Abstract
In the present study, we used a comprehensive panel of in vitro assays to evaluate the efficacy and safety of stilbazulenyl nitrone (STAZN) as a lead compound to treat acute ischemic stroke. First, we measured neuroprotection in vitro using two different HT22 hippocampal nerve cell assays. Secondly, to de-risk drug development, we used CeeTox analysis with the H4IIE rat hepatoma cell line to determine the acute toxicity profile of STAZN. Third, STAZN was tested in microsomes from four species for measures of metabolic stability. Last, we determined the Ames test genotoxicity profile of STAZN using Salmonella typhimurium TA989 and TA100. In vitro, STAZN was neuroprotective against toxicity induced by iodoacetic acid, and oxytosis-induced glutathione depletion was initiated by glutamate, with an EC50 value of 1–5 μM. Secondly, using CeeTox analysis, the estimated CTox value (i.e., sustained concentration expected to produce toxicity in a rat 14-day repeat dose study) for STAZN was calculated to be 260 μM. Third, the half-life of STAZN in humans, dogs, and rats was 60–78 min. Last, the genotoxicity profile showed that STAZN did not induce bacterial colony growth under any conditions tested, indicating the lack of mutagenicity with this compound. STAZN appears to be a multi-target neuroprotective compound that has an excellent safety profile in both the CeeTox and Ames mutagenicity assays. STAZN may have significant potential as a novel neuroprotective agent to treat stroke and should be pursued in clinically relevant embolic stroke models.
Keywords: Antioxidant, Nitrone, Safety, Efficacy, Genotoxicity, CeeTox
Introduction
There is a critical medical need for new therapeutics to halt the debilitating effects of acute ischemic stroke (AIS), the third leading cause of mortality and leading cause of adult morbidity in the USA [1]. Annually, approximately 0.8 million victims suffer a stroke in the USA [2] and 15 million worldwide [2]. In over 60% of stroke patients, vascular “clot” deposition or focal cerebral ischemia is responsible for the interruption of cerebral blood flow (CBF) and triggering of a cascade of deleterious events including free radical formation (ROS) and blood–brain barrier (BBB) injury [3]. Reduced CBF and severe oxygen deficiency lead to ischemia and eventually to behavioral and functional deficits, morbidity, and mortality [3].
Recent mechanistic hypotheses continue to implicate free radicals in neuronal, glial, and vascular damage following an ischemic stroke [3]. Because diverse experimental evidence implicates oxidative stress in stroke-induced damage [4–7], it has been suggested that methods to attenuate or block oxidative stress should be pursued as a target to treat stroke [3]. However, several stroke clinical trials have evaluated neuroprotective agents which target free radicals [6, 8–11], but the results are mixed [4, 7, 11, 12]. Recently, the first-generation hydrophilic nitrone spin trap agent NXY-059 failed in a well-designed clinical trial [13]. After much debate, it is now well accepted that NXY-059, which is water soluble and does not readily cross the BBB, was an inferior compound to develop to treat stroke, and that much of the preclinical rodent and marmoset study data overestimated the efficacy of the drug [4, 7, 12, 14, 15]. Prior to the development of NXY-059, Tirilazad, a 21-aminosteroid that inhibits lipid peroxidation, was shown not to improve outcome in stroke patients [11] and also causes injection site toxicity (phlebitis). However, in contrast to the negative results with NXY-059 and Tirilazad, there have been several reports of beneficial effects and moderate success in AIS patients using the hydrophobic multi-target free radical scavenger, Edaravone [6, 16], when administered up to 24 h after a stroke, and Edaravone is approved in Japan for AIS [6]. They key, as proposed by Moskowitz et al. [3], may be the need to develop pleiotropic or multi-target antioxidants. Preclinical and clinical data with Edaravone [6] should be considered as important proof-of-concept data supporting an antioxidant strategy as a useful strategy to treat stroke as long as the drugs have multiple activities.
Stilbazulenyl nitrone (STAZN) is a second-generation nitrone-based antioxidant that has been studied preclinically in a rodent stroke model with great success [17]. There are numerous positive attributes to STAZN that make this compound a good, if not optimal, choice of drug in this class of nitrone-based drugs for further testing and evaluation. First, STAZN has a low oxidation potential and is 300 times more potent than NXY-059 at inhibiting free radical-induced peroxidation [18]. Second, as shown by Ginsberg and colleagues, in a primary screen in a rat intraluminal suture stroke model, STAZN reduced infarct volume in a dose- and time-dependent manner [17, 19]. STAZN neuroprotection is conferred with doses 300–600 times lower than NXY-059 [17, 20]. Third, STAZN readily crosses the BBB in normal rats following peripheral injection and has a long circulating half-life (t1/2) with a biexponential decline in blood levels (t1/2 of 28 min and 7 h)[19]. Fourth, and most important for effective clinical translation, in two different studies, Ginsberg and Belayev have shown STAZN improved behavior measured up to 30 days following ischemia [17, 19]. Thus, STAZN appears to be superior to NXY-059 in many ways and has some pharmacological activities similar to that of Edaravone, which receives extensive clinical use in Japan [6].
However, at this point in translational drug development for stroke, there is a need not only to test drugs in multiple animal models of stroke[12, 21, 22] but also to evaluate promising drug candidates for acute toxicity, metabolic stability, and genotoxicity prior to further development in multiple species. This strategy will have a substantial impact on reducing the cost of expensive and time-consuming in vivo evaluation. Based upon the limited amount of published information available on STAZN, it appears that STAZN may be a good choice of compound in this drug class (i.e., nitrone antioxidant) for further analysis and development. Thus, we used a series of in vitro analysis assays to determine the neuroprotection, toxicity, stability, and mutagenicity profiles of STAZN.
Materials and Methods
Figure 1 presents the chemical structure of STAZN, which has a CLogP value of 11.0. CLogP is the estimated log octanol/water partition coefficient. A higher value indicates increased lipophilicity and improved BBB penetration.
Fig. 1.
