thank Robert Cox, Sally Durgerian, and Barney Ward for technical assistance. The study was supported by grants from the National Science and Engineering Research Council of Canada and the Alberta Heritage Foundation for Medical Research to R.C., by grants from the National Institutes of Health to S.M.R. (P01 MH51358, R01 MH57836) and to D.L.S. (1RO1MH/NS60941 and RO1AG08441), and by grants from the General Clinical Research Center (M01 RR00058) and the W. M. Keck Foundation to the Medical College of Wisconsin.
Abbreviations
- fMRI
functional MRI
- MTL
medial temporal lobe
- PFC
prefrontal cortex
- PET
positron emission tomography
- HRF
hemodynamic response functions
References
- 1.Tulving E. Elements of Episodic Memory. Oxford: Oxford Univ. Press; 1983. [Google Scholar]
- 2.Squire L R. Psychol Rev. 1992;99:195–231. doi: 10.1037/0033-295x.99.2.195. [DOI] [PubMed] [Google Scholar]
- 3.Cabeza R, Nyberg L. J Cognit Neurosci. 2000;12:1–47. doi: 10.1162/08989290051137585. [DOI] [PubMed] [Google Scholar]
- 4.Düzel E, Cabeza R, Picton T W, Yonelinas A P, Scheich H, Heinze H-J, Tulving E. Proc Natl Acad Sci USA. 1999;96:1794–1799. doi: 10.1073/pnas.96.4.1794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Eldridge L L, Knowlton B J, Furmanski C S, Bookheimer S Y, Engle S A. Nat Neurosci. 2000;3:1149–1152. doi: 10.1038/80671. [DOI] [PubMed] [Google Scholar]
- 6.Nyberg L, Tulving E, Habib R, Nilsson L-G, Kapur S, Houle S, Cabeza R, McIntosh A R. NeuroReport. 1995;7:249–252. [PubMed] [Google Scholar]
- 7.Rugg M D, Fletcher P C, Frith C D, Frackowiak R S J, Dolan R J. NeuroReport. 1997;8:1283–1287. doi: 10.1097/00001756-199703240-00045. [DOI] [PubMed] [Google Scholar]
- 8.Schacter D L, Alpert N M, Savage C R, Rauch S L, Albert M S. Proc Natl Acad Sci USA. 1996;93:321–325. doi: 10.1073/pnas.93.1.321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Stark C E L, Squire L R. J Neurosci. 2000;20:7776–7781. doi: 10.1523/JNEUROSCI.20-20-07776.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Stark C E L, Squire L R. Hippocampus. 2000;10:329–337. doi: 10.1002/1098-1063(2000)10:3<329::AID-HIPO13>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- 11.Nyberg L, McIntosh A R, Houle S, Nilson L-G, Tulving E. Nature (London) 1996;380:715–717. doi: 10.1038/380715a0. [DOI] [PubMed] [Google Scholar]
- 12.Schacter D L, Uecker A, Reiman E, Yun L S, Bandy D, Chen K, Cooper L A, Curran T. NeuroReport. 1997;8:3993–3998. doi: 10.1097/00001756-199712220-00028. [DOI] [PubMed] [Google Scholar]
- 13.Schacter D L, Reiman E, Curran T, Yun L S, Bandy D, McDermott K B, Roediger H L., III Neuron. 1996;17:267–274. doi: 10.1016/s0896-6273(00)80158-0. [DOI] [PubMed] [Google Scholar]
- 14.Schacter D L, Buckner R L, Koutstaal W, Dale A M, Rosen B R. NeuroImage. 1997;6:259–269. doi: 10.1006/nimg.1997.0305. [DOI] [PubMed] [Google Scholar]
- 15.Brainerd C J, Reyna V F. Dev Rev. 1990;10:3–47. [Google Scholar]
- 16.Rugg M D, Fletcher P C, Frith C D, Frackowiak R S, Dolan R J. Brain. 1996;119:2073–2083. doi: 10.1093/brain/119.6.2073. [DOI] [PubMed] [Google Scholar]
- 17.Buckner R L, Koustaal W, Schacter D L, Dale A M, Rotte M, Rosen B R. NeuroImage. 1998;7:163–175. doi: 10.1006/nimg.1998.0328. [DOI] [PubMed] [Google Scholar]
- 18.Stadler M A, Roediger H L I, McDermott K B. Mem Cognit. 1999;27:494–500. doi: 10.3758/bf03211543. [DOI] [PubMed] [Google Scholar]
- 19.Cox R W. Comp Biochem Res. 1996;29:162–173. doi: 10.1006/cbmr.1996.0014. [DOI] [PubMed] [Google Scholar]
- 20.Talairach J, Tournoux P. A Coplanar Sterotactic Atlas of the Human Brain. Stuttgart: Thieme; 1988. [Google Scholar]
