Abstract
Within a dry inner Alpine valley in the Eastern Central Alps (750 m a.s.l., Tyrol, Austria) the influence of climate variables (precipitation, air humidity, temperature) and soil water content on intra-annual dynamics of tree-ring development was determined in Scots pine (Pinus sylvestris L.) at two sites differing in soil water availability (xeric and dry-mesic site). Radial stem development was continuously followed during 2007 and 2008 by band dendrometers and repeated micro-sampling of the developing tree rings of mature trees. Daily and seasonal fluctuations of the stem radius, which reached almost half of total annual increment, primarily reflected changes in tree water status and masked radial stem growth especially during drought periods in spring. However, temporal dynamics of intra-annual radial growth determined by both methods were found to be quite similar, when onset of radial growth in dendrometer traces was defined by the occurrence of first enlarging xylem cells. Radial increments during the growing period, which lasted from early April through early August showed statistically significant relationships with precipitation (Kendall τ = 0.234, p < 0.01, and τ = 0.184, p < 0.05, at the xeric and dry-mesic site, respectively) and relative air humidity (Pearson r = 0.290, p < 0.05, and r = 0.306, p < 0.05 at the xeric and dry-mesic site, respectively). Soil water content and air temperature had no influence on radial stem increment. Culmination of radial stem growth was detected at both study plots around mid-May, prior to occurrence of more favourable climatic conditions, i.e. an increase in precipitation during summer. We suggest that the early decrease in radial growth rate is due to a high belowground demand for carbohydrates to ensure adequate resource acquisition on the drought prone substrate.
Keywords: Dendrometer, Drought, Dry inner Alpine valley, Pinus sylvestris, Radial growth, Xylem cell analysis
Introduction
To identify the climatic factors most closely associated with variations in tree-ring parameters, dendroclimatological methods are frequently applied (for review see Hughes 2002). These methods include the calculation of response functions, i.e. multiple regression techniques and/or Pearson-product-moment correlation analysis (Fritts 1976; Blasing et al. 1984; Cook and Kairiukstis 1990), which reveal statistically significant relationships between climate variables (e.g., monthly mean temperature and total precipitation for individual months of the year prior to growth and of the current year) and ring width time series. Based on several dendroclimatological studies conducted within dry, inner Alpine valleys, it is well established that radial growth of trees is primarily limited by spring precipitation (e.g., Kienast 1987; Oberhuber et al. 1998; Oberhuber and Kofler 2000; Rigling et al. 2002; Jolly et al. 2005), and severe drought triggers both, temporary declines and the mortality of susceptible or less competitive species in temperate forests (e.g., Oberhuber 2001; Rebetez and Dobbertin 2004; Bigler et al. 2006; Waldboth and Oberhuber 2008). Several authors recommend a combination of dendroclimatological and intra-annual growth studies to assess long- and short-term climatic influences, respectively, on radial tree growth (e.g., Camarero et al. 1998; Gartner et al. 2002; Deslauriers et al. 2003a; Rossi et al. 2006c; Gruber et al. 2009).
Automatic dendrometers have been used traditionally for continuous monitoring of stem radial variation throughout the year and determination of seasonal tree growth (e.g., Fritts 1961; Herzog et al. 1995; Carrer et al. 1998; Tardif et al. 2001; Bouriaud et al. 2005). However, dendrometer measurements are strongly influenced by the changing water status of the stem, which may mask crucial phenological events such as onset and ending of wood formation and timing of maximum growth (e.g., Kozlowski and Winget 1964; Downes et al. 1999; Zweifel and Häsler 2001; Mäkinen et al. 2003).
Alternatively, temporal dynamics of cambial activity and xylem development can be monitored throughout the growing season by repeatedly extracting wood samples at short intervals (e.g., Loris 1981; Antonova and Stasova 1993; Bäucker et al. 1998; Deslauriers et al. 2003a; Rossi et al. 2006b). This method enables a direct observation of the periodic process of cambial activity and the seasonal formation of xylem (Plomion et al. 2001; Vaganov et al. 2006) and is regarded as one of the most reliable techniques for monitoring xylem cell development (Mäkinen et al. 2008). Studies that compared dynamics of wood formation monitored by automatic dendrometers with histological analysis of the developing xylem (micro-coring), were primarily conducted in conifers in cold environments, i.e., at sites where radial tree growth is predominantly controlled by summer temperature (e.g., Mäkinen et al. 2003; Rossi et al. 2006c; Gruber et al. 2009). While Rossi et al. (2006c) found that both methods were able to detect the course of intra-annual radial growth, Zweifel and Häsler (2001) and Mäkinen et al. (2003) reported that replenishment of dehydrated bark after winter masked onset of radial stem growth in slow-growing boreal and subalpine tree species. Gruber et al. (2009), however, showed that growth-induced radial expansion of the stem within the Alpine treeline ecotone can be distinguished from re-hydration of the bark by histological analysis of wood formation.
In this study conducted within a dry inner Alpine environment dominated by Scots pine (Pinus sylvestris L.) we investigate the influence of environmental variables (climate and soil moisture) on intra-annual radial growth during two contrasting years. Whereas climate in 2007 was characterized by exceptionally warm and dry conditions at the beginning of the growing season in April, at the same time in 2008 cool-moist conditions corresponding to the long-term average prevailed. After determination of onset of radial stem growth in dendrometer traces by histological analysis of xylogenesis, daily changes in stem radius and extracted radial increments were correlated with environmental variables, and time of maximum increment growth was determined by applying Gompertz modelled growth functions (e.g., Zeide 1993; Deslauriers and Morin 2005). Two sites differing in soil moisture availability (xeric vs. dry-moist) were selected, to detect whether local site conditions exert a modulating effect on intra-annual tree growth.
Materials and methods
Study area
The study site (for the geographical location see Oberhuber et al. 1998) is part of a postglacial rock-slide area situated in the montane belt (c. 750 m a.s.l.) within the inner Alpine dry valley of the Inn River (Tyrol, Austria, 47°14′00″N, 10°50′20″E) and has a relatively continental climate with mean annual precipitation and temperature of 715 mm and 7.3 °C, respectively (long-term mean during 1911-2007 at Ötz, 812 m a.s.l., 5 km from the study area). The widespread plant community in the study area is a Spring Heath-Pine wood (Erico-Pinetum typicum, Ellenberg 1988). Human impact in this area was generally restricted to sporadic gathering of firewood and livestock grazing.
Because it has been found that trees within the study area respond quite differently to identical climatic conditions depending on the interaction of soil condition and topographic features on water availability (cf. Oberhuber and Kofler 2000; Oberhuber 2001) and there is evidence that trees at xeric sites are better adapted to water deficits than those at mesic sites (Orwig and Abrams 1997; Martín-Benito et al. 2008), the study was carried out at two sites differing in water availability. A more xeric open south-facing stand growing on shallow stony soil and a dry-mesic site with deeper soil and higher stand density in a hollow were selected (Table 1). Shallow soils, predominantly of protorendzina type, i.e. rendzic and lithic leptosols according to the FAO classification system (FAO 1998) are developed and consist of unconsolidated, coarse-textured materials with low water holding capacity. Distinct soil horizons are hardly ever developed and are restricted to small-scale areas within deep hollows. On the xeric site pioneer vegetation prevails in the ground flora, whereas crowberry (Vaccinium vitis-idaea L.) and a thick moss layer dominate the understory in the hollow, which indicates slightly moist conditions at the latter site. All measurements were carried out on dominant trees to reduce the influence of competition on radial growth. Whereas mean tree age at both study plots was quite similar (c. 155 yr), trees were twice as tall at the dry-mesic compared to the xeric site, which indicates more favourable soil moisture conditions at the former site (Table 1).