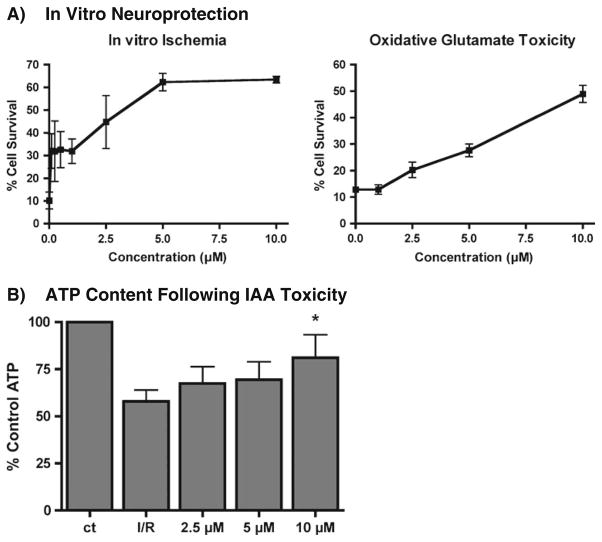
a Dose–response profile for STAZN neuroprotection in HT22 hippocampal cells: The data are presented as mean ± SD. STAZN was neuroprotective when applied using either the IAA or oxidative glutamate toxicity assays. EC50 approximately 1–5 μM. b Effect of STAZN on HT22 cell ATP content using the in vitro ischemia assay. STAZN maintains ATP in response to in vitro ischemia. HT22 cells were treated with 20 μM IAA alone or with the indicated doses of STAZN for 2 h followed by 2 h in fresh medium alone (IAA) or in the presence of STAZN. Total ATP levels were measured by a chemiluminescent assay (n=3–5 experiments; p<0.05 compared to I/R (IAA/change of medium)

Experimental Protocol: Drug Synthesis and Preparation for Assay: Since STAZN was first described by Becker et al. in [18] in 2002, it has not become commercially available. Therefore, we accessed the scientific [18] and patent literature [23] in order to develop a synthetic scheme so that STAZN could be synthesized for characterization using multiple in vitro analyses prior to extensive in vivo development. STAZN was synthesized and purified to >97.5% purity by Combi-Blocks (San Diego, CA). NMR analysis in CDCL3 confirmed the structure of STAZN, and high performance liquid chromatography (HPLC) analysis using a methanol–water gradient was used to determine the purity of the compound.
-
In Vitro Neuroprotection Analysis and ATP Measurement: Fetal calf serum (FCS) and dialyzed FCS (DFCS) were from Hyclone (Logan, UT). Dulbecco’s Modified Eagle’s Medium (DMEM) was purchased from Invitrogen (Carlsbad, CA). HT22 cells [24, 25] were grown in DMEM supplemented with 10% FCS.
In Vitro Ischemia Assay: The in vitro ischemia assay was performed using HT22 hippocampal neurons as described previously [26, 27]. Briefly, cells were seeded onto 96-well microtiter plates at a density of 5×103 cells per well. The next day, the medium was replaced with DMEM supplemented with 7.5% DFCS, and the cells were treated with 20 μM iodoacetic acid (IAA) alone or in the presence of STAZN. After 2 h, the medium in each well was aspirated and replaced with fresh medium without IAA but containing STAZN. Twenty hours later, the medium in each well was aspirated and replaced with fresh medium containing 5 μg/ml 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT). After 4 h of incubation at 37°C, cells were solubilized with 100 μl of a solution containing 50% dimethylformamide and 20% SDS (pH 4.7). The absorbance at 570 nm was measured on the following day. Total intracellular ATP was determined by a chemiluminescent assay as described previously [26, 27].Oxidative Glutamate Toxicity Assay: As described previously [26], HT22 cells were treated with the excitotoxic amino acid glutamate (5 mM), which induces cell death mediated by the depletion of intracellular glutathione (GSH) via inhibition of the cystine/glutamate antiporter [28, 29]. Thirty minutes after STAZN addition, 5 mM glutamate was added to initiate the cell death cascade, and 24 h later, cell survival was measured [26]. The in vitro cell death assays and the biochemical assays were repeated at least three times in triplicate each time and analyzed using Instat software. The data are presented as the mean ± SD. Statistical analysis was done by ANOVA followed by Bonferroni’s test. P<0.05 was considered statistically significant. CeeTox Analysis: The CeeTox assay was done in a blinded manner by CeeTox Inc. (Kalamazoo, MI, Study no. 9066-100035) according to methodology described previously [30, 31]. Since STAZN is a highly lipophilic compound, dimethylsulfoxide (DMSO) was used to prepare a 20-mM stock solution. All experiments that used DMSO as the drug solvent also included a DMSO negative control [31]. A CTox value was generated by CeeTox Inc. using a patented proprietary algorithm [32].
Metabolic Stability: The in vitro metabolic stability of STAZN (1 μM) in human and animal liver microsomes (0.5 mg protein/ml) was estimated by liquid chromatography tandem mass spectrometry (LC-MS/MS) by Absorption Systems LP (Exton, PA, Study no. 10CEDAP1). HPLC was run using solvent A (0.1% acetic acid in water) and solvent B (0.1%AcOH in MeOH/MeCN) 1:1 using a Luna C18 2.5 μm 30×2 mm column at a flow rate of 0.4–0.5 ml/min.
Genotoxicity Analysis: The genotoxicity studies were done in a blinded manner by Apredica Inc. (Watertown, MA, Study PALCSMC1111) using two strains of histidine incompetent cells (+/−) S9 liver fraction in order to measure the effects of metabolic activation of STAZN on genotoxicity. Thus, either Salmonella typhimurium TA989: hisD3052, rfa, uvrB/pKM101, which detects frameshift mutations or S. typhimurium TA100: hisG45, rfa, uvrB/pKM101, which detects base pair substitutions, was incubated in the presence of STAZN, with colony appearance on histidine-negative medium indicating point mutation reversal as originally described by Ames [33–35] and subsequently by [36, 37].
