Abstract
Leptin, discovered through positional cloning 15 years ago, is an adipocyte-secreted hormone with pleiotropic effects in the physiology and pathophysiology of energy homeostasis, endocrinology, and metabolism. Studies in vitro and in animal models highlight the potential for leptin to regulate a number of physiological functions. Available evidence from human studies indicates that leptin has a mainly permissive role, with leptin administration being effective in states of leptin deficiency, less effective in states of leptin adequacy, and largely ineffective in states of leptin excess. Results from interventional studies in humans demonstrate that leptin administration in subjects with congenital complete leptin deficiency or subjects with partial leptin deficiency (subjects with lipoatrophy, congenital or related to HIV infection, and women with hypothalamic amenorrhea) reverses the energy homeostasis and neuroendocrine and metabolic abnormalities associated with these conditions. More specifically, in women with hypothalamic amenorrhea, leptin helps restore abnormalities in hypothalamic-pituitary-peripheral axes including the gonadal, thyroid, growth hormone, and to a lesser extent adrenal axes. Furthermore, leptin results in resumption of menses in the majority of these subjects and, in the long term, may increase bone mineral content and density, especially at the lumbar spine. In patients with congenital or HIV-related lipoatrophy, leptin treatment is also associated with improvements in insulin sensitivity and lipid profile, concomitant with reduced visceral and ectopic fat deposition. In contrast, leptin's effects are largely absent in the obese hyperleptinemic state, probably due to leptin resistance or tolerance. Hence, another emerging area of research pertains to the discovery and/or usefulness of leptin sensitizers. Results from ongoing studies are expected to further increase our understanding of the role of leptin and the potential clinical applications of leptin or its analogs in human therapeutics.
Keywords: adipokines, adipose tissue, leptin resistance, leptin deficiency, hypoleptinemia
studies of genetically obese mice serendipitously found in the Jackson Laboratories revealed that their phenotypes derive from homozygous mutations of either the obese (ob) or diabetic (db) genes that result in obesity and insulin resistance or diabetes as well as endocrine and immune dysfunction (53, 54, 115, 117, 183, 261). The gene mutation in the ob/ob mouse results in a complete deficiency of or a truncated and biologically inactive ob gene product (287); the latter subsequently was given the name leptin (95), from the Greek word “leptos” (meaning “thin”), because when this protein was given to the obese ob/ob mice they lost significant amounts of body weight. It was then recognized that the db gene codes for the leptin receptor (140). Consequently, exogenously administered leptin reduces body weight and resolves the metabolic, endocrine, and immune disturbances in ob/ob mice but has no effects in db/db mice (100, 111, 287). In the 15 years following these initial observations, a series of studies have been carried out, and over 19,000 papers on leptin published in Medline have considerably increased our knowledge regarding the biology of leptin and its role in human endocrinology and metabolism. In this review, we focus on the role of leptin in regulating neuroendocrine function, insulin sensitivity, immune function, and bone metabolism and highlight the role of leptin in the pathophysiology and therapeutics of human disease.
LEPTIN IN HUMAN PHYSIOLOGY
Leptin is a 167-amino acid peptide with a four-helix bundle motif similar to that of a cytokine (24, 286). Leptin is produced predominantly in the adipose tissue but is also expressed in a variety of other tissues, including placenta, ovaries, mammary epithelium, bone marrow, and lymphoid tissues (178). Leptin binds to leptin receptors (ObRs) located throughout the central nervous system and peripheral tissues (71), with at least six receptor isoforms identified (ObRa, ObRb, ObRc, ObRd, ObRe, and ObRf) (140). These have homologous domains, but, due to alternative mRNA splicing, each receptor has a unique sequence and length (140, 258). The short isoforms ObRa and ObRc are thought to have important roles in transporting leptin across the blood-brain barrier (15, 105). The long isoform ObRb is ubiquitously expressed throughout the central nervous system and is primarily responsible for leptin signaling (15, 140, 258). The ObRb receptor is particularly important in the hypothalamus, where it regulates energy homeostasis and neuroendocrine function (65, 71, 79). In the db/db mouse model, the ObRb receptor is mutated and dysfunctional, resulting in the obese diabetic phenotype (274). The soluble leptin receptor isoform ObRe is the extracellular cleaved part of the long isoform ObRb and the main circulating leptin-binding protein (108) and may antagonize leptin transport by inhibiting surface binding and endocytosis of leptin (264).
In humans, the release of leptin into the circulation is pulsatile, and leptin concentrations follow a circadian rhythm (233), are affected by sleep patterns (196, 85), and display highest levels between midnight and early morning and lowest levels in the early to mid-afternoon (16, 149, 248). The pulsatile nature of leptin's secretion pattern (243) is similar in obese and lean individuals, but the obese have higher pulse amplitudes (16, 149, 248) and overall greater leptin concentrations than lean subjects due to their greater amount of body fat (57, 282). Furthermore, for the same age and body mass index (BMI), women have greater leptin concentrations than men (174). Leptin concentrations in women may be higher in the luteal phase of the menstrual cycle (8, 64, 157), whereas menopause is associated with a decline in circulating leptin (131). Renal failure also results in higher leptin levels (163). These observations suggest that sex differences in leptin concentration are likely also the result of differences in sex hormones, e.g., estrogen and testosterone (35, 153, 245), besides those in body fat mass and/or distribution (131, 137, 188, 194, 224, 229).
Progressive fasting results in a rapid decline in leptin concentration, which occurs before any loss of fat mass (20), thereby triggering an adaptive mechanism to conserve energy (37). In mice and humans, the neuroendocrine response to food deprivation includes decreasing reproductive hormone concentrations (thereby reducing fertility and preventing pregnancy) (38, 165, 194), decreasing thyroid hormone concentrations (thereby slowing metabolic rate), increasing growth hormone (GH) concentration (thereby mobilizing energy stores), and decreasing insulin-like growth factor I (IGF-I) concentration (thereby slowing energy-demanding growth-related processes) as well as increasing adrenocorticotropic hormone (ACTH) and cortisol (3, 184, 268).
To assess the role of leptin in regulating the neuroendocrine response to fasting in humans, we first performed studies on the pharmacokinetics of leptin in humans and determined the doses required to achieve specific levels of circulating leptin in blood (45, 46, 278). We then studied healthy volunteers in the fed (141) and fasting states (37, 40). In acutely energy-deprived lean men (i.e., fasted for 3 days) we observed a fall in leptin concentration concomitant with a spectrum of neuroendocrine abnormalities; most of these abnormalities were reversed with physiological replacement doses of leptin (37). In particular, leptin replacement during fasting prevented the starvation-induced changes in the hypothalamic-pituitary-gonadal axis and, in part, the hypothalamic-pituitary-thyroid axis and IGF-I binding capacity in serum but did not reverse the changes in the hypothalamic-pituitary-adrenal axis at an appreciable level and/or the renin-aldosterone system, and GH/IGF-I axes (37). We also found similar neuroendocrine abnormalities in normal-weight women after caloric deprivation; however, replacement doses of leptin only modestly restored the changes in luteinizing hormone (LH) pulsatility and did not affect any other neuroendocrine axes (40). A major difference between these studies was the extent of the fall in leptin concentrations during fasting. In the former study on lean men, leptin concentration dropped from 2.24 ng/ml to clearly hypoleptinemic levels of 0.27 ng/ml (37). In contrast, in the latter study on normal-weight women, leptin concentration fell from 14.7 ± 2.6 ng/ml to low physiological levels of 2.8 ± 0.3 ng/ml (40). These data suggest that there may be a leptin threshold necessary for leptin's regulation of the hypothalamic-pituary-peripheral axes (170) (167). We thus proposed that the discrepancies between these two studies were due to leptin's permissive role in regulating neuroendocrine function, and we put forth the hypothesis that leptin replacement exerts a physiologically important effect only when the circulating leptin concentration falls below a certain threshold (Fig. 1). This apparently has important implications in states of energy deficiency such as anorexia nervosa, lipoatrophy, and/or strenuously exercising athletes who are in negative energy balance (9, 123, 161, 162, 164). We will review below the results from several studies on the effects of leptin on physiological functions in these disease states supporting this notion.
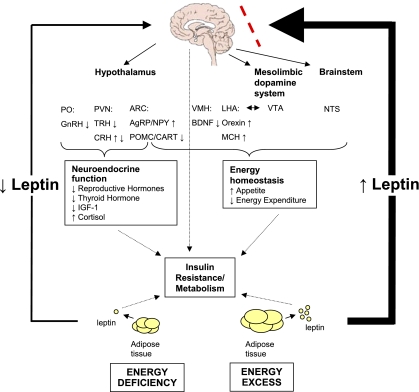
Fig. 1.
States of energy excess are associated with hyperleptinemia, but the hypothalamus is resistant or tolerant to the effects of increased leptin (dashed line). Energy deficiency results in hypoleptinemia. As a result, a complex neural circuit comprising orexigenic and anorexigenic signals is activated to increase food intake (220). In response to decreased leptin levels, there is increased expression of orexigenic neuropeptides AgRP and NPY in the ARC (59) and orexin and MCH in the LHA. Furthermore, there is decreased expression of anorexigenic neuropeptides POMC and CART in the ARC (59) and BDNF in the VMH. In addition to neurons that project from the LHA to the VTA, leptin also acts at the VTA of the mesolimbic dopamine system to regulate motivation for and reward of feeding. Leptin activation of the NTS of the brain stem also contributes to satiety. In addition, leptin has direct and/or downstream effects on the PVN and PO that are important for neuroendocrine responses to energy deprivation, including reducing reproductive and thyroid hormones. For the sake of comparison, leptin acts only indirectly on the GnRH-secreting neurons in the hypothalamus, and it can act directly and indirectly on TRH-secreting neurons (220). The effect of leptin on cortisol levels during starvation differs in mice and humans. Unlike in normal mice (102), leptin administration does not reverse the elevated adrenocorticotropin levels associated with starvation in humans (37). The mechanism of leptin's effect on the growth hormone axis is unclear. Dashed arrows indicate how leptin directly and indirectly influences metabolism and insulin resistance. AgRP, agouti-related protein; ARC, arcuate nucleus; BDNF, brain-derived neurotropic factor; CART, cocaine- and amphetamine-regulated transcript; CRH, corticotropin-releasing hormone; GnRH, gonadotropin-releasing hormone; IGF-I, insulin-like growth factor I; LHA, lateral hypothalamic area; MCH, melanin-concentrating hormone; NPY, neuropeptide Y; NTS, nucleus of the solitary tract; PO, preoptic area; POMC, proopiomelanocortin; PVN, paraventricular nucleus; TRH, thyrotropin-releasing hormone; VMH, ventromedial hypothalamic nucleus; VTA, ventral tegmental area.
Physiological Role of Leptin in the Regulation of Neuroendocrine Function
Hypothalamic-pituitary-gonadal axis.
Although leptin has been shown to stimulate LH secretion in rodents both in vitro and in vivo (285), hypothalamic gonadotropin-releasing hormone (GnRH) neurons do not express ObRb (72, 94, 217). Therefore, leptin must regulate the reproductive axis via indirect mechanisms. Recent studies have indicated that leptin regulates reproductive function by activating neurons that project afferent input to GnRH neurons in the preoptic area and other hypothalamic areas (106). Several neurons involved in energy homeostasis are anatomically associated with GnRH neurons [e.g., agouti-related peptide/neuropeptide Y (AgRP/NPY) and proopiomelanocortin (POMC) neurons] and may thus link changes in energy balance with subsequent alterations in reproductive function (106). There is also evidence suggesting that leptin may mediate the reproductive axis through regulation of kisspeptins, products of the Kiss1 gene, as well as dynorphin and neurokinin B. Mutations causing dysfunction of the kisspeptin receptor gene GPR54 results in hypogonadotropic hypogonadism in both mice and humans (61, 236), and various kisspeptins have been shown to stimulate the release of GnRH and increase plasma levels of LH, follicle stimulating hormone (FSH), and total testosterone in vivo in animals (91, 118, 240, 260). Approximately 40% of KiSS-1 mRNA-expressing cells in the arcuate nucleus coexpress ObRb mRNA (249). Moreover, ob/ob mice have lower levels of KiSS-1 mRNA than wild-type mice, and when ob/ob mice are treated with leptin, levels of KiSS-1 mRNA increase, suggesting that the reproductive dysfunction associated with leptin deficiency may be partially due to subnormal levels of kisspeptins (249). Similarly, human mutations in the gene encoding neurokinin B or its receptor lead to defective GnRH release and subsequent hypogonadism (145). Furthermore, a subpopulation of neurons in the arcuate nucleus coexpress kisspeptin, neurokinin B, and dynorphin, another peptide that is implicated in the feedback regulation of GnRH neurons (145). These neurons also contain leptin receptors (249) and may thus mediate the effects of nutritional status and stress on the hypothalamic-pituitary-gonadal axis (145). There is, in fact, compelling evidence from basic and clinical studies indicating that leptin is an important mediator of the hypothalamic-pituitary-gonadal axis (150), even though the exact brain areas and molecular mechanisms involved remain to be fully elucidated (106).
Ob/ob mice are infertile, and their fertility can be restored with exogenous leptin administration independently of weight loss (47). Similarly to humans, in otherwise healthy but acutely energy-deprived rodents, fasting results in decreased gonadotropin pulsatility and secretion and blunts reproductive function (3). Leptin administration to these rodents normalizes LH levels and the estrous cycle in females and restores testosterone levels in males (3). Similarly, a crucial permissive role for leptin in human reproductive function was confirmed by our study of caloric deprivation with leptin replacement in healthy volunteers studied in our General Clinical Research Center. We showed that caloric deprivation blunts LH pulsatility and testosterone secretion in healthy lean men and that leptin replacement can restore these hormones to prefasting baseline levels (37). In normal-weight women, caloric deprivation decreases LH peak frequency, which is also fully corrected with leptin replacement (40). We then studied whether leptin's regulation of the hypothalamic-pituary-gonadal axis could possibly play a role in the initiation of puberty, since R. Frisch had long suggested (76) that puberty in girls occurs when a certain amount of body weight is attained. We found in the context of longitudinal observational studies that rising leptin levels are associated with the onset of puberty in normal boys (166, 171). Sex differences in leptin levels during and after puberty are related to differences in subcutaneous fat depots and sex steroid levels (151, 221). Administration of leptin has resulted in increased circulating LH levels in a young prepubertal boy with leptin deficiency (66) and induced phenotypic and hormonal changes consistent with puberty in adults with congential leptin deficiency who had remained prepubertal (148). These findings extend similar observations in mice (2) and provide experimental evidence that normal leptin levels may have a permissive role and thus be necessary for normal progression of puberty and reproductive maturity.
Hypothalamic-pituitary-thyroid axis.
Leptin influences the thyroidal axis by regulating the expression of thyrotropin-releasing hormone (TRH) (230). In vitro and in vivo studies in rodents have shown that leptin directly stimulates TRH-expressing neurons in the paraventricular nucleus (PVN) of the hypothalamus to upregulate proTRH gene expression (144). Leptin also indirectly influences TRH neurons in the PVN through signals from the arcuate nucleus, as melanocortins (induced by leptin) stimulate and AgRP (suppressed by leptin) inhibits the thyroid axis (133, 211). Leptin also blunts the fasting-induced decrease in prohormone convertase-1 and -2 (PC1 and PC2), which cleave TRH from proTRH (230).
Leptin deficiency in the ob/ob mouse is associated with hypothalamic hypothyroidism from birth (266). Normal mice experience a drop in thyroxine concentration with fasting-induced hypoleptinemia (3) due to rapid suppression of TRH expression in the PVN, leading to decreased thyroxine and triiodothyronine concentrations (74). Leptin replacement reverses these changes (3).
In healthy humans, thyroid-stimulating hormone (TSH) is secreted in a pulsatile fashion similar to that of leptin, reaching a peak in the early morning hours and nadir in late morning (175). Individuals with congenital leptin deficiency have a highly disorganized TSH secretion pattern, suggesting that leptin may regulate TSH pulsatility and circadian rhythm (175). Despite altered circadian rhythm of TSH, however, the thyroid phenotype varies among humans with congenital leptin deficiency, with most cases having normal thyroid function (144, 211).
Acute starvation-induced hypoleptinemia is also associated with abnormalities in the hypothalamic-pituitary-thyroid axis in humans. We have shown that leptin administration in replacement doses prevents the fasting-induced changes in TSH pulsatility and increases free thyroxine concentration without affecting the fasting-induced fall in triiodothyronine concentration in healthy lean men with starvation-induced leptin deficiency (37). In another, intermediate study, in which we studied seven normal-weight women, replacement doses of leptin had no effect on fasting-induced changes in TSH pulsatility or thyroid hormone concentrations (40). We hypothesized that the fall in serum leptin concentration in these women did not reach the aforementioned threshold (40), beyond which we suggest that leptin replacement exerts a physiological effect. Taken together, these data indicate that decreasing leptin levels in states of energy deprivation may induce physiologically appropriate changes in the thyroid axis and through changes in thyroid possibly also on metabolism.
Hypothalamic-pituitary-growth hormone axis.
Both ob/ob and db/db mice have stunted growth curves and fragile growth plates (80, 81). Leptin enhances growth hormone-releasing hormone (GHRH)-induced GH secretion in rat anterior pituitary cells in vitro (216). Furthermore, it has been shown that leptin blunts the fasting-induced decrease in GH and increases longitudinal bone growth in normal rodents (81).
