Abstract
The optimal dose of in vivo-administrated alemtuzumab in the allogeneic transplantation setting has not been defined. We report our experience on 37 patients with high-risk diseases, mainly acute leukemia (AML 23, ALL 10 patients), who underwent sibling (49%) or unrelated (51%) PBSCT (35 patients), and received a total dose of only 10–20 mg Campath-1H as part of the conditioning, and post-transplant CYA without MTX. The neutrophil and especially the platelet engraftment were rapid. There were only two grade III–IV acute GvHD cases, which occurred in unrelated transplants in the Campath-10 cohort. Chronic GvHD developed in six cases (17%) and was limited to skin in five of them. After a median follow-up of 371 days (59–1191), 70% patients are alive and in CR (Karnofsky 100%), and 11 died (TRM n=6, relapse n=5). From the five patients relapsed, three were at advanced stage at transplant and four underwent sibling HCT with the higher (20 mg) alemtuzumab dose. With the 10 mg alemtuzumab schedule (5 mg/day at days −2 and −1) we achieve at day of transplantation low but still lymphotoxic alemtuzumab serum concentrations (176 ng/mL), whereas levels declined fast thereafter, and at engraftment nearly no Campath antibody remained in the patient's serum.
Keywords: alemtuzumab, pharmacokinetics, allogeneic hematopoietic cell transplantation, GVHD, donor lymphocyte infusion
Introduction
The optimal dose and schedule of in vivo-administered humanized Campath-1H (alemtuzumab) in the allo-SCT setting has not been defined. A total dose of 100 mg alemtuzumab divided over 5 days before transplant was found to be highly efficient in reducing GvHD, however with the cost of impaired immune reconstitution, increased infections and increased relapse rates.1, 2 Further studies have shown that 50 mg alemtuzumab is adequate for preventing GvHD when combined with CsA/MTX.3, 4 Recently, two single-center studies in Italy and Germany demonstrated that the total alemtuzumab dose can be reduced to 10–20 mg in reduced intensity conditioning allo-SCT for lymphoma5 or in elderly patients with AML.6 However, experience on the use of lower doses alemtuzumab, especially in the context of myeloablative transplantation for acute leukemia is sparse and pharmacokinetics are lacking.
With this background, we started a prospective observational study in Patras/Greece aiming to evaluate the feasibility and pharmacokinetics of very low-dose alemtuzumab in allo-SCT. Here, we report our experience in 37 patients with high-risk diseases, mainly acute leukemia, who underwent sibling or unrelated PBSCT and received before transplant 10–20 mg alemtuzumab and after transplant CsA without MTX as GvHD prophylaxis. Furthermore, we measured serum alemtuzumab levels and report the unique pharmacokinetics of the 10 mg alemtuzumab schedule.
Patients and methods
Overview of the study protocol
The protocol was reviewed and approved by the local institutional review board (Nr. 344/04.08.06) and institutional ethics committee (Nr. 525/07.09.06) and all patients gave written informed consent. Patients received a uniform GvHD prophylaxis with 10–20 mg alemtuzumab (MabCampath, Genzyme Europe B.V., Naarden, The Netherlands) and post-transplant CsA without MTX. CsA tapering started at day +50. High-risk patients were allowed to receive prophylactic donor lymphocyte infusions (DLI). The first 10 patients received a total dose of 20 mg alemtuzumab (10 mg/day at days −2 and −1), after prednisolone and dimetindene premedication. According to the protocol, engraftment kinetics, occurrence of acute GvHD>II and TRM were assessed in real time, and de-escalation of alemtuzumab was performed if ⩽4 patients experienced severe side-effects. Therefore, the next six patients received 15 mg alemtuzumab, and thereafter patients received a total dose of 10 mg alemtuzumab (5 mg/day at days −2 and −1). Three patients transplanted during the last time frame received 20 mg alemtuzumab because of low risk of relapse (aplastic anemia, paroxysmal nocturnal hemoglobinuria) or because of CML with high Gratwohl/EBMT score (6/7). Although this was not a dose-finding study, we focused on patients who received the lower 10 mg dose, and therefore besides presenting the clinical results of the entire patient population, we also show separately our observations of the 18 patients who received the lower dose (Campath-10 cohort) and of the 19 patients who received 20 or 15 mg alemtuzumab (Campath-20/15 cohort).