Briefly, approximately ten million bacteria are exposed in triplicate to test agent (six concentrations between 0.001953 and 0.0625 mg/ml), a negative control (DMSO) and a positive control (2-aminoanthracene [38–41]) for 90 min in a medium containing a low concentration of histidine (sufficient for about two doublings.) The cultures are then diluted into indicator medium lacking histidine, and dispensed into a 48-well plate. The plate is incubated for 48 h, and cells that have undergone a reversion will grow in a well, resulting in a color change in wells with growth. The number of wells showing growth is counted and compared to the vehicle control. An increase in the number of colonies of at least twofold over baseline (mean + SD of the vehicle control) indicates a positive response. An unpaired, one-sided Student’s t test is used to identify conditions that are significantly different from the vehicle control. When S9 fraction is included in the experiment, S9 fraction from the livers of Aroclor 1254-treated rats [42, 43] is included in the incubation at a final concentration of 4.5%. An NADPH-regenerating system is included as well to ensure a steady supply of reducing equivalents.
Results
The goal of this in vitro analysis study was to systematically determine the biological activity, toxicity profile, and genotoxicity profile of STAZN. The purpose of the studies using HT22 cells was twofold. First, to ensure that the compound that we had synthesized and chemically confirmed by NMR and HPLC analyses had biological activity, we used an in vitro model system that we routinely use for drug development of neuroprotective compounds [26, 27, 29, 44–46]. Second, we wanted to determine if the drug was effective against two different toxic insults and obtain a dose–response profile.
-
In Vitro Neuroprotection: For the in vitro ischemia model, HT22 cells were treated with IAA iodoacetic acid (IAA), an irreversible inhibitor of glyceraldehyde 3-phosphate dehydrogenase (G3PDH). G3PDH is an enzyme of the glycolytic pathway, which catalyzes the synthesis of 1,3-bisphosphoglycerate, a “high energy” intermediate used for the synthesis of ATP [26, 27, 44]. We also determined the effects of STAZN on HT22 cell survival treated with the excitotoxic amino acid glutamate, which in this cell line, due to the absence of high affinity NMDA receptors, induces cell death mediated by the depletion of intracellular glutathione (GSH) via inhibition of the cystine/glutamate antiporter [28, 29]. This model is referred to as the oxidative glutamate toxicity or oxytosis assay because of cell death initiated by GSH depletion.
As shown in Fig. 1a, STAZN effectively attenuated HT22 cell death mediated by either IAA (left panel) or glutamate (right panel). The beneficial effect of STAZN in the IAA assay was significant at concentrations between 1 and 10 μM, with an estimated EC50 of 1 μM, whereas in the oxidative glutamate toxicity assay, significance was not achieved until 5 μM. Thus, the STAZN that was synthesized for this study was neuroprotective in vitro and was able to significantly attenuate toxicity related to either IAA or glutamate.
Previously, it has been shown that select pharmacological compounds that protect from in vitro ischemia do so at least in part by preventing the loss of [26, 27]. In this study, we determined whether the neuroprotective effect of STAZN against IAA toxicity correlated with a change in cellular ATP levels. As shown in Fig. 1b, STAZN did significantly attenuate IAA-induced ATP loss at the highest dose tested. However, even with 10 μM STAZN, there was not complete neuroprotection against cell death nor was there complete prevention of ATP loss.
-
CeeTox Analysis: STAZN was soluble up to and including 100 μM in the culture medium system, but was not completely soluble at 300 μM. The solubility profile will later be discussed in reference to the cell toxicity results in Fig. 2a–c.
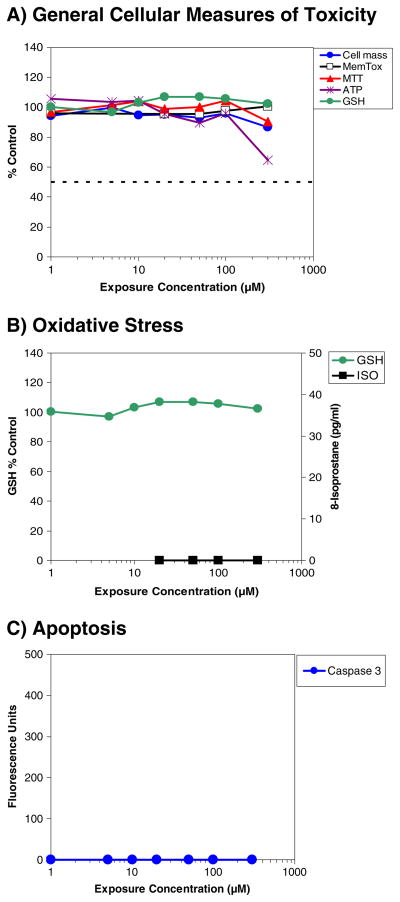
Figure 2a–c shows dose–response profiles for the effects of STAZN on cellular toxicity using a 24-h analysis endpoint. In general, for STAZN (Fig. 2 and Table 1), the TC50 (micromolar) for all general toxicity measures was >300, indicating an extreme level of safety using the in vitro toxicity assay.
As shown in Fig. 2a, there was a modest decrease in cellular ATP content when cells were incubated with 300-μM STAZN. There was no overt toxicity measured using any of the other parameters. The reduction of ATP content, which amounted to approximately a 35% decrease, was only observed at the highest exposure concentration and was measured in the absence of cell death, suggesting some level of membrane leakage. Figure 2b shows a comparison of two markers used to determine the effects of STAZN on markers of oxidative stress. For this measure, we used intracellular GSH content and lipid peroxidation measured as 8-isoprostane [31]. STAZN did not alter either marker (Table 1b). Figure 2c shows the lack of effect of STAZN on apoptosis measured by caspase-3 activity [31], which is a key mediator of apoptosis in neuronal cells, but also has non-apoptotic functions [47].
Table 1c summarizes the effects of STAZN on P-glycoprotein (PgP) Binding. There was an interaction of STAZN with PgP; however, it was a low-level interaction with the PgP transporters at the 50 μM concentration. Because of this, the CTox value and overall toxicity effects may be underestimated due to this possible observed interaction with the PgP transporter. Compounds with increased toxicity in the presence of cyclosporin A (CSA) have a high probability of binding to PgP proteins. However, compounds of low toxicity will typically not show a difference relative to the addition of CSA, regardless of whether they bind to PgP.