The GH axis in humans, however, is not affected by leptin in the same manner or to the same extent as it is in rodents. Children with congenital leptin deficiency have normal linear growth (66, 67), but children with the leptin receptor mutation can experience an early growth delay with subnormal concentrations of GH, IGF-I, and insulin'like growth factor-binding protein-3 (IGFBP3) (51). In healthy men, leptin replacement attenuates the starvation-induced fall in IGF-I but has no detectable short-term effect on circulating GH levels (37). In normal-weight women, in whom the starvation-induced fall in serum leptin is much less than that observed among men, leptin replacement does not prevent the reduction in IGF-I and IGFBP3 levels (40). Thus, it appears that in humans leptin may regulate not GH secretion per se but mainly the effect of GH (44) to regulate secretion of IGF-I and its binding proteins in the periphery. Mechanisms underlying these effects need to be studied in more detail in the future.
Hypothalamic-pituitary-adrenal axis.
Corticotropin-releasing hormone (CRH) is synthesized in the PVN, and leptin causes a dose-dependent stimulation of CRH release in vitro (58). ob/ob Mice exhibit increased adrenal stimulation by ACTH (22), and leptin administration blunts the stress-mediated increase in ACTH and cortisol in normal mice (102). From our analysis of leptin's pulse parameters, we have identified an inverse relationship between circulating leptin and ACTH in healthy men (149). However, studies in humans with mutations in the leptin or leptin receptor genes reveal that, despite abnormal leptin function, normal adrenal function is maintained (51, 66, 67, 69). Our earlier open-label studies of relative leptin deficiency in men with acute energy deprivation (i.e., starvation) and women with chronic energy deprivation (i.e., hypothalamic amenorrhea) (37, 273) showed no major effect of leptin replacement; but detailed studies were not performed. Similar results were reported in women with lipoatrophy and hypoleptinemia (202) studied in the context of an open-label study. The effects of leptin on the hypothalamic-pituitary-adrenal axis were more pronounced and achieved statistical significance in a randomized, placebo-controlled study with metreleptin administration in physiological doses to leptin-deficient subjects (see below under Hypothalamic amenorrhea) (49). Thus, further investigation is needed to define whether the effects of leptin deficiency are counterbalanced by the activation of compensatory mechanisms or whether there is redundancy of the regulatory pathways.
Physiological Role of Leptin in the Regulation of Insulin Sensitivity
In the leptin-deficient ob/ob mouse, leptin injections led to significantly greater reductions in serum glucose levels in a dose-dependent manner compared with pair-fed ob/ob control animals even before any significant reduction in body weight (95, 213, 234), indicating that leptin may have direct effects in regulating insulin sensitivity independently of changes in body weight (130, 135, 142). Schwartz et al. (234) calculated that 42% of leptin's hypoglycemic action was independent of weight reduction. In contrast, the effects of leptin on whole body glucose metabolism in nonobese, nondiabetic animals remain inconsistent, but in general, short-term administration of leptin does not affect glucose metabolism (100, 227, 275). Similarly, the effects of leptin administration in animal models of diet-induced obesity, a model that provides important insights into understanding the effects of leptin on glucose metabolism in obese humans, also reveal largely null data (27, 283).
Mouse models of lipoatrophy, such as the AZIP/F-1 mouse, completely lack white adipose tissue and are therefore unable to produce adequate amounts of leptin. This results in a nearly 20-fold reduction in leptin concentration (24, 187). These mice also have a wide array of metabolic abnormalities, such as dyslipidemia, insulin resistance, and fatty infiltration of the liver. Leptin has therefore been investigated as a regulator of insulin sensitivity (52). Transplantation of white adipose tissue in physiological quantities (86, 132), as well as exogenous leptin administration (246), improves insulin sensitivity in these mice. Other studies have shown that insulin sensitivity is fully restored with administration of the combination of leptin and adiponectin to lipoatrophic mice and only partially restored with either agent alone (281).
Interestingly, also, leptin treatment restores euglycemia and normalizes peripheral insulin sensitivity in animal models of type 1 diabetes, (48, 62, 77, 87, 104, 271). The mechanisms responsible for the effects of leptin in type 1 diabetic animals remain to be fully elucidated. Although leptin replacement normalizes food intake in animals with type 1 diabetes, pair-feeding studies indicate that decreased food intake cannot account for the restoration of normoglycemia in leptin-treated type 1 diabetic animals (104). Leptin may affect insulin sensitivity either through centrally mediated mechanisms or through direct peripheral effects of leptin in insulin-sensitive tissues. Thus, leptin may regulate glucose metabolism through other peripheral and/or central pathways. Peripherally, leptin treatment may activate signaling pathways that are similar albeit not identical with those activated by insulin and may alleviate the increase in plasma glucagon and growth hormone, which may contribute to the improvement in insulin sensitivity and mediate the restoration of euglycemia (62). Leptin infusion, probably acting through a central mechanism, inhibits hepatic glucose production, an effect potentially related to the downregulation of the expression of gluconeogenic genes in the liver such as G-6-Pase and PEPCK (87).
The presence of the long and short forms of the ObR in peripheral tissues supports the notion that leptin exerts direct physiological effects independently of signaling through the central nervous system (272). Several studies have reported that leptin increases glucose uptake in isolated soleus muscle in vivo and in animal models (13, 14, 33). These findings suggest that leptin exerts insulin-like effects on skeletal muscle. Leptin has also been shown to increase skeletal muscle glucose and fatty acid oxidation both in vitro and in vivo (185, 197, 250). These effects of leptin may be especially important because excessive deposition of lipids in skeletal muscle has been implicated in the development of insulin resistance (92). The mechanism by which leptin can directly affect glucose and fatty acid metabolism in skeletal muscle remains to be elucidated. One proposed mechanism involves cross-talk between the leptin and insulin-signaling pathways, especially phosphatidylinositol 3-kinase (PI3K) (129, 179). Another candidate for mediating the effect of leptin action on skeletal muscle is activation of 5-AMP-activated protein kinase (AMPK). Leptin directly stimulated fatty acid oxidation via AMPK-dependent pathways in isolated soleus muscle from FVB mice (185).
Leptin also directly affects glucose metabolism in the liver, which seems to be one of the primary tissues where leptin acts. Although leptin treatment did not elicit any changes in glucose production in hepatocytes under basal conditions, leptin significantly decreased the production of glucose in incubated hepatocytes in the presence of several gluconeogenic precursors (32). These effects appear to be mediated through PI3K-dependent activation of phosphodiesterase 3B (PDE3B) (288). Leptin also affects hepatic lipid metabolism via activation of PI3K rather than activation of AMPK (112). In human liver cells, leptin alone had no effects on the insulin-signaling pathway, but leptin pretreatment transiently enhanced insulin-induced tyrosine phosphorylation and PI3K binding to IRS-1 (256). These findings are consistent with animal studies and suggest complex interactions between the leptin and insulin-signaling pathways on glucose metabolism in liver.
Leptin may alter the action of insulin in isolated adipocytes (214, 270) and may promote glucose and fatty acid oxidation and lipolysis (34). The observations from in vitro and in vivo studies suggest that leptin promotes energy dissipation and decreases lipid deposition in adipose tissue. Nevertheless, in human obesity and in some animal models of obesity, excessive lipid deposition in adipose tissues cannot be corrected despite the elevated circulating leptin levels. Apparently, there is a suppressive mechanism that impairs leptin action in adipose tissue. As expected, in studies using human preadipocytes and adipocytes from lean and obese subjects, leptin had no effect on basal and insulin-stimulated glucose uptake or on basal and isoproterenol-stimulated lipolysis (5). Recently, we performed detailed interventional and mechanistic signaling studies in human adipose tissue in vivo, ex vivo, and in vitro (190). Leptin administration induced activation of STAT3 and AMPK signaling in human adipose tissue, and there was no difference between male and female and obese and lean subjects. Although there is convincing evidence indicating that leptin has favorable effects on adipose tissue metabolism, its effect in humans still needs to be fully elucidated.
It is also generally accepted that leptin significantly reduces insulin release from pancreatic β-cells under physiological conditions (4, 101, 119, 208). There are several mechanisms by which leptin may suppress insulin secretion by acting directly on the pancreas. Leptin affects the ATP-sensitive K+ channels through PI3K-dependent activation of PDE3B (101) and reduces glucose transport into β-cells (139). Leptin has also been shown to suppress preproinsulin mRNA and insulin promoter activity in vitro and in vivo (136, 237, 238). In these studies, leptin reduced preproinsulin mRNA expression only under conditions with stimulation by incretin hormones or high glucose concentrations. Recently, several studies demonstrated that leptin effectively inhibits glucagon secretion from pancreatic α-cells (180, 265). These findings suggest that leptin may modulate glucose homeostasis by inhibiting not only β-cell insulin release but also α-cell glucagon secretion. These observations also suggest that leptin represents a signaling molecule from adipose tissue to the endocrine pancreas that suppresses insulin secretion according to the needs dictated by body fat stores, and this connection establishes a classic endocrine feedback loop, the so-called “adipoinsular axis.”
The effects of leptin on glucose metabolism are much less pronounced in human skeletal muscle compared with results from animal studies. Leptin had no effects on either basal or insulin-stimulated glucose uptake in human skeletal muscle cells from normal subjects, obese subjects, and patients with type 2 diabetes (218). Also, leptin directly increases fatty acid oxidation in skeletal muscle from lean subjects but not from obese subjects (251). These findings provide more direct evidence of peripheral leptin resistance in obese humans.
Humans with congenital or human immunodeficiency virus (HIV)-associated lipoatrophy also have very low leptin levels (200, 210) and present with a profound lack of subcutaneous fat, insulin resistance, fatty infiltration of the liver, and increased cardiovascular risk (30, 93). As described below, leptin administration improves metabolic parameters in these patients (73, 263). Although leptin may directly affect pancreatic β-cell function (143), act as an insulin sensitizer, and improve metabolism in leptin-deficient subjects, it does not improve insulin sensitivity in obese human subjects (186) and does not activate intracellular signaling pathways and/or improve diabetes and glycemic control in obese humans with type 2 diabetes (191). In a human trial of leptin treatment in type 2 diabetic patients, glycemic control and body weight were not changed appreciably after leptin treatment even though serum leptin concentrations increased 150-fold compared with baseline in the high-dose group (103). Together, these observations imply that modulation of insulin sensitivity by leptin is also likely permissive, with diminished efficacy above a certain threshold (25).
In summary, activation of ObRb by leptin sets off a cascade of intracellular signal transduction pathways, including mainly the Janus kinase 2 (JAK2)/STAT3 pathway (79), but also the PI3K pathway (134), mitogen-activated protein kinase (MAPK) pathways including extracellular signal-related kinases 1 and 2 (ERK1/2), c-Jun amino-terminal kinases (JNK), and p38 (239, 242), and 5′-AMP-activated protein kinase (AMPK) pathways (1, 152, 185). Activation of individual pathways in the leptin-signaling network appears to be differentially regulated, and these pathways are also likely to be regulated by various other hormonal, neuronal, and metabolic signals that cross-talk with leptin. We have recently demonstrated, utilizing in vitro, ex vivo, and in vivo signaling studies, that leptin administration activates the above-mentioned signaling pathways in a dose-response manner in several peripheral tissues, including fat, muscle, and peripheral mononuclear cells (190, 191). Moreover, we have shown that endoplasmic reticulum stress induces resistance to leptin signaling and that activation of signaling pathways is saturable at leptin levels of ∼50 ng/ml, which are levels seen in obese humans. These data can be interpreted as pointing to factors underlying leptin resistance in humans and need to replicated and extended.
Physiological Role of Leptin in the Regulation of Immune Function
Hypoleptinemic states are associated with increased risk of infection (24). A variety of immune cells express ObRb, and it is likely that leptin directly affects immune function (24, 155, 209). In ex vivo studies, leptin has been shown to enhance phagocytic activity in macrophages (160); promote production of proinflammatory cytokines such as tumor necrosis factor-α (TNF-α), interleukin-6, and interleukin-12 (154); and stimulate chemotaxis in polymorphonuclear cells (24, 29). In addition, we have shown that leptin promotes lymphocyte survival in vitro by suppressing Fas-mediated apoptosis (209). Overall, leptin promotes Th1 cell differentiation and cytokine production (182). An in vivo study showed that leptin administration improved the immune dysfunction of ob/ob mice and protected against immune dysfunction in acutely starved wild-type mice (110). Obese mice, in contrast, are tolerant to any effects of leptin administration (209).
The role of leptin in immune function also seems to be a permissive one in humans (16). Leptin-deficient children develop infections early in life, and many die because of these abnormalities (149). Leptin exogenously administered to children with congenital leptin deficiency greatly improves their immune function (67). In normal-weight women with a starvation-induced decrease in leptin to 2.8 ng/ml, leptin replacement had no major effect on fasting-induced changes on most immunophenotypes and did not affect in vitro proliferative and cytokine-producing capacity of T cells, suggesting that this degree of acute leptin deficiency only minimally disrupts immune function (40). Our group also showed that raising circulating leptin in lean men to levels between high physiological (as seen in obesity) and low pharmacological (as seen in extreme obesity) resulted in no significant increase in cytokine and inflammatory marker concentrations, including those known to be important in insulin resistance and cardiovascular disease, such as interferon-γ (IFNγ), interleukin-10, and TNF-α (36), a fact that, in part, may be due to saturable leptin signaling pathways in humans (190, 191). We found similar results in obese diabetic subjects after 4–16 weeks of leptin administration (36). Therefore, although leptin in replacement doses may restore the Th1/Th2 immune dysfunction in leptin-deficient animals and humans, leptin does not seem to be involved in the pathogenesis of an obesity-related proinflammatory state in humans. Thus, the role of leptin in the regulation of immune function seems to also be permissive in humans. It remains to be seen whether leptin administration can provide clinical benefits to subjects with negative energy balance such as cachexia or malnutrition, who are prone to and suffer from increased morbidity and mortality from infectious diseases (181).
Physiological Role of Leptin in the Regulation of Bone Metabolism
Early studies in mice suggested that leptin might decrease bone mass through mechanisms linking the central regulation of bone remodeling and energy metabolism (126). Initial radiographic and histological analyses revealed that ob/ob mice have a high bone mass phenotype, and intracerebroventricular infusion of leptin reduced bone mass in ob/ob and wild-type mice independently of weight loss (63). However, recent evidence suggests that leptin's action in murine bone varies among skeletal regions (50). ob/ob mice have significantly greater bone mineral density (BMD) and trabecular bone volume in the lumbar spine but lower BMD, trabecular bone volume, and cortical thickness in the femur than wild-type mice (50). In terms of whole body volume, ob/ob mice have lower bone mass than normal lean mice since the appendicular skeleton comprises 80% of total bone volume and consists primarily of cortical bone (50). There is a 20–25% decrease in total bone mass in the ob/ob and db/db mouse models, primarily at the site of cortical bone (98, 156, 252). Peripheral leptin administration has been shown to increase bone mineral content and density in ob/ob mice (98, 252).
Leptin affects bone metabolism through both central and peripheral pathways. In mice, leptin appears to regulate the formation of cortical bone via sympathetic activation (97). ob/ob mice have diminished sympathetic tone (257), and leptin action in the ventromedial hypothalamus (VMH) can increase sympathetic activity (97). Furthermore, compared with wild-type mice, β1- and β2-adrenergic receptor knockout mice exhibit decreased cortical thickness and femoral mass (97) and low leptin levels (176). Data from rodent studies also suggest that leptin may mediate cortical bone formation by regulating the expression of several hypothalamic neuropeptides. One study found that mice lacking the NPY receptor Y2 have femurs with increased cortical bone mass and density (10), indicating that NPY inhibits cortical bone formation (97). However, leptin injection into the PVN has been shown to increase expression of neuromedin U (279), which may decrease both cortical and trabecular bone mass (231). Moreover, Yadav et al. (280) have shown that brainstem-derived serotonin binds to Htr2c receptors on VMH neurons, favoring bone mass accrual, and that leptin inhibits this effect by reducing serotonin synthesis and firing of serotonergic neurons. Thus, it appears that leptin affects the expression of many neuropeptides that positively or negatively affect bone metabolism.
Peripherally administered leptin increases bone mass through interactions with bone marrow stromal cells, osteoblasts, and osteoclasts. In stromal cells, leptin increases expression of osteogenic genes and directs them to the osteogenic instead of the adipogenic pathway (98, 99, 259). Furthermore, leptin increases osteoblast proliferation, de novo collagen synthesis, and mineralization in in vitro and in vivo mouse studies (89). Finally, leptin decreases osteoclastogenesis by stimulating stromal cells to increase osteoprotegerin expression and by decreasing receptor activator of nuclear factor-κB (RANK) ligand (107).
It remains unclear whether data from rodent studies also apply to humans. For example, we have shown that, in contrast to findings in mice, leptin does not regulate short-term fasting-induced autonomic changes and/or catecholamine levels (41). Indeed, the contrasting effects of leptin in the axial and appendicular skeleton of ob/ob mice do not appear to manifest in humans, as individuals with leptin deficiency have decreased bone mass and density throughout the skeleton (97). Using dual-energy X-ray absorptiometry, we assessed bone mineral content (BMC) and BMD in normal-weight children and adolescents (222). We found no relationship between leptin and BMD or BMC for either total body or body regions, after adjusting for age, fat mass, bone-free fat mass, and serum IGF-I and estradiol concentrations (222). It has also been observed that leptin levels do not correlate with BMD or BMC in healthy postmenopausal women after adjustment for body composition (124). These data seem to indicate that leptin's effect on bone metabolism reflects that of fat mass and may be mediated through changes in estradiol and IGF-I or other hormone levels. This is consistent with our recent findings that in leptin (and thus estradiol and IGF-I) -deficient women with hypothalamic amenorrhea there might be a weak association between circulating leptin levels and BMD/BMC (7).
