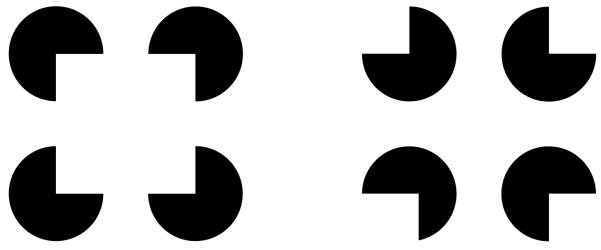Abstract
Introduction
Schizophrenia is currently diagnosed on the basis of patient reports and clinical observations. A diagnosis based on aetiology is inherently more reliable due to being closer to the disease process than the overt clinical manifestations. Accordingly, recent research in schizophrenia has focused on the development of biomarkers in a bit to improve the reliability and neurobiological relevance of the diagnosis. Visual information processing is one of these promising fields of recent biomarker research.
Areas covered
This article provides an overview of the available literature regarding deficits in schizophrenia detectable through psychophysical (contrast and motion sensitivity, visual backward-masking), ERP (P1 and N1 visual evoked potentials) and oscillatory (signal power and phase-locking factor of evoked oscilations) measures and their validity as trait or state biomarkers of the disease. The methodology included a search on articles related to visual information processing in schizophrenia on the PubMed database.
Expert opinion
Biomarker research in schizophrenia is a rapidly expanding area. Evidence exists to suggest that both psychotic and manic symptoms are associated with visual processing abnormalities. A specific impairment confined to the magnocellular component of the visual system might be a trait biomarker of schizophrenia.
Keywords: schizophrenia, diagnosis, early detection, trait biomarkers, state biomarkers, P1, oscillations, magnocellular pathway
1. Introduction
1.1 Current approach to diagnosis
Schizophrenia is a severe mental health disorder characterized by positive (delusions, hallucinations), negative (affective flattening, lack of drive, poverty of speech) and cognitive symptoms. No laboratory tests are currently available to confirm its diagnosis. Instead, the diagnosis is based on the patients’ symptoms and clinical observations according to sets of standardized criteria laid out in the fourth edition of the Diagnostic and Statistical Manual (DSM-IV) and the tenth edition of the International Classification of Diseases (ICD-10). Despite providing a much needed consensus regarding the characteristic features of schizophrenia [1, 2], DSM-IV and ICD-10 diagnoses have been shown to have only moderate reliability [3]. This is probably due to the overreliance on psychotic symptoms which have been demonstrated to be poor at predicting the course of the disease and response to drugs [3]. As a result the manifestations of schizophrenia are extremely heterogeneous, to the extent that this heterogeneity has come to be regarded as part of the schizophrenia core. In addition delusions and hallucinations can occur in a large number of mental health disorders which makes the nosological boundaries of schizophrenia indistinct [4]. The drawbacks of the operational schizophrenia diagnosis have wide ranging implications, affecting the reliability of fundamental and clinical research. In respect to genetic studies for example, none of the identified risk genes are specific to schizophrenia, but rather indicate general vulnerability to mental health disorders or personality psychopathology [5].
1.2 Biomarkers in schizophrenia: rationale
The shortcomings of the syndrome-based approach to the diagnosis of schizophrenia have stimulated research into biological correlates of the main disease process (biomarkers) that could be used to provide aetiological diagnosis. The NIH Biomarker Definitions Working Group defines biomarkers as quantifiable outcomes which are modified by the disease and therapeutic intervention. Such methods could help confirm or even reorganize the mental health disorders classification. It has been argued that schizophrenia is likely not a singular entity [6, 7] with various aetiological processes leading to a similar phenomenological manifestation. A successfully developed biomarker could tease out the underlying factors and delineate the psychiatric disorders that are currently grouped together by the DSM-IV and ICD-10 criteria of schizophrenia. It has been argued that such subtyping of schizophrenia could lead to significant empowerment of genetic and other basic research [8].
The potential of biomarkers to define schizophrenia aetiologically has been recognized in their inclusion as a key part of the dimensional approach to schizophrenia which may supersede the categorical approach in the next edition of DSM, DSM-V. While this approach does not argue for the abandonment of the construct of schizophrenia, it suggests that the features of schizophrenia can be decomposed into a range of dimensions (positive, negative, mood, motor, cognitive) that may be more relevant to the aetiology of schizophrenia than the operational criteria [9, 10]. Biomarkers can then act as “footholds” to define the pathophysiological mechanisms that underpin the different dimensions and ultimately validate their aetiological relevance [6]. The identified neural processes could then be used to generate novel drug targets which would be assessed according to their efficacy in treating specific symptom domains rather than categorical psychiatric entities.
The benefits of an ideal biomarker go further than improving diagnosis and include predictive power for disease course and treatment outcome. Such property will improve patient management by informing the time, type and course of interventions. In specific cases, biomarkers might also allow the identification of individuals at risk for schizophrenia. Identifying these populations will allow the efficient management of early intervention and can guide the discovery of preventive treatments. Last but not least, a successfully developed biomarker would provide an insight into the key pathophysiological mechanisms of the disease providing novel targets for therapeutic interventions.
The validation of a biomarker is complex process one aspect of which is the assessment of its criterion validity. It provides an estimate of the biomarker and disease process correlation and is characterized by the sensitivity and specificity of the test [11]. Sensitivity is “the proportion of truly diseased persons in a screened population who are identified as being diseased by the test” [12]. Therefore this is a measure of the likelihood of correctly diagnosing the target condition. Specificity is “the proportion of truly non-diseased persons who are so identified by the screening test” [12]. Specificity relates to the probability of correctly identifying a person who is healthy or has a different condition from the target one. Sensitivity and specificity therefore tell us about the accuracy of a test, but not the probability of disease. Predictive values (positive and negative) are used to calculate this by taking into account the prevalence of the disorder. Positive predictive value (PPV) is the percentage of people with a positive test who are correctly diagnosed. This value gives information about the probability of the disease being present if the test is positive taking into account its prevalence. Conversely, negative predictive value (NPV) is the percentage of people with a negative test who do not have the disease [11]. In this review we focus on biomarkers that have undergone explorative and unsystematic evaluation, so we will report the findings in the broader meaning of sensitivity (positive findings using the test in schizophrenia spectrum disorders) and specificity (positive findings using the test in other disorders or healthy individuals).
1.3 Trait and state biomarkers
Biomarkers have generally been divided into two categories: trait and state. Trait biomarkers reflect vulnerability to the disease and are typically the result of genetic factors. In the context of schizophrenia those biomarkers reflect the properties of biological and behavioural processes that have a causal role in the pathophysiology of the disorder. They are present before the onset of the disease and are independent of changes in the clinical and medication status. They are also found in at-risk populations for schizophrenia, such as individuals with schizotypal personality traits and biological relatives of schizophrenia patients. Trait biomarkers hold the potential of clarifying the borders between the different mental health disorder entities based on differential aetiology. Linking those biomarkers to a specific pathophysiological process could also guide the development and assessment of efficacy of potentially disease-modifying therapeutic agents. Finally, these biological characteristics could be used for the reliable identification of ultra high-risk populations which would improve the efficacy and benefit of early interventions.
In contrast to trait biomarkers, state biomarkers correlate with symptom severity and return to or approach normal values with remission. They are useful in exploring the mechanisms underpinning the acute phases of the disease and are well suited to assess the efficacy of symptomatic treatment. Such biomarkers, being more closely related to the disease pathophysiology than overt clinical symptoms, could be used as surrogates for traditional clinical endpoints (such as clinician-administered scales for the assessment of various symptoms of schizophrenia). State biomarkers that are found to predict long-term outcomes of the treatment (such as mortality, quality of life or functioning), could replace these endpoints reducing significantly the duration of studies aimed at detecting long-term benefit.
1.4 Biomarkers in visual information processing: rationale
As it was pointed out earlier, cognition is one of the main dimensions of schizophrenia. Evidence that cognitive deficits are highly prevalent [13, 14], present before the onset of the disease [15] and persist longitudinally [16, 17] suggest that they are probably related to the core of the disorder [18]. Their generalized nature has argued against a localized brain abnormality but instead favours an aberration of distributed neuronal processing[19, 20]. Therefore, biomarkers to provide objective measure of the pathophysiology of this core dimension in schizophrenia should focus on information processing patterns.