Conditioning regimen, donors, grafts and supportive care
Patients with AML received chemotherapy based- and those with ALL TBI based-myeloablative conditioning. Patients over 55 years of age or patients with severe comorbidities (HCT-specific comorbidity index ⩾3) received a fludarabine-based reduced toxicity regimen, as indicated. Donors were HLA-identical siblings or unrelated with at least seven out of eight (A, B, C and DRB1) HLA allele match. Patients were managed in laminar airflow rooms and received leukodepleted and irradiated blood products, and standard nursing and supportive care. CMV reactivation screening was done weekly by quantitative CMV-PCR. Chimerism analyses were carried out by PCR of informative microsatellite markers, as previously described in detail.7
Definitions and statistical analysis
Data were evaluated as of April 2010. The day of engraftment was defined as the first of three consecutive days with leucocytes>1 × 109 cells/L. Platelet recovery was defined as a platelet count over 20 × 109 cells/L without transfusion. Acute and chronic GvHD were staged and graded according to consensus criteria. The severity of chronic GvHD was assessed according to functional impairment and response to first-line therapy as mild, moderate or severe.8 Post-DLI GVHD is defined as the onset of GvHD after the delivery of DLI. OS was measured until death due to any cause, and PFS until disease progression or death due to any cause. Relapse and TRM were considered to be competing risks. Survival curves were generated by the Kaplan–Meier method with Graph-Pad-PrismTM software (La Jolla, CA, USA).
Alemtuzumab serum levels by a sensitive ELISA
Frozen serum samples were shipped on dry ice to BioAnaLab Ltd, Oxford, UK. Serum levels of alemtuzumab were measured by a sensitive ELISA assay developed at BioAnaLab (limit of detection 31.25 ng/mL) and results were the mean of duplicate analyses with a precision between duplicates <30%. Briefly, the Campath antigen, CD52 fusion protein (TFYO.CAMG2A.A6.1F2) is absorbed onto a microtitre plate at a concentration of 2.5 μg/mL (100 μL/well). Following blocking with 2% (w/v) BSA, test samples, calibration standards and quality control samples are applied. Following incubation and washing to remove any nonspecific binding, the bound Campath is detected using a monoclonal mouse anti-human IgG1-HRP conjugate. Visualization of the binding is achieved using TMB as the assay substrate. After stopping the reaction with 5% (v/v) HCl, the absorbance is measured at 450 nm with background correction at 54 nm. The blank and background-corrected absorbances are plotted against the log of the Campath concentration in the calibration standards, and the calibration curve is fitted using a 4-parameter logistic model. The concentration of quality control samples and unknown samples is determined from the blank and background-corrected absorbance values and the calibration curve parameters. The result was the mean of duplicate analyses with a precision between duplicates <30%.
Results
Engraftment, infections and TRM
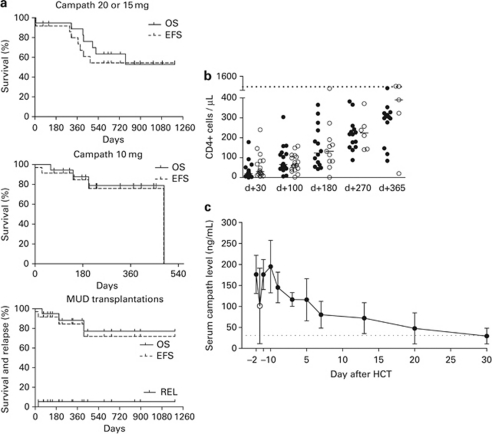
Characteristics of the 37 consecutive, mostly leukemia patients, are shown in Table 1. At transplant, 35% of the patients had advanced disease (>CR-1), whereas 16% patients were transplanted with active myeloid disease. The graft source was PBSC in all, except two patients. Alemtuzumab was well tolerated. The median time to recover leukocyte counts >1000 × 109 cells/L was 14 days (range 10–21), and the median time to achieve untransfused platelets above 20 × 109 cells/L was 12 days (7–29) and above 50 × 109 cells/L was 13 days (10–95). Seven patients revealed low percentage (<10%) T-cell restricted mixed chimerism at median day +100 (78–149), which all converted to full-donor chimerism after CsA was withdrawn or with DLI. The reconstitution of T-cell subsets is shown in Figure 1. A median absolute CD4 count, higher than 200 cells/μL, was reached at 9 months after transplantation. CMV reactivations occurred frequently and were managed successfully with preemptive antiviral therapy. Severe early- and late-opportunistic infections were: HHV-6 infection on day +58, pneumonitis after heroin inhalation on day +305, HBV reactivation on day +581, H1N1 infection on day +304 and CNS toxoplasmosis on day +61. The low incidence of an early- or late-severe opportunistic infection is reflected to the low day −100 TRM (3%) and 1-year TRM (14%). Causes of nonrelapse deaths were: CNS hemorrhage (n=1), infections in the context of acute GvHD (n=2) and post-DLI GvHD related (n=3).