Hypotheses raised by these animal and observational studies in humans on the effects of leptin on bone metabolism in humans, including bone macro- and microarchitecture, as well as the underlying signaling pathways, have just started to be studied in the context of interventional studies in humans and merit further investigation. The observations to date collectively suggest that leptin's effect on bone metabolism may also be permissive and that leptin may have a distinct therapeutic role in treating the low bone density of several disease states associated with negative energy balance (see below, Hypothalamic Amenorrhea).
LEPTIN DEFICIENCY AND LEPTIN THERAPY IN HUMAN DISEASE
Leptin replacement has been investigated for its physiological role in several conditions characterized by leptin deficiency. The main conditions that have been studied are congenital leptin deficiency, lipodystrophy, hypothalamic amenorrhea, and weight loss (39, 128).
Congenital Leptin Deficiency
Congenital leptin deficiency is a rare autosomal recessive disease caused by mutations in the leptin gene. In addition to marked obesity mainly due to hyperphagia, congenital leptin deficiency is associated with inadequate secretion of GnRH, manifesting in hypogonadotropic hypogonadism and, in most cases, failure to reach puberty, including absence of growth spurt, secondary sex characteristics, and menarche (253).
Leptin replacement reverses several of the changes seen with congenital leptin deficiency. There is usually marked weight loss. For example, in three adult patients treated with leptin, the average BMI dropped from 51.2 to 29.5 kg/m2 (212). Leptin replacement can also improve the hyperinsulinemia and dyslipidemia seen in these individuals (67, 88). In terms of neuroendocrine function, leptin replacement permits the appropriate progression through puberty (66, 67, 148). In an uncontrolled study of leptin replacement in three children with congenital leptin deficiency (67), a gradual increase in gonadotropins and estradiol and normalization of pulsatile secretion of LH and FSH were observed after 24 mo of leptin therapy in one child who was of appropriate pubertal age (67). In a similar study in adults with congenital leptin deficiency, it was observed that leptin replacement in men increased testosterone levels, and facial, pubic, and axillary hair, promoted penile and testicular growth, and permitted normal ejaculatory patterns (148).
Less dramatic changes were seen in the other neuroendocrine axes. A small, uncontrolled interventional study reported no major change in TSH after leptin replacement in children with congenital leptin deficiency, although participants did exhibit increased levels of triiodothyronine and thyroxine (67). Another uncontrolled study of three adults and one boy with congenital leptin deficiency observed normal thyroid function and no changes with leptin replacement (144, 211). Despite the fact that patients with congenital leptin deficiency have age- and sex-appropriate BMC and BMD, leptin treatment increases their skeletal maturation (67).
Finally, in terms of immune function, individuals with congenital leptin deficiency have a greater incidence of infection than the general population (205) due to decreased proliferation and function of CD4+ T cells, which normalizes with exogenous leptin administration (67). Leptin is currently available for life-long treatment of subjects with congenital leptin deficiency through an Amylin-sponsored compassionate leptin access program (73, 168).
Lipodystrophy
Lipodystrophy and lipoatrophy are disorders of adipose tissue characterized by loss of subcutaneous adipose tissue, usually associated with an increase in visceral adipose tissue. Congenital lipoatrophy is a rare autosomal recessive condition often associated with consanguineous marriage (201, 210). Leptin replacement has been studied in approximately 100 subjects with this condition in the context of small, uncontrolled interventional studies. These showed that leptin replacement therapy dramatically improves dyslipidemia and insulin sensitivity in these individuals and reduces glycosylated hemoglobin and hepatic gluconeogenesis and intrahepatic fat content (121, 122, 203, 215). Hb A1c is reduced by ∼3% in these subjects who are not responsive to other antihyperglycemic medications and/or high doses of insulin. Furthermore, leptin also normalizes testosterone levels and restores menstrual function in women with lipoatrophy. An open-label study in individuals with congenital lipoatrophy found that leptin replacement increased LH response to GnRH in women and increased testosterone levels in men (198). In addition, all eight women who had been amenorrheic prior to treatment resumed normal menses (198). Studies in both rodents and humans have shown that the improvement in glucose metabolism may be a direct effect of leptin replacement or an indirect effect due to reduction in ectopic fat, i.e., fat deposited in tissues other than adipose tissue such as the liver and muscle (24). Indeed, we have recently shown that leptin administration activated, in vitro, ex vivo, and in vivo, intracellular signaling pathways that overlap with those activated by insulin (190, 191). Thus, in addition to its lipolytic effect, which may indirectly improve metabolism and insulin resistance, leptin may also have direct effects in metabolically important tissues by directly activating signaling pathways that overlap, but are not completely identical, with signaling pathways activated by insulin (190, 191).
HIV-associated lipoatrophy is a much more prevalent condition, affecting an estimated 15–36% of HIV-infected patients (120) and is closely linked with insulin resistance and dysregulation of peripheral adipose tissue metabolism (159, 235, 262). In our initial randomized, placebo-controlled, interventional study, we found that leptin in physiological doses improves insulin resistance, hyperlipidemia, and truncal fat mass in these patients (23, 141). These results were confirmed by a longer independent study of open-label leptin treatment for 6 mo, which further demonstrated that the improvement in whole body insulin resistance in lipoatrophic patients is predominantly due to improved hepatic (i.e., suppression of endogenous glucose production) but not skeletal muscle (i.e., stimulation of glucose uptake) insulin sensitivity (195). Recently, we also demonstrated that leptin treatment in HIV lipoatrophic patients treated with pioglitazone also improves postprandial glucose metabolism (158). The favorable effects of leptin on insulin sensitivity in these patients could in part be due to potentiating the effect of pioglitazone (a thiazolidinedione) (84) on adiponectin secretion and plasma concentration (158), because leptin administration alone does not alter adiponectin levels (83, 141).
Studies performed to date indicate that leptin's ability to improve metabolic control, lipid levels, and truncal fat mass is comparable to or better than that induced by insulin sensitizers (169). Leptin may have beneficial effects through its lipolytic effect and/or by altering levels of other peripherally secreted molecules important in regulating insulin resistance and metabolism (6, 43, 82, 96, 125, 232, 254, 276). Nevertheless, leptin improves insulin resistance not only by decreasing body weight and fat mass, especially ectopic fat or intra-abdominal fat (192, 195), but also by activating insulin-sensitive tissues, including adipose tissue and liver (134). Leptin activates signaling pathways that overlap with those of insulin, including STAT3, MAPK, and PI3K in mice (134). As mentioned above, we have confirmed these findings and have extended them by performing in vivo, ex vivo, and in vitro studies in humans, revealing that leptin activated signaling pathways overlapping with those activated by insulin (190). Further study of these signaling pathways and possibly the identification of loci of leptin resistance may be of therapeutic significance, since it may identify areas where future intervention may improve leptin tolerance or resistance and thus help treat insulin resistance in obese, hyperleptinemic subjects who are currently tolerant of or resistant to the insulin-sensitizing effect of leptin (255).
Hypothalamic Amenorrhea
Hypothalamic amenorrhea is a common cause of absent menstrual periods and infertility. It is typically seen in women who are in a state of relative energy deficiency, such as those who exercise vigorously or have a low body fat mass such as in anorexia nervosa. These women are hypoleptinemic (19, 127).
Leptin replacement may be a promising treatment for infertility in women with hypothalamic amenorrhea (18, 273). In our proof-of-concept trial of leptin replacement, we found that leptin can normalize LH concentrations and pulse frequency within weeks of treatment and can restore ovulatory function after only months of treatment (273). Since many participants were amenorrheic for years, these results are remarkable and were independent of changes in body weight and fat mass (273). We have recently confirmed these findings in a larger double-blinded, randomized, placebo-controlled study of leptin replacement for 9 mo (49). Together, our results indicate that leptin therapy resulted in resumption of menses with irregular but sustained menstrual cycles in ∼70% of the subjects. Additionally, ∼60% of the subjects who menstruated also ovulated. Menstruation appeared at various stages of leptin therapy, ranging from 4 to 8 wk after initiation of treatment in the open-label 10-wk study and from 4 to 32 wk in the randomized, placebo-controlled 9-mo study (49, 273). Importantly, increasing the administered dose of leptin in some women who did not menstruate with the lower leptin dose turned out to be a feasible way to resolve amenorrhea (49).
Leptin replacement in these women may also affect other neuroendocrine axes (17). The effects on thyroid hormones were modest. In women with hypothalamic amenorrhea and average baseline leptin concentration of 3.4 ng/ml, leptin treatment significantly increased triiodothyronine and free thyroxine levels but did not affect TSH levels (273). Lesser effects were observed in another group of such women with baseline leptin concentration of 4.6 ng/ml, in whom leptin replacement mildly increased free triiodothyronine and did not affect free thyroxine or TSH concentrations (49).
We also observed beneficial changes in the growth hormone axis with leptin replacement. There was a significant increase in IGF-I and IGFBP3 levels after leptin administration for 10 wk (273), whereas 9 mo of leptin replacement only marginally affected IGF-I and IGFBP3 concentrations but led to increased ratio of IGF-I to IGFBP3 (49), indicating that leptin augments the availability of free IGF-I. We found no change in the adrenal axis in our earlier open-label study of leptin replacement (37, 273), although there was a small but statistically significant decrease in cortisol concentration after leptin replacement in our recent randomized, placebo-controlled trial (49). These data in leptin-deficient women are in contrast to data demonstrating a lack of an effect of leptin administration to improve bone metabolism in obese and thus presumably leptin-tolerant or -resistant women (55, 90). Finally, leptin replacement in women with hypothalamic amenorrhea and chronic relative leptin deficiency leads to greater activation of the TNF-α system (42), but the full effects on the immune system remain to be elucidated.
In women with hypothalamic amenorrhea, we also reported a significant increase in bone formation markers (osteocalcin and bone-specific alkaline phosphatase) after leptin administration for 10 wk but no change in total body and regional BMC and BMD, which was not surprising given the short duration of the study (273). Moreover, we were unable to determine whether this increase in bone formation markers is a direct effect of leptin or an indirect effect mediated by restoration of estradiol and/or IGF-I levels. In a recent randomized, placebo-controlled study (247), we confirmed the leptin-induced increase in markers of bone formation after 9 mo of leptin treatment in women with hypothalamic amenorrhea. Importantly, we documented a significant increase in lumbar spine BMC and BMD by 4–6% in six of those women who continued beyond the first 9 mo of double-blinded treatment to an open-label leptin replacement study for an additional year (247). These data indicate that long-term (2 yr) leptin replacement has a favorable effect on bone (predominantly trabecular bone), although we cannot rule out the possibility that changes would also manifest at the other sites examined (hip, radius) with more prolonged treatment. Interestingly, after 2 yr of leptin replacement, we observed a decrease in markers of bone resorption such as CTX (cross-linked COOH-terminal telopeptide of type 1 collagen), as opposed to the increase in markers of bone formation earlier into treatment (49, 273).
Weight Loss
As described above, leptin levels are higher in most obese subjects (173) and reduced in the setting of acute starvation. The reduction of fat mass occurring during weight loss results in decreased leptin concentrations (189, 172). Emerging evidence suggests that leptin may play a more important role in weight loss maintenance rather than weight loss per se. As leptin levels fall, energy expenditure, sympathetic nervous system tone, and thyroid hormones decrease to collectively drive patients to regain weight (223).
In a study of lean and obese subjects with relative hypoleptinemia (10–60 ng/ml) due to moderate weight loss, leptin replacement to increase serum leptin concentration to pre-weight loss levels over an average period of 8 wk resulted in an increase in circulating concentrations of triiodothyronine and thyroxine but no change in TSH, which was reduced compared with baseline (223). This effect of leptin may be especially important because the mechanisms through which body weight returns to baseline are thought to involve a compensatory decrease in energy expenditure (146) due to decreasing thyroid hormone levels resulting in increased skeletal muscle work efficiency (223, 226). However, other studies in overweight and obese subjects during severe (113) and mild (244) caloric restriction found no effects of leptin administration in pharmacological doses on circulating thyroid hormone levels. The lack of a significant change in TSH levels in most studies may be due to the pulsatile nature of TSH, requiring careful timing of sampling. It has been proposed that leptin may directly stimulate thyroxine release from the thyroid gland and/or increase the bioactivity of TSH (223).
On the other hand, leptin administration does not have major effects on other physiological processes that have been studied. Pharmacological leptin administration in overweight and obese subjects during weight loss induced by severe (113) and mild (244) hypocaloric diet does not affect IGF-I and IGFBP3 concentrations or their ratio. Furthermore, a recent study demonstrated that leptin replacement in normal-weight, overweight, and obese subjects after moderate weight loss (in whom leptin concentration dropped relative to baseline but did not reach clearly hypoleptinemic levels) failed to affect circulating markers of bone formation and resorption (55). In addition, functional magnetic resonance imaging data also suggest that leptin treatment can prevent the changes in brain activity involved in the regulatory, emotional, and cognitive control of food intake following weight loss (225).
The role of leptin in weight loss maintenance is an area of active investigation, and there are currently several clinical trials under way that aim at further evaluating the role of leptin treatment in weight loss maintenance (ClinicalTrials.gov: NCT00265980, NCT01155180, NCT00073242), the results of which are eagerly awaited. It is expected, however, that leptin alone will not be sufficient to overcome the problem of leptin tolerance or resistance associated with obesity and that the development of novel medication with the ability to boost leptin's efficiency will eventually be needed.
LEPTIN EXCESS DUE TO LEPTIN RESISTANCE OR TOLERANCE IN HUMAN DISEASE
In contrast to obese subjects with congenital leptin deficiency, garden-variety obese individuals have greater leptin concentrations than lean individuals and are resistant or tolerant to the effects of leptin (57, 191). Initial attempts to utilize leptin as a monotherapy for obesity included using supraphysiological doses of leptin (75, 103, 147); however, data from these trials revealed modest, if any, weight loss and with significant variability among the participants. In a small, proof-of-concept, phase II clinical trial, Heymsfield et al. (103) administered leptin in escalating doses to obese participants for up to 24 wk. Although a dose-dependent response was noted, participants exhibited only modest weight loss despite supraphysiological doses of leptin (103). With the highest dose of leptin at 0.30 mg·kg−1·day−1, obese participants who completed the study lost a mean of 7.1 kg; however, when participants who withdrew from the study were included in the analysis, there was only a mean weight loss of 3.3 kg (103). Furthermore, there was significant variability among the participants in the response to leptin treatment (103). Hukshorn et al. (114) have also tried pegylated leptin, which has a much longer half-life. In their randomized, double-blinded trial in obese men receiving weekly injections of pegylated leptin for 12 wk, there were no significant differences in weight loss or percent body fat (114). Generally, only at supraphysiological doses does leptin induce some degree of weight loss in obese subjects, and even then the effect is very mild and likely clinically insignificant (103, 113). In accordance with the concept of leptin resistance or tolerance in obesity, the effects of leptin on weight loss and other physiological parameters appear to be most pronounced during states of relative leptin deficiency, e.g., fasting and hypothalamic amenorrhea or lipoatrophy (26, 141, 195).
Leptin resistance or tolerance was first thought to be due to mutations of the leptin receptor (51) and other rare monogenic obesity syndromes (68). However, most instances appear to be multifactorial; only a few cases of human obesity are due to monogenic syndromes (69). Although it was determined that the exact defect in the leptin receptor present in db/db mice is not present in obese humans, several genetic variants are associated with hyperleptinemia (56), including the Lys109Arg or Gln223Arg mutation in the leptin receptor (LEPR) gene (138), Mutations of other genes downstream of leptin, including POMC and the melanocortin 4 receptor (MC4R), also result in an obese phenotype with associated neuroendocrine dysfunction (70). Additional variants include the 241G/A variant (the Val18 missense mutation) of the melanocortin 3 receptor (MC3R) (284) and the two single-nucleotide polymorphisms (SNPs) rs17817449 and rs1421085 found in the fatso/fat mass- and obesity-associated gene (FTO) (267). Other genetic variants that may predispose patients to develop leptin resistance or tolerance remain to be further investigated.
Leptin transport across the blood-brain barrier is impaired in obesity. This is partially due to saturation of the transporter as a result of hyperleptinemia and a subsequent decrease in transport activity (11, 28, 269). Moreover, different brain regions may saturate at different concentrations. Banks et al. (12) used a brain perfusion method to deliver leptin at certain concentrations in mice. By use of a concentration seen in hypoleptinemic lean humans (1 ng/ml), the hypothalamus contained a higher concentration of leptin than any other brain region. At the concentration representing obese humans (30 ng/ml), the hypothalamus contained less leptin than other regions (12). The selective activation of brain regions at certain concentrations implies that there can be different thresholds for different actions of leptin (12). In addition, the soluble leptin receptor isoform ObRe antagonizes leptin transport by inhibiting surface binding and endocytosis of leptin (264).
Leptin signaling is subject to negative feedback regulation, which may be more pronounced in the obese state and associated hyperleptinemia (204). The expression of SOCS3, a major inhibitor of leptin signaling, is induced by the JAK2/STAT3 pathway, and SOCS3 acts to inhibit the phosphorylation and activation of JAK2 and ObRb tyrosine residue Tyr985 (199). Leptin administration leads to increased STAT3 expression and greater weight loss in SOCS3 knockout mice (109, 193). Another negative regulator of STAT3-mediated gene induction is SHP2, which is also activated by leptin (31). Finally, protein-tyrosine phosphatase-1B (PTP1B) is another molecule that may inhibit leptin signaling by dephosphorylating JAK2. PTP1B knockout mice have increased leptin sensitivity and reduced fatness (204). These feedback mechanisms may explain why excessive leptin can actually result in the same disturbances as leptin deficiency (e.g., insulin resistance and infertility) (24). We (190, 191) have recently confirmed that similar signaling pathways are activated by leptin in humans, but it remains currently unknown whether leptin signaling inhibition does exist in humans in vivo or whether leptin signaling is simply saturable at relatively higher leptin levels. This remains an area of active investigation in our laboratory.