One way of probing information processing is to examine the characteristics of the neural response to perception. Perception is a complex process that requires the synchronized activity of lower and higher order brain structures [21]. A disruption in connectivity is likely to affect the local and long-range processes that necessitate successful stimulus encoding. In accordance with this line of thinking, evidence from has accumulated for purely perceptual abnormalities in schizophrenia. In this review we will provide an overview of the available literature regarding visual processing abnormalities in schizophrenia and their relevance as biomarkers. We focus on the vision because we believe that it is a particularly useful modality to search for such abnormal patterns of neuronal activity as we now have extensive knowledge on its normal physiology. To place the findings in schizophrenia in context, we will begin with a short overview of the organization of the human visual system.
2. The visual system: a dual pathway
2.1 Anatomy and physiology
The visual system is organized along the lines of two separate pathways that carry qualitatively different information from the retina to the primary visual cortex (V1). The magnocellular or M-pathway is stimulated by low contrast images, low spatial frequency information (LSF - blurry images) and motion. The parvocellular or P-pathway on the other hand responds to high luminance contrast, high spatial frequencies (HSF - detailed images) and color. Beyond V1 the two pathways feed into the dorsal and ventral visual cortical streams which respectively receive primarily M- and P-pathway inputs ensuring that the two streams continue to process distinct types of information [22, 23].
2.2 Separating the visual pathways: methods
In humans, the M- (dorsal) and P- (ventral) pathways can be studied relatively independently by manipulating the characteristics of the visual stimulus, commonly a grating (Figure 1A) to stimulate predominantly one or the other pathway. Commonly used manipulations to produce M- vs P-biased stimuli are: low vs. high luminance contrast; low vs. high spatial frequency (number of dark and bright bars per visual degree, Figure 1A); high vs. low temporal frequency (the alternation rate of the grating measured in Hz; Figure 1B); large vs. small size; moving vs. stationary stimulus; requirement of the task – stimulus location vs. stimulus identification (Table 1).
Figure 1.

Spatially and temporally modulated gratings. A. Low and high spatial frequency stimuli on the left- and right-hand side respectively. B. In the temporally modulated conditions, the dark and bright bars change their positions at a constant rate.
Table 1.
Functional differences between the magnocellular and parvocellular pathways.
| Characteristics of the stimulus | M-pathway | P-pathway |
|---|---|---|
| Contrast | Low | High |
| Spatial frequency | Low | High |
| Temporal frequency | High | Low |
| Motion | Yes | No |
| Color | No | Yes |
| Size | Large | Small |
| Task requirement | Location | Object identification |
The interaction between the two pathways can be probed using tasks such as Visual-Backward Masking (VBM). In this paradigm two stimuli are presented in rapid succession. The recognition of the first stimulus (target) is interrupted by the second (mask) if the inter-stimulus interval (IS) is short enough. When the ISI is short it is thought that the fast M-pathway response to the mask overtakes the slow P-pathway information regarding the target which leads to failure to detect the target. It has been hypothesized that dysfunctions of the M-pathway would lead to increased interruption of the P-pathway.
3. Visual information processing performance biomarkers in schizophrenia
Deficits in the visual perception have been demonstrated with a number of behavioural paradigms and we will proceed to review those that hold the greatest diagnostic potential (Table 2).
Table 2.
Sensitivity and specificity of visual processing biomarkers to the schizophrenia spectrum phenotype in comparison with bipolar disorder
| Schiziophrenia patients |
Schizophrenia relatives |
Schizotypes | Bipolar disoder |
|
|---|---|---|---|---|
| Contrast sensitivity |
+/− | +/− | − | ? |
| Motion detection |
+ | + | − | − |
| Motion integration |
+ | − | ? | − |
| VBM | + | + | + | + |
| P1 | + | + | + | + |
| Power/PLF of evoked oscillations |
+ | ? | + | +/− |
3.1 Contrast sensitivity
The simplest method to assess visual perception is by measuring contrast sensitivity, the minimum level of contrast that is needed to detect the presence of a spatial frequency (SF) grating. SF refers to the number of alternating dark and white bars of grating per visual angle (expressed in cycles per degree). Some [24-26] but not all [27, 28] studies have reported increased contrast sensitivity threshold in schizophrenia (i.e. patients need higher contrast in order to perceive a stimulus). This trend was particularly pronounced when using low SF [29-31] but see [32, 33]. Slaghuis et al. compared the contrast sensitivity at low and high SF and found that patients with deficit/negative syndrome have significantly worse CS on the low SF than controls or patients with predominantly positive symptomatology [26, 34]. Another study reported a correlation between deficits in detection of low spatial frequency and another sign of cortical dysfunction (sensory neurological soft signs) [29]. This data has been interpreted as evidence for a possible M-pathway dysfunction in schizophrenia. Modulating the stimuli in the temporal domain lends support to this hypothesis. In these paradigms the grating stimuli flicker with a low or high frequency to engage the M- and P- pathways, respectively (Figure 1B). Schizophrenia patients perform poorly on the high temporal frequency, M-biased condition [26, 34, 35]. Also, the introduction of grating flicker impaired the ability of patients to detect high spatial frequency P-biased gratings which they normally perceive [25]. However the available data from this type of experiments does not fully comply with an isolated M-pathway abnormality, as in some experiments deficits were noted in respect to P-biased stimuli as well (low temporal and high spatial frequency) [26, 34].
Whether the contrast sensitivity abnormalities reflect state or trait abnormalities in schizophrenia is not clear. In one study contrast sensitivity was unimpaired in patients receiving atypical (second generation) antipsychotics whereas those on typical (first generation) antipsychotics had impaired CS [32]. The same study found no abnormality in respect to CS in a group of unaffected relatives which contradicts the findings from a different report [36]. Two studies have found no evidence for disruption in individuals with schizotypal personality disorder [37, 38]. These discrepancies as well as the equivocal results regarding the presence of the deficit in patients could be due to the variability in the degree of temporal and spatial modulation of the stimuli used in different studies. The introduction of a standardized method for the detection of CS abnormalities, as well as larger sample studies could help resolve these issues.
Another limitation of the use of CS impairment as a biomarker of schizophrenia is its lack of specificity, as similar aberrations are reported in autism [39], dyslexia [40, 41], body dysmorphic disorder [42] and in normal ageing [43].
3.2 Motion sensitivity
The preferential involvement of the M-pathway in motion perception has prompted researchers to explore the sensitivity to motion in schizophrenia patients. Two basic processes have been investigated: motion discrimination and motion integration. Paradigms probing the former measure the ability to identify the direction of motion of objects moving at various velocities. Schizophrenia patients exhibit significant impairment on these tasks, particularly in respect to higher velocities [27, 44-46]. In tasks that assess motion integration, participants have to detect the direction of movement of a target array of dots intermixed among distractor dots moving in the opposite direction [47]. Schizophrenia patients are consistently impaired on these tasks as well [48-51]. The case for a primary M-pathway abnormality was strengthened by two studies showing that the motion sensitivity abnormality in schizophrenia is exacerbated when the moving stimuli are biased towards the M- but not P-pathway [45, 52].
Data from studies in relatives of schizophrenia patients have indicated that two measures of motion sensitivity are differentially affected in relatives of schizophrenia patients. Specifically, it was demonstrated that the relatives’ ability to discriminate motion is abnormal [27] but their performance on motion integration tasks is intact [47]. This has prompted suggestions that while motion integration is state-dependent, motion sensitivity is a trait biomarker. Therefore, the M-pathway mediated process that is involved in differentiating motion signals is probably part of the initial, vulnerability profile of schizophrenia while the development of psychosis leads to deterioration in the ability to integrate motion signals contextually.
Motion discrimination appears to delineate schizophrenia spectrum from bipolar disorder, as bipolar patients have normal performance [49]. The implications of these data are that neither psychosis, nor mood symptoms affect the motion discrimination process. Instead, motion discrimination is a trait abnormality that is limited to the risk for schizophrenia.