CTox Ranking: For STAZN, a CTox value was generated by CeeTox Inc. using a patented proprietary algorithm [32]. The CTox value was caclulated from the TC50 values documented in Table 1. The CTox ranking for STAZN, which is an estimate of a sustained concentration expected or necessary to produce toxicity in a rat 14-day repeat dose study, was 260 μM. Per CeeTox criteria, STAZN is thus considered to have a low probability of in vivo toxicity effects. Metabolic Stability: The half-life (t1/2) values for STAZN are summarized in Table 2. The half-life varied quite a lot between the four species tested, with mouse having the lowest half-life. The half-life in humans was similar to both dog and rat and was in the range of 60–78 min. In addition, clearance was similar in human, dog, and rat, but also differed in mouse much as the calculated half-life measure did (not shown). The half-life of testosterone (positive control) measured in parallel demonstrated that testosterone was significantly metabolized, indicating that the human and animal liver microsomes used in this study were metabolically active (t1/2 <10–32 min; data not shown).
Genotoxicity: Genotoxicity studies were done using two strains of bacteria, specifically S. typhimurium TA989: hisD3052, rfa, uvrB/pKM101, which detects frameshift mutations and S. typhimurium TA100: hisG45, rfa, uvrB/pKM101, which detects base pair substitutions either in the presence or absence of an S9 fraction from the livers of Aroclor 1254-treated rats clearly showed that STAZN was not mutagenic. As shown in Table 3, under all conditions tested, there was no significant (greater than or equal to twofold) increase in the number of colonies formed in the presence of STAZN up to and including a concentration of 0.0625 mg/ml (0.106 mM).
Fig. 2.
Effects of STAZN on a cellular toxicity, b oxidative stress, and c apoptosis following incubation of rat hepatoma-derived H4IIE cells with STAZN. Data are expressed as percent control for cell mass (blue line), membrane toxicity (black line open square), MTT assay (red line), ATP (purple line), GSH content (green line) or 8-isoprostane (picogram per milliliter; black line closed square), and fluorescence units for caspase-3. STAZN produced significant *p<0.05 decreases in ATP levels using a test concentration of 300 μM
Table 1.
Summary of CeeTox assay results
| A) General toxicity measures | |||
| Cell number TC50 (μM) | MemTox TC50 (μM) | MTT TC50 (μM) | ATP TC50 (μM) |
| >300 | >300 | >300 | >300 |
| B) Oxidative stress and apoptosis measures | |||
| Total GSH TC50 (μM) | Percent change in Total GSH | Membrane lipid peroxidation | Caspase 3 activity (index/dose) |
| >300 | NC | 0 | NC |
| C) P-glycoprotein (PgP) binding | |||
| % Control (compound) | % Control (compound + CSA) | % Difference | |
| 100.3 | 83.4 | 16.9 | |
For general toxicity measures, oxidative stress and apoptosis, the TC50 value, which is the concentration that produced a half-maximal response, was extrapolated from the graphs presented in Fig. 1a–c. The H4IIE cells possess high levels of PgP protein in the cell membrane. Cells are incubated with and without cyclosporin A (CSA; a PgP inhibitor) at a single exposure concentration (50 μM), and the difference in toxicity is determined with the MTT assay. Compounds with increased toxicity in the presence of CSA have a high probability of binding to PgP proteins. In this assay, STAZN had low interaction with the PgP transporter protein
MTT 3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyltetrazolium bromide, ATP adenosine triphosphate, NC no change. MemTox membrane toxicity measure
Table 2.
Differential metabolic stability in four species
| Species | Percent remaining of initial compounda |
Half-lifeb | ||||
|---|---|---|---|---|---|---|
| 0 min | 10 min | 20 min | 30 min | 60 min | t1/2 (min) | |
| Dog | 100 | 56.2 | 65.5 | 54.5 | 47.6 | 71 |
| Human | 100 | 57.7 | 39.7 | 46.9 | 49.1 | 78 |
| Mouse | 100 | 17.2 | 19.8 | 10.0 | 13.8 | <10c (27) |
| Rat | 100 | 82.4 | 76.6 | 80.3 | 122d | >60 (95) |
Percent remaining of test compound was calculated based on the peak area ratio of the test compound to the internal standard
Half-life was calculated based on half-life=0.693/k, where k is the elimination rate constant based on the slope of the plot of natural logarithm percent remaining versus incubation time
When the percent remaining was <50% at the first incubation time, the half-life was expressed as less than the shortest incubation time, and the calculated half-life was also listed
The rat data point with underscored characters was excluded from the calculation of half-life
Table 3.
STAZN genotoxicity using TA989 and TA100 S. typhimurium
| TA989±S9 | |||||
|---|---|---|---|---|---|
| TA989−S9 | TA989+S9 | ||||
| Conc (mg/ml) | N | Mean no. of positive wells | SD | Mean no. of positive wells | SD |
| 0 | 9 | 1.00 | 0.87 | 2.00 | 1.32 |
| .0625 | 3 | 0.67 | 0.58 | 2.33 | 0.58 |
| .03125 | 3 | 0.00 | 0.00 | 4.00 | 0.00 |
| .015625 | 3 | 0.67 | 0.58 | 3.00 | 0.00 |
| .007813 | 3 | 0.33 | 0.58 | 5.00 | 0.00 |
| .003906 | 3 | 1.33 | 0.58 | 1.33 | 2.31 |
| .001963 | 3 | 1.33 | 1.53 | 2.33 | 1.53 |
| Positive | 3 | 36.00 | 3.61 | 48.00 | 0.00 |
| TA100±S9 | |||||
| TA100−S9 | TA100+S9 | ||||
| 0 | 9 | 5.44 | 1.01 | 6.78 | 1.79 |
| .0625 | 3 | 5.67 | 2.08 | 8.67 | 3.06 |
| .03125 | 3 | 5.00 | 0.00 | 10.67 | 1.15 |
| .015625 | 3 | 7.33 | 4.04 | 6.33 | 1.15 |
| .007813 | 3 | 6.33 | 0.58 | 6.67 | 0.58 |
| .003906 | 3 | 3.67 | 2.52 | 6.33 | 2.31 |
| .001963 | 3 | 5.00 | 3.00 | 7.33 | 1.53 |
| Positive | 3 | 48.00 | 0.00 | 48.00 | 0.00 |
Baseline for TA989−S9=1.87; baseline for TA989+S9=3.32; Baseline for TA100−S9=6.46; Baseline for TA100+S9=8.57. Data shown are mean number positive wells ± SD (n=3 for each drug dose, n=9 for control). Significant fold increase in the number of colonies over baseline values (greater than or equal to twofold) are considered positive in the Ames test. All measurements in the presence of STAZN were p>0.05. However, all measurements in the presence of the positive control compound 2-aminoanthracene were significant, p<0.05
Discussion
Translational stroke research has evolved past basic pharmacological testing in models appropriate for the target disease [12, 21, 22, 31]. There is a need to understand not only the neuroprotective effects of a drug but also evaluate the drug for acute toxicity and genotoxicity. Moreover, in order to effectively translate preclinical information into successful clinical trials, knowledge of the metabolic stability of a drug will assist with the design of a suitable dosing regimen.