Table 1.
Site description and characteristics of selected stands (A = aspect, CC = canopy coverage, Prz = Protorendzina (rendzic leptosol), Rw = raw humus, S = soil depth, SD = standard deviation, SDM = stem diameter, Syr = Syrosem (lithic leptosol), TH = tree height, Xm = xeromoder)
| Site | A | Slope (°) |
Soil type |
Humus type |
S (cm) |
CC (%) |
TH (m) |
SDM1 (cm) mean ± SD |
Tree age1 (yr) mean ± SD |
|---|---|---|---|---|---|---|---|---|---|
| xeric | SW | 40 | Syr | Xm | 0-10 | 33 | 4-5 | 24.4 ± 4.9 | 150 ± 30 |
| dry-mesic | N | <10 | Prz | Rw | 20-30 | 66 | 10 | 29.3 ± 3.2 | 167 ± 23 |
Stem diameter and tree age were determined at 1 m height.
Microclimate records
During the study period, daily precipitation, relative air humidity and air temperature were collected automatically at 2 m height (ONSET, Pocasset, MA, USA) at the xeric site on an open ridge, i.e., in a non-vegetated area. This site was chosen to record (i) total rainfall by minimising interception loss, and (ii) to measure relative air humidity closely representing conditions in the canopy, rather than in the understory. Because the dry-mesic plot was located at the same elevation and within less than 200 m in linear distance, records of precipitation and relative air humidity from this plot were regarded as representative for the whole study area. To determine seasonal differences in air temperature between study sites caused by varying topography and canopy coverage, air temperature sensors (HOBO, ONSET, Pocasset, MA, USA) shielded against solar radiation were installed at 2 m height within both stands in 2009. Mean air temperature recorded throughout April-October was 0.59 °C lower at the dry-mesic site than at the xeric site, but due to seasonal variation in insolation some variability in mean monthly temperature differences between sites occurred. During the major growing period, i.e., in May and June, mean air temperature differed by 0.40 °C and 0.04 °C, respectively, whereby higher values were recorded at the xeric site. The largest difference in air temperature between sites was recorded in September, when monthly mean air temperature at the xeric site was 0.97 °C higher than at the dry-mesic site. Long-term records (LTM) of total monthly precipitation and mean monthly temperatures since 1911 were available from a nearby meteorological station (Ötz, 812 m a.s.l., 5 km from the study area).
Soil moisture dynamics (volumetric water content) and soil temperature in the top 5-10 cm soil layer were continuously monitored. Moisture sensors are based on a capacitive method (Cyclobios, proprietary development at University of Innsbruck, Austria). Due to small-scale variability of soil structure with soil depth, records of three soil moisture and temperature sensors placed at each plot were averaged. Measuring intervals for all sensors were 30 min. Mean daily air and soil temperature and soil water content (Vol. %) were calculated by averaging all measurements (48 values/day).
Xylem sampling and determination of wood formation
Seasonal xylem cell dynamics (XCD) were monitored during the growing seasons 2007 and 2008 by taking micro-cores from 5 trees/site of the outermost tree rings with a diameter and length of 2.5 mm and c. 2 cm, respectively (Rossi et al. 2006a). To determine the variability in intra-annual wood formation between trees at each plot (i.e., xeric and dry-mesic site), individual trees were randomly selected. However, trees with major stem or crown anomalies due to high mistletoe infection were excluded from the analysis. Micro-cores were taken at all study plots during March through October in c. 10 day intervals to include the whole dynamic of xylem formation. Because c. 20 samples were taken throughout the growing season from each selected tree, micro-cores were sampled from different trees at both study plots in 2007 and 2008, to avoid effects of wounding on wood formation dynamics. Samples were taken on the slope-perpendicular side of the stem following a spiral trajectory up the stem starting at c. 1 m stem height. A distance of c. 2 cm in tangential and longitudinal direction was kept to avoid lateral influence of wound reactions on adjacent sampling positions.
Immediately after extraction, cores were fixed in a solution of 70 % ethanol, propionic acid and 40 % formaldehyde (mixing ratio: 90/5/5), subsequently embedded in glycolmethacrylate (Technovit 7100) and polymerized after adding an accelerator. Transverse sections c. 12 μm thick were cut with a rotary microtome, stained with a water solution of 0.05 % cresyl fast violet and observed under a light microscope with polarised light to differentiate the development of xylem cells, i.e., the discrimination between tracheids in enlarging and cell-wall thickening phase (Antonova and Stasova 1993; Deslauriers et al. 2003a; Rossi et al. 2006b). The number of radial enlarging cells, cells undergoing secondary wall thickening and mature xylem cells were counted on all sampled cores in three radial rows. Xylem formation was considered to have begun when one horizontal row of cells was detected in the enlarging phase. The increase in tree-ring width over time was determined in three radial rows to the nearest 0.001 mm by image analysis (ProgRes CapturePro 2.5, Jenoptik, Germany) and included cells in radial enlargement, cell-wall thickening and mature xylem cells. Measurements of 5 cores (trees) per date and for each site were averaged (cf. Deslauriers et al. 2003a; Rossi et al. 2006c).
Standardization of cell number and fitting of xylem growth
Because circumferential variability in ring width exists at different positions of the stem (e.g., Creber and Chaloner 1984) and hence among different samples, standardization is required (Rossi et al. 2003). Ring width of the previous three tree rings were recorded in every sample and used for a correction of intra-annual increase in ring width for each tree. Ring width development including cells in radial enlargement, cell wall thickening and mature xylem cells in each i-sample (i.e. micro-cores taken throughout growing seasons 2007 and 2008) was corrected as follows:
where:
rwi = corrected ring width
rwmi = measured intra-annual ring width
rwm= mean ring width of previous rings of all i-samples
rws = ring width of previous rings for each i-sample
Short-term variation in intra-annual increase in ring width based on dendrometer records and micro-core sampling (i.e. xylem development) was modelled with a Gompertz function using the nonlinear regression procedure included in the Origin software package (OriginLab Corporation, Northampton, MA, USA).
Time of bud break in the upper crown was recorded at the same trees selected for micro-coring. Five additional trees per site were included to account for the large within-tree variability observed. Bud break was assessed weekly and defined as readily identifiable swelling of buds. At this time, bud scales still covered the new needles.