It is noteworthy, that motion sensitivity abnormalities are found also in children and adults with dyslexia [53-55]. This may be accounted for by evidence showing a strong association between cognitive-perceptual schizotypy and adult dyslexia [56], as well as increased incidence of dyslexia among first-degree relatives of schizophrenia patients [57]. Therefore, dyslexia and the schizophrenia spectrum disorders may share a genetic vulnerability [58] that extends to trait biomarkers such as motion sensitivity.
3.3 Visual-backward masking task
Abnormal M- and P-pathway interaction has been investigated using the visual backward masking task described earlier. There is consistent evidence for VBM abnormalities in patients[59]. For example, Butler et al. have shown that controls need approximately 120 ms of interval between the target and the mask for successful recognition, while patients require around 300 ms[60].
These abnormalities were found to be primarily M-related when using designs that discriminate between the two visual pathways. A study by Cadenhead et al. found that VBM deficits in patients are more pronounced when participants are required to identify the location of the probe, as opposed to its recognition[61]. A different study that used M- and P-biased masks and targets (modulated through color and luminance contrast) demonstrated that M-masks led to significant lengthening of the masking effect [62]. These results suggest that the M-pathway is aberrantly hyperfunctional, causing larger interruption to the slow P-pathway signal. The lack of difference between LSF and HSF masks reported in other studies[60, 63] have been interpreted in the context of the inability of spatially modulated stimuli to differentially engage the two pathways[62].
Reports of VBM abnormalities in unaffected relatives suggest that the task might be probing a trait vulnerability factor for schizophrenia [64-67]. Similarly to patients, there was a trend for more pronounced deficits when the tasks placed greater emphasis on M-pathway input (e.g. location vs. object identification). Another group used the propensity of red light to suppress the M-pathway in relatives of schizophrenia patients. They found that VBM performance of the relatives was not influenced by the change of the neutral background color to red [67]. In contrast, the controls did worse in the red light condition. The lack of modulation of performance in the relatives was interpreted as additional evidence for M-pathway dysfunction. Some data is also available to suggest subtle VBM abnormalities in schizotypal personality disorder[68].
The VBM abnormality also does not appear to be the result of medication, as antipsychotic medication either reduced it or was not found to influence the performance [60, 62, 63]. In addition one study has reported aberrant VBM in unmedicated patients in remission[69].
Abnormal VBM may not be specific for schizophrenia because impairments have been reported in bipolar patients[70] and schizoaffective disorder[71]. Unaffected relatives of bipolar patients are generally spared VBM deficits[66] unless they have developed at least one mood episode[72]. This suggests that VBM probes a process that mediates the vulnerability to schizophrenia spectrum disorders. A similar deficit occurs in developed bipolar disorder though probably through a different pathophysiological mechanism[73]. Dyslexic individuals have also been reported to have aberrant VBM performance [74]. As it was argued earlier, this could be due to the possible overlap between schizotypy and dyslexia [58].
In addition, Alzheimer’s patients also have VBM abnormalities [43, 75]. This confirms the relevance of the VBM abnormality to organic neuronal damage but also demonstrates the limitations of the test in terms of specificity.
4. Visual information processing biomarkers: Electrophysiology experiments
The finding of visual processing deficits in schizophrenia has suggested that the disorder may be characterized by dysfunction during the stage of early information encoding. EEG (electroencephalography) and MEG (magnetoencephalography) are the most suitable tools to further investigate this process due to their excellent temporal resolution. They are also particularly promising biomarkers due to their non-invasiveness, low cost and lower susceptibility to the non-specific motivational effects of schizophrenia. In these settings perception is most commonly investigated by event-related paradigms, in which sensory stimuli are repeatedly presented on a computer screen while the EEG is recorded. Set epochs around the onset of the stimuli (e.g. −400 to 1000 ms) are then averaged together to isolate the evoked signals. The result is a representation of the amplitude fluctuations of the electrical signal (event-related potentials, ERP). The data can be analyzed further by determining the power and phase of the neural oscillations that make up the ERPs. Ample evidence has now accumulated for ERP and oscillatory abnormalities during visual information processing in the schizophrenia spectrum and this is discussed in more detail below
4.1 Event-related potentials
Two early visual evoked potentials have been studied extensively as indicators of early information processing in schizophrenia. The first one, P1, is a positive potential that peaks at 110-140 ms and is generated by the lateral extrastriate cortex (part of the dorsal stream) and the ventral fusiform gyrus (part of the ventral stream)[76]. The second peak, N1, appears in the 150 – 200 ms range and consists of several phases originating from parts of the ventral stream. The contributions of the two visual pathways to the formation of the two potentials have been clarified by data showing that M-biased stimuli (LSF) elicit an enlarged P1, while P-biased images (HSF) produce a dominant N1[77]. In addition, the response of P1 and N1 amplitudes to contrast change is consistent with M- and P-pathway contrast sensitivity curves respectively [78]. This has led authors to conclude that P1 and N1 are reliable indicators of the functioning of the M- and P-pathway respectively, but see Skottun et al. for criticism [79].
Studies in schizophrenia patients have consistently reported a nearly 50% reduction in the amplitude of the P1 peak [80]. The P1 deficit is demonstrable both in simple perceptual paradigms that require minimum attention, as well as in more complex cognitive tasks. In contrast, most studies report normal N1 amplitude. One study explored this effect by administering an illusory contour (Kanitsa-type, Figure 2) task to patients [81]. It has already been established that the perception of coherent illusory forms augments the N1 but not the P1 peaks [82]. The hypothesis was that such a task would elicit any subtle N1 abnormalities in patients that are not readily demonstrable in conventional paradigms. In both studies there was no significant difference between patients and controls in respect to the N1 amplitude in response to the illusory and non-illusory stimuli suggesting intact P-pathway processing. The two groups however differed in respect to their P1 amplitude, the schizophrenia patients exhibiting lower values than the controls. The abnormality in the primarily M-pathway driven P1 potential but not the P-biased N1 argues again in favor of a specific disturbance in the former visual pathway.
Figure 2.
Kanitsa Illusion. Inducer disks are rotated in order to create (left hand-side) an illusory contour figure. Control images on the right hand-side.
Schechter et al. extended these findings by demonstrating that the P1 deficit in patients was detectable using M-biased (low contrast) but not P-biased (chromatic) stimuli [83]. The hypothesized M-deficit was also investigated in two steady-state ERP studies [84, 85]. In this experimental setting the stimuli are presented in rapid succession at a constant rate. This leads to habituation at the higher cortical levels and increases the sensitivity for the early, lower level visual deficits [24]. The two studies reported abnormal response in schizophrenia patients during the M-biased but not P-biased condition.
P1 deficits have been recently described in unaffected relatives of schizophrenia patients [86], as well as in a group of healthy volunteers with high schizotypal personality traits [87].and in hallucination-prone individuals [88]. The trait status of the P1 abnormality is supported by a study that found no relationship between P1 reduction and disease chronicity, medication or current clinical state [80]. Also, P1 deficits were described in a sample of first-episode never-medicated psychosis patients [89].
The P1 abnormality may however not be specific to the schizophrenia phenotype, as patients with bipolar disorder [90] and Alzhemer’s disease [91] also have similar deficits. Moreover, P1 amplitude reductions are found in prefrontal cortex lesions [92], and during the acute stages of optic neuritis associated with Multiple Sclerosis [93, 94] highlighting the dependence of P1 on top-down and bottom-up factors respectively. This leads to the conclusion that similar to the deficit on the VBM task, the P1 abnormality is probably a primarily M-pathway mediated trait biomarker for schizophrenia that however is present in a number of other psychiatric and somatic diseases that involve frank neuronal damage or aberrant connectivity.
4.2 Oscillatory abnormalities
It is now widely accepted that neural oscillations are a fundamental mechanism that underpin coordinated brain activity [95, 96]. A variety of cognitive tasks reliably evoke synchronized oscillations. This suggests that they are functionally important in acts such as perception, motor control, memory formation and others [97]. Higher-frequency oscillations (beta and gamma) have been hypothesized to facilitate local synchronization, while lower frequency oscillations (theta, alpha) coordinate long-distance coordination between different regions of cortex [21, 98]. Oscillations are a potentially important biomarker of neural connectivity in schizophrenia, as they not only give rise to the previously reviewed P1 and N1 potential [99] but also provide a more detailed perspective on the integrity of information processing [100].