The treatment of AIS with a neuroprotective compound has still not been achieved due to poor choice of compound, lack of efficacy or overestimated efficacy, and dose-limiting toxicity [12, 21, 48–51]. In order to continue to advance the development of STAZN, we systematically tested the drug using a series of in vitro assays to predict bioactivity, toxicity, and stability.
Using hippocampal HT22 nerve cells, a standard model to detect molecules with neuroprotective activity [26, 27, 29, 44–46], we found that STAZN was neuroprotective, achieving an EC50 of 1–5 μM. However, even though STAZN was neuroprotective, it was weakly so and did not completely block either the effects of IAA or glutamate. It should be noted that the EC50 values with STAZN are 1- to 100-fold higher than the most effective drugs assessed in the two assays [26, 29], but similar in efficacy to the polyphenol fisetin [27] and the lipoxygenase inhibitor baicalein [44]. The maximal level of protection achieved by STAZN reached approximately 60% and 52% of total cell survival, respectively. Nevertheless, in the IAA assay, this represented a 621% increase in survival that was related to significant attenuation of IAA-induced cellular ATP loss and a 416% increase in survival in the oxidative glutamate toxicity assay. Since STAZN was effective against two different insults that cause cell death by two distinct mechanisms, ATP depletion and GSH depletion, STAZN may be considered a multi-target nitrone. Previous studies using the HT22 cell assay have shown that antioxidants and polyphenolic compounds with diverse mechanisms of action can attenuate injury-induced cell death, thereby increasing cell survival [26, 27, 29, 44–46]. Our new data suggest that STAZN may have other important neuroprotective activities independent of its basic antioxidant potential that was the impetus for the preclinical development by Becker, Ginsberg, and Belayev [17–19].
Using the CeeTox screening system, a general cellular health panel, the TC50 value for all assays was >300 μM. There was only one occurrence of “toxicity” measured, a decrease in ATP levels, and that was only observed at a dose of 300 μM. Since all other measures were not adversely affected, it appears that a very high concentration of STAZN may cause membrane leakage that is sublethal or has an adverse effect on mitochondria, thus reducing ATP production. From a drug development perspective, it is interesting to note that STAZN is efficacious in vitro using HT22 cells with an EC50 value in the range of 1–5 μM. Based upon in vitro analysis, the efficacy/toxicity ratio for STAZN indicates that there is a significant therapeutic safety window of 60–150-fold. STAZN has also been studied in vivo in a preclinical stroke model [17, 19], where it was shown to produce significant histochemical (i.e., reduced infarct volume) and behavioral improvement without any signs of acute toxicity. However, the studies were not specifically designed to detect acute toxicity of the drugs on any measure.
The metabolic stability of STAZN was measured using microsomal preparations from four different species. The basic assay measured disappearance of the parent molecule, but did not measure metabolites of the compound, which was detected using the LC-MS/MS system. The assay shows that STAZN is metabolized in a similar way by three of the four species, including human, dog, and rat, with mouse being the outlier. There are two main points of interest that can be gleaned from the metabolism studies. First, STAZN was metabolically unstable in all species, with approximately 50% of the drug metabolized within 60 min in both human and dog and 86% metabolized in the mouse. The data from the rat microsomal preparation assay at 60 min was inconclusive because there was assay interference, but 20% of the drug was metabolized within 30 min. It is important to note and compare the in vitro data with that documented by the Ginsberg group previously. They reported that in rats in vivo, STAZN has a long circulating t1/2, with a biexponential decline in blood levels (t1/2 of 28 min and 7 h) [19]. Thus, there are differences in estimated t1/2 values derived from in vitro to in vivo analyses. Clearly, both in vitro and in vivo stability and biodistribution studies are required to fully understand the kinetics of a drug candidate.
Based upon metabolism and the calculated t1/2, the in vivo bioavailability may be low after single dosing, and this must be taken into consideration when developing the compound to treat stroke. The results of the microsomal preparation analyses must be addressed in the context of the general cellular toxicity assays done using H4IIE cells. During incubation with microsomes, a STAZN metabolite was detected, but not identified. Additionally, it should be noted that H4IIE cells express several key cytochrome P (CYP) 450 enzymes including CYP1A, CYP2B, CYP2C, and CYP3A that can metabolize and conjugate (glucuronide and glutathione) drugs during the 24-h incubation period. Per McKim [31], results obtained with H4IIE cells usually reflect “toxicity” due to the non-metabolized forms of the test compounds. However, since acute toxicity of STAZN was extremely low, there is no concern that the metabolite formed in vitro was toxic.