Dendrometer records
At three trees per plot, we installed electronic band dendrometers (DMS dendrometer type D-6 with measuring amplifier t8.MV, UMS, Munich, Germany) about 1 m above ground in early March 2007. To avoid influence of frequent wounding on dendrometer traces, different sample populations for determination of XCD and dendrometer measurements were selected. Dendrometers consisted of a clip sensor sliding on a teflon-pad, whereby the manufacturer gives a temperature coefficient of the dendrometer of < 4 μm K−1. Accordingly, dendrometer traces were corrected for temperature sensitivity (4 μm K−1). The measuring band consisted of Invar-steel, which shows a temperature coefficient of linear expansion < 1 μm m−1 K−1. Dead outermost layers (periderm) of the bark were scraped away to reduce the influence of hygroscopic swelling and shrinkage of the bark on dendrometer records (DMR) and to ensure close contact with the stem (cf. Zweifel and Häsler 2001). Data were recorded with a DT-6 data logger programmed to record measurements taken every 30 minutes, and daily increment of stem radius was calculated by averaging all daily measurements (48 values/day). Site-specific mean stem radius changes were determined by averaging three trees/site.
The daily stem radius variation from late March through end of October was then determined by calculating the difference between mean values of two consecutive days (“daily mean approach”, Deslauriers et al. 2007), which represents a combination of water- and growth-induced radius expansion (e.g., Herzog et al. 1995; Daudet et al. 2005; Steppe et al. 2006). Additionally, we extracted radial stem increments from DMR based on the methodology described by Downes et al. (1999) and Deslauriers et al. (2003b). Radial stem increment was defined as that part of the stem’s circadian cycle when the stem radius exceeded the morning maximum until the subsequent maximum. Only consecutive maximum values were used in correlations between stem increment and climate variables. Occasionally, stem shrinkage lasting from a few days up to about three weeks was recorded during drought periods. When > 3 days were required until the previous cycle maximum was exceeded, the difference between maximum values was allocated to the last three days before the maximum was reached. A uniform distribution of increments to corresponding number of days was regarded as not appropriate, considering minor annual increments within the study area amounting to 0.3 - 0.5 mm/yr. Cell enlargement was regarded the major driving force for radial stem increase (cf. Deslauriers et al. 2003b). Cambial cell division represents only a small amount of radial change (cf. Gruber et al. 2010) and secondary wall thickening and lignification take place inside enlarged cells and therefore are not expressed as a radial increase. Therefore, dendrometer traces were set to zero at the day of the year when the first row of enlarging cells (minus half standard deviation to account for actual onset of growth) was detected. Half standard deviation approximately corresponded to difference in sampling intervals at the start of the growing season (cf. Gruber et al. 2010).
Pearson correlation coefficients were calculated between environmental variables (relative air humidity, air temperature, soil water content) and daily stem radius variations and radial stem increments extracted from DMR, whereby time lags from one to three days were considered. Kolmogorov-Smirnov tests were applied to check for normal distribution of selected variables. Since precipitation records were not normally distributed, Kendall’s rank correlation coefficient (τ) was determined (Sheskin 2007). The main period of radial stem increase used for calculating relationships between extracted increments and climate-parameters ranged from early April to early July 2007 and end of April to early August 2008.
Results
Environmental variables during growing seasons 2007 and 2008
Climate in 2007 was characterized by the occurrence of exceptionally mild temperatures in spring (Fig. 1, Table 2). An almost continuous drought period was recorded from 20 March to 6 May 2007, when total monthly precipitation in April reached < 2 mm (LTM 39 mm) and mean monthly air temperature was 13.9 °C, i.e. c. 6 °C above LTM (Table 2). In contrast to 2007 climate at the beginning of the growing season in 2008 was cool and wet, whereby air temperature in April corresponded to LTM and precipitation exceeded LTM by almost 50 % (Fig. 1, Table 2). While mean daily air temperatures recorded in summer 2008 were similar to temperature records of 2007, total summer precipitation was c. 25 % lower than in 2007 (Table 2).
Fig. 1.
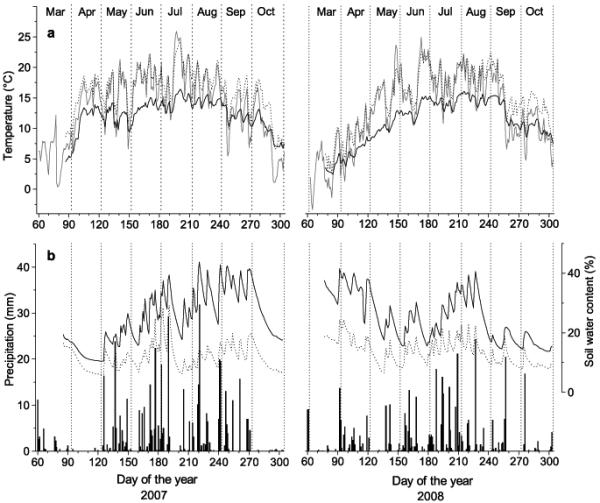
Climate variables and soil water content recorded during the growing seasons 2007 and 2008 within the study area. (a) Mean daily air (grey line) and soil temperature (b) Daily precipitation sum (bars) and soil water content. Study sites are denoted by dotted and solid lines for the xeric and dry-mesic site, respectively.
Table 2.
Monthly and summer (June-August) mean daily air temperature, precipitation sum and mean soil water content during 2007 and 2008 growing seasons recorded within the study area. Long-term mean values (LTM, 1911 - 2006) of air temperature and precipitation were available from a nearby meteorological station (Oetz, 5 km from the study area). Mean values ± standard deviation (SD) are shown
| Air temperature (°C) | Precipitation (mm) | Soil water content (%) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||
| xeric | dry-mesic | xeric | dry-mesic | |||||||
| LTM | 2007 | 2008 | LTM | 2007 | 2008 | 2007 | 2008 | |||
| April | 7.5 ± 1.7 | 13.9 ± 3.4 | 7.6 ± 3.2 | 39 ± 19 | 2 | 58 | 9.9 ± 3.2 | 13.5 ± 2.4 | 18.2 ± 3.3 | 35.4 ± 4.4 |
| May | 12.0 ± 1.8 | 14.7 ± 3.8 | 15.1 ± 3.7 | 64 ± 26 | 86 | 28 | 10.1 ± 2.7 | 18.0 ± 4.5 | 11.6 ± 3.2 | 25.4 ± 5.8 |
| Summer | 15.8 ± 1.0 | 17.8 ± 3.3 | 17.7 ± 3.2 | 316 ± 65 | 308 | 236 | 13.1 ± 5.0 | 28.6 ± 6.8 | 14.6 ± 3.4 | 25.9 ± 6.7 |
In April 2007, soil temperatures reached 15.6 and 10.9 °C at the xeric and dry-mesic site, respectively, which exceeded soil temperature records in April 2008 by c. 5 °C (Fig. 1). Soil temperatures in mid July 2007 and end of June 2008 reached maximum values of c. 25 and c. 17 °C at the xeric and dry-mesic site, respectively. During the drought period in spring 2007 soil water content dropped to 10.8 Vol. % at the dry-mesic and 6.3 Vol. % at the xeric site (Fig. 1, Table 2). Starting with rainfall events in May 2007, soil moisture at the dry-mesic site reached 30 Vol. % in June. During a drought in mid-July, which lasted c. 10-days, soil moisture sharply decreased but was replenished and stayed at high values until early October, when missing precipitation caused increasing dryness of the soil. In contrast to 2007, frequent rainfall events in spring 2008 caused high soil moisture at the start of the growing season in April and May. Throughout both growing seasons studied, records of mean soil water content were predominantly c. 10 – 15 % lower at the xeric compared to dry-mesic site (Fig. 1, Table 2).