Specifically, in addition to the measure of power of the oscillations, they are also characterized by their phase-locking factor (PLF). While the former provides information about activity in different frequency bands, the latter indicates the degree of synchronization of neural oscillations to the stimulus presentation, irrespective of power [100]. It provides information regarding the variability of the neural response and reductions in PLF have been interpreted as an indication of increased “cortical noise” [101, 102].
Studies that have explored oscillatory patterns in schizophrenia report various abnormalities [103]. Reduced stimulus induced synchrony (PLF) and altered power in different frequency bands have been reported in schizophrenia patients [104-107]. This suggests that individuals affected by schizophrenia do not exhibit the same degree of synchronized and coordinated neural activity during perception when compared to healthy controls. No studies so far have directly investigated the M- and P-pathway contributions to these impairments. Tentative evidence for normal P-pathway function in respect to oscillations comes from two studies that explored gamma band patterns during the execution of a VBM task [108],[109]. The authors explored gamma band activity in patients during a VBM task and reported normal initial gamma bursts (around 100 ms, representative of early sensory registration), but aberrant later burst (200-400ms), which have been suggested to be associated with perceptual organization and hence later, P-pathway driven processes [110].
Oscillatory abnormalities in schizophrenia do not appear to be affected by medication suggesting they are trait abnormalities [103]. Furthermore, a recently submitted report from our group found evidence for occipital power and PLF abnormalities in the theta, beta and gamma frequency bands in a group of healthy volunteers with high levels of schizotypal personality traits.
One study so far has explored evoked oscillations in response to visual stimuli in bipolar disorder and reported altered occipital alpha (decrease) and beta (increase) signal power [111]. This pattern showed a trend for normalization after treatment with the mood stabilising agent sodium valproate. Another study in bipolar patients however did not find difference in respect to the occipital oscillations [112]. Even if the oscillatory abnormalities distinguish between schizophrenia and bipolar disorder, its proposed role as a key mechanism in neuronal synchronization would mean that it is unlikely to be specific to schizophrenia in relationship to other mental health and neurological disorders which involve aberrant connectivity. In demonstration of this, aberrations in oscillatory activity have been reported in autism and William’s Syndrome [113]. The promise for specificity may lie in identifying oscillatory abnormalities in concrete neural systems that are involved in the pathophysiology of the different conditions. For example, an auditory study using MEG described a down-regulated top-down network during perception in schizophrenia and high-risk individuals that may be more specific to the schizophrenia spectrum than oscillatory abnormalities alone [114].
5. Expert opinion
Abnormal visual information processing in schizophrenia is a rapidly expanding area of biomarker research. The rationale for biomarker research is the possibility to separate more clearly the diagnostic entities that are currently lumped together by the syndrome-based approach to the diagnosis of schizophrenia [8]. One of the currently discussed approaches to be included in DSM-V involves decomposing the presenting features of schizophrenia into several dimensions that may be more closely related to the underlying pathology than the operational diagnosis of schizophrenia [6]. Information processing abnormalities could account for a number of those features, so biomarkers probing connectivity could provide “footholds” to validate the pathophysiological mechanisms that underpin the different dimensions [6].
Visual information processing is particularly well-suited to test information processing in schizophrenia as normal perception requires integrity of between- and within-region connectivity. In addition, vision is one the best characterized sensory modality in humans thus improving the chances of detection of an abnormality in schizophrenia.
Evidence of visual abnormalities in schizophrenia has mounted in the recent years. Despite this progress we are still far away from definitive tests to either form etiology-based diagnostic refinement or to reliably detect at-risk populations. However, certain patterns have begun to emerge.
Performance–based psychophysical measures of visual processing have been widely used in schizophrenia research. They have the advantage of being low-cost, as well as easy to administer and analyze. The results show a relatively consistent impairment in schizophrenia patients. The conflicting findings from studies that used spatially and temporally modulated stimuli could be attributed to the large variability in the designs of the reported tasks. Standardization and validation of these paradigms are therefore needed in order to assess their significance as biomarkers. In contrast VBM tasks are well-validated and are perhaps better placed to establish the presence of general information processing deficits. They show abnormal performance in schizophrenia and bipolar patients, the relatives of schizophrenia patients but not bipolar ones. These findings suggest that VBM probably probes a general attention-related abnormality that characterizes the vulnerability to schizophrenia but which also appears in established bipolar disorder. The VBM test could be used specifically in the evaluation of cognition enhancing drugs in patients and in unaffected relatives of schizophrenia patients. Its easy application means that it might be useful in detecting populations at-risk for schizophrenia in large screening programmes.
Tasks that place greater emphasis on the M-pathway (such as motion perception or M-biased VBM) also produce robust abnormalities that appear to be specific to schizophrenia when compared to bipolar disorder. This suggests that the general information processing deficit in the schizophrenia spectrum is due to a magnocellular pathway dysfunction which is not present in other disorders. It is thus feasible that further sophistication and standardization of the M-biased psychophysical tasks could lead to the advent of a practical biomarker to reliably detect the presence of a schizophrenia phenotype and serve as trait-biomarker.
It should be noted that M-pathway abnormalities appear in dyslexia as well. However this might not be a concern for the specificity of the M-pathway abnormality to schizoprhenia, as strong evidence has now accumulated pointing at association between dyslexia and psychopathological or genetic risk for schizophrenia.
Electrophysiological measures show considerable promise as biomarkers in schizophrenia research especially in view of their low invasiveness, cost and requirement for cooperation in comparison for example to expensive brain imaging. ERP abnormalities appear to produce more robust findings in schizophrenia research than psychophysical experiments [80]. Similar to VBM, ERP abnormalities are found in schizophrenia and bipolar patients, as well as populations at-risk for schizophrenia. They are unaffected by currently available medication and predict cognitive performance [115, 116] and thus show promise as biomarkers for effective cognition-enhancing drugs, disease stratification and the identification of at-risk populations. Furthermore, EEG is an objective way of assessing visual information processing in in animal models of disease that potentially does not require complicated behavioural paradigms. ERPs could therefore play a key part in translating compounds from the preclinical to clinical stages of drug development using similar paradigms. The detection of drug efficacy in phase I proof of concept studies could be enhanced by selecting healthy volunteers with ERP abnormalities as found in schizotypal individuals and unaffected relatives.
Oscillatory biomarkers potentially have added benefits over ERP measures because they provide more in-depth evaluation of connectivity or visual pathway functioning. This comes at the expense of more complicated and less automatic analysis. Two measures are most commonly reported in schizophrenia: signal power and phase-locking factor (PLF). While deficits have been reported in both, signal power tends to have larger variation, as both abnormal increases and decreases have been noted. In contrast, PLF is consistently reduced in patients. The robustness of the PLF measure could be useful in defining the schizophrenia phenotype, especially when used with paradigms that reliably dissect the M- and P-pathway. At present this remains a speculation, as the specificity of PLF oscillatory deficits for schizophrenia compared to other disorders remains unclear. Another strategy for pursuing of specificity of the oscillatory abnormalities to schizophrenia may be to focus on the temporal and spatial characteristics of the signal in the separate disorders. As it was mentioned earlier, one MEG study demonstrated aberrant coupling between the sensory and parietal cortex during perception in the schizophrenia spectrum [114]. The aberration in such specific networks may be more sensitive and specific to the separate disorder rather than general biomarekrs of connectivity.
In conclusion, visual information processing deficits in schizophrenia provide a number of opportunities for biomarker research in schizophrenia. The practical and cost-effective psychophysical experiments could be developed into precise outcome measures to assess the efficacy of treatments that enhance cognition. ERP and oscillatory measures may become main-stream biomarker in translational medicine in schizophrenia. Finally, the robustness of the oscillatory deficits in schizophrenia along with our improved understanding of the specific M-pathway aberrations in this disorder could be a promising approach to refining the diagnosis of schizophrenia and its related disorders.
