Abstract
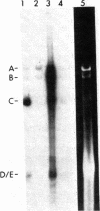
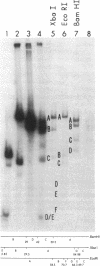
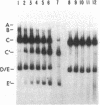
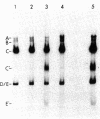
Adenovirus (Ad) type 2 DNA synthesis can be initiated in the presence of a soluble extract of uninfected HeLa cell nuclei, a 25-60% saturated ammonium sulfate fraction of infected cytoplasm and viral DNA covalently linked to a 5′-terminal protein (Ad DNA-prot). As the purification, from either the nuclei or cytoplasm, of factors active in DNA replication proceeded, various nonreplicative reactions which incorporate labeled deoxynucleotides were uncovered. In order to distinguish replicative from repair reactions, an assay was developed in which the Ad DNA-prot was digested with Xba I, all of the fragments so produced were used (without separation) in a replication reaction, and the products were assayed by electrophoresis on neutral agarose gels. In replicative reactions, most of the radioactivity was incorporated into the terminal fragments, with the internal fragments remaining unlabeled. Infected cytoplasm contains a “discrimination” function in addition to specific factors for Ad DNA replication. The discrimination factors inhibit the nonspecific nucleotide incorporation by uninfected HeLa nuclear extracts on Ad DNA-prot. The specific replicative incorporation into the terminal Ad DNA-prot fragments has also allowed an independent assay for reinitiation of progeny molecules synthesized in vitro. After the first round of replication, the 5′ strand of the progeny duplex from each end is labeled. These same labeled strands will be displaced during the second round of replication and appear in new bands which have been shown to be the single-strand equivalents of the terminal fragments. Thus, at least two rounds of Ad DNA synthesis can initiate at each terminus in vitro. The appearance of displaced single strands requires DNA replication because the addition of dideoxycytidine triphosphate after the first round of synthesis prevents the displacement reaction. Both the progeny single- and double-stranded DNA appear to be linked to protein.
Keywords: eukaryotic DNA replication, soluble nuclear extracts, soluble cytoplasmic extracts, DNA-protein interaction
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brison O., Kedinger C., Wilhelm J. Enzymatic properties of viral replication complexes isolated from adenovirus type 2-infected HeLa cell nuclei. J Virol. 1977 Nov;24(2):423–435. doi: 10.1128/jvi.24.2.423-435.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Challberg M. D., Kelly T. J., Jr Adenovirus DNA replication in vitro. Proc Natl Acad Sci U S A. 1979 Feb;76(2):655–659. doi: 10.1073/pnas.76.2.655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horwitz M. S. Bidirectional replication of adenovirus type 2 DNA. J Virol. 1976 Apr;18(1):307–315. doi: 10.1128/jvi.18.1.307-315.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horwitz M. S., Kaplan L. M., Abboud M., Maritato J., Chow L. T., Broker T. R. Adenovirus DNA replication in soluble extracts of infected cell nuclei. Cold Spring Harb Symp Quant Biol. 1979;43(Pt 2):769–780. doi: 10.1101/sqb.1979.043.01.084. [DOI] [PubMed] [Google Scholar]
- Ikeda J. E., Longiaru M., Horwitz M. S., Hurwitz J. Elongation of primed DNA templates by eukaryotic DNA polymerases. Proc Natl Acad Sci U S A. 1980 Oct;77(10):5827–5831. doi: 10.1073/pnas.77.10.5827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan L. M., Ariga H., Hurwitz J., Horwitz M. S. Complementation of the temperature-sensitive defect in H5ts125 adenovirus DNA replication in vitro. Proc Natl Acad Sci U S A. 1979 Nov;76(11):5534–5538. doi: 10.1073/pnas.76.11.5534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan L. M., Kelinman R. E., Horwitz M. S. Replication of adenovirus type 2 DNA in vitro. Proc Natl Acad Sci U S A. 1977 Oct;74(10):4425–4429. doi: 10.1073/pnas.74.10.4425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lechner R. L., Kelly T. J., Jr The structure of replicating adenovirus 2 DNA molecules. Cell. 1977 Dec;12(4):1007–1020. doi: 10.1016/0092-8674(77)90165-9. [DOI] [PubMed] [Google Scholar]
- Maizel J. V., Jr, White D. O., Scharff M. D. The polypeptides of adenovirus. I. Evidence for multiple protein components in the virion and a comparison of types 2, 7A, and 12. Virology. 1968 Sep;36(1):115–125. doi: 10.1016/0042-6822(68)90121-9. [DOI] [PubMed] [Google Scholar]
- Mulder C., Delius H. Specificity of the break produced by restricting endonuclease R 1 in Simian virus 40 DNA, as revealed by partial denaturation mapping. Proc Natl Acad Sci U S A. 1972 Nov;69(11):3215–3219. doi: 10.1073/pnas.69.11.3215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharp P. A., Moore C., Haverty J. L. The infectivity of adenovirus 5 DNA-protein complex. Virology. 1976 Dec;75(2):442–456. doi: 10.1016/0042-6822(76)90042-8. [DOI] [PubMed] [Google Scholar]
- Stillman B. W., Bellett A. J. Replication of DNA in adenovirus-infected cells. Cold Spring Harb Symp Quant Biol. 1979;43(Pt 2):729–739. doi: 10.1101/sqb.1979.043.01.081. [DOI] [PubMed] [Google Scholar]
- Weingärtner B., Winnacker E. L., Tolun A., Pettersson U. Two complementary strand-specific termination sites for adenovirus DNA replication. Cell. 1976 Oct;9(2):259–268. doi: 10.1016/0092-8674(76)90117-3. [DOI] [PubMed] [Google Scholar]
- Winnacker E. L. Adenovirus DNA: structure and function of a novel replicon. Cell. 1978 Aug;14(4):761–773. doi: 10.1016/0092-8674(78)90332-x. [DOI] [PubMed] [Google Scholar]
- Yamashita T., Arens M., Green M. Adenovirus deoxyribonucleic acid replication. Isolation of a soluble replication system and analysis of the in vitro DNA product. J Biol Chem. 1977 Nov 25;252(22):7940–7946. [PubMed] [Google Scholar]