Endoplasmic reticulum stress has recently been shown to play a role in the development of leptin resistance. Obese diabetic animals and humans have been shown to have higher levels of endoplasmic reticulum stress in the liver, adipose tissue, and pancreatic β-cells (21, 177, 207, 241). Studies in obese mice have shown that increased endoplasmic reticulum stress inhibits leptin receptor signaling in the hypothalamus, and chemical chaperones that decrease endoplasmic reticulum stress have been shown to increase leptin sensitivity (206). Furthermore, induction of endoplasmic reticulum stress via intracerebroventricular administration of thapsigargin results in increased food intake and body weight (277). We have recently performed a series of in vivo, ex vivo, and in vitro signaling studies in lean and obese nondiabetic and type 2 diabetic men and women and found that endoplasmic reticulum stress attenuates or completely inhibits leptin signaling (190, 191). Thus, the negative effect of endoplasmic reticulum stress on leptin sensitivity may also contribute to insulin resistance.
Targeting these mechanisms of leptin resistance will be important in the treatment for obesity and has led to development of leptin sensitizers, including the chemical chaperones mentioned above. Roth et al. (228) have also proposed that amylin may act synergistically with leptin to induce fat-specific weight loss, and the amylin analog pramlintide has been tried in conjunction with leptin in clinical trials for weight loss with modest effects (219). In a recent phase II clinical trial, the combination of pramlintide and human recombinant leptin in obese and overweight human subjects produced significantly more weight loss than either treatment alone (219). The results, however, appeared to be additive rather than synergistic, implying that amylin may have independent effects on weight loss but does not likely improve sensitivity to leptin. We have recently shown that leptin administration does not influence circulating amylin levels (116) and subsequently we have performed ex vivo and in vitro signaling studies that are consistent with these clinical observations (143, 292). We demonstrated that leptin and amylin alone and in combination activate STAT3, AMP-activated protein kinase, Akt, and extracellular signal-regulated kinase signaling pathways in human adipose tissue ex vivo and human primary adipocytes and peripheral blood mononuclear cells in vitro; all phosphorylation events were saturable at leptin and amylin concentrations of ∼50 and 20 ng/ml, respectively (190). These data indicate that leptin and amylin activate overlapping intracellular signaling pathways in humans and have additive, but not synergistic, effects. Moreover, the saturable nature of leptin signaling may underly the development of tolerance to leptin's effects at levels above ∼50 ng/ml (190, 191). Results of studies on combination treatments or the development of leptin “sensitizers” that would enhance leptin's efficacy are awaited with great interest.
CONCLUSIONS AND FUTURE DIRECTIONS
Observational and interventional studies in animals and humans have shown that leptin contributes to the regulation of energy homeostasis, neuroendocrine function, metabolism, immune function, and bone metabolism (78). Both complete (e.g., congenital leptin deficiency) and partial (e.g., hypothalamic amenorrhea, lipoatrophy) leptin deficiency states present with dysfunction in these systems that can be reversed, in part or in whole, with leptin treatment. Although the vast majority of obese individuals are hyperleptinemic and resistant or tolerant to exogenous leptin, further research is needed to elucidate whether leptin sensitizers will be useful and if leptin has a role in weight loss maintenance. For the time being, available data suggest that leptin deficiency is not so different from other hormone deficiency states and can thus be treated accordingly (60). Thus, it is anticipated that large double-blinded phase III clinical trials in humans will prove in the not so distant future the role of leptin administration in replacement doses to correct abnormalities in leptin-deficient disease states such as lipodystrophy or hypothalamic amenorrhea. By contrast, states of leptin excess, such as obesity and type 2 diabetes, appear to be states associated with leptin tolerance, in which leptin alone is not expected to have appreciable clinical significance.
DISCLOSURES
No conflicts of interest are reported by the author(s).
ACKNOWLEDGMENTS
The Mantzoros group is supported by Grants DK-58785, DK-079929, and DK-081913 from the National Institute of Diabetes and Digestive and Kidney Diseases and a VA Merit Review Award.
REFERENCES
- 1. Ahima RS. Adipose tissue as an endocrine organ. Obesity (Silver Spring) 14, Suppl 5: 242S–249S, 2006 [DOI] [PubMed] [Google Scholar]
- 2. Ahima RS, Dushay J, Flier SN, Prabakaran D, Flier JS. Leptin accelerates the onset of puberty in normal female mice. J Clin Invest 99: 391–395, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Ahima RS, Prabakaran D, Mantzoros C, Qu D, Lowell B, Maratos-Flier E, Flier JS. Role of leptin in the neuroendocrine response to fasting. Nature 382: 250–252, 1996 [DOI] [PubMed] [Google Scholar]
- 4. Ahren B, Havel PJ. Leptin inhibits insulin secretion induced by cellular cAMP in a pancreatic B cell line (INS-1 cells). Am J Physiol Regul Integr Comp Physiol 277: R959–R966, 1999 [DOI] [PubMed] [Google Scholar]
- 5. Aprath-Husmann I, Rohrig K, Gottschling-Zeller H, Skurk T, Scriba D, Birgel M, Hauner H. Effects of leptin on the differentiation and metabolism of human adipocytes. Int J Obes Relat Metab Disord 25: 1465–1470, 2001 [DOI] [PubMed] [Google Scholar]
- 6. Aronis KN, Diakopoulos KN, Fiorenza CG, Chamberland JP, Mantzoros CS. Leptin administered in physiological or pharmacological doses does not regulate circulating angiogenesis factors in humans. Diabetologia 54: 2358–2367, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Aronis KN, Kilim H, Chamberland JP, Breggia A, Rosen C, Mantzoros CS. Preadipocyte factor-1 levels are higher in women with Hypothalamic Amenorrhea and are associated with bone mineral content and bone mineral density through a mechanism independent of leptin. J Clin Endocrinol Metab July 27 [Epub ahead of print] PMID: 21795455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Asimakopoulos B, Milousis A, Gioka T, Kabouromiti G, Gianisslis G, Troussa A, Simopoulou M, Katergari S, Tripsianis G, Nikolettos N. Serum pattern of circulating adipokines throughout the physiological menstrual cycle. Endocr J 56: 425–433, 2009 [DOI] [PubMed] [Google Scholar]
- 9. Audi L, Mantzoros CS, Vidal-Puig A, Vargas D, Gussinye M, Carrascosa A. Leptin in relation to resumption of menses in women with anorexia nervosa. Mol Psychiatry 3: 544–547, 1998 [DOI] [PubMed] [Google Scholar]
- 10. Baldock PA, Allison S, McDonald MM, Sainsbury A, Enriquez RF, Little DG, Eisman JA, Gardiner EM, Herzog H. Hypothalamic regulation of cortical bone mass: opposing activity of Y2 receptor and leptin pathways. J Bone Miner Res 21: 1600–1607, 2006 [DOI] [PubMed] [Google Scholar]
- 11. Banks WA. Leptin transport across the blood-brain barrier: implications for the cause and treatment of obesity. Curr Pharm Des 7: 125–133, 2001 [DOI] [PubMed] [Google Scholar]
- 12. Banks WA, Clever CM, Farrell CL. Partial saturation and regional variation in the blood-to-brain transport of leptin in normal-weight mice. Am J Physiol Endocrinol Metab 278: E1158–E1165, 2000 [DOI] [PubMed] [Google Scholar]
- 13. Bates SH, Gardiner JV, Jones RB, Bloom SR, Bailey CJ. Acute stimulation of glucose uptake by leptin in l6 muscle cells. Horm Metab Res 34: 111–115, 2002 [DOI] [PubMed] [Google Scholar]
- 14. Berti L, Kellerer M, Capp E, Haring HU. Leptin stimulates glucose transport and glycogen synthesis in C2C12 myotubes: evidence for a PI3-kinase mediated effect. Diabetologia 40: 606–609, 1997 [DOI] [PubMed] [Google Scholar]
- 15. Bjorbaek C, Elmquist JK, Michl P, Ahima RS, van Bueren A, McCall AL, Flier JS. Expression of leptin receptor isoforms in rat brain microvessels. Endocrinology 139: 3485–3491, 1998 [DOI] [PubMed] [Google Scholar]
- 16. Bluher S, Mantzoros CS. Leptin in humans: lessons from translational research. Am J Clin Nutr 89: 991S–997S, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Bluher S, Mantzoros CS. Leptin in reproduction. Curr Opin Endocrinol Diabetes Obes 14: 458–464, 2007 [DOI] [PubMed] [Google Scholar]
- 18. Bluher S, Mantzoros CS. The role of leptin in regulating neuroendocrine function in humans. J Nutr 134: 2469S–2474S, 2004 [DOI] [PubMed] [Google Scholar]
- 19. Bluher S, Shah S, Mantzoros CS. Leptin deficiency: clinical implications and opportunities for therapeutic interventions. J Investig Med 57: 784–788, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Boden G, Chen X, Mozzoli M, Ryan I. Effect of fasting on serum leptin in normal human subjects. J Clin Endocrinol Metab 81: 3419–3423, 1996 [DOI] [PubMed] [Google Scholar]
- 21. Boden G, Duan X, Homko C, Molina EJ, Song W, Perez O, Cheung P, Merali S. Increase in endoplasmic reticulum stress-related proteins and genes in adipose tissue of obese, insulin-resistant individuals. Diabetes 57: 2438–2444, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Bray GA, York DA. Hypothalamic and genetic obesity in experimental animals: an autonomic and endocrine hypothesis. Physiol Rev 59: 719–809, 1979 [DOI] [PubMed] [Google Scholar]
- 23. Brennan AM, Lee JH, Tsiodras S, Chan JL, Doweiko J, Chimienti SN, Wadhwa SG, Karchmer AW, Mantzoros CS. r-metHuLeptin improves highly active antiretroviral therapy-induced lipoatrophy and the metabolic syndrome, but not through altering circulating IGF and IGF-binding protein levels: observational and interventional studies in humans. Eur J Endocrinol 160: 173–176, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Brennan AM, Mantzoros CS. Drug Insight: the role of leptin in human physiology and pathophysiology—emerging clinical applications. Nat Clin Pract Endocrinol Metab 2: 318–327, 2006 [DOI] [PubMed] [Google Scholar]
- 25. Brennan AM, Mantzoros CS. Leptin and adiponectin: their role in diabetes. Curr Diab Rep 7: 1–2, 2007 [DOI] [PubMed] [Google Scholar]
- 26. Brinkoetter M, Magkos F, Vamvini M, Mantzoros CS. Leptin treatment reduces body fat but does not affect lean body mass or the myostatin-follistatin-activin axis in lean hypoleptinemic women. Am J Physiol Endocrinol Metab 301: E99–E104, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Buettner R, Newgard CB, Rhodes CJ, O'Doherty RM. Correction of diet-induced hyperglycemia, hyperinsulinemia, and skeletal muscle insulin resistance by moderate hyperleptinemia. Am J Physiol Endocrinol Metab 278: E563–E569, 2000 [DOI] [PubMed] [Google Scholar]
- 28. Burguera B, Couce ME, Curran GL, Jensen MD, Lloyd RV, Cleary MP, Poduslo JF. Obesity is associated with a decreased leptin transport across the blood-brain barrier in rats. Diabetes 49: 1219–1223, 2000 [DOI] [PubMed] [Google Scholar]
- 29. Caldefie-Chezet F, Poulin A, Vasson MP. Leptin regulates functional capacities of polymorphonuclear neutrophils. Free Radic Res 37: 809–814, 2003 [DOI] [PubMed] [Google Scholar]
- 30. Capeau J, Magre J, Lascols O, Caron M, Bereziat V, Vigouroux C, Bastard JP. Diseases of adipose tissue: genetic and acquired lipodystrophies. Biochem Soc Trans 33: 1073–1077, 2005 [DOI] [PubMed] [Google Scholar]
- 31. Carpenter LR, Farruggella TJ, Symes A, Karow ML, Yancopoulos GD, Stahl N. Enhancing leptin response by preventing SH2-containing phosphatase 2 interaction with Ob receptor. Proc Natl Acad Sci USA 95: 6061–6066, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Ceddia RB, Lopes G, Souza HM, Borba-Murad GR, William WN, Jr, Bazotte RB, Curi R. Acute effects of leptin on glucose metabolism of in situ rat perfused livers and isolated hepatocytes. Int J Obes Relat Metab Disord 23: 1207–1212, 1999 [DOI] [PubMed] [Google Scholar]
- 33. Ceddia RB, William WN, Jr, Curi R. Comparing effects of leptin and insulin on glucose metabolism in skeletal muscle: evidence for an effect of leptin on glucose uptake and decarboxylation. Int J Obes Relat Metab Disord 23: 75–82, 1999 [DOI] [PubMed] [Google Scholar]
- 34. Ceddia RB, William WN, Jr, Lima FB, Curi R. Leptin inhibits insulin-stimulated incorporation of glucose into lipids and stimulates glucose decarboxylation in isolated rat adipocytes. J Endocrinol 158: R7–R9, 1998 [DOI] [PubMed] [Google Scholar]
- 35. Chan JL, Bluher S, Yiannakouris N, Suchard MA, Kratzsch J, Mantzoros CS. Regulation of circulating soluble leptin receptor levels by gender, adiposity, sex steroids, and leptin: observational and interventional studies in humans. Diabetes 51: 2105–2112, 2002 [DOI] [PubMed] [Google Scholar]
- 36. Chan JL, Bullen J, Stoyneva V, Depaoli AM, Addy C, Mantzoros CS. Recombinant methionyl human leptin administration to achieve high physiologic or pharmacologic leptin levels does not alter circulating inflammatory marker levels in humans with leptin sufficiency or excess. J Clin Endocrinol Metab 90: 1618–1624, 2005 [DOI] [PubMed] [Google Scholar]
- 37. Chan JL, Heist K, DePaoli AM, Veldhuis JD, Mantzoros CS. The role of falling leptin levels in the neuroendocrine and metabolic adaptation to short-term starvation in healthy men. J Clin Invest 111: 1409–1421, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Chan JL, Mantzoros CS. Leptin and the hypothalamic-pituitary regulation of the gonadotropin-gonadal axis. Pituitary 4: 87–92, 2001 [DOI] [PubMed] [Google Scholar]
- 39. Chan JL, Mantzoros CS. Role of leptin in energy-deprivation states: normal human physiology and clinical implications for hypothalamic amenorrhoea and anorexia nervosa. Lancet 366: 74–85, 2005 [DOI] [PubMed] [Google Scholar]
- 40. Chan JL, Matarese G, Shetty GK, Raciti P, Kelesidis I, Aufiero D, De Rosa V, Perna F, Fontana S, Mantzoros CS. Differential regulation of metabolic, neuroendocrine, and immune function by leptin in humans. Proc Natl Acad Sci USA 103: 8481–8486, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Chan JL, Mietus JE, Raciti PM, Goldberger AL, Mantzoros CS. Short-term fasting-induced autonomic activation and changes in catecholamine levels are not mediated by changes in leptin levels in healthy humans. Clin Endocrinol (Oxf) 66: 49–57, 2007 [DOI] [PubMed] [Google Scholar]
- 42. Chan JL, Moschos SJ, Bullen J, Heist K, Li X, Kim YB, Kahn BB, Mantzoros CS. Recombinant methionyl human leptin administration activates signal transducer and activator of transcription 3 signaling in peripheral blood mononuclear cells in vivo and regulates soluble tumor necrosis factor-alpha receptor levels in humans with relative leptin deficiency. J Clin Endocrinol Metab 90: 1625–1631, 2005 [DOI] [PubMed] [Google Scholar]
- 43. Chan JL, Stoyneva V, Kelesidis T, Raciti P, Mantzoros CS. Peptide YY levels are decreased by fasting and elevated following caloric intake but are not regulated by leptin. Diabetologia 49: 169–173, 2006 [DOI] [PubMed] [Google Scholar]