Acknowledgments
Declaration of Interest The Manchester Wellcome Trust Clinical Research Facility (grant no. 052820) and P1vital Ltd provide facility and financial support. The authors also are supported by the University of Manchester. Specifically, I Koychev is supported by means of a PhD studentship by the University of Manchester and through P1vital Ltd. JFW Deakin’s work has also been supported by the Manchester Biomedical Research Centre. He has also carried out paid consultancy work and speaking work for Servier, Merck Sharp & Dohme, AstraZeneca, Janssen-Cilag and Eli Lilly. JFW Deakin also own shares in P1vital Ltd. W El-Deredy has no conflicts of interest and has received no payment in the preparation of this manuscript.
References
- 1.Kendler KS, Gruenberg AM, Kinney DK. Independent diagnoses of adoptees and relatives as defined by DSM-III in the provincial and national samples of the Danish Adoption Study of Schizophrenia. Arch Gen Psychiatry. 1994;51(6):456–68. doi: 10.1001/archpsyc.1994.03950060020002. [DOI] [PubMed] [Google Scholar]
- 2.Kety SS, Wender PH, Jacobsen B, et al. Mental illness in the biological and adoptive relatives of schizophrenic adoptees. Replication of the Copenhagen Study in the rest of Denmark. Arch Gen Psychiatry. 1994;51(6):442–55. doi: 10.1001/archpsyc.1994.03950060006001. [DOI] [PubMed] [Google Scholar]
- 3.Jansson LB, Parnas J. Competing definitions of schizophrenia: what can be learned from polydiagnostic studies? Schizophr Bull. 2007;33(5):1178–200. doi: 10.1093/schbul/sbl065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tandon R, Keshavan MS, Nasrallah HA. Schizophrenia, “Just the Facts”: what we know in 2008 part 1: overview. Schizophr Res. 2008;100(1-3):4–19. doi: 10.1016/j.schres.2008.01.022. [DOI] [PubMed] [Google Scholar]
- 5.Allan CL, Cardno AG, McGuffin P. Schizophrenia: from genes to phenes to disease. Curr Psychiatry Rep. 2008;10(4):339–43. doi: 10.1007/s11920-008-0054-x. [DOI] [PubMed] [Google Scholar]
- 6.Tandon R, Nasrallah HA, Keshavan MS. Schizophrenia, “just the facts” 4. Clinical features and conceptualization. Schizophr Res. 2009;110(1-3):1–23. doi: 10.1016/j.schres.2009.03.005. [DOI] [PubMed] [Google Scholar]
- 7.Kendell RE. Diagnosis and classification of functional psychoses. Br Med Bull. 1987;43(3):499–513. doi: 10.1093/oxfordjournals.bmb.a072198. [DOI] [PubMed] [Google Scholar]
- 8.Stober G, Ben-Shachar D, Cardon M, et al. Schizophrenia: from the brain to peripheral markers. A consensus paper of the WFSBP task force on biological markers. World J Biol Psychiatry. 2009;10(2):127–55. doi: 10.1080/15622970902898980. [DOI] [PubMed] [Google Scholar]
- 9.Fiedorowicz JG, Epping EA, Flaum M. Toward defining schizophrenia as a more useful clinical concept. Curr Psychiatry Rep. 2008;10(4):344–51. doi: 10.1007/s11920-008-0055-9. [DOI] [PubMed] [Google Scholar]
- 10.Regier DA. Time for a fresh start? Rethinking psychosis in DSM-V. Schizophr Bull. 2007;33(4):843–5. doi: 10.1093/schbul/sbm055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mayeux R. Biomarkers: potential uses and limitations. NeuroRx. 2004;1(2):182–8. doi: 10.1602/neurorx.1.2.182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Last JM. Dictionary of Epidemiology. 4th ed OUP; USA: 2001. [Google Scholar]
- 13.Keefe RS, Eesley CE, Poe MP. Defining a cognitive function decrement in schizophrenia. Biol Psychiatry. 2005;57(6):688–91. doi: 10.1016/j.biopsych.2005.01.003. [DOI] [PubMed] [Google Scholar]
- 14.Saykin AJ, Gur RC, Gur RE, et al. Neuropsychological function in schizophrenia. Selective impairment in memory and learning. Arch Gen Psychiatry. 1991;48(7):618–24. doi: 10.1001/archpsyc.1991.01810310036007. [DOI] [PubMed] [Google Scholar]
- 15.Woodberry KA, Giuliano AJ, Seidman LJ. Premorbid IQ in schizophrenia: a meta-analytic review. Am J Psychiatry. 2008;165(5):579–87. doi: 10.1176/appi.ajp.2008.07081242. [DOI] [PubMed] [Google Scholar]
- 16.Rund BR. A review of longitudinal studies of cognitive functions in schizophrenia patients. Schizophr Bull. 1998;24(3):425–35. doi: 10.1093/oxfordjournals.schbul.a033337. [DOI] [PubMed] [Google Scholar]
- 17.Hoff AL, Svetina C, Shields G, et al. Ten year longitudinal study of neuropsychological functioning subsequent to a first episode of schizophrenia. Schizophr Res. 2005;78(1):27–34. doi: 10.1016/j.schres.2005.05.010. [DOI] [PubMed] [Google Scholar]
- 18.Heinrichs RW. The primacy of cognition in schizophrenia. Am Psychol. 2005;60(3):229–42. doi: 10.1037/0003-066X.60.3.229. [DOI] [PubMed] [Google Scholar]
- 19.Andreasen NC. A unitary model of schizophrenia: Bleuler’s “fragmented phrene” as schizencephaly. Arch Gen Psychiatry. 1999;56(9):781–7. doi: 10.1001/archpsyc.56.9.781. [DOI] [PubMed] [Google Scholar]
- 20.Friston K. Disconnection and cognitive dysmetria in schizophrenia. Am J Psychiatry. 2005;162(3):429–32. doi: 10.1176/appi.ajp.162.3.429. [DOI] [PubMed] [Google Scholar]
- 21.Engel AK, Fries P, Singer W. Dynamic predictions: oscillations and synchrony in top-down processing. Nat Rev Neurosci. 2001;2(10):704–16. doi: 10.1038/35094565. [DOI] [PubMed] [Google Scholar]
- 22.Merigan W, Freeman A, Meyers SP. Parallel processing streams in human visual cortex. Neuroreport. 1997;8(18):3985–91. doi: 10.1097/00001756-199712220-00027. [DOI] [PubMed] [Google Scholar]
- 23.Van Essen DC, Gallant JL. Neural mechanisms of form and motion processing in the primate visual system. Neuron. 1994;13(1):1–10. doi: 10.1016/0896-6273(94)90455-3. [DOI] [PubMed] [Google Scholar]
- 24.Butler PD, Javitt DC. Early-stage visual processing deficits in schizophrenia. Curr Opin Psychiatry. 2005;18(2):151–7. doi: 10.1097/00001504-200503000-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Keri S, Antal A, Szekeres G, et al. Spatiotemporal visual processing in schizophrenia. J Neuropsychiatry Clin Neurosci. 2002;14(2):190–6. doi: 10.1176/jnp.14.2.190. [DOI] [PubMed] [Google Scholar]
- 26.Slaghuis WL. Contrast sensitivity for stationary and drifting spatial frequency gratings in positive- and negative-symptom schizophrenia. J Abnorm Psychol. 1998;107(1):49–62. doi: 10.1037//0021-843x.107.1.49. [DOI] [PubMed] [Google Scholar]
- 27.Chen Y, Nakayama K, Levy DL, et al. Psychophysical isolation of a motion-processing deficit in schizophrenics and their relatives and its association with impaired smooth pursuit. Proc Natl Acad Sci U S A. 1999;96(8):4724–9. doi: 10.1073/pnas.96.8.4724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chen Y, Palafox GP, Nakayama K, et al. Motion perception in schizophrenia. Arch Gen Psychiatry. 1999;56(2):149–54. doi: 10.1001/archpsyc.56.2.149. [DOI] [PubMed] [Google Scholar]
- 29.Cimmer C, Szendi I, Csifcsak G, et al. Abnormal neurological signs, visual contrast sensitivity, and the deficit syndrome of schizophrenia. Prog Neuropsychopharmacol Biol Psychiatry. 2006;30(7):1225–30. doi: 10.1016/j.pnpbp.2006.03.021. [DOI] [PubMed] [Google Scholar]