Dynamics of tree ring growth and relationship with xylogenesis
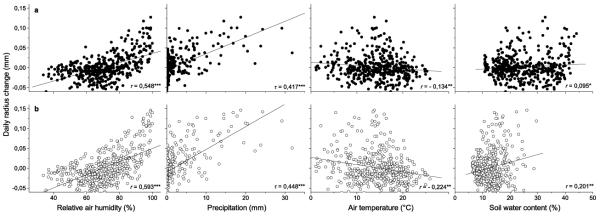
Typical diurnal cycles of stem shrinking and swelling during a drought period in 2008 are depicted in Fig. 2. At the dry mesic site stem radius reached maximum and minimum values in the morning (between 6 and 9 a.m.) and afternoon (between 4 and 6 p.m.), respectively, and were closely related to relative air humidity. At the xeric site, maximum and minimum values were reached c. 150 and 30 min earlier, respectively, than at the dry-mesic site. Mean amplitude of diurnal fluctuations in stem radius during the drought period varied between c. 150 μm at the xeric and c. 75 μm at the dry-mesic site (Fig. 2), whereby mean standard deviation in diurnal amplitudes between trees at the same site was 59 and 28 μm at the xeric and dry-mesic site, respectively. As depicted in Fig. 3, daily radius change showed highest direct correlations calculated over the whole measurement period with relative air humidity (r = 0.548, p < 0.001, and r = 0.593, p < 0.001, at the dry-mesic and xeric site, respectively) and precipitation (Kendall τ = 0.417, p < 0.001, and τ = 0.448, p < 0.001, at the dry-mesic and xeric site, respectively). Significant indirect relationships were observed between daily radius change and air temperature at both study plots (r = −0.134, p < 0.01, and r = −0.224, p < 0.01, at the dry-mesic and xeric site, respectively). Low but statistically significant coefficients were also found with soil water content (r = 0.095, p < 0.05, and r = 0.201, p < 0.01, at the dry-mesic and xeric site, respectively), when considering a time lag of one day (Fig. 3). Without accounting for a time lag, correlation coefficients amounted to −0.007 (p = 0.884) at the dry-mesic and 0.131 (p = 0.071) at the xeric site (data not shown).
Fig. 2.
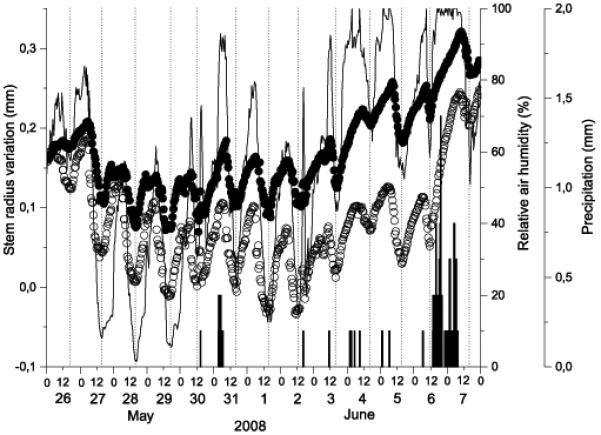
Diurnal cycles of stem radius variation during a dry period in 2008 compared to relative air humidity (solid line) and precipitation (bars). Study sites are denoted by open and filled circles for the xeric and dry-mesic site, respectively. Dotted lines mark daily minima in stem radius.
Fig. 3.
Correlations between daily radius change and climate parameters (relative air humidity, precipitation, air temperature) and soil water content at the dry-mesic (a, filled circles) and xeric site (b, open circles) from April through October 2007 and 2008 (n = 424). Pearson correlation coefficient (r) and Kendall’s tau coefficient (τ) were calculated. A one-day lag in stem radius change was considered in correlation with soil water content. *** p < 0.001; ** p < 0.01; * p < 0.05.
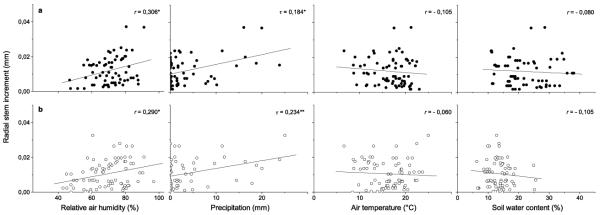
Relationships between stem radial increments extracted from DMR and environmental variables (climate variables and soil water content) at the study plots are depicted in Fig. 4. During the growing period 2007 and 2008 stem radial increments at the dry-mesic site were directly related to relative air humidity (r = 0.306, p < 0.05) and precipitation (Kendall τ = 0.184, p < 0.05). At the xeric site, the corresponding correlation coefficient for air humidity was 0.290 (p < 0.05) and Kendall’s τ coefficient for precipitation was 0.234 (p < 0.01). No statistically significant relationships were found between radial stem increments and air temperature and soil water content at either study plot (Fig. 4). Correlation coefficients decreased or showed only minor changes when time lags in growth response from one to three days were considered (data not shown).
Fig. 4.
Correlations between radial stem increment and climate parameters (relative air humidity, precipitation, air temperature) and soil water content at the dry-mesic (a, filled circles) and xeric site (b, open circles). Period of radial stem increase used in calculations ranged from early April to early July 2007 and end of April to early August 2008. Pearson correlation coefficient (r) and Kendall’s tau coefficient (τ) were calculated. Number of samples was 74 and 78 for the dry-mesic and xeric site, respectively. ** p < 0.0 1; * p < 0.05.
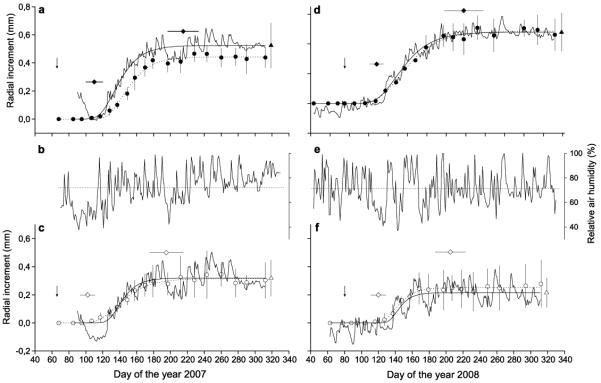
In Fig. 5 intra-annual radial growth in 2007 and 2008 is compared between continuous DMR (daily means) and XCD that allowed measurement of increase in ring width over 1-2 week periods. As a baseline for the comparison, dendrometer traces were set to zero at the day of the year, when first row of enlarging cells were detected (for details see Materials and Methods). Exceptionally mild spring temperatures in spring 2007 caused early bud break by the end of March (85 d), whereas in 2008 bud break in the upper crown occurred two weeks later (99 d). Mean onset of radial enlargement at the xeric site in 2007 and 2008 occurred on 12 and 30 April (102 and 120 d of the year, respectively). At the dry-mesic site start of radial cell enlargement was delayed in 2007 and 2008 by c. 1 week.