- 44. Chan JL, Williams CJ, Raciti P, Blakeman J, Kelesidis T, Kelesidis I, Johnson ML, Thorner MO, Mantzoros CS. Leptin does not mediate short-term fasting-induced changes in growth hormone pulsatility but increases IGF-I in leptin deficiency states. J Clin Endocrinol Metab 93: 2819–2827, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Chan JL, Wong SL, Mantzoros CS. Pharmacokinetics of subcutaneous recombinant methionyl human leptin administration in healthy subjects in the fed and fasting states: regulation by gender and adiposity. Clin Pharmacokinet 47: 753–764, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Chan JL, Wong SL, Orlova C, Raciti P, Mantzoros CS. Pharmacokinetics of recombinant methionyl human leptin after subcutaneous administration: variation of concentration-dependent parameters according to assay. J Clin Endocrinol Metab 92: 2307–2311, 2007 [DOI] [PubMed] [Google Scholar]
- 47. Chehab FF, Lim ME, Lu R. Correction of the sterility defect in homozygous obese female mice by treatment with the human recombinant leptin. Nat Genet 12: 318–320, 1996 [DOI] [PubMed] [Google Scholar]
- 48. Chinookoswong N, Wang JL, Shi ZQ. Leptin restores euglycemia and normalizes glucose turnover in insulin-deficient diabetes in the rat. Diabetes 48: 1487–1492, 1999 [DOI] [PubMed] [Google Scholar]
- 49. Chou SH, Chamberland JP, Liu X, Matarese G, Gao C, Stefanakis R, Brinkoetter MT, Gong H, Arampatzi K, Mantzoros CS. Leptin is an effective treatment for hypothalamic amenorrhea. Proc Natl Acad Sci USA 108: 6585–6590, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Cirmanova V, Bayer M, Starka L, Zajickova K. The effect of leptin on bone: an evolving concept of action. Physiol Res 57, Suppl 1: S143–S151, 2008 [DOI] [PubMed] [Google Scholar]
- 51. Clement K, Vaisse C, Lahlou N, Cabrol S, Pelloux V, Cassuto D, Gourmelen M, Dina C, Chambaz J, Lacorte JM, Basdevant A, Bougneres P, Lebouc Y, Froguel P, Guy-Grand B. A mutation in the human leptin receptor gene causes obesity and pituitary dysfunction. Nature 392: 398–401, 1998 [DOI] [PubMed] [Google Scholar]
- 52. Cohen SE, Kokkotou E, Biddinger SB, Kondo T, Gebhardt R, Kratzsch J, Mantzoros CS, Kahn CR. High circulating leptin receptors with normal leptin sensitivity in liver-specific insulin receptor knock-out (LIRKO) mice. J Biol Chem 282: 23672–23678, 2007 [DOI] [PubMed] [Google Scholar]
- 53. Coleman DL. Effects of parabiosis of obese with diabetes and normal mice. Diabetologia 9: 294–298, 1973 [DOI] [PubMed] [Google Scholar]
- 54. Coleman DL. A historical perspective on leptin. Nat Med 16: 1097–1099, 2010 [DOI] [PubMed] [Google Scholar]
- 55. Conroy R, Girotra M, Shane E, McMahon DJ, Pavlovich KH, Leibel RL, Rosenbaum M, Korner J. Leptin administration does not prevent the bone mineral metabolism changes induced by weight loss. Metabolism, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Considine RV, Considine EL, Williams CJ, Hyde TM, Caro JF. The hypothalamic leptin receptor in humans: identification of incidental sequence polymorphisms and absence of the db/db mouse and fa/fa rat mutations. Diabetes 45: 992–994, 1996 [DOI] [PubMed] [Google Scholar]
- 57. Considine RV, Sinha MK, Heiman ML, Kriauciunas A, Stephens TW, Nyce MR, Ohannesian JP, Marco CC, McKee LJ, Bauer TL, et al. Serum immunoreactive-leptin concentrations in normal-weight and obese humans. N Engl J Med 334: 292–295, 1996 [DOI] [PubMed] [Google Scholar]
- 58. Costa A, Poma A, Martignoni E, Nappi G, Ur E, Grossman A. Stimulation of corticotrophin-releasing hormone release by the obese (ob) gene product, leptin, from hypothalamic explants. Neuroreport 8: 1131–1134, 1997 [DOI] [PubMed] [Google Scholar]
- 59. Cowley MA, Smart JL, Rubinstein M, Cerdan MG, Diano S, Horvath TL, Cone RD, Low MJ. Leptin activates anorexigenic POMC neurons through a neural network in the arcuate nucleus. Nature 411: 480–484, 2001 [DOI] [PubMed] [Google Scholar]
- 60. Dardeno TA, Chou SH, Moon HS, Chamberland JP, Fiorenza CG, Mantzoros CS. Leptin in human physiology and therapeutics. Front Neuroendocrinol 31: 377–393, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. de Roux N, Genin E, Carel JC, Matsuda F, Chaussain JL, Milgrom E. Hypogonadotropic hypogonadism due to loss of function of the KiSS1-derived peptide receptor GPR54. Proc Natl Acad Sci USA 100: 10972–10976, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Denroche HC, Levi J, Wideman RD, Sequeira RM, Huynh FK, Covey SD, Kieffer TJ. Leptin therapy reverses hyperglycemia in mice with streptozotocin-induced diabetes, independent of hepatic leptin signaling. Diabetes 60: 1414–1423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Ducy P, Amling M, Takeda S, Priemel M, Schilling AF, Beil FT, Shen J, Vinson C, Rueger JM, Karsenty G. Leptin inhibits bone formation through a hypothalamic relay: a central control of bone mass. Cell 100: 197–207, 2000 [DOI] [PubMed] [Google Scholar]
- 64. Einollahi N, Dashti N, Nabatchian F. Serum leptin concentrations during the menstrual cycle in Iranian healthy women. Acta Med Iran 48: 300–303, 2010 [PubMed] [Google Scholar]
- 65. Elmquist JK, Bjorbaek C, Ahima RS, Flier JS, Saper CB. Distributions of leptin receptor mRNA isoforms in the rat brain. J Comp Neurol 395: 535–547, 1998 [PubMed] [Google Scholar]
- 66. Farooqi IS, Jebb SA, Langmack G, Lawrence E, Cheetham CH, Prentice AM, Hughes IA, McCamish MA, O'Rahilly S. Effects of recombinant leptin therapy in a child with congenital leptin deficiency. N Engl J Med 341: 879–884, 1999 [DOI] [PubMed] [Google Scholar]
- 67. Farooqi IS, Matarese G, Lord GM, Keogh JM, Lawrence E, Agwu C, Sanna V, Jebb SA, Perna F, Fontana S, Lechler RI, DePaoli AM, O'Rahilly S. Beneficial effects of leptin on obesity, T cell hyporesponsiveness, and neuroendocrine/metabolic dysfunction of human congenital leptin deficiency. J Clin Invest 110: 1093–1103, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Farooqi IS, O'Rahilly S. Monogenic obesity in humans. Annu Rev Med 56: 443–458, 2005 [DOI] [PubMed] [Google Scholar]
- 69. Farooqi IS, Wangensteen T, Collins S, Kimber W, Matarese G, Keogh JM, Lank E, Bottomley B, Lopez-Fernandez J, Ferraz-Amaro I, Dattani MT, Ercan O, Myhre AG, Retterstol L, Stanhope R, Edge JA, McKenzie S, Lessan N, Ghodsi M, De Rosa V, Perna F, Fontana S, Barroso I, Undlien DE, O'Rahilly S. Clinical and molecular genetic spectrum of congenital deficiency of the leptin receptor. N Engl J Med 356: 237–247, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Farooqi S. Insights from the genetics of severe childhood obesity. Horm Res 68, Suppl 5: 5–7, 2007 [DOI] [PubMed] [Google Scholar]
- 71. Fei H, Okano HJ, Li C, Lee GH, Zhao C, Darnell R, Friedman JM. Anatomic localization of alternatively spliced leptin receptors (Ob-R) in mouse brain and other tissues. Proc Natl Acad Sci USA 94: 7001–7005, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Finn PD, Cunningham MJ, Pau KY, Spies HG, Clifton DK, Steiner RA. The stimulatory effect of leptin on the neuroendocrine reproductive axis of the monkey. Endocrinology 139: 4652–4662, 1998 [DOI] [PubMed] [Google Scholar]
- 73. Fiorenza CG, Chou SH, Mantzoros CS. Lipodystrophy: pathophysiology and advances in treatment. Nat Rev Endocrinol 7: 137–150, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Flier JS, Harris M, Hollenberg AN. Leptin, nutrition, and the thyroid: the why, the wherefore, and the wiring. J Clin Invest 105: 859–861, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Fogteloo AJ, Pijl H, Frolich M, McCamish M, Meinders AE. Effects of recombinant human leptin treatment as an adjunct of moderate energy restriction on body weight, resting energy expenditure and energy intake in obese humans. Diabetes Nutr Metab 16: 109–114, 2003 [PubMed] [Google Scholar]
- 76. Frisch RE, McArthur JW. Menstrual cycles: fatness as a determinant of minimum weight for height necessary for their maintenance or onset. Science 185: 949–951, 1974 [DOI] [PubMed] [Google Scholar]
- 77. Fujikawa T, Chuang JC, Sakata I, Ramadori G, Coppari R. Leptin therapy improves insulin-deficient type 1 diabetes by CNS-dependent mechanisms in mice. Proc Natl Acad Sci USA 107: 17391–17396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Gale SM, Castracane VD, Mantzoros CS. Energy homeostasis, obesity and eating disorders: recent advances in endocrinology. J Nutr 134: 295–298, 2004 [DOI] [PubMed] [Google Scholar]
- 79. Gao Q, Horvath TL. Cross-talk between estrogen and leptin signaling in the hypothalamus. Am J Physiol Endocrinol Metab 294: E817–E826, 2008 [DOI] [PubMed] [Google Scholar]
- 80. Garris DR, Burkemper KM, Garris BL. Influences of diabetes (db/db), obese (ob/ob) and dystrophic (dy/dy) genotype mutations on hind limb bone maturation: a morphometric, radiological and cytochemical indices analysis. Diabetes Obes Metab 9: 311–322, 2007 [DOI] [PubMed] [Google Scholar]
- 81. Gat-Yablonski G, Phillip M. Leptin and regulation of linear growth. Curr Opin Clin Nutr Metab Care 11: 303–308, 2008 [DOI] [PubMed] [Google Scholar]
- 82. Gavrila A, Chan JL, Miller LC, Heist K, Yiannakouris N, Mantzoros CS. Circulating melanin-concentrating hormone, agouti-related protein, and alpha-melanocyte-stimulating hormone levels in relation to body composition: alterations in response to food deprivation and recombinant human leptin administration. J Clin Endocrinol Metab 90: 1047–1054, 2005 [DOI] [PubMed] [Google Scholar]
- 83. Gavrila A, Chan JL, Yiannakouris N, Kontogianni M, Miller LC, Orlova C, Mantzoros CS. Serum adiponectin levels are inversely associated with overall and central fat distribution but are not directly regulated by acute fasting or leptin administration in humans: cross-sectional and interventional studies. J Clin Endocrinol Metab 88: 4823–4831, 2003 [DOI] [PubMed] [Google Scholar]
- 84. Gavrila A, Hsu W, Tsiodras S, Doweiko J, Gautam S, Martin L, Moses AC, Karchmer AW, Mantzoros CS. Improvement in highly active antiretroviral therapy-induced metabolic syndrome by treatment with pioglitazone but not with fenofibrate: a 2 × 2 factorial, randomized, double-blinded, placebo-controlled trial. Clin Infect Dis 40: 745–749, 2005 [DOI] [PubMed] [Google Scholar]
- 85. Gavrila A, Peng CK, Chan JL, Mietus JE, Goldberger AL, Mantzoros CS. Diurnal and ultradian dynamics of serum adiponectin in healthy men: comparison with leptin, circulating soluble leptin receptor, and cortisol patterns. J Clin Endocrinol Metab 88: 2838–2843, 2003 [DOI] [PubMed] [Google Scholar]
- 86. Gavrilova O, Marcus-Samuels B, Leon LR, Vinson C, Reitman ML. Leptin and diabetes in lipoatrophic mice. Nature 403: 850; discussion 850–851, 2000 [DOI] [PubMed] [Google Scholar]
- 87. German JP, Thaler JP, Wisse BE, Oh IS, Sarruf DA, Matsen ME, Fischer JD, Taborsky GJ, Jr, Schwartz MW, Morton GJ. Leptin activates a novel CNS mechanism for insulin-independent normalization of severe diabetic hyperglycemia. Endocrinology 152: 394–404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Gibson WT, Farooqi IS, Moreau M, DePaoli AM, Lawrence E, O'Rahilly S, Trussell RA. Congenital leptin deficiency due to homozygosity for the Delta133G mutation: report of another case and evaluation of response to four years of leptin therapy. J Clin Endocrinol Metab 89: 4821–4826, 2004 [DOI] [PubMed] [Google Scholar]
- 89. Gordeladze JO, Drevon CA, Syversen U, Reseland JE. Leptin stimulates human osteoblastic cell proliferation, de novo collagen synthesis, and mineralization: Impact on differentiation markers, apoptosis, and osteoclastic signaling. J Cell Biochem 85: 825–836, 2002 [DOI] [PubMed] [Google Scholar]
- 90. Gordon CM. Leptin and the skeleton—where is the fat? Metabolism 2011, July 8 [Epub ahead of print] No abstract available. PMID21742353 [DOI] [PubMed] [Google Scholar]
- 91. Gottsch ML, Cunningham MJ, Smith JT, Popa SM, Acohido BV, Crowley WF, Seminara S, Clifton DK, Steiner RA. A role for kisspeptins in the regulation of gonadotropin secretion in the mouse. Endocrinology 145: 4073–4077, 2004 [DOI] [PubMed] [Google Scholar]
- 92. Greco AV, Mingrone G, Giancaterini A, Manco M, Morroni M, Cinti S, Granzotto M, Vettor R, Camastra S, Ferrannini E. Insulin resistance in morbid obesity: reversal with intramyocellular fat depletion. Diabetes 51: 144–151, 2002 [DOI] [PubMed] [Google Scholar]
- 93. Grinspoon S, Carr A. Cardiovascular risk and body-fat abnormalities in HIV-infected adults. N Engl J Med 352: 48–62, 2005 [DOI] [PubMed] [Google Scholar]
- 94. Hakansson ML, Brown H, Ghilardi N, Skoda RC, Meister B. Leptin receptor immunoreactivity in chemically defined target neurons of the hypothalamus. J Neurosci 18: 559–572, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Halaas JL, Gajiwala KS, Maffei M, Cohen SL, Chait BT, Rabinowitz D, Lallone RL, Burley SK, Friedman JM. Weight-reducing effects of the plasma protein encoded by the obese gene. Science 269: 543–546, 1995 [DOI] [PubMed] [Google Scholar]
- 96. Hamnvik OP, Liu X, Petrou M, Gong H, Chamberland JP, Kim EH, Christophi CA, Kales SN, Christiani DC, Mantzoros CS. Soluble leptin receptor and leptin are associated with baseline adiposity and metabolic risk factors, and predict adiposity, metabolic syndrome, and glucose levels at 2-year follow-up: the Cyprus Metabolism Prospective Cohort Study. Metabolism 60: 987–993, 2011 [DOI] [PubMed] [Google Scholar]
- 97. Hamrick MW. Leptin and bone: a consensus emerging? BoneKEy-Osteovision 4: 99–107, 2007 [Google Scholar]
- 98. Hamrick MW, Della-Fera MA, Choi YH, Pennington C, Hartzell D, Baile CA. Leptin treatment induces loss of bone marrow adipocytes and increases bone formation in leptin-deficient ob/ob mice. J Bone Miner Res 20: 994–1001, 2005 [DOI] [PubMed] [Google Scholar]
- 99. Hamrick MW, Della Fera MA, Choi YH, Hartzell D, Pennington C, Baile CA. Injections of leptin into rat ventromedial hypothalamus increase adipocyte apoptosis in peripheral fat and in bone marrow. Cell Tissue Res 327: 133–141, 2007 [DOI] [PubMed] [Google Scholar]
- 100. Harris RB, Zhou J, Redmann SM, Jr, Smagin GN, Smith SR, Rodgers E, Zachwieja JJ. A leptin dose-response study in obese (ob/ob) and lean (+/?) mice. Endocrinology 139: 8–19, 1998 [DOI] [PubMed] [Google Scholar]
- 101. Harvey J, Ashford ML. Insulin occludes leptin activation of ATP-sensitive K+ channels in rat CRI-G1 insulin secreting cells. J Physiol 511: 695–706, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Heiman ML, Ahima RS, Craft LS, Schoner B, Stephens TW, Flier JS. Leptin inhibition of the hypothalamic-pituitary-adrenal axis in response to stress. Endocrinology 138: 3859–3863, 1997 [DOI] [PubMed] [Google Scholar]
- 103. Heymsfield SB, Greenberg AS, Fujioka K, Dixon RM, Kushner R, Hunt T, Lubina JA, Patane J, Self B, Hunt P, McCamish M. Recombinant leptin for weight loss in obese and lean adults: a randomized, controlled, dose-escalation trial. JAMA 282: 1568–1575, 1999 [DOI] [PubMed] [Google Scholar]
- 104. Hidaka S, Yoshimatsu H, Kondou S, Tsuruta Y, Oka K, Noguchi H, Okamoto K, Sakino H, Teshima Y, Okeda T, Sakata T. Chronic central leptin infusion restores hyperglycemia independent of food intake and insulin level in streptozotocin-induced diabetic rats. FASEB J 16: 509–518, 2002 [DOI] [PubMed] [Google Scholar]