- 30.Keri S, Kiss I, Kelemen O, et al. Anomalous visual experiences, negative symptoms, perceptual organization and the magnocellular pathway in schizophrenia: a shared construct? Psychol Med. 2005;35(10):1445–55. doi: 10.1017/S0033291705005398. [DOI] [PubMed] [Google Scholar]
- 31.O’Donnell BF, Potts GF, Nestor PG, et al. Spatial frequency discrimination in schizophrenia. J Abnorm Psychol. 2002;111(4):620–5. doi: 10.1037//0021-843x.111.4.620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chen Y, Levy DL, Sheremata S, et al. Effects of typical, atypical, and no antipsychotic drugs on visual contrast detection in schizophrenia. Am J Psychiatry. 2003;160(10):1795–801. doi: 10.1176/appi.ajp.160.10.1795. [DOI] [PubMed] [Google Scholar]
- 33.Schwartz BD, Winstead DK. Icon formation in chronic schizophrenics. Biol Psychiatry. 1985;20(9):1015–8. doi: 10.1016/0006-3223(85)90200-8. [DOI] [PubMed] [Google Scholar]
- 34.Slaghuis WL. Spatio-temporal luminance contrast sensitivity and visual backward masking in schizophrenia. Exp Brain Res. 2004;156(2):196–211. doi: 10.1007/s00221-003-1771-3. [DOI] [PubMed] [Google Scholar]
- 35.Schwartz BD, McGinn T, Winstead DK. Disordered spatiotemporal processing in schizophrenics. Biol Psychiatry. 1987;22(6):688–98. doi: 10.1016/0006-3223(87)90200-9. [DOI] [PubMed] [Google Scholar]
- 36.Keri S, Kelemen O, Benedek G, et al. Vernier threshold in patients with schizophrenia and in their unaffected siblings. Neuropsychology. 2004;18(3):537–42. doi: 10.1037/0894-4105.18.3.537. [DOI] [PubMed] [Google Scholar]
- 37.Farmer CM, O’Donnell BF, Niznikiewicz MA, et al. Visual perception and working memory in schizotypal personality disorder. Am J Psychiatry. 2000;157(5):781–8. doi: 10.1176/appi.ajp.157.5.781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.O’Donnell BF, Bismark A, Hetrick WP, et al. Early stage vision in schizophrenia and schizotypal personality disorder. Schizophr Res. 2006;86(1-3):89–98. doi: 10.1016/j.schres.2006.05.016. [DOI] [PubMed] [Google Scholar]
- 39.Vlamings PH, Jonkman LM, van Daalen E, et al. Basic abnormalities in visual processing affect face processing at an early age in autism spectrum disorder. Biol Psychiatry. 2010;68(12):1107–13. doi: 10.1016/j.biopsych.2010.06.024. [DOI] [PubMed] [Google Scholar]
- 40.Lehmkuhle S, Garzia RP, Turner L, et al. A defective visual pathway in children with reading disability. N Engl J Med. 1993;328(14):989–96. doi: 10.1056/NEJM199304083281402. [DOI] [PubMed] [Google Scholar]
- 41.Livingstone MS, Rosen GD, Drislane FW, et al. Physiological and anatomical evidence for a magnocellular defect in developmental dyslexia. Proc Natl Acad Sci U S A. 1991;88(18):7943–7. doi: 10.1073/pnas.88.18.7943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Feusner JD, Moody T, Hembacher E, et al. Abnormalities of visual processing and frontostriatal systems in body dysmorphic disorder. Arch Gen Psychiatry. 2010;67(2):197–205. doi: 10.1001/archgenpsychiatry.2009.190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Schlotterer G, Moscovitch M, Crapper-McLachlan D. VISUAL PROCESSING DEFICITS AS ASSESSED BY SPATIAL FREQUENCY CONTRAST SENSITIVITY AND BACKWARD MASKING IN NORMAL AGEING AND ALZHEIMER’S DISEASE. Brain. 1983;107(1):309–324. doi: 10.1093/brain/107.1.309. [DOI] [PubMed] [Google Scholar]
- 44.Schwartz BD, Maron BA, Evans WJ, et al. High velocity transient visual processing deficits diminish ability of patients with schizophrenia to recognize objects. Neuropsychiatry Neuropsychol Behav Neurol. 1999;12(3):170–7. [PubMed] [Google Scholar]
- 45.Kim D, Wylie G, Pasternak R, et al. Magnocellular contributions to impaired motion processing in schizophrenia. Schizophr Res. 2006;82(1):1–8. doi: 10.1016/j.schres.2005.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Clementz BA, McDowell JE, Dobkins KR. Compromised speed discrimination among schizophrenia patients when viewing smooth pursuit targets. Schizophr Res. 2007;95(1-3):61–4. doi: 10.1016/j.schres.2007.05.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chen Y, Bidwell LC, Holzman PS. Visual motion integration in schizophrenia patients, their first-degree relatives, and patients with bipolar disorder. Schizophr Res. 2005;74(2-3):271–81. doi: 10.1016/j.schres.2004.04.002. [DOI] [PubMed] [Google Scholar]
- 48.Chen Y, Nakayama K, Levy D, et al. Processing of global, but not local, motion direction is deficient in schizophrenia. Schizophr Res. 2003;61(2-3):215–27. doi: 10.1016/s0920-9964(02)00222-0. [DOI] [PubMed] [Google Scholar]
- 49.Chen Y, Levy DL, Sheremata S, et al. Bipolar and schizophrenic patients differ in patterns of visual motion discrimination. Schizophr Res. 2006;88(1- 3):208–16. doi: 10.1016/j.schres.2006.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Stuve TA, Friedman L, Jesberger JA, et al. The relationship between smooth pursuit performance, motion perception and sustained visual attention in patients with schizophrenia and normal controls. Psychol Med. 1997;27(1):143–52. doi: 10.1017/s0033291796004230. [DOI] [PubMed] [Google Scholar]
- 51.Li CS. Impaired detection of visual motion in schizophrenia patients. Prog Neuropsychopharmacol Biol Psychiatry. 2002;26(5):929–34. doi: 10.1016/s0278-5846(02)00207-5. [DOI] [PubMed] [Google Scholar]
- 52.Tadin D, Kim J, Doop ML, et al. Weakened center-surround interactions in visual motion processing in schizophrenia. J Neurosci. 2006;26(44):11403–12. doi: 10.1523/JNEUROSCI.2592-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Cornelissen P, Bradley L, Fowler S, et al. What children see affects how they spell. Dev Med Child Neurol. 1994;36(8):716–26. doi: 10.1111/j.1469-8749.1994.tb11914.x. [DOI] [PubMed] [Google Scholar]
- 54.Stein J, Talcott J, Walsh VV. Controversy about the visual magnocellular deficit in developmental dyslexics. Trends Cogn Sci. 2000;4(6):209–211. doi: 10.1016/s1364-6613(00)01484-4. [DOI] [PubMed] [Google Scholar]
- 55.Talcott JB, Hansen PC, Assoku EL, et al. Visual motion sensitivity in dyslexia: evidence for temporal and energy integration deficits. Neuropsychologia. 2000;38(7):935–43. doi: 10.1016/s0028-3932(00)00020-8. [DOI] [PubMed] [Google Scholar]
- 56.Richardson AJ. Dyslexia, handedness and syndromes of psychosis-proneness. Int J Psychophysiol. 1994;18(3):251–63. doi: 10.1016/0167-8760(94)90011-6. [DOI] [PubMed] [Google Scholar]
- 57.Fish B. Infant predictors of the longitudinal course of schizophrenic development. Schizophr Bull. 1987;13(3):395–409. doi: 10.1093/schbul/13.3.395. [DOI] [PubMed] [Google Scholar]
- 58.Yeo RA, Gangestad SW, Edgar C, et al. The evolutionary genetic underpinnings of schizophrenia: the developmental instability model. Schizophr Res. 1999;39(3):197–206. doi: 10.1016/s0920-9964(99)00074-2. [DOI] [PubMed] [Google Scholar]
- 59.McClure RK. The visual backward masking deficit in schizophrenia. Prog Neuropsychopharmacol Biol Psychiatry. 2001;25(2):301–11. doi: 10.1016/s0278-5846(00)00166-4. [DOI] [PubMed] [Google Scholar]