Table 1. Transplant characteristics, engraftment and GvHD.
| Campath-20/15 group | Campath-10 group | All | |
|---|---|---|---|
| No. of pts | 19 | 18 | 37 |
| Age, median years (range) | 39 (17–59) | 38 (20–65) | 38 (17–65) |
| Male (M)/female (F) (in %) | 74/26 | 45/55 | 59/40 |
| Disease | |||
| AML | 11 (58%) | 12 (67%) | 23 (62%) |
| De novo/sec-, t-AML | 8/3 | 8/4 | 16/7 |
| CR-1 | 9 | 6 | 15 |
| CR-2 | 0 | 3 | 3 |
| REL/refractory | 2/0 | 0/3 | 5 |
| ALL | 4 (21%) | 6 (33%) | 10 (27%) |
| B-ALL/T-ALL | 3/1 | 5/1 | 8/2 |
| CR-1 | 3a | 5 | 8 |
| CR-2 | 1 | 1 | 2 |
| MDS RAEB-2 (untreated) | 1 (5%) | — | 1 (3%) |
| CML blast crisis, 2nd CP | 1 (5%) | — | 1 (3%) |
| AA/PNH | 2 (10%) | — | 2 (5%) |
| Risk status at HCTb | |||
| Standard/advanced (in %) | 70/30 | 61/39 | 65/35 |
| Stem cell source | |||
| PBSC/BM (in %) | 95/5 | 94/6 | 95/5 |
| Donor | |||
| Sibling | 11 (58%) | 7 (39%) | 18 (49%) |
| VUD | 8 (42%) | 11 (61%) | 19 (51%) |
| 8/8 match | 3 | 6 | 9 |
| 7/8 match | 5 | 5 | 10 |
| Class I mismatch (A/B/C) | −/1/4 | 3/−/2 | 3/1/6 |
| Class II mismatch (DRB1/DQB1/DPB1) | −/−/3 | −/−/6 | −/−/9 |
| Cell dose (median, range) | |||
| CD34+ ( × 106 cells/kg) | 5, 2 (2, 5–15) | 5, 1 (1, 5–10,3) | 5, 2 (1, 5–15) |
| CD3+ ( × 107 cells/kg) | 27 (7–71) | 25 (5–64) | 25 (5–71) |
| Conditioning regimenc | |||
| Busi/Cy | 9 (47%) | 7 (39%) | 16 (43%) |
| TBI/VP16/Cy | 4 (21%) | 4 (22%) | 8 (22%) |
| Flu/BCNU/MEL or TT | 6 (32%) | 7 (39%) | 13 (35%) |
| Precond. HD-ARA-C | 1 (5%) | 3 (17%) | 4 (11%) |
| Sex mismatch | |||
| (M → F/F → M) | 5/− (26%) | 4/3 (39%) | 9/3 (32%) |
| ABO incompatibility | |||
| (Major/minor/bi-directional) | 3/3/− (32%) | 5/3/3 (61%) | 8/6/3 (46%) |
| CMV (donor → recipient) | |||
| D+ → R+ | 11 (58%) | 14 (78%) | 25 (68%) |
| D+ → R− | 2 (10%) | 1 (6%) | 3 (8%) |
| D− → R+ | 3 (16%) | 3 (17%) | 6 (16%) |
| D− → R− | 3 (16%) | — | 3 (8%) |
| Engraftment | |||
| WBC>1000 × 109 cells/L | 14 (10–19) | 14 (11–21) | 14 (10–21) |
| plt>20 × 109 cells/L | 11 (8–19) | 12 (7–27) | 12 (7–27) |
| plt>50 × 109 cells/L | 12 (10–95) | 13 (10–27) | 13 (10–95) |
| T-cell mixed chimerism (in %) | 17 | 22 | 19 |
| CsA discontinuation | |||
| Median day (range) | 150 (70–250) | 109 (30–202) | 126 (30–250) |
| Acute GvHD | |||
| No. of evaluable pts | 18 | 18 | 36 |
| 0 | 12 (67%) | 11 (61%) | 23 (64%) |
| I | 6 (33%) | 3 (17%) | 9 (25%) |
| II | 0 | 2 (11%) | 2 (5%) |
| III–IV | 0 | 2 (11%) | 2 (5%) |
| Chronic GvHD | |||
| No. of evaluable pts | 18 | 17 | 35 |
| No | 14 (78%) | 15 (88%) | 30 (83%) |
| Limited | 4 (22%) | 1 (6%) | 5 (14%) |
| Extensive | 0 | 1 (6%) | 1 (3%) |
| Severity chronic GvHDd | |||
| Mild | 3 (17%) | 0 | 3 (9%) |
| Moderate | 1 (5%) | 1 (6%) | 2 (6%) |
| Severe | 0 | 1 (6%) | 1 (3%) |
Abbreviations: AA=aplastic anemia; PNH=paroxysmal nocturnal hemoglobinuria; REL=relapse rate