- 105. Hileman SM, Pierroz DD, Masuzaki H, Bjorbaek C, El-Haschimi K, Banks WA, Flier JS. Characterizaton of short isoforms of the leptin receptor in rat cerebral microvessels and of brain uptake of leptin in mouse models of obesity. Endocrinology 143: 775–783, 2002 [DOI] [PubMed] [Google Scholar]
- 106. Hill JW, Elmquist JK, Elias CF. Hypothalamic pathways linking energy balance and reproduction. Am J Physiol Endocrinol Metab 294: E827–E832, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Holloway WR, Collier FM, Aitken CJ, Myers DE, Hodge JM, Malakellis M, Gough TJ, Collier GR, Nicholson GC. Leptin inhibits osteoclast generation. J Bone Miner Res 17: 200–209, 2002 [DOI] [PubMed] [Google Scholar]
- 108. Houseknecht KL, Mantzoros CS, Kuliawat R, Hadro E, Flier JS, Kahn BB. Evidence for leptin binding to proteins in serum of rodents and humans: modulation with obesity. Diabetes 45: 1638–1643, 1996 [DOI] [PubMed] [Google Scholar]
- 109. Howard JK, Cave BJ, Oksanen LJ, Tzameli I, Bjorbaek C, Flier JS. Enhanced leptin sensitivity and attenuation of diet-induced obesity in mice with haploinsufficiency of Socs3. Nat Med 10: 734–738, 2004 [DOI] [PubMed] [Google Scholar]
- 110. Howard JK, Lord GM, Matarese G, Vendetti S, Ghatei MA, Ritter MA, Lechler RI, Bloom SR. Leptin protects mice from starvation-induced lymphoid atrophy and increases thymic cellularity in ob/ob mice. J Clin Invest 104: 1051–1059, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Hsu A, Aronoff DM, Phipps J, Goel D, Mancuso P. Leptin improves pulmonary bacterial clearance and survival in ob/ob mice during pneumococcal pneumonia. Clin Exp Immunol 150: 332–339, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Huang W, Dedousis N, Bhatt BA, O'Doherty RM. Impaired activation of phosphatidylinositol 3-kinase by leptin is a novel mechanism of hepatic leptin resistance in diet-induced obesity. J Biol Chem 279: 21695–21700, 2004 [DOI] [PubMed] [Google Scholar]
- 113. Hukshorn CJ, Menheere PP, Westerterp-Plantenga MS, Saris WH. The effect of pegylated human recombinant leptin (PEG-OB) on neuroendocrine adaptations to semi-starvation in overweight men. Eur J Endocrinol 148: 649–655, 2003 [DOI] [PubMed] [Google Scholar]
- 114. Hukshorn CJ, Saris WH, Westerterp-Plantenga MS, Farid AR, Smith FJ, Campfield LA. Weekly subcutaneous pegylated recombinant native human leptin (PEG-OB) administration in obese men. J Clin Endocrinol Metab 85: 4003–4009, 2000 [DOI] [PubMed] [Google Scholar]
- 115. Hummel KP, Dickie MM, Coleman DL. Diabetes, a new mutation in the mouse. Science 153: 1127–1128, 1966 [DOI] [PubMed] [Google Scholar]
- 116. Hwang JJ, Chan JL, Ntali G, Malkova D, Mantzoros CS. Leptin does not directly regulate the pancreatic hormones amylin and pancreatic polypeptide: interventional studies in humans. Diabetes Care 31: 945–951, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Ingalls AM, Dickie MM, Snell GD. Obese, a new mutation in the house mouse. J Hered 41: 317–318, 1950 [DOI] [PubMed] [Google Scholar]
- 118. Irwig MS, Fraley GS, Smith JT, Acohido BV, Popa SM, Cunningham MJ, Gottsch ML, Clifton DK, Steiner RA. Kisspeptin activation of gonadotropin releasing hormone neurons and regulation of KiSS-1 mRNA in the male rat. Neuroendocrinology 80: 264–272, 2004 [DOI] [PubMed] [Google Scholar]
- 119. Ishida K, Murakami T, Mizuno A, Iida M, Kuwajima M, Shima K. Leptin suppresses basal insulin secretion from rat pancreatic islets. Regul Pept 70: 179–182, 1997 [DOI] [PubMed] [Google Scholar]
- 120. Jacobson DL, Knox T, Spiegelman D, Skinner S, Gorbach S, Wanke C. Prevalence of, evolution of, and risk factors for fat atrophy and fat deposition in a cohort of HIV-infected men and women. Clin Infect Dis 40: 1837–1845, 2005 [DOI] [PubMed] [Google Scholar]
- 121. Javor ED, Cochran EK, Musso C, Young JR, Depaoli AM, Gorden P. Long-term efficacy of leptin replacement in patients with generalized lipodystrophy. Diabetes 54: 1994–2002, 2005 [DOI] [PubMed] [Google Scholar]
- 122. Javor ED, Ghany MG, Cochran EK, Oral EA, DePaoli AM, Premkumar A, Kleiner DE, Gorden P. Leptin reverses nonalcoholic steatohepatitis in patients with severe lipodystrophy. Hepatology 41: 753–760, 2005 [DOI] [PubMed] [Google Scholar]
- 123. Jimerson DC, Mantzoros C, Wolfe BE, Metzger ED. Decreased serum leptin in bulimia nervosa. J Clin Endocrinol Metab 85: 4511–4514, 2000 [DOI] [PubMed] [Google Scholar]
- 124. Jurimae J, Jurimae T, Leppik A, Kums T. The influence of ghrelin, adiponectin, and leptin on bone mineral density in healthy postmenopausal women. J Bone Miner Metab 26: 618–623, 2008 [DOI] [PubMed] [Google Scholar]
- 125. Kang ES, Magkos F, Sienkiewicz E, Mantzoros CS. Circulating vaspin and visfatin are not affected by acute or chronic energy deficiency or leptin administration in humans. Eur J Endocrinol 164: 911–917, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Karsenty G, Oury F. The central regulation of bone mass, the first link between bone remodeling and energy metabolism. J Clin Endocrinol Metab 95: 4795–4801, 2010 [DOI] [PubMed] [Google Scholar]
- 127. Kelesidis T, Kelesidis I, Chou S, Mantzoros CS. Narrative review: the role of leptin in human physiology: emerging clinical applications. Ann Intern Med 152: 93–100, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Kelesidis T, Mantzoros CS. The emerging role of leptin in humans. Pediatr Endocrinol Rev 3: 239–248, 2006 [PubMed] [Google Scholar]
- 129. Kellerer M, Koch M, Metzinger E, Mushack J, Capp E, Haring HU. Leptin activates PI-3 kinase in C2C12 myotubes via janus kinase-2 (JAK-2) and insulin receptor substrate-2 (IRS-2) dependent pathways. Diabetologia 40: 1358–1362, 1997 [DOI] [PubMed] [Google Scholar]
- 130. Khan A, Narangoda S, Ahren B, Holm C, Sundler F, Efendic S. Long-term leptin treatment of ob/ob mice improves glucose-induced insulin secretion. Int J Obes Relat Metab Disord 25: 816–821, 2001 [DOI] [PubMed] [Google Scholar]
- 131. Kiess W, Petzold S, Topfer M, Garten A, Bluher S, Kapellen T, Korner A, Kratzsch J. Adipocytes and adipose tissue. Best Pract Res Clin Endocrinol Metab 22: 135–153, 2008 [DOI] [PubMed] [Google Scholar]
- 132. Kim JK, Gavrilova O, Chen Y, Reitman ML, Shulman GI. Mechanism of insulin resistance in A-ZIP/F-1 fatless mice. J Biol Chem 275: 8456–8460, 2000 [DOI] [PubMed] [Google Scholar]
- 133. Kim MS, Small CJ, Stanley SA, Morgan DG, Seal LJ, Kong WM, Edwards CM, Abusnana S, Sunter D, Ghatei MA, Bloom SR. The central melanocortin system affects the hypothalamo-pituitary thyroid axis and may mediate the effect of leptin. J Clin Invest 105: 1005–1011, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Kim YB, Uotani S, Pierroz DD, Flier JS, Kahn BB. In vivo administration of leptin activates signal transduction directly in insulin-sensitive tissues: overlapping but distinct pathways from insulin. Endocrinology 141: 2328–2339, 2000 [DOI] [PubMed] [Google Scholar]
- 135. Koch C, Augustine RA, Steger J, Ganjam GK, Benzler J, Pracht C, Lowe C, Schwartz MW, Shepherd PR, Anderson GM, Grattan DR, Tups A. Leptin rapidly improves glucose homeostasis in obese mice by increasing hypothalamic insulin sensitivity. J Neurosci 30: 16180–16187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Kulkarni RN, Wang ZL, Wang RM, Hurley JD, Smith DM, Ghatei MA, Withers DJ, Gardiner JV, Bailey CJ, Bloom SR. Leptin rapidly suppresses insulin release from insulinoma cells, rat and human islets and, in vivo, in mice. J Clin Invest 100: 2729–2736, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Lagiou P, Signorello LB, Mantzoros CS, Trichopoulos D, Hsieh CC, Trichopoulou A. Hormonal, lifestyle, and dietary factors in relation to leptin among elderly men. Ann Nutr Metab 43: 23–29, 1999 [DOI] [PubMed] [Google Scholar]
- 138. Lahlou N, Issad T, Lebouc Y, Carel JC, Camoin L, Roger M, Girard J. Mutations in the human leptin and leptin receptor genes as models of serum leptin receptor regulation. Diabetes 51: 1980–1985, 2002 [DOI] [PubMed] [Google Scholar]
- 139. Lam NT, Cheung AT, Riedel MJ, Light PE, Cheeseman CI, Kieffer TJ. Leptin reduces glucose transport and cellular ATP levels in INS-1 beta-cells. J Mol Endocrinol 32: 415–424, 2004 [DOI] [PubMed] [Google Scholar]
- 140. Lee GH, Proenca R, Montez JM, Carroll KM, Darvishzadeh JG, Lee JI, Friedman JM. Abnormal splicing of the leptin receptor in diabetic mice. Nature 379: 632–635, 1996 [DOI] [PubMed] [Google Scholar]
- 141. Lee JH, Chan JL, Sourlas E, Raptopoulos V, Mantzoros CS. Recombinant methionyl human leptin therapy in replacement doses improves insulin resistance and metabolic profile in patients with lipoatrophy and metabolic syndrome induced by the highly active antiretroviral therapy. J Clin Endocrinol Metab 91: 2605–2611, 2006 [DOI] [PubMed] [Google Scholar]
- 142. Lee JW, Romsos DR. Leptin administration normalizes insulin secretion from islets of Lep(ob)/Lep(ob) mice by food intake-dependent and -independent mechanisms. Exp Biol Med (Maywood) 228: 183–187, 2003 [DOI] [PubMed] [Google Scholar]
- 143. Lee YH, Magkos F, Mantzoros CS, Kang ES. Effects of leptin and adiponectin on pancreatic beta-cell function. Metabolism, 2011, May 30 [Epub ahead of print] PMID: 21632069 [DOI] [PubMed] [Google Scholar]
- 144. Legradi G, Emerson CH, Ahima RS, Flier JS, Lechan RM. Leptin prevents fasting-induced suppression of prothyrotropin-releasing hormone messenger ribonucleic acid in neurons of the hypothalamic paraventricular nucleus. Endocrinology 138: 2569–2576, 1997 [DOI] [PubMed] [Google Scholar]
- 145. Lehman MN, Coolen LM, Goodman RL. Minireview. Kisspeptin/neurokinin B/dynorphin (KNDy) cells of the arcuate nucleus: a central node in the control of gonadotropin-releasing hormone secretion. Endocrinology 151: 3479–3489, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146. Leibel RL, Rosenbaum M, Hirsch J. Changes in energy expenditure resulting from altered body weight. N Engl J Med 332: 621–628, 1995 [DOI] [PubMed] [Google Scholar]
- 147. Lejeune MP, Hukshorn CJ, Saris WH, Westerterp-Plantenga MS. Effect of dietary restraint during and following pegylated recombinant leptin (PEG-OB) treatment of overweight men. Int J Obes Relat Metab Disord 27: 1494–1499, 2003 [DOI] [PubMed] [Google Scholar]
- 148. Licinio J, Caglayan S, Ozata M, Yildiz BO, de Miranda PB, O'Kirwan F, Whitby R, Liang L, Cohen P, Bhasin S, Krauss RM, Veldhuis JD, Wagner AJ, DePaoli AM, McCann SM, Wong ML. Phenotypic effects of leptin replacement on morbid obesity, diabetes mellitus, hypogonadism, and behavior in leptin-deficient adults. Proc Natl Acad Sci USA 101: 4531–4536, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149. Licinio J, Mantzoros C, Negrao AB, Cizza G, Wong ML, Bongiorno PB, Chrousos GP, Karp B, Allen C, Flier JS, Gold PW. Human leptin levels are pulsatile and inversely related to pituitary-adrenal function. Nat Med 3: 575–579, 1997 [DOI] [PubMed] [Google Scholar]
- 150. Licinio J, Negrao AB, Mantzoros C, Kaklamani V, Wong ML, Bongiorno PB, Mulla A, Cearnal L, Veldhuis JD, Flier JS, McCann SM, Gold PW. Synchronicity of frequently sampled, 24-h concentrations of circulating leptin, luteinizing hormone, and estradiol in healthy women. Proc Natl Acad Sci USA 95: 2541–2546, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151. Licinio J, Negrao AB, Mantzoros C, Kaklamani V, Wong ML, Bongiorno PB, Negro PP, Mulla A, Veldhuis JD, Cearnal L, Flier JS, Gold PW. Sex differences in circulating human leptin pulse amplitude: clinical implications. J Clin Endocrinol Metab 83: 4140–4147, 1998 [DOI] [PubMed] [Google Scholar]
- 152. Lim CT, Kola B, Korbonits M. AMPK as a mediator of hormonal signalling. J Mol Endocrinol 44: 87–97, 2009 [DOI] [PubMed] [Google Scholar]
- 153. Lin KC. Increase of leptin levels following exogenous administration of estrogen in women with normal menstruation. Kaohsiung J Med Sci 16: 13–19, 2000 [PubMed] [Google Scholar]
- 154. Loffreda S, Yang SQ, Lin HZ, Karp CL, Brengman ML, Wang DJ, Klein AS, Bulkley GB, Bao C, Noble PW, Lane MD, Diehl AM. Leptin regulates proinflammatory immune responses. FASEB J 12: 57–65, 1998 [PubMed] [Google Scholar]
- 155. Lord GM, Matarese G, Howard JK, Baker RJ, Bloom SR, Lechler RI. Leptin modulates the T-cell immune response and reverses starvation-induced immunosuppression. Nature 394: 897–901, 1998 [DOI] [PubMed] [Google Scholar]
- 156. Lorentzon R, Alehagen U, Boquist L. Osteopenia in mice with genetic diabetes. Diabetes Res Clin Pract 2: 157–163, 1986 [DOI] [PubMed] [Google Scholar]
- 157. Ludwig M, Klein HH, Diedrich K, Ortmann O. Serum leptin concentrations throughout the menstrual cycle. Arch Gynecol Obstet 263: 99–101, 2000 [DOI] [PubMed] [Google Scholar]
- 158. Magkos F, Brennan A, Sweeney L, Kang ES, Doweiko J, Karchmer AW, Mantzoros CS. Leptin replacement improves postprandial glycemia and insulin sensitivity in human immunodeficiency virus-infected lipoatrophic men treated with pioglitazone: a pilot study. Metabolism 60: 1045–1049, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159. Magkos F, Mantzoros CS. Body fat redistribution and metabolic abnormalities in HIV-infected patients on highly active antiretroviral therapy: novel insights into pathophysiology and emerging opportunities for treatment. Metabolism 60: 749–753, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160. Mancuso P, Gottschalk A, Phare SM, Peters-Golden M, Lukacs NW, Huffnagle GB. Leptin-deficient mice exhibit impaired host defense in Gram-negative pneumonia. J Immunol 168: 4018–4024, 2002 [DOI] [PubMed] [Google Scholar]
- 161. Mantzoros C, Flier JS, Lesem MD, Brewerton TD, Jimerson DC. Cerebrospinal fluid leptin in anorexia nervosa: correlation with nutritional status and potential role in resistance to weight gain. J Clin Endocrinol Metab 82: 1845–1851, 1997 [DOI] [PubMed] [Google Scholar]
- 162. Mantzoros CS. Leptin and the hypothalamus: neuroendocrine regulation of food intake. Mol Psychiatry 4: 8–12, 16–17, 1999 [DOI] [PubMed] [Google Scholar]
- 163. Mantzoros CS. Leptin in renal failure. J Ren Nutr 9: 122–125, 1999 [DOI] [PubMed] [Google Scholar]
- 164. Mantzoros CS. Obesity, eating disorders and restrained eating: is leptin the missing link? Mol Psychiatry 2: 377–380, 1997 [DOI] [PubMed] [Google Scholar]
- 165. Mantzoros CS. The role of leptin and hypothalamic neuropeptides in energy homeostasis: update on leptin in obesity. Growth Horm IGF Res 11, Suppl A: S85–S89, 2001 [DOI] [PubMed] [Google Scholar]
- 166. Mantzoros CS. The role of leptin in human obesity and disease: a review of current evidence. Ann Intern Med 130: 671–680, 1999 [DOI] [PubMed] [Google Scholar]
- 167. Mantzoros CS. Role of leptin in reproduction. Ann NY Acad Sci 900: 174–183, 2000 [DOI] [PubMed] [Google Scholar]
- 168. Mantzoros CS. W(h)ither metreleptin for lipodystrophy and the metabolic syndrome? Endocr Pract 16: 162–166, 2010 [DOI] [PubMed] [Google Scholar]