- 60.Butler PD, Harkavy-Friedman JM, Amador XF, et al. Backward masking in schizophrenia: relationship to medication status, neuropsychological functioning, and dopamine metabolism. Biol Psychiatry. 1996;40(4):295–8. doi: 10.1016/0006-3223(96)00007-8. [DOI] [PubMed] [Google Scholar]
- 61.Cadenhead KS, Serper Y, Braff DL. Transient versus sustained visual channels in the visual backward masking deficits of schizophrenia patients. Biol Psychiatry. 1998;43(2):132–8. doi: 10.1016/S0006-3223(97)00316-8. [DOI] [PubMed] [Google Scholar]
- 62.Schechter I, Butler PD, Silipo G, et al. Magnocellular and parvocellular contributions to backward masking dysfunction in schizophrenia. Schizophr Res. 2003;64(2-3):91–101. doi: 10.1016/s0920-9964(03)00008-2. [DOI] [PubMed] [Google Scholar]
- 63.Butler PD, DeSanti LA, Maddox J, et al. Visual backward-masking deficits in schizophrenia: relationship to visual pathway function and symptomatology. Schizophr Res. 2003;59(2-3):199–209. doi: 10.1016/s0920-9964(01)00341-3. [DOI] [PubMed] [Google Scholar]
- 64.Green MF, Nuechterlein KH, Breitmeyer B. Backward masking performance in unaffected siblings of schizophrenic patients. Evidence for a vulnerability indicator. Arch Gen Psychiatry. 1997;54(5):465–72. doi: 10.1001/archpsyc.1997.01830170091012. [DOI] [PubMed] [Google Scholar]
- 65.Green MF, Nuechterlein KH, Breitmeyer B, et al. Forward and backward visual masking in unaffected siblings of schizophrenic patients. Biol Psychiatry. 2006;59(5):446–51. doi: 10.1016/j.biopsych.2005.06.035. [DOI] [PubMed] [Google Scholar]
- 66.Keri S, Kelemen O, Benedek G, et al. Different trait markers for schizophrenia and bipolar disorder: a neurocognitive approach. Psychol Med. 2001;31(5):915–22. doi: 10.1017/s0033291701004068. [DOI] [PubMed] [Google Scholar]
- 67.Bedwell JS, Brown JM, Miller LS. The magnocellular visual system and schizophrenia: what can the color red tell us? Schizophr Res. 2003;63(3):273–84. doi: 10.1016/s0920-9964(02)00356-0. [DOI] [PubMed] [Google Scholar]
- 68.Cadenhead K, Kumar C, Braff D. Clinical and experimental characteristics of “hypothetically psychosis prone” college students. J Psychiatr Res. 1996;30(5):331–40. doi: 10.1016/0022-3956(96)00020-9. [DOI] [PubMed] [Google Scholar]
- 69.Green MF, Nuechterlein KH, Breitmeyer B, et al. Backward masking in unmedicated schizophrenic patients in psychotic remission: possible reflection of aberrant cortical oscillation. Am J Psychiatry. 1999;156(9):1367–73. doi: 10.1176/ajp.156.9.1367. [DOI] [PubMed] [Google Scholar]
- 70.Green MF, Nuechterlein KH, Mintz J. Backward masking in schizophrenia and mania. I. Specifying a mechanism. Arch Gen Psychiatry. 1994;51(12):939–44. doi: 10.1001/archpsyc.1994.03950120011003. [DOI] [PubMed] [Google Scholar]
- 71.McClure RK. The visual backward masking deficit in schizoaffective disorder. Prog Neuropsychopharmacol Biol Psychiatry. 1999;23(5):785–90. doi: 10.1016/s0278-5846(99)00040-8. [DOI] [PubMed] [Google Scholar]
- 72.Duffy A, Hajek T, Alda M, et al. Neurocognitive functioning in the early stages of bipolar disorder: visual backward masking performance in high risk subjects. Eur Arch Psychiatry Clin Neurosci. 2009;259(5):263–9. doi: 10.1007/s00406-008-0862-3. [DOI] [PubMed] [Google Scholar]
- 73.Green MF, Nuechterlein KH, Mintz J. Backward masking in schizophrenia and mania. II. Specifying the visual channels. Arch Gen Psychiatry. 1994;51(12):945–51. doi: 10.1001/archpsyc.1994.03950120017004. [DOI] [PubMed] [Google Scholar]
- 74.Bowen R, Wright B, Zecker G, et al. Investigative Ophthalmology and Visual Science. Journal of the Optical Society of America. 1999;40(533) [Google Scholar]
- 75.Mendola JD, Cronin-Golomb A, Corkin S, et al. Prevalence of visual deficits in Alzheimer’s disease. Optom Vis Sci. 1995;72(3):155–67. doi: 10.1097/00006324-199503000-00003. [DOI] [PubMed] [Google Scholar]
- 76.Di Russo F, Martinez A, Hillyard SA. Source analysis of event-related cortical activity during visuo-spatial attention. Cereb Cortex. 2003;13(5):486–99. doi: 10.1093/cercor/13.5.486. [DOI] [PubMed] [Google Scholar]
- 77.Martinez A, Russo F. Di, Anllo-Vento L, et al. Electrophysiological analysis of cortical mechanisms of selective attention to high and low spatial frequencies. Clin Neurophysiol. 2001;112(11):1980–98. doi: 10.1016/s1388-2457(01)00660-5. [DOI] [PubMed] [Google Scholar]
- 78.Ellemberg D, Hammarrenger B, Lepore F, et al. Contrast dependency of VEPs as a function of spatial frequency: the parvocellular and magnocellular contributions to human VEPs. Spat Vis. 2001;15(1):99–111. doi: 10.1163/15685680152692042. [DOI] [PubMed] [Google Scholar]
- 79.Skottun BC, Skoyles JR. On identifying magnocellular and parvocellular responses on the basis of contrast-response functions. Schizophr Bull. 2011;37(1):23–6. doi: 10.1093/schbul/sbq114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Yeap S, Kelly SP, Sehatpour P, et al. Visual sensory processing deficits in Schizophrenia and their relationship to disease state. Eur Arch Psychiatry Clin Neurosci. 2008;258(5):305–16. doi: 10.1007/s00406-008-0802-2. [DOI] [PubMed] [Google Scholar]
- 81.Foxe JJ, Murray MM, Javitt DC. Filling-in in schizophrenia: a high-density electrical mapping and source-analysis investigation of illusory contour processing. Cereb Cortex. 2005;15(12):1914–27. doi: 10.1093/cercor/bhi069. [DOI] [PubMed] [Google Scholar]
- 82.Murray MM, Foxe DM, Javitt DC, et al. Setting boundaries: brain dynamics of modal and amodal illusory shape completion in humans. J Neurosci. 2004;24(31):6898–903. doi: 10.1523/JNEUROSCI.1996-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Schechter I, Butler PD, Zemon VM, et al. Impairments in generation of early-stage transient visual evoked potentials to magno- and parvocellular-selective stimuli in schizophrenia. Clin Neurophysiol. 2005;116(9):2204–15. doi: 10.1016/j.clinph.2005.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Butler PD, Zemon V, Schechter I, et al. Early-stage visual processing and cortical amplification deficits in schizophrenia. Arch Gen Psychiatry. 2005;62(5):495–504. doi: 10.1001/archpsyc.62.5.495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Kim D, Zemon V, Saperstein A, et al. Dysfunction of early-stage visual processing in schizophrenia: harmonic analysis. Schizophr Res. 2005;76(1):55–65. doi: 10.1016/j.schres.2004.10.011. [DOI] [PubMed] [Google Scholar]
- 86.Yeap S, Kelly SP, Sehatpour P, et al. Early visual sensory deficits as endophenotypes for schizophrenia: high-density electrical mapping in clinically unaffected first-degree relatives. Arch Gen Psychiatry. 2006;63(11):1180–8. doi: 10.1001/archpsyc.63.11.1180. [DOI] [PubMed] [Google Scholar]
- 87.Koychev I, El-Deredy W, Haenschel C, et al. Visual information processing deficits as biomarkers of vulnerability to schizophrenia: an event-related potential study in schizotypy. Neuropsychologia. 2010;48(7):2205–14. doi: 10.1016/j.neuropsychologia.2010.04.014. [DOI] [PubMed] [Google Scholar]