Fig. 5.
Comparison between mean radial increment determined by dendrometers (n = 3 trees/site) and measurement of the developing xylem (including enlarging, wall thickening and mature xylem cells; n = 5 trees/site) in 2007 (a, c) and 2008 (d, f). Mean standard deviation between dendrometer records are indicated by triangles. Study sites are denoted by closed and open circles for the dry-mesic and xeric site, respectively. Daily variation in relative air humidity in 2007 and 2008 are depicted in (b) and (e), respectively, and horizontal dotted lines indicate mean values. Radial increment curves were modelled by applying the Gompertz function for each site and year (for parameters see Table 3), whereby thin and dotted lines are models fitted for radius change and cell growth, respectively. SD for tree-ring width determined by XCD are indicated (n = 5 trees/site). Diamonds indicate day of the year ± SD, when one horizontal row of cells in the enlarging phase was detected at the start and end of the growing season. Arrows point to time of bud break.
Relative air humidity was closely related not only to daily radius change (cf. Fig. 2), but also to prolonged radial stem shrinkage during drought periods (Fig. 5). At the xeric site, changes in stem radius detected by DMR were more pronounced than at the dry-mesic site and amounted to c. 50 % of total annual increment. Records of intra-annual radial growth determined by DMR and XCD were quite similar except for the dry-mesic site in 2007 (Fig. 5), where the first row of enlarging tracheids was detected in mid-April when drought caused pronounced stem shrinkage.
In 2007 maximum daily radial growth determined by DMR and XCD peaked around 18th May (138 d of the yr) at both study plots. Compared to 2007, maximum growth in 2008 was delayed at the dry-mesic site by about 10 d (Table 3). Enlarging cells assumed to indicate radial stem growth were detected at the xeric site until 195 (14th July) and 205 d of the year (24th August) in 2007 and 2008, respectively, and until 215 (2nd August) and 218 d of the year (5th August), respectively, at the dry-mesic site (Fig. 5).
Table 3.
Parameters of the Gompertz function for intra-annual radial growth in 2007 and 2008 (see Fig. 5) based on dendrometer records (DMR) and analysis of xylem cell development (XCD) at study plots and R2 of the model (A = upper asymptote, Ip = inflection point, d yr = day of the year, κ = rate of change parameter, mean values ± SD)
| Year | Site | Method | A (μm) |
Ip (d yr) |
κ | R2 |
|---|---|---|---|---|---|---|
| 2007 | xeric | DMR | 320 ± 7 | 139 ± 1.7 | 0.079 ± 0.014 | 0.789 |
| XCD | 316 ± 8 | 136 ± 2.0 | 0.0477 ± 0.006 | 0.982 | ||
| dry-mesic | DMR | 525 ± 5 | 133 ± 0.9 | 0.059 ± 0.004 | 0.910 | |
| XCD | 425 ± 8 | 143 ± 1.4 | 0.0610 ± 0.007 | 0.989 | ||
| 2008 | xeric | DMR | 215 ± 6 | 142 ± 2.1 | 0.104 ± 0.028 | 0.737 |
| XCD | 251 ± 5 | 139 ± 1.3 | 0.0793 ± 0.010 | 0.989 | ||
| dry-mesic | DMR | 480 ± 4 | 148 ± 0.8 | 0.0512 ± 0.003 | 0.968 | |
| XCD | 486 ± 8 | 151 ± 1.2 | 0.0455 ± 0.004 | 0.994 |
Discussion
Growth and climate relationship
Timing of bud break and onset of cell differentiation processes differed by almost 3 weeks between 2007 and 2008 at both study plots, suggesting marked effects of early spring temperature on resumption of shoot growth, cambial activity and xylogenesis. Oribe et al. (2001) and Gričar et al. (2006) demonstrated that heating of stems of evergreen conifers induced reactivation of the cambium during the quiescent stage, which indicates that cambium activity is highly responsive to temperature. Influence of temperature on wood formation in early spring can also be deduced from earlier onset of cell differentiation processes during both growing seasons at the xeric compared to the dry-mesic site. Similarly, at several timberline sites, warm spring temperatures were found to result in earlier onset of cambial activity (Rossi et al. 2007; Deslauriers et al. 2008; Gruber et al. 2009). However, daily variations in stem radius reflect changes in stem hydration, which was reported to occur mainly in the elastic tissues outside the cambium (e.g., Dobbs and Scott 1971; Parlange et al. 1975; Zweifel et al. 2000), rather than changes in actual radial growth. Similar findings were observed by Mäkinen et al. (2003) and Gruber et al. (2009) in studies on seasonal changes in radial increment of Picea abies and Pinus cembra, respectively. Our finding that enlargement of tracheids in April 2007 occurred during an extensive drought period lasting from late March through early May 2007 indicates that at the start of the growing season, radial cell expansion was not hindered by stem water storage. On the other hand, during 2007 and 2008 tracheid enlargement stopped c. 2 - 3 weeks earlier at the drought-prone xeric site compared to the dry-mesic site, indicating that duration of radial growth is adversely affected by water deficits in summer (cf. Gruber et al. 2010). An early cessation of cambial activity and radial growth in conifers was also found in years when extraordinary hot and dry conditions prevailed during the growing season within the study area (Pichler and Oberhuber 2007) and elsewhere (e.g., Rigling et al. 2003; Levanič et al. 2009; Thabeet et al. 2009) .
Records of daily variations in stem radius revealed that stem re-hydration primarily occurred after stem radial minima were developed in the afternoon, when water supply was adequate, which agrees with findings of several authors (e.g., Hsiao and Acevedo 1974; Hinckley and Lassoie 1981; Zweifel et al. 2006). Lower amplitude of diurnal stem fluctuations and later occurrence of daily maximum and minimum values at the dry-mesic compared to the xeric site can be explained by more favourable soil water conditions at the former site. On the other hand, at the south-facing xeric site, where open canopy allows radiative heating of the soil surface, higher rates of evapotranspiration lead to more distinct diurnal stem fluctuations. Distinct reductions in stem diameter during an extensive drought period in spring 2007 also masked determination of onset of radial growth in DMR at both sites. Previously, we reported a close relationship between daily radial increment based on DMR and number of enlarging tracheids in cembran pine (Pinus cembra L.) throughout the treeline ecotone (Gruber et al. 2009), which allowed proper timing of radial growth onset in dendrometer traces. In this study we found a close agreement in intra-annual radial growth determined by DMR and XCD in majority of the cases by applying histological analysis of developing tracheids to define the baseline in DMR. Therefore, we suggest that onset of radial growth in DMR of coniferous trees, which show water-related swelling or shrinkage of the stem at the start of the growing season, can be detected in most cases by taking micro-cores in weekly intervals at the time of budburst and determining first enlarging tracheids.