The genotoxicity studies of STAZN using two histidine incompetent bacterial strains S. typhimurium (TA989) and (TA100), which are extremely useful in the detection of frameshift mutations and base pair substitutions induced by carcinogenic compounds[34, 35, 37–41], were negative. The studies were negative whether in the presence or absence of an S9 liver fraction from Aroclor 1254-treated rats [42, 43], a method commonly used to provide metabolic activation of the test drug. S9 metabolic activation is representative of phase I metabolism primarily due to the presence of CYP450 and phase II metabolism due to transferase activity in the cytosolic portion. Thus, even though STAZN is a complex heterocyclic compound, it is not mutagenic like other heterocyclic azulene compounds [52, 53] or the positive control 2-aminoanthracene [39], which was used in the study.
The pharmacological effects of STAZN should be directly compared to NXY-059, which failed in the SAINT 2 clinical trial [13]. There are numerous basic differences between the two molecules. First, STAZN has a low oxidation potential, suggesting that it will be an effective antioxidant in vivo, whereas NXY-059 has a high oxidation potential and thus would have limited antioxidant activity under physiological conditions [54]. Second, STAZN is 300 times more potent than NXY-059 at inhibiting free radical-induced peroxidation in vitro [18, 54]. Third, STAZN is a highly lipophilic compound, whereas NXY-059 is a polar molecule, highly water-soluble nonlipophilic molecule [19, 54]. However, using CeeTox analysis, STAZN had a CTox value of 260 μM, whereas the CTox value for NXY-059 was >300 μM. Thus, both nitrones were characterized as having the potential for low toxicity in vivo.
In conclusion, the novel combination of multiple neuroprotection assays, CeeTox analysis, microsomal stability assays, and genotoxocity analysis has allowed us to investigate many aspects of STAZN pharmacology and toxicity, de-risking steps that are now mandatory for drug development. The primary observation that STAZN can attenuate IAA and oxytosis-induced cell death is important because it suggests that STAZN is more than an antioxidant, and this pleiotropy would be beneficial to treat stroke. The CTox value >260 μM calculated for STAZN during the initial stages of drug de-risking, in addition to the lack of mutagenic activity, indicates that STAZN has a good safety profile and should continue to be developed as a lead compound to treat stroke.
Acknowledgments
This work was supported by a U01 Translational research grant NS060685 to PAL.
Footnotes
Conflicts of interest There are no conflicts of interest to disclose.
Contributor Information
Paul A. Lapchak, Email: Paul.Lapchak@cshs.org, Department of Neurology, Cedars-Sinai Medical Center, Davis Research Building, D-2091, 110 N. George Burns Road, Los Angeles, CA 90048, USA
David R. Schubert, Cellular Neurobiology Laboratories, The Salk Institute, 10010 North Torrey Pines Road, La Jolla, San Diego, CA 92037-1099, USA
Pamela A. Maher, Cellular Neurobiology Laboratories, The Salk Institute, 10010 North Torrey Pines Road, La Jolla, San Diego, CA 92037-1099, USA
References
- 1.Ingall T. Stroke—incidence, mortality, morbidity and risk. J Insur Med. 2004;36(2):143–52. [PubMed] [Google Scholar]
- 2.Lloyd-Jones D, Adams R, Carnethon M, De Simone G, Ferguson TB, Flegal K, et al. Heart disease and stroke statistics—2009 update: a report from the American Heart Association Statistics Committee to Stroke Statistics Subcommittee. Circulation. 2009;119(3):480–6. doi: 10.1161/CIRCULATIONAHA.108.191259. [DOI] [PubMed] [Google Scholar]
- 3.Moskowitz MA, Lo EH, Iadecola C. The science of stroke: mechanisms in search of treatments. Neuron. 2010;67(2):181–98. doi: 10.1016/j.neuron.2010.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bath PM, Gray LJ, Bath AJ, Buchan A, Miyata T, Green AR. Effects of NXY-059 in experimental stroke: an individual animal meta-analysis. Br J Pharmacol. 2009;157(7):1157–71. doi: 10.1111/j.1476-5381.2009.00196.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dirnagl U, Iadecola C, Moskowitz MA. Pathobiology of ischaemic stroke: an integrated view. Trends Neurosci. 1999;22(9):391–7. doi: 10.1016/s0166-2236(99)01401-0. [DOI] [PubMed] [Google Scholar]
- 6.Lapchak PA. A critical assessment of edaravone acute ischemic stroke efficacy trials: is edaravone an effective neuroprotective therapy? Expert Opin Pharmacother. 2010;11(10):1753–63. doi: 10.1517/14656566.2010.493558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Savitz SI. A critical appraisal of the NXY-059 neuroprotection studies for acute stroke: a need for more rigorous testing of neuroprotective agents in animal models of stroke. Exp Neurol. 2007;205(1):20–5. doi: 10.1016/j.expneurol.2007.03.003. [DOI] [PubMed] [Google Scholar]
- 8.Lees KR, Zivin JA, Ashwood T, Davalos A, Davis SM, Diener HC, et al. NXY-059 for acute ischemic stroke. N Engl J Med. 2006;354(6):588–600. doi: 10.1056/NEJMoa052980. [DOI] [PubMed] [Google Scholar]
- 9.Otomo E. Effect of a novel free radical scavenger, edaravone (MCI-186), on acute brain infarction. Randomized, placebo-controlled, double-blind study at multicenters. Cerebrovasc Dis. 2003;15(3):222–9. doi: 10.1159/000069318. [DOI] [PubMed] [Google Scholar]