- 88.Schwartzman D, Maravic K, Kranczioch C, et al. Altered early visual processing components in hallucination-prone individuals. Neuroreport. 2008;19(9):933–7. doi: 10.1097/WNR.0b013e328301a640. [DOI] [PubMed] [Google Scholar]
- 89.Yeap S, Kelly SP, Thakore JH, et al. Visual sensory processing deficits in first-episode patients with Schizophrenia. Schizophr Res. 2008;102(1-3):340–3. doi: 10.1016/j.schres.2008.03.026. [DOI] [PubMed] [Google Scholar]
- 90.Yeap S, Kelly SP, Reilly RB, et al. Visual sensory processing deficits in patients with bipolar disorder revealed through high-density electrical mapping. J Psychiatry Neurosci. 2009;34(6):459–64. [PMC free article] [PubMed] [Google Scholar]
- 91.Grayson AS, Weiler EM, Sandman DE. Visual evoked potentials in early Alzheimer’s dementia: an exploratory study. J Gen Psychol. 1995;122(1):113–29. doi: 10.1080/00221309.1995.9921226. [DOI] [PubMed] [Google Scholar]
- 92.Barcelo F, Suwazono S, Knight RT. Prefrontal modulation of visual processing in humans. Nat Neurosci. 2000;3(4):399–403. doi: 10.1038/73975. [DOI] [PubMed] [Google Scholar]
- 93.Weinstock-Guttman B, Baier M, Stockton R, et al. Pattern reversal visual evoked potentials as a measure of visual pathway pathology in multiple sclerosis. Mult Scler. 2003;9(5):529–34. doi: 10.1191/1352458503ms935rr. [DOI] [PubMed] [Google Scholar]
- 94.Fulgente T, Thomas A, Lobefalo L, et al. Are VEP abnormalities in optic neuritis (ON) dependent on plaque size? A reappraisal of the physiopathology of ON based on improved MRI and multiple-lead recordings. Ital J Neurol Sci. 1996;17(1):43–54. doi: 10.1007/BF01995708. [DOI] [PubMed] [Google Scholar]
- 95.Fries P. Neuronal gamma-band synchronization as a fundamental process in cortical computation. Annu Rev Neurosci. 2009;32:209–24. doi: 10.1146/annurev.neuro.051508.135603. [DOI] [PubMed] [Google Scholar]
- 96.Singer W. Neuronal synchrony: a versatile code for the definition of relations? Neuron. 1999;24(1):49–65. 111–25. doi: 10.1016/s0896-6273(00)80821-1. [DOI] [PubMed] [Google Scholar]
- 97.Varela F, Lachaux JP, Rodriguez E, et al. The brainweb: phase synchronization and large-scale integration. Nat Rev Neurosci. 2001;2(4):229–39. doi: 10.1038/35067550. [DOI] [PubMed] [Google Scholar]
- 98.von Stein A, Chiang C, Konig P. Top-down processing mediated by interareal synchronization. Proc Natl Acad Sci U S A. 2000;97(26):14748–53. doi: 10.1073/pnas.97.26.14748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Gruber WR, Klimesch W, Sauseng P, et al. Alpha phase synchronization predicts P1 and N1 latency and amplitude size. Cereb Cortex. 2005;15(4):371–7. doi: 10.1093/cercor/bhh139. [DOI] [PubMed] [Google Scholar]
- 100.Roach BJ, Mathalon DH. Event-related EEG time-frequency analysis: an overview of measures and an analysis of early gamma band phase locking in schizophrenia. Schizophr Bull. 2008;34(5):907–26. doi: 10.1093/schbul/sbn093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Winterer G, Coppola R, Goldberg TE, et al. Prefrontal broadband noise, working memory, and genetic risk for schizophrenia. Am J Psychiatry. 2004;161(3):490–500. doi: 10.1176/appi.ajp.161.3.490. [DOI] [PubMed] [Google Scholar]
- 102.Winterer G, Ziller M, Dorn H, et al. Schizophrenia: reduced signal-to-noise ratio and impaired phase-locking during information processing. Clin Neurophysiol. 2000;111(5):837–49. doi: 10.1016/s1388-2457(99)00322-3. [DOI] [PubMed] [Google Scholar]
- 103.Uhlhaas PJ, Singer W. Abnormal neural oscillations and synchrony in schizophrenia. Nat Rev Neurosci. 2010;11(2):100–13. doi: 10.1038/nrn2774. [DOI] [PubMed] [Google Scholar]
- 104.Haenschel C, Bittner RA, Waltz J, et al. Cortical oscillatory activity is critical for working memory as revealed by deficits in early-onset schizophrenia. J Neurosci. 2009;29(30):9481–9. doi: 10.1523/JNEUROSCI.1428-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Haenschel C, Linden DE, Bittner RA, et al. Alpha Phase Locking Predicts Residual Working Memory Performance in Schizophrenia. Biol Psychiatry. 2010 doi: 10.1016/j.biopsych.2010.06.013. [DOI] [PubMed] [Google Scholar]
- 106.Spencer KM, Nestor PG, Perlmutter R, et al. Neural synchrony indexes disordered perception and cognition in schizophrenia. Proc Natl Acad Sci U S A. 2004;101(49):17288–93. doi: 10.1073/pnas.0406074101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Spencer KM, Niznikiewicz MA, Shenton ME, et al. Sensory-evoked gamma oscillations in chronic schizophrenia. Biol Psychiatry. 2008;63(8):744–7. doi: 10.1016/j.biopsych.2007.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Green MF, Mintz J, Salveson D, et al. Visual masking as a probe for abnormal gamma range activity in schizophrenia. Biol Psychiatry. 2003;53(12):1113–9. doi: 10.1016/s0006-3223(02)01813-9. [DOI] [PubMed] [Google Scholar]
- 109.Wynn JK, Light GA, Breitmeyer B, et al. Event-related gamma activity in schizophrenia patients during a visual backward-masking task. Am J Psychiatry. 2005;162(12):2330–6. doi: 10.1176/appi.ajp.162.12.2330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Tallon-Baudry C, Bertrand O. Oscillatory gamma activity in humans and its role in object representation. Trends Cogn Sci. 1999;3(4):151–162. doi: 10.1016/s1364-6613(99)01299-1. [DOI] [PubMed] [Google Scholar]
- 111.Ozerdem A, Guntekin B, Tunca Z, et al. Brain oscillatory responses in patients with bipolar disorder manic episode before and after valproate treatment. Brain Res. 2008;1235:98–108. doi: 10.1016/j.brainres.2008.06.101. [DOI] [PubMed] [Google Scholar]
- 112.Lee PS, Chen YS, Hsieh JC, et al. Distinct neuronal oscillatory responses between patients with bipolar and unipolar disorders: a magnetoencephalographic study. J Affect Disord. 2010;123(1-3):270–5. doi: 10.1016/j.jad.2009.08.020. [DOI] [PubMed] [Google Scholar]
- 113.Grice SJ, Spratling MW, Karmiloff-Smith A, et al. Disordered visual processing and oscillatory brain activity in autism and Williams syndrome. Neuroreport. 2001;12(12):2697–700. doi: 10.1097/00001756-200108280-00021. [DOI] [PubMed] [Google Scholar]
- 114.Koh Y, Shin KS, Kim JS, et al. An MEG study of alpha modulation in patients with schizophrenia and in subjects at high risk of developing psychosis. Schizophr Res. 2010 doi: 10.1016/j.schres.2010.10.001. [DOI] [PubMed] [Google Scholar]
- 115.Butler PD, Abeles IY, Weiskopf NG, et al. Sensory contributions to impaired emotion processing in schizophrenia. Schizophr Bull. 2009;35(6):1095–107. doi: 10.1093/schbul/sbp109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Haenschel C, Bittner RA, Haertling F, et al. Contribution of impaired early-stage visual processing to working memory dysfunction in adolescents with schizophrenia: a study with event-related potentials and functional magnetic resonance imaging. Arch Gen Psychiatry. 2007;64(11):1229–40. doi: 10.1001/archpsyc.64.11.1229. [DOI] [PubMed] [Google Scholar]