Daily radial increments extracted from DMR during the growing period in 2007 and 2008 have been found to be significantly related to precipitation. This is consistent with previous ecophysiological and dendroclimatological studies conducted within the study area (e.g., Oberhuber et al. 1998) and at other dry inner Alpine environments (Rigling et al. 2002; Zweifel et al. 2006). Low correlation coefficients are explained by the overall minor annual increments within the study area and the use of water reserves in the stem for cell enlargement and hence growth, although no precipitation concurrently occurred. Missing relationships between radial increment and soil water content can be explained by the low water holding capacity of shallow, stony soils, i.e., a fast drainage of the rooting zone after rainfall. This is supported by recorded abrupt fluctuations in soil water content following precipitation events. Zweifel et al. (2006) also reported a decoupling between radial growth of Pinus sylvestris exposed to drought and actual soil water potential, whereby the authors suggested that precipitation resulted in some increase in leaf water potential, which led to an improvement in tree water status, i.e., an increase in cell turgor and enlargement of xylem cells. Breshears et al. (2008) observed that foliar absorption of intercepted rainfall led to substantial improvement in water status of Juniperus monosperma during drought. Several studies also revealed that wetting of leaves with fog and dew positively affected plant water balance without noticeably increasing soil wetness (e.g., Katz et al. 1989; Boucher et al. 1995; Burgess and Dawson 2004; Limm et al. 2009). Correspondingly, direct statistically significant relationships between relative air humidity and extracted radial increments at both study plots, might indicate improved tree water status due to a decrease in transpiration rates when vapour pressure deficits of the air were low.
Temporal dynamic of radial growth
At both study plots, culmination in radial growth in 2007 and 2008 was found in late spring (mid-May) prior to increase in precipitation and soil water content during summer. Zweifel et al. (2006) and Eilmann et al. (2009) suggested that cambial activity of drought-exposed Pinus sylvestris is directly controlled by water availability. A similar reasoning can be deduced from an extended growing period found at dry-mesic sites within the study area (Gruber et al. 2010). However, Zweifel et al. (2006) also reported that the main period of radial growth in Pinus sylvestris at dry sites covered about 30 – 70 % of productive days, which left a long time period of carbon assimilation when no radial growth occurred. We therefore suggest that extreme environmental conditions prevailing within the study area, i.e., low precipitation and recurring drought periods in spring combined with limited water holding capacity and nutrient deficiency of shallow, stony soils (cf. Krapfenbauer 1969), might cause elevated belowground carbohydrate demand to ensure adequate water and nutrient supply for shoot growth. An inherent high priority for within-tree carbon allocation to fine roots under low moisture conditions is supported in results of Brunner et al. (2009), who reported that for Pinus sylvestris dominating in a comparable dry inner-Alpine environment, increased irrigation could not further increase fine root biomass. Additionally, in ectomycorrhizal symbiosis, which is the dominating type of mycorrhizal symbiosis among trees including members of the genus Pinus and a way to overcome nutrient deficiency of the substrate, up to 1/3 of the photoassimilates are estimated to be necessary for mycorrhizal functioning (for a review see Nehls et al. 2007).
Hence, early achievement of maximum growth rate in spring can be regarded as an adaptation to cope with poor site conditions within the study area, which requires an early switch of carbon allocation to belowground organs including need to defend against biotic stress to sustain tree physiology (carbon assimilation, water and nutrient uptake) and resist attack by pathogens (insects, fungi) and semiparasitic mistletoe (Viscum album L. ssp. austriacum). To what extent radial growth of drought-exposed Pinus sylvestris is directly controlled by water availability or indirectly by belowground carbon demand needs to be analysed in future studies.
Conclusion
Results of our study provide insight on tree-ring development in Pinus sylvestris exposed to soil dryness. The detected culmination of radial growth rate in spring, prior to occurrence of more favourable growing conditions in summer deserves further investigation, e.g., by comparing intra-annual growth dynamics of shoots and roots (cf. Thibeault-Martel et al. 2008) and/or by determining carbon partitioning to above- and belowground organs throughout the year (cf. Li et al. 2008). Although drought periods in spring masked growth-induced radial expansion of the stem, determination of radial growth onset in dendrometer traces of extremely slow-growing Pinus sylvestris could be accomplished by histological analysis of wood formation.
Acknowledgements
This work was supported by the Austrian Science Fund (FWF Project No. P19563-B16 “Dynamics of cambial activity and wood formation of Scots pine (Pinus sylvestris L.) exposed to soil dryness”. Special thanks are to I. Swidrak for technical support in histological analysis of wood formation. We also thank the communicating editor and anonymous reviewers for their valuable suggestions and comments to improve the manuscript. Climate data were provided by Hydrographischer Dienst, Innsbruck, which is greatly acknowledged.
References
- Antonova GF, Stasava VV. Effects of environmental factors on wood formation in Scots pine stems. Trees. 1993;7:214–219. [Google Scholar]
- Bäucker E, Bues C, Vogel M. Radial growth dynamics of spruce (Picea abies) measured by micro-cores. IAWA J. 1998;3:301–309. [Google Scholar]
- Bigler C, Bräker OU, Bugmann H, Dobbertin M, Rigling A. Drought as an inciting mortality factor in Scots pine stands of the Valais, Switzerland. Ecosystems. 2006;9:330–343. [Google Scholar]
- Blasing TJ, Solomon AM, Duvick DN. Response functions revisited. Tree-Ring Bull. 1984;44:1–15. [Google Scholar]
- Boucher JF, Munson AD, Bernier PY. Foliar absorption of dew influences shoot water potential and root-growth in Pinus strobus seedlings. Tree Physiol. 1995;15:819–823. [Google Scholar]
- Bouriaud O, Leban J-M, Bert D, Deleuze C. Intra-annual variations in climate influence growth and wood density of Norway spruce. Tree Physiol. 2005;25:651–660. doi: 10.1093/treephys/25.6.651. [DOI] [PubMed] [Google Scholar]