- 21.Forman S D, Cohen J D, Fitzgerald M, Eddy W F, Mintun M A, Noll D C. Magn Reson Med. 1995;33:636–647. doi: 10.1002/mrm.1910330508. [DOI] [PubMed] [Google Scholar]
- 22.Schacter D L, Norman K A, Koutstaal W. Annu Rev Psychol. 1998;49:289–318. doi: 10.1146/annurev.psych.49.1.289. [DOI] [PubMed] [Google Scholar]
- 23.Roediger H L, McDermott K B, Robinson K J. In: Theories of Memory II. Conway M A, Gathercole S E, Cornoldi C, editors. Vol. 2. Hove, Sussex, U.K.: Psychology Press; 1998. pp. 187–245. [Google Scholar]
- 24.Roediger H L, McDermott K B. J Exp Psychol Learn Mem Cognit. 1995;21:803–814. [Google Scholar]
- 25.Mather M, Henkel L A, Johnson M K. Mem Cognit. 1997;25:826–837. doi: 10.3758/bf03211327. [DOI] [PubMed] [Google Scholar]
- 26.Norman K A, Schacter D L. Mem Cognit. 1997;25:838–848. doi: 10.3758/bf03211328. [DOI] [PubMed] [Google Scholar]
- 27.Lepage M, Habib R, Tulving R. Hippocampus. 1998;8:313–322. doi: 10.1002/(SICI)1098-1063(1998)8:4<313::AID-HIPO1>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- 28.Schacter D L, Wagner A D. Hippocampus. 1999;9:7–24. doi: 10.1002/(SICI)1098-1063(1999)9:1<7::AID-HIPO2>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 29.Ungerleider L G. Science. 1995;270:769–775. doi: 10.1126/science.270.5237.769. [DOI] [PubMed] [Google Scholar]
- 30.Suzuki W A, Amaral D G. J Comp Neurol. 1994;350:497–533. doi: 10.1002/cne.903500402. [DOI] [PubMed] [Google Scholar]
- 31.Aggleton J P, Brown M W. Behav Brain Sci. 1999;22:425–489. [PubMed] [Google Scholar]
- 32.Alvarez P, Squire L R. Proc Natl Acad Sci USA. 1994;91:7041–7045. doi: 10.1073/pnas.91.15.7041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Damasio A R. Cognition. 1989;33:25–62. doi: 10.1016/0010-0277(89)90005-x. [DOI] [PubMed] [Google Scholar]
- 34.Nyberg L, Habib R, McIntosh A, Tulving E. Proc Natl Acad Sci USA. 2000;97:11120–11124. doi: 10.1073/pnas.97.20.11120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wheeler M E, Petersen S E, Buckner R L. Proc Natl Acad Sci USA. 2000;97:11125–11129. doi: 10.1073/pnas.97.20.11125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Saykin A J, Johnson S C, Flashman L A, McAllister T W, Sparling M, Darcey T M, Moritz C H, Guerin S J, Weaver J, Mamourian A. Brain. 1999;122:1963–1971. doi: 10.1093/brain/122.10.1963. [DOI] [PubMed] [Google Scholar]
- 37.Rugg M D, Henson R N A. In: The Cognitive Neuroscience of Memory, Encoding and Retrieval. Parker A E, Wilding E L, Bussey T, editors. Hove, Sussex, U.K.: Psychology Press; 2001. , in press. [Google Scholar]
- 38.Tulving E, Kapur S, Markowitsch H J, Craik F I M, Habib R, Houle S. Proc Natl Acad Sci USA. 1994;91:2012–2015. doi: 10.1073/pnas.91.6.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tulving E, Markowitsch H J, Craik F I M, Habib R, Houle S. Cereb Cortex. 1996;6:71–79. doi: 10.1093/cercor/6.1.71. [DOI] [PubMed] [Google Scholar]
- 40.Konishi S, Wheeler M E, Donaldson D I, Buckner R L. NeuroImage. 2000;12:276–286. doi: 10.1006/nimg.2000.0614. [DOI] [PubMed] [Google Scholar]
- 41.Henson R N, Rugg M D, Shallice T, Josephs O, Dolan R J. J Neurosci. 1999;19:3962–3972. doi: 10.1523/JNEUROSCI.19-10-03962.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.McDermott K B, Jones T C, Petersen S E, Lageman S K, Roediger H L., III J Cognit Neurosci. 2000;12:965–976. doi: 10.1162/08989290051137503. [DOI] [PubMed] [Google Scholar]
- 43.Cabeza R, Grady C L, Nyberg L, McIntosh A R, Tulving E, Kapur S, Jennings J M, Houle S, Craik F I M. J Neurosci. 1997;17:391–400. doi: 10.1523/JNEUROSCI.17-01-00391.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cabeza, R., Anderson, N. D., Kester, J., Lennartsson, E. R. & McIntosh, A. R. (2001) Brain Cognit, in press.
