Abstract
Recent studies have explored the potential of central nervous system-derived neural stem cells (CNS-NSC) to repopulate the enteric nervous system. However, the exact phenotypic fate of gut-transplanted CNS-NSC has not been characterized. The aim of this study was to investigate the effect of the gut microenvironment on phenotypic fate of CNS-NSC in vitro. With the use of Transwell culture, differentiation of mouse embryonic CNS-NSC was studied when cocultured without direct contact with mouse intestinal longitudinal muscle-myenteric plexus preparations (LM-MP) compared with control noncocultured cells, in a differentiating medium. Differentiated cells were analyzed by immunocytochemistry and quantitative RT-PCR to assess the expression of specific markers and by whole cell patch-clamp studies for functional characterization of their phenotype. We found that LM-MP cocultured cells had a significant increase in the numbers of cells that were immune reactive against the panneuronal marker β-tubulin, neurotransmitters neuronal nitric oxide synthase (nNOS), choline acetyltransferase (ChAT), and neuropeptide vasoactive intestinal peptide (VIP) and showed an increase in expression of these genes, compared with control cells. Whole cell patch-clamp analysis showed that coculture with LM-MP decreases cell excitability and reduces voltage-gated Na+ currents but significantly enhances A-current and late afterhyperpolarization (AHP) and increases the expression of the four AHP-generating Ca2+-dependent K+ channel genes (KCNN), compared with control cells. In a separate experiment, differentiation of LM-MP cocultured CNS-NSC produced a significant increase in the numbers of cells that were immune reactive against the neurotransmitters nNOS, ChAT, and the neuropeptide VIP compared with CNS-NSC differentiated similarly in the presence of neonatal brain tissue. Our results show that the gut microenvironment induces CNS-NSC to produce neurons that share some of the characteristics of classical enteric neurons, further supporting the therapeutic use of these cells for gastrointestinal disorders.
Keywords: central nervous system, enteric nervous system, longitudinal muscle-myenteric plexus, afterhyperpolarization, neuronal nitric oxide synthase, Ca2+-dependent K+ channel genes
neuronal dysfunction is an important cause of gastrointestinal dysmotility. It may be due to a global or a selective loss of neurons or neurotransmitters and is part of the pathophysiological spectrum of many disorders including Hirschsprung's disease, achalasia, hypertrophic pyloric stenosis, and diabetic gastropathy (2, 5, 8, 11, 13, 17–19, 26). The treatment of such disorders is far from satisfactory and remains palliative at best. Simple pharmacological replacement of enteric neurotransmitters constitutes a crude and ineffective therapy for most of these disorders because of an inability of therapy to address temporal- and spatial-specific patterns of neuronal activation in healthy organs. Transplantation of the affected region of the gut with neural stem cells (NSC), which can differentiate into healthy neurons and restore functionality to the dysfunctional gut, is therefore an attractive option for these disorders.
We have hypothesized that, on transplantation into the gut, neural stem cells derived from developmentally distinct organs such as the brain can either mimic or assume the behavior of enteric nervous system (ENS) neurons and that the microenvironment of the gut secretes certain gut-specific factors that guide the plasticity of such neurons to assume ENS behavior. To this end, we previously transplanted NSC from embryonic mouse central nervous system (CNS) into the dysfunctional and dysmotile region of the adult gut and examined their short-term differentiation and functional properties (20, 22). Although these in vivo studies provided proof of principle of the feasibility of this approach, there remains much to be learned about the abilities of CNS-NSC (or NSC from other sources) to assume a true enteric neuronal phenotype. A recent study reports the effects of environmental cues from ENS that regulate CNS progenitor differentiation (4). However, the physiological behavior of these differentiated neurons has not been characterized.
In this study, we established an in vitro model system that enabled us to study the molecular and electrophysiological phenotype of CNS-NSC derivatives in response to putative gut-derived signals. The system consists of differentiating CNS-NSC in a Transwell culture system in the presence of organotypic preparations of longitudinal muscle containing myenteric plexus (LM-MP) from the adult gut. Comparing the cells derived from LM-MP co-cultured CNS-NSC to cells derived from brain cocultured CNS-NSC as well as noncocultured CNS-NSC, we show that this model system of the gut microenvironment induces CNS-NSC to differentiate into neurons that mimic enteric behavior, reinforcing their therapeutic potential for gastrointestinal neural disorders.