All patients received post-transplant CsA only, except one patient in the Camapth-20/15 group who received additional low-dose MTX (5 mg/m2 on day +1, day +3).
One with persistent extramedullary disease, one with active hemophagocytosis.
Standard risk: AML or ALL in CR-1, AA or PNH.
Busi/Cy and TBI/VP16/Cy, standard myeloablative conditionings; Flu/BCNU/MEL or TT, fludarabine-based reduced toxicity regimens. Precond HD-ARA-C, patients with active disease received 1 week before conditioning high-dose Ara-C.
Severity was assessed according to National Institutes of Health consensus (Biol Blood Marrow Transplant 2005; 11(12): 945–956).
Figure 1.
(a) Kaplan–Meier curves OS, EFS and relapse rate for patients who received Campath 20 or 15 mg (n=19), Campath 10 mg (n=18) or who underwent matched unrelated donor transplantation (n=19). (b) CD4+ reconstitution in the Campath-20, Campath-15 (filled symbols) and Campath-10 (open symbols) cohort. The short solid line represents median value and the horizontal dotted lines show 5th percentile of normal distribution. (c) Serum alemtuzumab levels (median±s.d.) in patients (n=8) who received Campath-1H 10 mg. Samples were collected 15 min after the end of the first infusion of 5 mg Campath-1H (day −2), before the second 5 mg infusion (open circle), after the end of the second infusion (day −1), at day of transplantation (day 0) and at various time points after allo-SCT. Horizontal dotted line denotes the limit of detection of the ELISA technique.
GvHD
We did not observe any grade II or higher acute GvHD after sibling transplantation (Table 1). Two grade II (5%) and two grade III (5%) acute GvHD cases occurred in matched unrelated donor recipients of the Campath-10 cohort. It must be noted that both grade III acute GvHD patients had active disease at transplant and CsA was discontinued early (day +30, day +95). Chronic GvHD developed in only six cases (cumulative incidence 17%) and was limited to skin in five of them. The single case of severe GvHD beyond day +100 observed in an HbsAg+ patient/donor pair (fulminant acute-like liver GvHD on day +143). The low-chronic GvHD incidence is reflected in the very good performance status of the 26 patients who are alive (median Karnofsky 100%, 80–100).
DLI
Three patients received DLI's for relapse but none of them responded. A total of 10 recipients received prophylactic DLI at a median of 159 days after allo-SCT (78–426), 5 because of mixed chimerism and 5 preemptive. Notably, six of them (four sibling, two matched unrelated donor) developed GvHD. In three cases, the post-DLI GvHD was mild and resolved, however further three recipients (two volunteer unrelated donor (VUD), one sibling) experienced severe and fatal post-DLI GvHD (liver, liver/gut and bronchiolitis obliterans), even though the total CD3+ dose was relatively low (median 2 × 106 cells/kg, 0.96–2.5 × 106 cells/kg).