- 45.Wagner A D, Poldrack R A, Eldridge L L, Desmond J E, Glover G H, Gabrieli J D E. NeuroReport. 1998;9:3711–3717. doi: 10.1097/00001756-199811160-00026. [DOI] [PubMed] [Google Scholar]
- 46.Petrides M. In: Handbook of Neuropsychology. Boller F, Grafman J, editors. Vol. 9. Amsterdam: Elsevier; 1994. pp. 59–82. [Google Scholar]
- 47.Owen A M. Eur J Neurosci. 1997;9:1329–1339. doi: 10.1111/j.1460-9568.1997.tb01487.x. [DOI] [PubMed] [Google Scholar]
- 48.D'Esposito M. In: Handbook of Functional Neuroimaging of Cognition. Cabeza R, Kingstone A, editors. Cambridge, MA: MIT Press; 2001. pp. 293–327. [Google Scholar]
- 49.Raichle M E, Fiez J A, Videen T O, MacLeod A-M K, Pardo J V, Fox P T, Petersen S E. Cereb Cortex. 1994;4:8–26. doi: 10.1093/cercor/4.1.8. [DOI] [PubMed] [Google Scholar]
- 50.Wagner A D, Desmond J E, Demb J B, Glover G H, Gabrieli J D E. J Cognit Neurosci. 1997;9:714–726. doi: 10.1162/jocn.1997.9.6.714. [DOI] [PubMed] [Google Scholar]
- 51.Wagner A D, Maril A, Schacter D L. J Cognit Neurosci. 2000;12, Suppl. 2:52–60. doi: 10.1162/089892900564064. [DOI] [PubMed] [Google Scholar]
- 52.Demb J B, Desmond J E, Wagner A D, Vaidya C J, Glover G H, Gabrieli J D. J Neurosci. 1995;15:5870–5878. doi: 10.1523/JNEUROSCI.15-09-05870.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gabrieli J D E, Desmond J E, Demb J B, Wagner A D, Stone M V, Vaidya C J, Glover G H. Psychol Sci. 1996;7:278–283. [Google Scholar]
- 54.Blaxton T A, Bookheimer S Y, Zeffiro T A, Figlozzi C M, Gaillard W D, Theodore W H. Can J Exp Psychol. 1996;50:42–56. doi: 10.1037/1196-1961.50.1.42. [DOI] [PubMed] [Google Scholar]
- 55.Wagner A D, Schacter D L, Rotte M, Koutstaal W, Maril A, Dale A M, Rosen B R, Buckner R L. Science. 1998;281:1188–1191. doi: 10.1126/science.281.5380.1188. [DOI] [PubMed] [Google Scholar]
- 56.Buckner, R. L., Wheeler, M. & Sheridan, M. (2001) J. Cognit. Neurosci., in press. [DOI] [PubMed]
- 57.Nyberg L, Cabeza R, Tulving E. Psychon Bull Rev. 1996;3:135–148. doi: 10.3758/BF03212412. [DOI] [PubMed] [Google Scholar]
- 58.Kapur S, Craik F I M, Jones C, Brown G M, Houlse S, Tulving E. NeuroReport. 1995;6:1880–1884. doi: 10.1097/00001756-199510020-00014. [DOI] [PubMed] [Google Scholar]
- 59.Henson R N A, Rugg M D, Shallice T, Josephs O, Dolan R J. J Neurosci. 1999;19:3962–3972. doi: 10.1523/JNEUROSCI.19-10-03962.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Allan K, Wilding E L, Rugg M D. Acta Psychol. 1998;98:231–252. doi: 10.1016/s0001-6918(97)00044-9. [DOI] [PubMed] [Google Scholar]
- 61.Curran T, Schacter D L, Norman K A, Galluccio L. Neuropsychologia. 1997;35:1035–1049. doi: 10.1016/s0028-3932(97)00029-8. [DOI] [PubMed] [Google Scholar]
- 62.Moscovitch M, Melo B. Neuropsychologia. 1997;35:1017–1034. doi: 10.1016/s0028-3932(97)00028-6. [DOI] [PubMed] [Google Scholar]