- Breshears DD, McDowell NG, Goddard KL, Dayem KE, Martens SN, Meyer CW, Brown KM. Foliar absorption of intercepted rainfall improves woody plant water status most during drought. Ecology. 2008;89(1):41–47. doi: 10.1890/07-0437.1. [DOI] [PubMed] [Google Scholar]
- Brunner I, Pannatier EG, Frey B, Rigling A, Landolt W, Zimmermann S, Dobbertin M. Morphological and physiological responses of Scots pine fine roots to water supply in a dry climatic region in Switzerland. Tree Physiol. 2009;29:541–550. doi: 10.1093/treephys/tpn046. [DOI] [PubMed] [Google Scholar]
- Burgess SSO, Dawson TE. The contribution of fog to the water relations of Sequoia sempervirens (D. Don): foliar uptake and prevention of dehydration. Plant Cell Environ. 2004;27:1023–1034. [Google Scholar]
- Camarero JJ, Guerrero-Campo J, Gutiérrez E. Tree-ring growth and structure of Pinus uncinata and Pinus sylvestris in the Central Spanish Pyrenees. Arct Alp Res. 1998;30(1):1–10. [Google Scholar]
- Carrer M, Anfodillo T, Urbinati C, Carraro V. High-altitude forest sensitivity to global warming: results from long-term and short-term analyses in the Eastern Italian Alps. In: Beninston M, Innes JL, editors. The Impacts of Climate Variability on Forests. Springer; Berlin: 1998. pp. 171–189. [Google Scholar]
- Cook ER, Kairiukstis LA. Methods of Dendrochronology. Applications in the Environmental Sciences. Kluwer; Dordrecht: 1990. [Google Scholar]
- Creber GT, Chaloner WO. Influence of environmental factors on the wood structure of living and fossil trees. Bot Rev. 1984;50:357–448. [Google Scholar]
- Daudet FA, Ameglio T, Cochard H, Archilla O, Lacointe A. Experimental analysis of the role of water and carbon in tree stem diameter variations. J Exp Bot. 2005;56:135–144. doi: 10.1093/jxb/eri026. [DOI] [PubMed] [Google Scholar]
- Deslauriers A, Morin H, Begin Y. Cellular phenology of annual ring formation of Abies balsamea in the Quebec boreal forest (Canada) Can J For Res. 2003a;33:190–200. [Google Scholar]
- Deslauriers A, Morin H, Urbinati C, Carrer M. Daily weather response of balsam fir (Abies balsamea (L.) Mill.) stem radius increment from dendrometer analysis in the boreal forests of Québec (Canada) Trees. 2003b;17:477–484. [Google Scholar]
- Deslauriers A, Morin H. Intra-annual tracheid production in balsam fir stems and the effect of meteorological variables. Trees. 2005;19:402–408. [Google Scholar]
- Deslauriers A, Rossi S, Anfodillo T. Dendrometer and intra-annual tree growth: What kind of information can be inferred? Dendrochronologia. 2007;25:113–124. [Google Scholar]
- Deslauriers A, Rossi S, Anfodillo T, Saracino A. Cambial phenology, wood formation and temperature thresholds in two contrasting years at high altitude in southern Italy. Tree Physiol. 2008;28:863–871. doi: 10.1093/treephys/28.6.863. [DOI] [PubMed] [Google Scholar]
- Dobbs RC, Scott DRM. Distribution of diurnal fluctuations in stem circumference of Douglas-fir. Can J For Res. 1971;1:80–83. [Google Scholar]
- Downes G, Beadle C, Worledge D. Daily stem growth patterns in irrigated Eucalyptus globulus and E. nitens in relation to climate. Trees. 1999;14:102–111. [Google Scholar]
- Eilmann B, Zweifel R, Buchmann N, Fonti P, Rigling A. Drought-induced adaptation of the xylem in Scots pine and pubescent oak. Tree Physiol. 2009;29:1011–1020. doi: 10.1093/treephys/tpp035. [DOI] [PubMed] [Google Scholar]
- Ellenberg H. Vegetation Ecology of Central Europe. Cambridge University Press; Cambridge: 1988. [Google Scholar]
- FAO . World reference base for soil resources. FAO; Rome: 1998. [Google Scholar]
- Fritts HC. An evaluation of three techniques for measuring radial tree growth. Bull Ecol Soc Am. 1961;42:54–55. [Google Scholar]
- Fritts HC. Tree Rings and Climate. Academic Press; London: 1976. [Google Scholar]
- Gartner BL, Aloni R, Funada R, Lichtfuss-Gautier AN, Roig FA. Clues for dendrochronology from studies of wood structure and function. Dendrochronologia. 2002;20(1-2):53–61. [Google Scholar]
- Gričar J, Zupančič M, Čufar K, Primož O. Regular cambial activity and xylem and phloem formation in locally heated and cooled stem portions of Norway spruce. Wood Sci Techn. 2006;41(6):463–475. [Google Scholar]
- Gruber A, Zimmermann J, Wieser G, Oberhuber W. Effects of climate variables on intra-annual stem radial increment in Pinus cembra (L.) along the alpine treeline ecotone. Ann For Sci. 2009;66:503. doi: 10.1051/forest/2009038. doi: 10.1051/forest/2009038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruber A, Strobl S, Veit B, Oberhuber W. Impact of drought on the temporal dynamics of wood formation in Pinus sylvestris. Tree Physiol. 2010 doi: 10.1093/treephys/tpq003. doi: 10.1093/treephys/tpq003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsiao TC, Acevedo E. Plant responses to water deficits, water-use efficiency, and drought resistance. Agric. Meteorol. 1974;14:9–84. [Google Scholar]
- Herzog KM, Häsler R, Thum R. Diurnal changes in the radius of a subalpine Norway spruce stem: their relation to the sap flow and their use to estimate transpiration. Trees. 1995;10:94–101. [Google Scholar]
- Hinckley TM, Lassoie JP. Radial growth in conifers and deciduous trees: a comparison. Mitt forstl Bundesvers Wien. 1981;142:17–56. [Google Scholar]
- Hughes MK. Dendrochronology in climatology – the state of the art. Dendrochronologia. 2002;20(1-2):95–116. [Google Scholar]
- Jolly WM, Dobbertin M, Zimmermann NE, Reichstein M. Divergent vegetation growth response to the 2003 heat wave in the Swiss Alps. Geophys Res Lett. 2005;32:L18409. doi:10.1029/2005GL023252. [Google Scholar]
- Katz C, Oren R, Schulze E-D, Milburn JA. Uptake of water and solutes through twigs of Picea abies (L.) Karst. Trees. 1989;3:33–37. [Google Scholar]
- Kienast F, Schweingruber FH, Bräker OU, Schär E. Tree-ring studies on conifers along ecological gradients and the potential of single-year analyses. Can J For Res. 1987;17:683–696. [Google Scholar]
- Kozlowski TT, Winget CH. Diurnal and seasonal variations in radii of tree stems. Ecology. 1964;45:149–155. [Google Scholar]
- Krapfenbauer A. Böden auf Dolomit und Serpentin in ihrer Auswirkung auf die Waldernährung. Cbl Ges Forstw. 1969;86:89–219. [Google Scholar]
- Levanič T, Gričar J, Gagen M, Jalkanen R, Loader NJ, McCarroll D, Oven P, Robertson I. The climatic sensitivity of Norway spruce [Picea abies (L.) Karst.] in the southeastern European Alps. Trees. 2009;23:169–180. [Google Scholar]
- Li M-H, Xiao W-F, Wang S-G, Cheng G-W, Cherubini P, Cai X-H, Liu X-L, Wang X-D, Zhu W-D. Mobile carbohydrates in Himalayan treeline trees I. Evidence for carbon gain limitation but not for growth limitation. Tree Physiol. 2008;28:1287–1296. doi: 10.1093/treephys/28.8.1287. [DOI] [PubMed] [Google Scholar]