- 10.Watanabe T, Tahara M, Todo S. The novel antioxidant edaravone: from bench to bedside. Cardiovasc Ther. 2008;26(2):101–14. doi: 10.1111/j.1527-3466.2008.00041.x. [DOI] [PubMed] [Google Scholar]
- 11.Tirilazad International Steering Committee. Tirilazad mesylate in acute ischemic stroke: a systematic review. Stroke. 2000;31 (9):2257–65. doi: 10.1161/01.str.31.9.2257. [DOI] [PubMed] [Google Scholar]
- 12.Lapchak PA. Translational stroke research using a rabbit embolic stroke model: a correlative analysis hypothesis for novel therapy development. Transl Stroke Res. 2010;1(2):96–107. doi: 10.1007/s12975-010-0018-4. Perspective Review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shuaib A, Lees KR, Lyden P, Grotta J, Davalos A, Davis SM, et al. NXY-059 for the treatment of acute ischemic stroke. N Engl J Med. 2007;357(6):562–71. doi: 10.1056/NEJMoa070240. [DOI] [PubMed] [Google Scholar]
- 14.Feuerstein GZ, Zaleska MM, Krams M, Wang X, Day M, Rutkowski JL, et al. Missing steps in the STAIR case: a Translational Medicine perspective on the development of NXY-059 for treatment of acute ischemic stroke. J Cereb Blood Flow Metab. 2008;28:217–19. doi: 10.1038/sj.jcbfm.9600516. [DOI] [PubMed] [Google Scholar]
- 15.Lapchak PA, Araujo DM, Song D, Wei J, Zivin JA. Neuroprotective effects of the spin trap agent disodium-[(tert- butylimino)methyl] benzene-1,3-disulfonate N-oxide (generic NXY-059) in a rabbit small clot embolic stroke model: combination studies with the thrombolytic tissue plasminogen activator. Stroke. 2002;33(5):1411–5. doi: 10.1161/01.str.0000015346.00054.8b. [DOI] [PubMed] [Google Scholar]
- 16.Otomo E, Tohgi H, Kogure K, Hirai S, Terashi A, Gotoh F, et al. Clinical efficacy of a free radical scavenger, MCI-186, on acute cerebral infarction: early phase II clinical trial. Ther Res. 1998;19:1311–32. [Google Scholar]
- 17.Ginsberg MD, Becker DA, Busto R, Belayev A, Zhang Y, Khoutorova L, et al. Stilbazulenyl nitrone, a novel antioxidant, is highly neuroprotective in focal ischemia. Ann Neurol. 2003;54 (3):330–42. doi: 10.1002/ana.10659. [DOI] [PubMed] [Google Scholar]
- 18.Becker DA, Ley JJ, Echegoyen L, Alvarado R. Stilbazulenyl nitrone (STAZN): a nitronyl-substituted hydrocarbon with the potency of classical phenolic chain-breaking antioxidants. J Am Chem Soc. 2002;124(17):4678–84. doi: 10.1021/ja011507s. [DOI] [PubMed] [Google Scholar]
- 19.Ley JJ, Vigdorchik A, Belayev L, Zhao W, Busto R, Khoutorova L, et al. Stilbazulenyl nitrone, a second-generation azulenyl nitrone antioxidant, confers enduring neuroprotection in experimental focal cerebral ischemia in the rat: neurobehavior, histopathology, and pharmacokinetics. J Pharmacol Exp Ther. 2005;313 (3):1090–100. doi: 10.1124/jpet.105.083386. [DOI] [PubMed] [Google Scholar]
- 20.Kuroda S, Tsuchidate R, Smith ML, Maples KR, Siesjo BK. Neuroprotective effects of a novel nitrone, NXY-059, after transient focal cerebral ischemia in the rat. J Cereb Blood Flow Metab. 1999;19(7):778–87. doi: 10.1097/00004647-199907000-00008. [DOI] [PubMed] [Google Scholar]
- 21.Lapchak PA, Zhang JH. Resolving the negative data publication dilemna in translational stroke research. Transl Stroke Research. 2011;2(1):1–6. doi: 10.1007/s12975-010-0057-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.STAIR. Recommendations for standards regarding preclinical neuroprotective and restorative drug development. Stroke. 1999;30(12):2752–8. doi: 10.1161/01.str.30.12.2752. [DOI] [PubMed] [Google Scholar]
- 23.Becker DA, Ley J, inventors. Azulenyl nitrone spin trapping agents, methods of making and using same. 0888290. US patent number. 2008
- 24.Davis JB, Maher P. Protein kinase C activation inhibits glutamate-induced cytotoxicity in a neuronal cell line. Brain Res. 1994;652:169–73. doi: 10.1016/0006-8993(94)90334-4. [DOI] [PubMed] [Google Scholar]
- 25.Maher P, Davis J. The role of monoamine metabolism in oxidative glutamate toxicity. J Neurosci. 1996;16:6394–401. doi: 10.1523/JNEUROSCI.16-20-06394.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lapchak PA, Schubert DR, Maher PA. Delayed treatment with a novel neurotrophic compound reduces behavioral deficits in rabbit ischemic stroke. J Neurochem. 2011;116(1):122–31. doi: 10.1111/j.1471-4159.2010.07090.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Maher P, Salgado KF, Zivin JA, Lapchak PA. A novel approach to screening for new neuroprotective compounds for the treatment of stroke. Brain Res. 2007;1173:117–25. doi: 10.1016/j.brainres.2007.07.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tan S, Schubert D, Maher P. Oxytosis: a novel form of programmed cell death. Curr Top Med Chem. 2001;1(6):497–506. doi: 10.2174/1568026013394741. [DOI] [PubMed] [Google Scholar]
- 29.Liu Y, Dargusch R, Maher P, Schubert D. A broadly neuroprotective derivative of curcumin. J Neurochem. 2008;105 (4):1336–45. doi: 10.1111/j.1471-4159.2008.05236.x. [DOI] [PubMed] [Google Scholar]
- 30.Lapchak PA, KcKim JM. CeeTox™ analysis of CNB-001 a novel curcumin-based neurotrophic/neuroprotective lead compound to treat stroke: comparison with NXY-059 and radicut. Transl Stroke Research. 2011;2(1):51–9. doi: 10.1007/s12975-010-0034-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.McKim JM., Jr Building a tiered approach to in vitro predictive toxicity screening: a focus on assays with in vivo relevance. Comb Chem High Throughput Screen. 2010;13(2):188–206. doi: 10.2174/138620710790596736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.McKim JM Jr, inventor. Ceetox, Inc. Toxicity screening methods. 7615361. US patent number. 2009