- 169. Mantzoros CS. Whither recombinant human leptin treatment for HIV-associated lipoatrophy and the metabolic syndrome? J Clin Endocrinol Metab 94: 1089–1091, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170. Mantzoros CS, Flier JS. Editorial: leptin as a therapeutic agent—trials and tribulations. J Clin Endocrinol Metab 85: 4000–4002, 2000 [DOI] [PubMed] [Google Scholar]
- 171. Mantzoros CS, Flier JS, Rogol AD. A longitudinal assessment of hormonal and physical alterations during normal puberty in boys. V. Rising leptin levels may signal the onset of puberty. J Clin Endocrinol Metab 82: 1066–1070, 1997 [DOI] [PubMed] [Google Scholar]
- 172. Mantzoros CS, Liolios AD, Tritos NA, Kaklamani VG, Doulgerakis DE, Griveas I, Moses AC, Flier JS. Circulating insulin concentrations, smoking, and alcohol intake are important independent predictors of leptin in young healthy men. Obes Res 6: 179–186, 1998 [DOI] [PubMed] [Google Scholar]
- 173. Mantzoros CS, Moschos S, Avramopoulos I, Kaklamani V, Liolios A, Doulgerakis DE, Griveas I, Katsilambros N, Flier JS. Leptin concentrations in relation to body mass index and the tumor necrosis factor-alpha system in humans. J Clin Endocrinol Metab 82: 3408–3413, 1997 [DOI] [PubMed] [Google Scholar]
- 174. Mantzoros CS, Moschos SJ. Leptin: in search of role(s) in human physiology and pathophysiology. Clin Endocrinol (Oxf) 49: 551–567, 1998 [DOI] [PubMed] [Google Scholar]
- 175. Mantzoros CS, Ozata M, Negrao AB, Suchard MA, Ziotopoulou M, Caglayan S, Elashoff RM, Cogswell RJ, Negro P, Liberty V, Wong ML, Veldhuis J, Ozdemir IC, Gold PW, Flier JS, Licinio J. Synchronicity of frequently sampled thyrotropin (TSH) and leptin concentrations in healthy adults and leptin-deficient subjects: evidence for possible partial TSH regulation by leptin in humans. J Clin Endocrinol Metab 86: 3284–3291, 2001 [DOI] [PubMed] [Google Scholar]
- 176. Mantzoros CS, Qu D, Frederich RC, Susulic VS, Lowell BB, Maratos-Flier E, Flier JS. Activation of beta(3) adrenergic receptors suppresses leptin expression and mediates a leptin-independent inhibition of food intake in mice. Diabetes 45: 909–914, 1996 [DOI] [PubMed] [Google Scholar]
- 177. Marchetti P, Bugliani M, Lupi R, Marselli L, Masini M, Boggi U, Filipponi F, Weir GC, Eizirik DL, Cnop M. The endoplasmic reticulum in pancreatic beta cells of type 2 diabetes patients. Diabetologia 50: 2486–2494, 2007 [DOI] [PubMed] [Google Scholar]
- 178. Margetic S, Gazzola C, Pegg GG, Hill RA. Leptin: a review of its peripheral actions and interactions. Int J Obes Relat Metab Disord 26: 1407–1433, 2002 [DOI] [PubMed] [Google Scholar]
- 179. Maroni P, Bendinelli P, Piccoletti R. Intracellular signal transduction pathways induced by leptin in C2C12 cells. Cell Biol Int 29: 542–550, 2005 [DOI] [PubMed] [Google Scholar]
- 180. Marroqui L, Vieira E, Gonzalez A, Nadal A, Quesada I. Leptin downregulates expression of the gene encoding glucagon in alphaTC1–9 cells and mouse islets. Diabetologia 54: 843–851 [DOI] [PubMed] [Google Scholar]
- 181. Matarese G, Mantzoros C, La Cava A. Leptin and adipocytokines: bridging the gap between immunity and atherosclerosis. Curr Pharm Des 13: 3676–3680, 2007 [DOI] [PubMed] [Google Scholar]
- 182. Matarese G, Moschos S, Mantzoros CS. Leptin in immunology. J Immunol 174: 3137–3142, 2005 [DOI] [PubMed] [Google Scholar]
- 183. Mayer J, Bates MW, Dickie MM. Hereditary diabetes in genetically obese mice. Science 113: 746–747, 1951 [DOI] [PubMed] [Google Scholar]
- 184. Miller KK, Parulekar MS, Schoenfeld E, Anderson E, Hubbard J, Klibanski A, Grinspoon SK. Decreased leptin levels in normal weight women with hypothalamic amenorrhea: the effects of body composition and nutritional intake. J Clin Endocrinol Metab 83: 2309–2312, 1998 [DOI] [PubMed] [Google Scholar]
- 185. Minokoshi Y, Kim YB, Peroni OD, Fryer LG, Muller C, Carling D, Kahn BB. Leptin stimulates fatty-acid oxidation by activating AMP-activated protein kinase. Nature 415: 339–343, 2002 [DOI] [PubMed] [Google Scholar]
- 186. Mittendorfer B, Horowitz JF, DePaoli AM, McCamish MA, Patterson BW, Klein S. Recombinant human leptin treatment does not improve insulin action in obese subjects with type 2 diabetes. Diabetes 60: 1474–1477, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187. Moitra J, Mason MM, Olive M, Krylov D, Gavrilova O, Marcus-Samuels B, Feigenbaum L, Lee E, Aoyama T, Eckhaus M, Reitman ML, Vinson C. Life without white fat: a transgenic mouse. Genes Dev 12: 3168–3181, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188. Montague CT, Prins JB, Sanders L, Digby JE, O'Rahilly S. Depot- and sex-specific differences in human leptin mRNA expression: implications for the control of regional fat distribution. Diabetes 46: 342–347, 1997 [DOI] [PubMed] [Google Scholar]
- 189. Monzillo LU, Hamdy O, Horton ES, Ledbury S, Mullooly C, Jarema C, Porter S, Ovalle K, Moussa A, Mantzoros CS. Effect of lifestyle modification on adipokine levels in obese subjects with insulin resistance. Obes Res 11: 1048–1054, 2003 [DOI] [PubMed] [Google Scholar]
- 190. Moon HS, Chamberland JP, Diakopoulos KN, Fiorenza CG, Ziemke F, Schneider B, Mantzoros CS. Leptin and amylin act in an additive manner to activate overlapping signaling pathways in peripheral tissues: in vitro and ex vivo studies in humans. Diabetes Care 34: 132–138, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191. Moon HS, Matarese G, Brennan AM, Chamberland JP, Liu X, Fiorenza CG, Mylvaganam GH, Abanni L, Carbone F, Williams CJ, De Paoli AM, Schneider BE, Mantzoros CS. Efficacy of metreleptin in obese patients with type 2 diabetes: cellular and molecular pathways underlying leptin tolerance. Diabetes 60: 1647–1656, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192. Moran SA, Patten N, Young JR, Cochran E, Sebring N, Reynolds J, Premkumar A, Depaoli AM, Skarulis MC, Oral EA, Gorden P. Changes in body composition in patients with severe lipodystrophy after leptin replacement therapy. Metabolism 53: 513–519, 2004 [DOI] [PubMed] [Google Scholar]
- 193. Mori H, Hanada R, Hanada T, Aki D, Mashima R, Nishinakamura H, Torisu T, Chien KR, Yasukawa H, Yoshimura A. Socs3 deficiency in the brain elevates leptin sensitivity and confers resistance to diet-induced obesity. Nat Med 10: 739–743, 2004 [DOI] [PubMed] [Google Scholar]
- 194. Moschos S, Chan JL, Mantzoros CS. Leptin and reproduction: a review. Fertil Steril 77: 433–444, 2002 [DOI] [PubMed] [Google Scholar]
- 195. Mulligan K, Khatami H, Schwarz JM, Sakkas GK, DePaoli AM, Tai VW, Wen MJ, Lee GA, Grunfeld C, Schambelan M. The effects of recombinant human leptin on visceral fat, dyslipidemia, and insulin resistance in patients with human immunodeficiency virus-associated lipoatrophy and hypoleptinemia. J Clin Endocrinol Metab 94: 1137–1144, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196. Mullington JM, Chan JL, Van Dongen HP, Szuba MP, Samaras J, Price NJ, Meier-Ewert HK, Dinges DF, Mantzoros CS. Sleep loss reduces diurnal rhythm amplitude of leptin in healthy men. J Neuroendocrinol 15: 851–854, 2003 [DOI] [PubMed] [Google Scholar]
- 197. Muoio DM, Dohm GL, Fiedorek FT, Jr, Tapscott EB, Coleman RA. Leptin directly alters lipid partitioning in skeletal muscle. Diabetes 46: 1360–1363, 1997 [DOI] [PubMed] [Google Scholar]
- 198. Musso C, Cochran E, Javor E, Young J, Depaoli AM, Gorden P. The long-term effect of recombinant methionyl human leptin therapy on hyperandrogenism and menstrual function in female and pituitary function in male and female hypoleptinemic lipodystrophic patients. Metabolism 54: 255–263, 2005 [DOI] [PubMed] [Google Scholar]
- 199. Myers MG, Cowley MA, Munzberg H. Mechanisms of leptin action and leptin resistance. Annu Rev Physiol 70: 537–556, 2008 [DOI] [PubMed] [Google Scholar]
- 200. Nagy GS, Tsiodras S, Martin LD, Avihingsanon A, Gavrila A, Hsu WC, Karchmer AW, Mantzoros CS. Human immunodeficiency virus type 1-related lipoatrophy and lipohypertrophy are associated with serum concentrations of leptin. Clin Infect Dis 36: 795–802, 2003 [DOI] [PubMed] [Google Scholar]
- 201. Nishiyama A, Yagi M, Awano H, Okizuka Y, Maeda T, Yoshida S, Takeshima Y, Matsuo M. Two Japanese infants with congenital generalized lipodystrophy due to BSCL2 mutations. Pediatr Int 51: 775–779, 2009 [DOI] [PubMed] [Google Scholar]
- 202. Oral EA, Ruiz E, Andewelt A, Sebring N, Wagner AJ, Depaoli AM, Gorden P. Effect of leptin replacement on pituitary hormone regulation in patients with severe lipodystrophy. J Clin Endocrinol Metab 87: 3110–3117, 2002 [DOI] [PubMed] [Google Scholar]
- 203. Oral EA, Simha V, Ruiz E, Andewelt A, Premkumar A, Snell P, Wagner AJ, DePaoli AM, Reitman ML, Taylor SI, Gorden P, Garg A. Leptin-replacement therapy for lipodystrophy. N Engl J Med 346: 570–578, 2002 [DOI] [PubMed] [Google Scholar]
- 204. Oswal A, Yeo G. Leptin and the control of body weight: a review of its diverse central targets, signaling mechanisms, and role in the pathogenesis of obesity. Obesity (Silver Spring) 18: 221–229, 2009 [DOI] [PubMed] [Google Scholar]
- 205. Ozata M, Ozdemir IC, Licinio J. Human leptin deficiency caused by a missense mutation: multiple endocrine defects, decreased sympathetic tone, and immune system dysfunction indicate new targets for leptin action, greater central than peripheral resistance to the effects of leptin, and spontaneous correction of leptin-mediated defects. J Clin Endocrinol Metab 84: 3686–3695, 1999 [DOI] [PubMed] [Google Scholar]
- 206. Ozcan L, Ergin AS, Lu A, Chung J, Sarkar S, Nie D, Myers MG, Jr, Ozcan U. Endoplasmic reticulum stress plays a central role in development of leptin resistance. Cell Metab 9: 35–51, 2009 [DOI] [PubMed] [Google Scholar]
- 207. Ozcan U, Cao Q, Yilmaz E, Lee AH, Iwakoshi NN, Ozdelen E, Tuncman G, Gorgun C, Glimcher LH, Hotamisligil GS. Endoplasmic reticulum stress links obesity, insulin action, and type 2 diabetes. Science 306: 457–461, 2004 [DOI] [PubMed] [Google Scholar]
- 208. Pallett AL, Morton NM, Cawthorne MA, Emilsson V. Leptin inhibits insulin secretion and reduces insulin mRNA levels in rat isolated pancreatic islets. Biochem Biophys Res Commun 238: 267–270, 1997 [DOI] [PubMed] [Google Scholar]
- 209. Papathanassoglou E, El-Haschimi K, Li XC, Matarese G, Strom T, Mantzoros C. Leptin receptor expression and signaling in lymphocytes: kinetics during lymphocyte activation, role in lymphocyte survival, and response to high fat diet in mice. J Immunol 176: 7745–7752, 2006 [DOI] [PubMed] [Google Scholar]
- 210. Pardini VC, Victoria IM, Rocha SM, Andrade DG, Rocha AM, Pieroni FB, Milagres G, Purisch S, Velho G. Leptin levels, beta-cell function, and insulin sensitivity in families with congenital and acquired generalized lipoatropic diabetes. J Clin Endocrinol Metab 83: 503–508, 1998 [DOI] [PubMed] [Google Scholar]
- 211. Paz-Filho G, Delibasi T, Erol HK, Wong ML, Licinio J. Congenital leptin deficiency and thyroid function. Thyroid Res 2: 11, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212. Paz-Filho G, Mastronardi C, Delibasi T, Wong ML, Licinio J. Congenital leptin deficiency: diagnosis and effects of leptin replacement therapy. Arq Bras Endocrinol Metabol 54: 690–697, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213. Pelleymounter MA, Cullen MJ, Baker MB, Hecht R, Winters D, Boone T, Collins F. Effects of the obese gene product on body weight regulation in ob/ob mice. Science 269: 540–543, 1995 [DOI] [PubMed] [Google Scholar]
- 214. Perez C, Fernandez-Galaz C, Fernandez-Agullo T, Arribas C, Andres A, Ros M, Carrascosa JM. Leptin impairs insulin signaling in rat adipocytes. Diabetes 53: 347–353, 2004 [DOI] [PubMed] [Google Scholar]
- 215. Petersen KF, Oral EA, Dufour S, Befroy D, Ariyan C, Yu C, Cline GW, DePaoli AM, Taylor SI, Gorden P, Shulman GI. Leptin reverses insulin resistance and hepatic steatosis in patients with severe lipodystrophy. J Clin Invest 109: 1345–1350, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216. Pombo M, Pombo CM, Garcia A, Caminos E, Gualillo O, Alvarez CV, Casanueva FF, Dieguez C. Hormonal control of growth hormone secretion. Horm Res 55, Suppl 1: 11–16, 2001 [DOI] [PubMed] [Google Scholar]
- 217. Quennell JH, Mulligan AC, Tups A, Liu X, Phipps SJ, Kemp CJ, Herbison AE, Grattan DR, Anderson GM. Leptin indirectly regulates gonadotropin-releasing hormone neuronal function. Endocrinology 150: 2805–2812, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218. Ranganathan S, Ciaraldi TP, Henry RR, Mudaliar S, Kern PA. Lack of effect of leptin on glucose transport, lipoprotein lipase, and insulin action in adipose and muscle cells. Endocrinology 139: 2509–2513, 1998 [DOI] [PubMed] [Google Scholar]
- 219. Ravussin E, Smith SR, Mitchell JA, Shringarpure R, Shan K, Maier H, Koda JE, Weyer C. Enhanced weight loss with pramlintide/metreleptin: an integrated neurohormonal approach to obesity pharmacotherapy. Obesity (Silver Spring) 17: 1736–1743, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220. Robertson SA, Leinninger GM, Myers MG., Jr Molecular and neural mediators of leptin action. Physiol Behav 94: 637–642, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221. Roemmich JN, Clark PA, Berr SS, Mai V, Mantzoros CS, Flier JS, Weltman A, Rogol AD. Gender differences in leptin levels during puberty are related to the subcutaneous fat depot and sex steroids. Am J Physiol Endocrinol Metab 275: E543–E551, 1998 [DOI] [PubMed] [Google Scholar]
- 222. Roemmich JN, Clark PA, Mantzoros CS, Gurgol CM, Weltman A, Rogol AD. Relationship of leptin to bone mineralization in children and adolescents. J Clin Endocrinol Metab 88: 599–604, 2003 [DOI] [PubMed] [Google Scholar]
- 223. Rosenbaum M, Goldsmith R, Bloomfield D, Magnano A, Weimer L, Heymsfield S, Gallagher D, Mayer L, Murphy E, Leibel RL. Low-dose leptin reverses skeletal muscle, autonomic, and neuroendocrine adaptations to maintenance of reduced weight. J Clin Invest 115: 3579–3586, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224. Rosenbaum M, Nicolson M, Hirsch J, Heymsfield SB, Gallagher D, Chu F, Leibel RL. Effects of gender, body composition, and menopause on plasma concentrations of leptin. J Clin Endocrinol Metab 81: 3424–3427, 1996 [DOI] [PubMed] [Google Scholar]
- 225. Rosenbaum M, Sy M, Pavlovich K, Leibel RL, Hirsch J. Leptin reverses weight loss-induced changes in regional neural activity responses to visual food stimuli. J Clin Invest 118: 2583–2591, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226. Rosenbaum M, Vandenborne K, Goldsmith R, Simoneau JA, Heymsfield S, Joanisse DR, Hirsch J, Murphy E, Matthews D, Segal KR, Leibel RL. Effects of experimental weight perturbation on skeletal muscle work efficiency in human subjects. Am J Physiol Regul Integr Comp Physiol 285: R183–R192, 2003 [DOI] [PubMed] [Google Scholar]
- 227. Rossetti L, Massillon D, Barzilai N, Vuguin P, Chen W, Hawkins M, Wu J, Wang J. Short term effects of leptin on hepatic gluconeogenesis and in vivo insulin action. J Biol Chem 272: 27758–27763, 1997 [DOI] [PubMed] [Google Scholar]