Disease response and outcome
After a median follow-up of 371 days (59–1191), 26 out of 37 patients are alive (70%) and are in CR, and 11 died (TRM n=6, relapse n=5). It is noteworthy that from the five patients relapsed in our study, three out of five were at advanced stage at transplant, four underwent sibling allo-SCT and four received the higher (20 mg) alemtuzumab dose. Outcome curves are shown in Figure 1. The estimated 1- and 2-year OS probabilities for all patients are 84% (95% CI, 9–19) and 57% (95% CI, 18–24), respectively. The estimated 1- and 2-year EFS probabilities are 75% (95% CI, 12–21) and 54% (95% CI, 18–23), respectively.
Alemtuzumab pharmacokinetics
We measured serum alemtuzumab levels in eight patients (five AML, three ALL) who received Campath-1H 10 mg with a sensitive ELISA technique (limit of detection 31.25 ng/mL). A total of 54 samples (median seven samples/patient) were tested from day −2 (15 min after the end of the first infusion of 5 mg Campath-1H) up to day +30 after transplantation (Figure 1c). The median serum peak level was 176 ng/mL (range 135–281) and was found at day of transplantation (day 0). Alemtuzumab levels declined slowly thereafter reaching a median serum level at day +7 of 78 ng/mL (41–114) and at day +20 just above the detection limit (median 42 ng/mL). Alemtuzumab was still detected only in one out of four patients at day +30. There was no difference in the alemtuzumab pharmacokinetics between AML and ALL patients. Campath levels were not influenced by body weight or body surface area (r2 test).
Discussion
Pharmacokinetic studies of alemtuzumab at a total dose of 100 mg (20 mg/day × 5 days) before reduced intensity conditioning transplantation have demonstrated that the median serum peak level was 13 700 ng/mL, the alemtuzumab concentration remained at high levels above 1000 ng/mL at least 4 weeks after the last infusion and the estimated time to achieve concentrations below the lymphotoxic level (100 ng/mL) was 60 days.9 At a total dose of 50 mg alemtuzumab (10 mg/day × 5 days), Rebello et al.10 have shown that the median serum peak level was 2500 ng/mL, and Campath-1H persisted above 500 ng/mL (limit of detection) at least 11 days after the last dose. To our knowledge, we present here for the first time pharmacokinetic studies of lower doses of alemtuzumab in the HCT setting. With our administration dose and timing (total dose 10 mg, 5 mg/day at days −2 and −1), we achieved the maximum peak level at day of stem cell infusion, which is lymphotoxic but still 1–2 log lower as compared with the 100 mg and the 50 mg schedules. It must be noted however, that the pharmacokinetics shown here may not apply to patients with bulky lymphoid malignancy due to binding of the antibody to CD52+ tumor cells. Khouri et al.11 have reported a fast drop of serum concentrations in patients with active ALL or CLL.
Despite the omission of post-transplant MTX and the early withdrawal of CsA (median day +126) the incidence of severe acute or chronic GvHD with our very low-dose alemtuzumab schedule was low. Although direct comparison is not possible, protocols using 50 mg alemtuzumab report similar low incidences of acute and chronic GvHD.3, 4, 11 Our GvHD incidence is in agreement with two recent reports of low-dose alemtuzumab (10–20 mg) when combined with CsA/MTX5 or CsA only.6 Most nonrelapse deaths (three out of six) were attributed to post-DLI GvHD. Though additional factors contributed to these deaths (iron overload, lost of follow-up) these events emphasize the need for careful selection of patients planed to receive prophylactic DLI. Lutz et al.12 reported also a high (69%) incidence of GvHD after prophylactic DLI, which is higher than in studies reported DLI for relapsed or progressive disease. Prospective randomized trials evaluating the value of prophylactic DLI are warranted.
The neutrophil, and especially the platelet engraftment was rapid and compares favorably with other reports in myeloablative PBSCT.13 We believe that both the omission of MTX as well as the use of Campath, instead of ATG, contributed to the rapid engraftment observed.14 Campath-1 antibodies do not react with hematopoietic stem cells in contrast with polyclonal ATG preparations, which contain antibodies specific for chemokine receptors (CXCR4, CCR5 and CCR7) and haematopoietic progenitor cells, and thus may interfere with engraftment and regeneration. In randomized studies, the addition of antithymocyte globulin15 or anti-Jurkat ATG-Fresenius13 resulted in delayed neutrophil and/or platelet engraftment. Although we initially postulated that low-dose alemtuzumab will not delay immune reconstitution after HCT, most patients failed to reach normal CD4+ counts within the first year and in 33% of them their number did not surpass 200 cells/μL. Similar immune reconstitution kinetics have been reported also with 100 mg and 50 mg alemtuzumab schedules,1, 2, 16 however these reports include mainly reduced intensity conditioning transplants and results in the myeloablative setting with Camapth-1H are lacking.3, 11 Although the incidence of CMV reactivation was high, in contrast with the 100 mg schedule we did not observe late CMV infections and no CMV disease occurred.