- 33.Ames B, Lee F, Durston W. An improved bacterial test system for the detection and classification of mutagens and carcinogens. Proc Natl Acad Sci USA. 1973;70:782–6. doi: 10.1073/pnas.70.3.782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ames BN. Carcinogens are mutagens: their detection and classification. Environ Health Perspect. 1973;6:115–8. doi: 10.1289/ehp.7306115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ames BN, Durston WE, Yamasaki E, Lee FD. Carcinogens are mutagens: a simple test system combining liver homogenates for activation and bacteria for detection. Proc Natl Acad Sci USA. 1973;70(8):2281–5. doi: 10.1073/pnas.70.8.2281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mortelmans K, Zeiger E. The Ames Salmonella/microsome mutagenicity assay. Mutat Res. 2000;455(1–2):29–60. doi: 10.1016/s0027-5107(00)00064-6. [DOI] [PubMed] [Google Scholar]
- 37.Zeiger E. Historical perspective on the development of the genetic toxicity test battery in the United States. Environ Mol Mutagen. 2010;51(8–9):781–91. doi: 10.1002/em.20602. [DOI] [PubMed] [Google Scholar]
- 38.Jemnitz K, Veres Z, Torok G, Toth E, Vereczkey L. Comparative study in the Ames test of benzo[a]pyrene and 2-aminoanthracene metabolic activation using rat hepatic S9 and hepatocytes following in vivo or in vitro induction. Mutagenesis. 2004;19(3):245–50. doi: 10.1093/mutage/geh026. [DOI] [PubMed] [Google Scholar]
- 39.Ayrton AD, Neville S, Ioannides C. Cytosolic activation of 2-aminoanthracene: implications in its use as diagnostic mutagen in the Ames test. Mutat Res. 1992;265(1):1–8. doi: 10.1016/0027-5107(92)90034-y. [DOI] [PubMed] [Google Scholar]
- 40.Hannan MA, Recio L, Deluca PP, Enoch H. Co-mutagenic effects of 2-aminoanthracene and cigarette smoke condensate on smoker’s urine in the Ames Salmonella assay system. Cancer Lett. 1981;13(3):203–12. doi: 10.1016/0304-3835(81)90019-7. [DOI] [PubMed] [Google Scholar]
- 41.Kawalek JC, Andrews AW. Effect of aromatic hydrocarbons on the metabolism of 2-aminoanthracene to mutagenic products in the Ames assay. Carcinogenesis. 1981;2(12):1367–9. doi: 10.1093/carcin/2.12.1367. [DOI] [PubMed] [Google Scholar]
- 42.Aly HA, Domenech O. Aroclor 1254 induced cytotoxicity and mitochondrial dysfunction in isolated rat hepatocytes. Toxicology. 2009;262(3):175–83. doi: 10.1016/j.tox.2009.05.018. [DOI] [PubMed] [Google Scholar]
- 43.Aly HA, Domenech O, Abdel-Naim AB. Aroclor 1254 impairs spermatogenesis and induces oxidative stress in rat testicular mitochondria. Food Chem Toxicol. 2009;47(8):1733–8. doi: 10.1016/j.fct.2009.03.019. [DOI] [PubMed] [Google Scholar]
- 44.Lapchak PA, Maher P, Schubert D, Zivin JA. Baicalein, an antioxidant 12/15-lipoxygenase inhibitor improves clinical rating scores following multiple infarct embolic strokes. Neuroscience. 2007;150(3):585–91. doi: 10.1016/j.neuroscience.2007.09.033. [DOI] [PubMed] [Google Scholar]
- 45.Maher P. A comparison of the neurotrophic activities of the flavonoid fisetin and some of its derivatives. Free Radic Res. 2006;40(10):1105–11. doi: 10.1080/10715760600672509. [DOI] [PubMed] [Google Scholar]
- 46.Maher P, Akaishi T, Schubert D, Abe K. A pyrazole derivative of curcumin enhances memory. Neurobiol Aging. 2010;31(4):706–9. doi: 10.1016/j.neurobiolaging.2008.05.020. [DOI] [PubMed] [Google Scholar]
- 47.D’Amelio M, Cavallucci V, Cecconi F. Neuronal caspase-3 signaling: not only cell death. Cell Death Differ. 2010;17 (7):1104–14. doi: 10.1038/cdd.2009.180. [DOI] [PubMed] [Google Scholar]
- 48.Legos JJ, Tuma RF, Barone FC. Pharmacological interventions for stroke: failures and future. Expert Opin Investig Drugs. 2002;11 (5):603–14. doi: 10.1517/13543784.11.5.603. [DOI] [PubMed] [Google Scholar]
- 49.Green AR. Why do neuroprotective drugs that are so promising in animals fail in the clinic? An industry perspective. Clin Exp Pharmacol Physiol. 2002;29(11):1030–4. doi: 10.1046/j.1440-1681.2002.03767.x. [DOI] [PubMed] [Google Scholar]
- 50.Liebeskind DS, Kasner SE. Neuroprotection for ischaemic stroke: an unattainable goal? CNS Drugs. 2001;15(3):165–74. doi: 10.2165/00023210-200115030-00001. [DOI] [PubMed] [Google Scholar]
- 51.O’Collins VE, Macleod MR, Donnan GA, Horky LL, van der Worp BH, Howells DW. 1,026 experimental treatments in acute stroke. Ann Neurol. 2006;59(3):467–77. doi: 10.1002/ana.20741. [DOI] [PubMed] [Google Scholar]
- 52.Yan J, Wang L, Fu PP, Yu H. Photomutagenicity of 16 polycyclic aromatic hydrocarbons from the US EPA priority pollutant list. Mutat Res. 2004;557(1):99–108. doi: 10.1016/j.mrgentox.2003.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wang L, Yan J, Fu PP, Parekh KA, Yu H. Photomutagenicity of cosmetic ingredient chemicals azulene and guaiazulene. Mutat Res. 2003;530(1–2):19–26. doi: 10.1016/s0027-5107(03)00131-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ginsberg MD. Life after cerovive: a personal perspective on ischemic neuroprotection in the post-NXY-059 era. Stroke. 2007;38(6):1967–72. doi: 10.1161/STROKEAHA.106.479170. [DOI] [PubMed] [Google Scholar]