MATERIALS AND METHODS
Animals
Staged-pregnant female mice (C57/BL6, Charles River, Wilmington, MA) at embryonic day 13.5 (E13.5) were used for the isolation of CNS-NSC. Adult male (4 wk to 5 mo old, C57/BL6; Charles River) were used for the small intestinal LM-MP isolations. Postnatal day 5 animals (P5, C57/BL6; Charles River) were used for brain slice culture preparations. Experimental protocols were approved by the Administrative Panel on Laboratory Animal Care (APLAC) at the Stanford University, California in accordance with the guidelines provided by the National Institutes of Health.
Isolation and In Vitro Culture of Mouse CNS-NSC
Staged-pregnant mice were anesthetized with isoflurane before being euthanized by cervical dislocation. A laparotomy was performed to expose the uteri and remove the embryos. Brains of these embryos were removed, and the subventricular zone was dissected out from each brain hemisphere. The dissected tissue was washed with ice-cold MEM-HEPES containing 100 U/ml penicillin-streptomycin (PS) (Invitrogen, Carlsbad, CA), treated with Accutase (Innovative Cell Technologies, San Diego, CA) for 5 min at 37°C and gently triturated using a 27.5-gauge needle and 1-ml syringe. The resulting single cell suspension was centrifuged, washed with sterile Dulbecco's PBS (DPBS), and resuspended in neural stem cell medium [neurobasal medium containing B27 without retinoic acid, 0.7% BSA, 2 mM l-glutamine, 50 μM β-mercaptoethanol, and 100 U/ml PS, plus 20 ng/ml fibroblast growth factor, and 20 ng/ml epidermal growth factor (Invitrogen)]. In culture, single neural stem cells proliferated to form neurospheres, which were dissociated using the protocol described above and passaged once every 4 days.
Small Intestinal LM-MP Preparations
Adult (4–20 wk old) male C57/BL6 mice were anesthetized as described above, and a laparotomy was performed to expose the small intestine. One-centimeter-long pieces of the small intestine were dissected out, flushed and cleaned with sterile HBSS containing 100 U/ml PS, and placed over a plastic rod. A small incision was made on the serosal surface and the longitudinal muscle with the adherent LM-MP peeled off from the underlying tissue using a wet sterile cotton swab. Individual LM-MP pieces were placed in ice-cold MEM-HEPES containing 100 U/ml PS and were cultured at 37°C and 5% CO2 atmosphere in DMEM containing 10% fetal bovine serum and 100 U/ml PS overnight, before being washed with DPBS and being placed in coculture with CNS-NSC.
Brain Slice Preparation
Long-term organotypic cultures of adult brain tissue are known to be less stable and more prone to disintegration than similar culture of neonatal brain tissue (6). Because our experimental setup needs long-term culture of a richly innervated tissue, we used P5 neonatal mouse brains as one of the required control. P5 mice heads were removed and dissected to remove their brains, which were irrigated in artificial cerebrospinal fluid (A-CSF) (28). With the use of a tissue slicer, the brains were sliced at a thickness of 250 μm with constant A-CSF irrigation. These brain slices were washed in ice-cold MEM-HEPES containing 100 U/ml PS and were cultured at 37°C in 5% CO2 in DMEM containing 10% fetal bovine serum and 100 U/ml PS overnight, before being washed with DPBS and being placed in coculture with CNS-NSC.