It is difficult to compare the outcomes of our high-risk leukemia patients with other series using Campath-1H, as most studies have reported on lymphoma patients receiving reduced intensity conditioning. Though the follow-up period is still relatively short, the estimated 2-year OS and EFS of 77% in unrelated allo-SCT (n=19) is very encouraging and compares favorably with other series of unrelated T-repleted myeloablative SCT for AML13 or T-cell depleted myeloablative SCT with higher doses Campath3, 4, 11 or ATG.13
Taken together, despite the single-center design of the study, and the relative low number and heterogeneity of treated patients, the results suggest that 10 mg alemtuzumab before transplant combined with only CsA after transplant may efficiently prevent severe acute and chronic GVHD, after sibling PBSCT and higher doses are probably not required. With the 10 mg alemtuzumab schedule (5 mg/day at days −2 and −1), we achieve at day of transplantation, low but still lymphotoxic alemtuzumab concentrations, whereas levels decline fast thereafter, and at the time of engraftment nearly no Campath antibody remains in the patient's serum. Such fast clearance of the low-dose alemtuzumab may prove beneficial regarding safety and GvL efficacy. The optimal minimal dose of alemtuzumab for preventing GvHD in the unrelated setting probably ranges between 10–20 mg, however prospective randomized trials evaluating also the influence of additional parameters like the presence of mismatches and the time of CsA withdrawn, are needed.
Acknowledgments
We acknowledge the physicians, fellows and nurses of the University Hospital of Patras for their dedication to the patients. We specially thank G Oikonomopolou whose invaluable contribution in the BMT laboratory made this work possible, E Kefala for data managing and N Zoudiari, G Lazana and D Kokkinou for help in sample processing for pharmacokinetic studies.
The authors declare no conflict of interest.
References
- Kottaridis PD, Milligan DW, Chopra R, Chakraverty RK, Chakrabarti S, Robinson S, et al. In vivo CAMPATH-1H prevents graft-versus-host disease following nonmyeloablative stem cell transplantation. Blood. 2000;96:2419–2425. [PubMed] [Google Scholar]
- Chakrabarti S, Mackinnon S, Chopra R, Kottaridis PD, Peggs K, O'Gorman P, et al. High incidence of cytomegalovirus infection after nonmyeloablative stem cell transplantation: potential role of Campath-1H in delaying immune reconstitution. Blood. 2002;99:4357–4363. doi: 10.1182/blood.v99.12.4357. [DOI] [PubMed] [Google Scholar]
- Das-Gupta EP, Russell NH, Shaw BE, Pearce RM, Byrne JL. Long-term outcome of unrelated donor transplantation for AML using myeloablative conditioning incorporating pretransplant Alemtuzumab. Biol Blood Marrow Transplant. 2007;13:724–733. doi: 10.1016/j.bbmt.2007.02.011. [DOI] [PubMed] [Google Scholar]
- Patel B, Kirkland KE, Szydlo R, Pearce RM, Clark RE, Craddock C, et al. Favorable outcomes with alemtuzumab-conditioned unrelated donor stem cell transplantation in adults with high-risk Philadelphia chromosome-negative acute lymphoblastic leukemia in first complete remission. Haematologica. 2009;94:1399–1406. doi: 10.3324/haematol.2009.008649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dodero A, Carrabba M, Milani R, Rizzo E, Raganato A, Montefusco V, et al. Reduced-intensity conditioning containing low-dose alemtuzumab before allogeneic peripheral blood stem cell transplantation: graft-versus-host disease is decreased but T-cell reconstitution is delayed. Exp Hematol. 2005;33:920–927. doi: 10.1016/j.exphem.2005.05.009. [DOI] [PubMed] [Google Scholar]