- Limm EB, Simonin KA, Bothman AG, Dawson TE. Foliar water uptake: a common water acquisition strategy for plants of the redwood forest. Oecologia. 2009;161:449–459. doi: 10.1007/s00442-009-1400-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loris K. Dickenwachstum von Zirbe, Fichte und Lärche an der alpinen Waldgrenze/Patscherkofel. Mitt Forstl Bundesvers Wien. 1981;142:417–441. [Google Scholar]
- Mäkinen H, Nöjd P, Saranpää P. Seasonal changes in stem radius and production of new tracheids in Norway spruce. Tree Physiol. 2003;23:959–968. doi: 10.1093/treephys/23.14.959. [DOI] [PubMed] [Google Scholar]
- Mäkinen H, Seo J-W, Nöjd P, Schmitt U, Jalkanen R. Seasonal dynamics of wood formation: a comparison between pinning, microcoring and dendrometer measurements. Eur J For Res. 2008;127:235–245. [Google Scholar]
- Martín-Benito D, Cherubini P, Río M, Canellas I. Growth response to climate and drought in Pinus nigra Arn. trees of different crown classes. Trees. 2008;22:363–373. [Google Scholar]
- Nehls U, Grunze N, Willmann M, Reich M, Küster H. Sugar for my honey: Carbohydrate partitioning in ectomycorrhizal symbiosis. Phytochemistry. 2007;68:82–91. doi: 10.1016/j.phytochem.2006.09.024. [DOI] [PubMed] [Google Scholar]
- Oberhuber W, Stumböck M, Kofler W. Climate-tree-growth relationships of Scots pine stands (Pinus sylvestris L.) exposed to soil dryness. Trees. 1998;13:19–27. [Google Scholar]
- Oberhuber W, Kofler W. Topographic influences on radial growth of Scots pine (Pinus sylvestris L.) at small spatial scales. Plant Ecol. 2000;146:229–238. [Google Scholar]
- Oberhuber W. The role of climate in the mortality of Scots pine (Pinus sylvestris L.) exposed to soil dryness. Dendrochronologia. 2001;19(1):45–55. [Google Scholar]
- Oribe Y, Funada R, Shibagaki M, Kubo T. Cambial reactivation in locally heated stems of evergreen conifer Abies sachalinensis (Schmidt) Masters. Planta. 2001;212:684–691. doi: 10.1007/s004250000430. [DOI] [PubMed] [Google Scholar]
- Orwig DA, Abrams MD. Variation in radial growth responses to drought among species, site, and canopy strata. Trees. 1997;11:474–484. [Google Scholar]
- Parlange JY, Turner NC, Waggoner PE. Water uptake, diameter change, and nonlinear diffusion in tree stems. Plant Physiol. 1975;55:247–250. doi: 10.1104/pp.55.2.247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pichler P, Oberhuber W. Radial growth response of coniferous forest trees in an inner Alpine environment to heat-wave in 2003. For Ecol Manage. 2007;242:688–699. [Google Scholar]
- Plomion C, Leprovost G, Stokes A. Wood formation in trees. Plant Physiol. 2001;127:1513–1523. [PMC free article] [PubMed] [Google Scholar]
- Rebetez M, Dobbertin M. Climate change may already threaten Scots pine stands in the Swiss Alps. Theor Appl Clim. 2004;79:1–9. [Google Scholar]
- Rigling A, Bräker OU, Schneiter G, Schweingruber FH. Intra-annual tree-ring parameters indicating differences in drought stress of Pinus sylvestris forests within the Erico-Pinion in the Valais (Switzerland) Plant Ecol. 2002;163:105–121. [Google Scholar]
- Rigling A, Brühlhart H, Bräker OU, Forster T, Schweingruber FH. Effects of irrigation on diameter growth and vertical resin duct production in Pinus sylvestris L. on dry sites in the central Alps, Switzerland. For Ecol Manage. 2003;175:285–296. [Google Scholar]
- Rossi S, Deslauriers A, Morin H. Application of the Gompertz equation for the study of xylem cell development. Dendrochronologia. 2003;21:33–39. [Google Scholar]
- Rossi S, Anfodillo T, Menardi R. Trephor: a new tool for sampling microcores from tree stems. IAWA J. 2006a;27:89–97. [Google Scholar]
- Rossi S, Deslauriers A, Anfodillo T. Assessment of cambial activity and xylogenesis by microsampling tree species: an example at the Alpine timberline. IAWA J. 2006b;27:383–394. [Google Scholar]
- Rossi S, Deslauriers A, Anfodillo T, Morin H, Saracino A, Motta R, Borghetti M. Conifers in cold environments synchronize maximum growth rate of tree-ring formation with day length. New Phytol. 2006c;170(2):301–310. doi: 10.1111/j.1469-8137.2006.01660.x. [DOI] [PubMed] [Google Scholar]
- Rossi S, Deslauriers A, Anfodillo T, Carraro V. Evidence of threshold temperatures for xylogenesis in conifers at high altitudes. Oecologia. 2007;152(1):1–12. doi: 10.1007/s00442-006-0625-7. [DOI] [PubMed] [Google Scholar]
- Sheskin D. Handbook of parametric and nonparametric statistical procedures. 4th ed. Chapman & Hall CRC; Boca Raton, FL: 2007. [Google Scholar]
- Steppe K, De Pauw DJW, Lemeur R, Vanrolleghem PA. A mathematical model linking tree sap flow dynamics to daily stem diameter fluctuations and radial stem growth. Tree Physiol. 2006;26:257–273. doi: 10.1093/treephys/26.3.257. [DOI] [PubMed] [Google Scholar]
- Tardif J, Flannigan M, Bergeron Y. An analysis of the daily radial activity of 7 boreal tree species, North-western Québec. Environ Monit Assess. 2001;67:141–160. doi: 10.1023/a:1006430422061. [DOI] [PubMed] [Google Scholar]
- Thabeet A, Vennetier M, Gadbin-Henry C, Denelle N, Roux M, Caraglio Y, Vila B. Response of Pinus sylvestris L. to recent climatic events in the French Mediterranean region. Trees. 2009;23:843–853. [Google Scholar]
- Thibeault-Martel M, Krause C, Morin H, Rossi S. Cambial activity and intra-annual xylem formation in roots and stems of Abies balsamea and Picea mariana. Ann Bot. 2008;102(5):667–674. doi: 10.1093/aob/mcn146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaganov EA, Hughes MK, Shashkin AV. Growth Dynamics of Conifer Tree Rings. Images of Past and Future Environments. Springer; Berlin: 2006. (Ecol. Studies 183). [Google Scholar]
- Waldboth M, Oberhuber W. Synergistic effect of drought and chestnut blight (Cryphonectria parasitica) on growth decline of European chestnut (Castanea sativa) For Pathol. 2009;39:43–55. [Google Scholar]
- Zeide B. Analysis of growth equations. For Sci. 1993;39:594–616. [Google Scholar]
- Zweifel R, Item H, Häsler R. Stem radius changes and their relation to stored water in stems of young Norway spruce trees. Trees. 2000;15:50–57. [Google Scholar]
- Zweifel R, Häsler R. Dynamics of water storage in mature subalpine Picea abies: temporal and spatial patterns of change in stem radius. Tree Physiol. 2001;21:561–569. doi: 10.1093/treephys/21.9.561. [DOI] [PubMed] [Google Scholar]
- Zweifel R, Zimmermann L, Zeugin F, Newberry DM. Intra-annual radial growth and water relations of trees: implications towards a growth mechanism. J Exp Bot. 2006;57(6):1445–1459. doi: 10.1093/jxb/erj125. [DOI] [PubMed] [Google Scholar]