- 228. Roth JD, Roland BL, Cole RL, Trevaskis JL, Weyer C, Koda JE, Anderson CM, Parkes DG, Baron AD. Leptin responsiveness restored by amylin agonism in diet-induced obesity: evidence from nonclinical and clinical studies. Proc Natl Acad Sci USA 105: 7257–7262, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229. Saad MF, Damani S, Gingerich RL, Riad-Gabriel MG, Khan A, Boyadjian R, Jinagouda SD, el-Tawil K, Rude RK, Kamdar V. Sexual dimorphism in plasma leptin concentration. J Clin Endocrinol Metab 82: 579–584, 1997 [DOI] [PubMed] [Google Scholar]
- 230. Sanchez VC, Goldstein J, Stuart RC, Hovanesian V, Huo L, Munzberg H, Friedman TC, Bjorbaek C, Nillni EA. Regulation of hypothalamic prohormone convertases 1 and 2 and effects on processing of prothyrotropin-releasing hormone. J Clin Invest 114: 357–369, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231. Sato S, Hanada R, Kimura A, Abe T, Matsumoto T, Iwasaki M, Inose H, Ida T, Mieda M, Takeuchi Y, Fukumoto S, Fujita T, Kato S, Kangawa K, Kojima M, Shinomiya K, Takeda S. Central control of bone remodeling by neuromedin U. Nat Med 13: 1234–1240, 2007 [DOI] [PubMed] [Google Scholar]
- 232. Scheer FA, Chan JL, Fargnoli J, Chamberland J, Arampatzi K, Shea SA, Blackburn GL, Mantzoros CS. Day/night variations of high-molecular-weight adiponectin and lipocalin-2 in healthy men studied under fed and fasted conditions. Diabetologia 53: 2401–2405, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233. Scheer FA, Hilton MF, Mantzoros CS, Shea SA. Adverse metabolic and cardiovascular consequences of circadian misalignment. Proc Natl Acad Sci USA 106: 4453–4458, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234. Schwartz MW, Baskin DG, Bukowski TR, Kuijper JL, Foster D, Lasser G, Prunkard DE, Porte D, Jr, Woods SC, Seeley RJ, Weigle DS. Specificity of leptin action on elevated blood glucose levels and hypothalamic neuropeptide Y gene expression in ob/ob mice. Diabetes 45: 531–535, 1996 [DOI] [PubMed] [Google Scholar]
- 235. Sekhar RV, Patel SG, D'Amico S, Shi J, Balasubramanyam A, Rehman K, Jahoor F, Visnegarwala F. Effects of rosiglitazone on abnormal lipid kinetics in HIV-associated dyslipidemic lipodystrophy: a stable isotope study. Metabolism 60: 754–760, 2011 [DOI] [PubMed] [Google Scholar]
- 236. Seminara SB, Messager S, Chatzidaki EE, Thresher RR, Acierno JS, Jr, Shagoury JK, Bo-Abbas Y, Kuohung W, Schwinof KM, Hendrick AG, Zahn D, Dixon J, Kaiser UB, Slaugenhaupt SA, Gusella JF, O'Rahilly S, Carlton MB, Crowley WF, Jr, Aparicio SA, Colledge WH. The GPR54 gene as a regulator of puberty. N Engl J Med 349: 1614–1627, 2003 [DOI] [PubMed] [Google Scholar]
- 237. Seufert J, Kieffer TJ, Habener JF. Leptin inhibits insulin gene transcription and reverses hyperinsulinemia in leptin-deficient ob/ob mice. Proc Natl Acad Sci USA 96: 674–679, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238. Seufert J, Kieffer TJ, Leech CA, Holz GG, Moritz W, Ricordi C, Habener JF. Leptin suppression of insulin secretion and gene expression in human pancreatic islets: implications for the development of adipogenic diabetes mellitus. J Clin Endocrinol Metab 84: 670–676, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239. Severin S, Ghevaert C, Mazharian A. The mitogen-activated protein kinase signalling pathways: role in megakaryocyte differentiation. J Thromb Haemost 8: 17–26, 2009 [DOI] [PubMed] [Google Scholar]
- 240. Shahab M, Mastronardi C, Seminara SB, Crowley WF, Ojeda SR, Plant TM. Increased hypothalamic GPR54 signaling: a potential mechanism for initiation of puberty in primates. Proc Natl Acad Sci USA 102: 2129–2134, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241. Sharma NK, Das SK, Mondal AK, Hackney OG, Chu WS, Kern PA, Rasouli N, Spencer HJ, Yao-Borengasser A, Elbein SC. Endoplasmic reticulum stress markers are associated with obesity in nondiabetic subjects. J Clin Endocrinol Metab 93: 4532–4541, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242. Sharma V, Mustafa S, Patel N, Wambolt R, Allard MF, McNeill JH. Stimulation of cardiac fatty acid oxidation by leptin is mediated by a nitric oxide-p38 MAPK-dependent mechanism. Eur J Pharmacol 617: 113–117, 2009 [DOI] [PubMed] [Google Scholar]
- 243. Shea SA, Hilton MF, Orlova C, Ayers RT, Mantzoros CS. Independent circadian and sleep/wake regulation of adipokines and glucose in humans. J Clin Endocrinol Metab 90: 2537–2544, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244. Shetty GK, Matarese G, Magkos F, Moon HS, Liu X, Brennan AM, Mylvaganam G, Sykoutri D, DePaoli AM, Mantzoros CS. Leptin administration to overweight and obese subjects for 6 months increases free leptin concentrations but does not alter circulating hormones of the thyroid and IGF axes during weight loss induced by a mild hypocaloric diet. Eur J Endocrinol 165: 249–254, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245. Shimizu H, Shimomura Y, Nakanishi Y, Futawatari T, Ohtani K, Sato N, Mori M. Estrogen increases in vivo leptin production in rats and human subjects. J Endocrinol 154: 285–292, 1997 [DOI] [PubMed] [Google Scholar]
- 246. Shimomura I, Hammer RE, Ikemoto S, Brown MS, Goldstein JL. Leptin reverses insulin resistance and diabetes mellitus in mice with congenital lipodystrophy. Nature 401: 73–76, 1999 [DOI] [PubMed] [Google Scholar]
- 247. Sienkiewicz E, Magkos F, Aronis KN, Brinkoetter M, Chamberland JP, Chou S, Arampatzi KM, Gao C, Koniaris A, Mantzoros CS. Long-term metreleptin treatment increases bone mineral density and content at the lumbar spine of lean hypoleptinemic women. Metabolism 2011. July 6 PMID: 21741057 [DOI] [PubMed] [Google Scholar]
- 248. Sinha MK, Ohannesian JP, Heiman ML, Kriauciunas A, Stephens TW, Magosin S, Marco C, Caro JF. Nocturnal rise of leptin in lean, obese, and non-insulin-dependent diabetes mellitus subjects. J Clin Invest 97: 1344–1347, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249. Smith JT, Acohido BV, Clifton DK, Steiner RA. KiSS-1 neurones are direct targets for leptin in the ob/ob mouse. J Neuroendocrinol 18: 298–303, 2006 [DOI] [PubMed] [Google Scholar]
- 250. Steinberg GR, Dyck DJ. Development of leptin resistance in rat soleus muscle in response to high-fat diets. Am J Physiol Endocrinol Metab 279: E1374–E1382, 2000 [DOI] [PubMed] [Google Scholar]
- 251. Steinberg GR, Parolin ML, Heigenhauser GJ, Dyck DJ. Leptin increases FA oxidation in lean but not obese human skeletal muscle: evidence of peripheral leptin resistance. Am J Physiol Endocrinol Metab 283: E187–E192, 2002 [DOI] [PubMed] [Google Scholar]
- 252. Steppan CM, Crawford DT, Chidsey-Frink KL, Ke H, Swick AG. Leptin is a potent stimulator of bone growth in ob/ob mice. Regul Pept 92: 73–78, 2000 [DOI] [PubMed] [Google Scholar]
- 253. Strobel A, Issad T, Camoin L, Ozata M, Strosberg AD. A leptin missense mutation associated with hypogonadism and morbid obesity. Nat Genet 18: 213–215, 1998 [DOI] [PubMed] [Google Scholar]
- 254. Sun Q, van Dam RM, Meigs JB, Franco OH, Mantzoros CS, Hu FB. Leptin and soluble leptin receptor levels in plasma and risk of type 2 diabetes in U.S. women: a prospective study. Diabetes 59: 611–618, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255. Sweeney LL, Brennan AM, Mantzoros CS. The role of adipokines in relation to HIV lipodystrophy. AIDS 21: 895–904, 2007 [DOI] [PubMed] [Google Scholar]
- 256. Szanto I, Kahn CR. Selective interaction between leptin and insulin signaling pathways in a hepatic cell line. Proc Natl Acad Sci USA 97: 2355–2360, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257. Takeda S, Elefteriou F, Levasseur R, Liu X, Zhao L, Parker KL, Armstrong D, Ducy P, Karsenty G. Leptin regulates bone formation via the sympathetic nervous system. Cell 111: 305–317, 2002 [DOI] [PubMed] [Google Scholar]
- 258. Tartaglia LA. The leptin receptor. J Biol Chem 272: 6093–6096, 1997 [DOI] [PubMed] [Google Scholar]
- 259. Thomas T, Gori F, Khosla S, Jensen MD, Burguera B, Riggs BL. Leptin acts on human marrow stromal cells to enhance differentiation to osteoblasts and to inhibit differentiation to adipocytes. Endocrinology 140: 1630–1638, 1999 [DOI] [PubMed] [Google Scholar]
- 260. Thompson EL, Patterson M, Murphy KG, Smith KL, Dhillo WS, Todd JF, Ghatei MA, Bloom SR. Central and peripheral administration of kisspeptin-10 stimulates the hypothalamic-pituitary-gonadal axis. J Neuroendocrinol 16: 850–858, 2004 [DOI] [PubMed] [Google Scholar]
- 261. Tritos NA, Mantzoros CS. Leptin: its role in obesity and beyond. Diabetologia 40: 1371–1379, 1997 [DOI] [PubMed] [Google Scholar]
- 262. Tsiodras S, Mantzoros C. Leptin and adiponectin in the HIV associated metabolic syndrome: physiologic and therapeutic implications. Am J Infect Dis 2: 141–152, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263. Tsiodras S, Perelas A, Wanke C, Mantzoros CS. The HIV-1/HAART associated metabolic syndrome—novel adipokines, molecular associations and therapeutic implications. J Infect 61: 101–113, 2010 [DOI] [PubMed] [Google Scholar]
- 264. Tu H, Kastin AJ, Hsuchou H, Pan W. Soluble receptor inhibits leptin transport. J Cell Physiol 214: 301–305, 2008 [DOI] [PubMed] [Google Scholar]
- 265. Tuduri E, Marroqui L, Soriano S, Ropero AB, Batista TM, Piquer S, Lopez-Boado MA, Carneiro EM, Gomis R, Nadal A, Quesada I. Inhibitory effects of leptin on pancreatic alpha-cell function. Diabetes 58: 1616–1624, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266. van der Kroon PH, Boldewijn H, Langeveld-Soeter N. Congenital hypothyroidism in latent obese (ob/ob) mice. Int J Obes 6: 83–90, 1982 [PubMed] [Google Scholar]
- 267. van Rossum CT, Hoebee B, van Baak MA, Mars M, Saris WH, Seidell JC. Genetic variation in the leptin receptor gene, leptin, and weight gain in young Dutch adults. Obes Res 11: 377–386, 2003 [DOI] [PubMed] [Google Scholar]
- 268. Vance ML, Thorner MO. Fasting alters pulsatile and rhythmic cortisol release in normal man. J Clin Endocrinol Metab 68: 1013–1018, 1989 [DOI] [PubMed] [Google Scholar]
- 269. Vila R, Adan C, Rafecas I, Fernandez-Lopez JA, Remesar X, Alemany M. Plasma leptin turnover rates in lean and obese Zucker rats. Endocrinology 139: 4466–4469, 1998 [DOI] [PubMed] [Google Scholar]
- 270. Walder K, Filippis A, Clark S, Zimmet P, Collier GR. Leptin inhibits insulin binding in isolated rat adipocytes. J Endocrinol 155: R5–R7, 1997 [DOI] [PubMed] [Google Scholar]
- 271. Wang MY, Chen L, Clark GO, Lee Y, Stevens RD, Ilkayeva OR, Wenner BR, Bain JR, Charron MJ, Newgard CB, Unger RH. Leptin therapy in insulin-deficient type I diabetes. Proc Natl Acad Sci USA 107: 4813–4819 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272. Wang Y, Kuropatwinski KK, White DW, Hawley TS, Hawley RG, Tartaglia LA, Baumann H. Leptin receptor action in hepatic cells. J Biol Chem 272: 16216–16223, 1997 [DOI] [PubMed] [Google Scholar]
- 273. Welt CK, Chan JL, Bullen J, Murphy R, Smith P, DePaoli AM, Karalis A, Mantzoros CS. Recombinant human leptin in women with hypothalamic amenorrhea. N Engl J Med 351: 987–997, 2004 [DOI] [PubMed] [Google Scholar]
- 274. White DW, Tartaglia LA. Leptin and OB-R: body weight regulation by a cytokine receptor. Cytokine Growth Factor Rev 7: 303–309, 1996 [DOI] [PubMed] [Google Scholar]
- 275. Widdowson PS, Upton R, Pickavance L, Buckingham R, Tadayyon M, Arch J, Williams G. Acute hyperleptinemia does not modify insulin sensitivity in vivo in the rat. Horm Metab Res 30: 259–262, 1998 [DOI] [PubMed] [Google Scholar]
- 276. Wolfe BE, Jimerson DC, Orlova C, Mantzoros CS. Effect of dieting on plasma leptin, soluble leptin receptor, adiponectin and resistin levels in healthy volunteers. Clin Endocrinol (Oxf) 61: 332–338, 2004 [DOI] [PubMed] [Google Scholar]
- 277. Won JC, Jang PG, Namkoong C, Koh EH, Kim SK, Park JY, Lee KU, Kim MS. Central administration of an endoplasmic reticulum stress inducer inhibits the anorexigenic effects of leptin and insulin. Obesity (Silver Spring) 17: 1861–1865, 2009 [DOI] [PubMed] [Google Scholar]
- 278. Wong SL, DePaoli AM, Lee JH, Mantzoros CS. Leptin hormonal kinetics in the fed state: effects of adiposity, age, and gender on endogenous leptin production and clearance rates. J Clin Endocrinol Metab 89: 2672–2677, 2004 [DOI] [PubMed] [Google Scholar]
- 279. Wren AM, Small CJ, Abbott CR, Jethwa PH, Kennedy AR, Murphy KG, Stanley SA, Zollner AN, Ghatei MA, Bloom SR. Hypothalamic actions of neuromedin U. Endocrinology 143: 4227–4234, 2002 [DOI] [PubMed] [Google Scholar]
- 280. Yadav VK, Oury F, Suda N, Liu ZW, Gao XB, Confavreux C, Klemenhagen KC, Tanaka KF, Gingrich JA, Guo XE, Tecott LH, Mann JJ, Hen R, Horvath TL, Karsenty G. A serotonin-dependent mechanism explains the leptin regulation of bone mass, appetite, and energy expenditure. Cell 138: 976–989, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281. Yamauchi T, Kamon J, Waki H, Terauchi Y, Kubota N, Hara K, Mori Y, Ide T, Murakami K, Tsuboyama-Kasaoka N, Ezaki O, Akanuma Y, Gavrilova O, Vinson C, Reitman ML, Kagechika H, Shudo K, Yoda M, Nakano Y, Tobe K, Nagai R, Kimura S, Tomita M, Froguel P, Kadowaki T. The fat-derived hormone adiponectin reverses insulin resistance associated with both lipoatrophy and obesity. Nat Med 7: 941–946, 2001 [DOI] [PubMed] [Google Scholar]
- 282. Yannakoulia M, Yiannakouris N, Bluher S, Matalas AL, Klimis-Zacas D, Mantzoros CS. Body fat mass and macronutrient intake in relation to circulating soluble leptin receptor, free leptin index, adiponectin, and resistin concentrations in healthy humans. J Clin Endocrinol Metab 88: 1730–1736, 2003 [DOI] [PubMed] [Google Scholar]
- 283. Yaspelkis BB, 3rd, Davis JR, Saberi M, Smith TL, Jazayeri R, Singh M, Fernandez V, Trevino B, Chinookoswong N, Wang J, Shi ZQ, Levin N. Leptin administration improves skeletal muscle insulin responsiveness in diet-induced insulin-resistant rats. Am J Physiol Endocrinol Metab 280: E130–E142, 2001 [DOI] [PubMed] [Google Scholar]
- 284. Yiannakouris N, Melistas L, Kontogianni M, Heist K, Mantzoros CS. The Val81 missense mutation of the melanocortin 3 receptor gene, but not the 1908c/T nucleotide polymorphism in lamin A/C gene, is associated with hyperleptinemia and hyperinsulinemia in obese Greek caucasians. J Endocrinol Invest 27: 714–720, 2004 [DOI] [PubMed] [Google Scholar]
- 285. Yu WH, Kimura M, Walczewska A, Karanth S, McCann SM. Role of leptin in hypothalamic-pituitary function. Proc Natl Acad Sci USA 94: 1023–1028, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 286. Zhang F, Basinski MB, Beals JM, Briggs SL, Churgay LM, Clawson DK, DiMarchi RD, Furman TC, Hale JE, Hsiung HM, Schoner BE, Smith DP, Zhang XY, Wery JP, Schevitz RW. Crystal structure of the obese protein leptin-E100. Nature 387: 206–209, 1997 [DOI] [PubMed] [Google Scholar]
- 287. Zhang Y, Proenca R, Maffei M, Barone M, Leopold L, Friedman JM. Positional cloning of the mouse obese gene and its human homologue. Nature 372: 425–432, 1994 [DOI] [PubMed] [Google Scholar]
- 288. Zhao AZ, Shinohara MM, Huang D, Shimizu M, Eldar-Finkelman H, Krebs EG, Beavo JA, Bornfeldt KE. Leptin induces insulin-like signaling that antagonizes cAMP elevation by glucagon in hepatocytes. J Biol Chem 275: 11348–11354, 2000 [DOI] [PubMed] [Google Scholar]