- Bertz H, Spyridonidis A, Wasch R, Grullich C, Egger M, Finke J. A novel GVHD-prophylaxis with low-dose alemtuzumab in allogeneic sibling or unrelated donor hematopoetic cell transplantation: the feasibility of deescalation. Biol Blood Marrow Transplant. 2009;15:1563–1570. doi: 10.1016/j.bbmt.2009.08.002. [DOI] [PubMed] [Google Scholar]
- Spyridonidis A, Zeiser R, Wasch R, Bertz H, Finke J. Capillary electrophoresis for chimerism monitoring by PCR amplification of microsatellite markers after allogeneic hematopoietic cell transplantation. Clin Transplant. 2005;19:350–356. doi: 10.1111/j.1399-0012.2005.00347.x. [DOI] [PubMed] [Google Scholar]
- Filipovich AH, Weisdorf D, Pavletic S, Socie G, Wingard JR, Lee SJ, et al. National Institutes of Health consensus development project on criteria for clinical trials in chronic graft-versus-host disease: I diagnosis and staging working group report. Biol Blood Marrow Transplant. 2005;11:945–956. doi: 10.1016/j.bbmt.2005.09.004. [DOI] [PubMed] [Google Scholar]
- Morris EC, Rebello P, Thomson KJ, Peggs KS, Kyriakou C, Goldstone AH, et al. Pharmacokinetics of alemtuzumab used for in vivo and in vitro T-cell depletion in allogeneic transplantations: relevance for early adoptive immunotherapy and infectious complications. Blood. 2003;102:404–406. doi: 10.1182/blood-2002-09-2687. [DOI] [PubMed] [Google Scholar]
- Rebello P, Cwynarski K, Varughese M, Eades A, Apperley JF, Hale G. Pharmacokinetics of CAMPATH-1H in BMT patients. Cytotherapy. 2001;3:261–267. doi: 10.1080/146532401317070899. [DOI] [PubMed] [Google Scholar]
- Khouri IF, Albitar M, Saliba RM, Ippoliti C, Ma YC, Keating MJ, et al. Low-dose alemtuzumab (Campath) in myeloablative allogeneic stem cell transplantation for CD52-positive malignancies: decreased incidence of acute graft-versus-host-disease with unique pharmacokinetics. Bone Marrow Transplant. 2004;33:833–837. doi: 10.1038/sj.bmt.1704435. [DOI] [PubMed] [Google Scholar]
- Lutz C, Massenkeil G, Nagy M, Neuburger S, Tamm I, Rosen O, et al. A pilot study of prophylactic donor lymphocyte infusions to prevent relapse in adult acute lymphoblastic leukemias after allogeneic hematopoietic stem cell transplantation. Bone Marrow Transplant. 2008;41:805–812. doi: 10.1038/sj.bmt.1705981. [DOI] [PubMed] [Google Scholar]
- Finke J, Bethge WA, Schmoor C, Ottinger HD, Stelljes M, Zander AR, et al. Standard graft-versus-host disease prophylaxis with or without anti-T-cell globulin in haematopoietic cell transplantation from matched unrelated donors: a randomised, open-label, multicentre phase 3 trial. Lancet Oncol. 2009;10:855–864. doi: 10.1016/S1470-2045(09)70225-6. [DOI] [PubMed] [Google Scholar]
- Juliusson G, Theorin N, Karlsson K, Frodin U, Malm C. Subcutaneous alemtuzumab vs ATG in adjusted conditioning for allogeneic transplantation: influence of Campath dose on lymphoid recovery, mixed chimerism and survival. Bone Marrow Transplant. 2006;37:503–510. doi: 10.1038/sj.bmt.1705263. [DOI] [PubMed] [Google Scholar]
- Bacigalupo A, Lamparelli T, Bruzzi P, Guidi S, Alessandrino PE, di Bartolomeo P, et al. Antithymocyte globulin for graft-versus-host disease prophylaxis in transplants from unrelated donors: 2 randomized studies from Gruppo Italiano Trapianti Midollo Osseo (GITMO) Blood. 2001;98:2942–2947. doi: 10.1182/blood.v98.10.2942. [DOI] [PubMed] [Google Scholar]
- Hill QA, Hill A, Collyns TA, Pearce RM, Cook G. Similar lymphocyte recovery and CMV reactivation profiles between reduced intensity conditioning with alemtuzumab and myeloablative allogeneic stem cell transplantation. Bone Marrow Transplant. 2008;41:749–751. doi: 10.1038/sj.bmt.1705974. [DOI] [PubMed] [Google Scholar]