Differentiation Experiments
To ascertain differentiation differences between cells cocultured with and without LM-MP, CNS-NSC (4th-passage proliferating cells) were differentiated under two conditions: Test, wherein cells were cocultured with LM-MP, and Controls, where cells were not cocultured with tissue. CNS-NSC were plated onto sterile poly-ornithine/laminin-coated 22-mm glass coverslips at a density of 2,000 cells/coverslip (to be analyzed by immunocytochemistry and whole cell patch clamp) and onto BD poly-d-lysine laminin-coated six-well plates at a density of 50,000 cells/well (plated at higher densities to yield enough RNA to be analyzed by quantitative RT-PCR) in DC medium (neurobasal medium containing B27 without retinoic acid, 0.7% BSA, 2 mM l-glutamine, 50 μM β-mercaptoethanol, and 100 U/ml PS) and allowed to adhere. LM-MP tissue pieces were then added to the respective wells by placing them into Transwells containing a 0.4-μm microporous membrane (Corning, Corning, NY). Cocultures were maintained in DC medium and cultured for 7 days for immunocytochemistry analysis and qRT-PCR and an additional 3 days for patch-clamp analysis. For noncoculture experiments, CNS-NSC were cultured in DC medium without the addition of LM-MP for coculture.
Similarly, for ascertaining the differences in effect exerted by LM-MP and brain tissue on differentiation, CNS-NSC (4th-passage proliferating cells) were plated on sterile poly-ornithine/laminin-coated 22-mm glass coverslips at a density of 2,000 cells/coverslip and, after adhering to the coverslips, were cocultured for 7 days in DC medium using similar experimental setup as described above. To ensure that the brain slices in culture do not disintegrate to interfere with the experiment and to further ensure that both LM-MP and brain tissue in culture are equally fresh, both were replaced with fresh tissue after 3 days of culture. After 7 days of culture, the coverslips were removed and analyzed by immunocytochemistry.
RNA Isolation and qRT-PCR
RNA from the cultured cells was extracted using Qiagen RNeasy mini kit to get total RNA from these cells. The RNA quality and quantity was checked using Agilent Bioanalyzer 2100 and Nanodrop 2000c. Quantification of expression of specific genes was carried out using ABI TaqMan probes and ABI StepOne plus real-time instrument. Gene expression analysis of βIII- tubulin (neurons; Mm00727586_s1), glial fibrillary acidic protein (GFAP) (glia; Mm01253033_m1), choline acetyl transferase (ChAT; cholinergic neurons; Mm01221882_m1), neuronal nitric oxide synthase (nNOS; nitrinergic neurons; Mm01208059_m1), vasoactive intestinal peptide (VIP; VIP+ neurons; Mm00660234_m1), RET protooncogene (Mm00436304_m1), and Ca2+-dependent K+ channel genes (KCNN1, Mm01349165_m1; KCNN2, Mm00446514_m1; KCNN3, Mm01212856_m1; KCNN4, Mm01149369_m1) was carried out in biological triplicates of each treatment. Expression of these genes for each treatment was normalized to the expression of their respective housekeeping gene GAPDH (Mm03302249_g1). Fold change in gene expression between the treatments was calculated using the Pfaffl method.
Immunofluorescence Analysis
Coverslips containing cells from treatments and control (3 from each category) were fixed in ice-cold 4% paraformaldehyde in 0.1 M (pH 7.4) for 15 min at room temperature. Fixed cells were blocked and permeabilized for 1 h at room temperature with PBS containing 0.3% Triton X-100 and 5% normal goat serum (NGS). After being washed in PBS, cells were incubated with primary antibodies diluted in PBS containing 1.5% NGS overnight at 4°C. The following antisera were used: βIII-tubulin (mouse 1:500; Abcam, Cambridge, MA), GFAP (rabbit 1:1,500; DAKO, Carpinteria, CA), ChAT (rabbit 1:400; Abcam), nNOS (rabbit 1:500; Zymed, San Francisco, CA), and VIP (rabbit 1:400; Abcam). After being washed with PBS, cells were incubated for 1 h at room temperature with Alexa-conjugated secondary antibodies (Invitrogen AlexaFluor anti-rabbit 594 and anti-mouse antibodies 649, 1:1,000). After two more washes, the coverslips were mounted onto the slides with Hardset DAPI-mounting media (Vector Laboratories, Burlingame, CA). Differentiated CNS-NSC were also stained with mouse and rabbit IgG (5 μg each, Vector) for isotype controls and were counterstained with the appropriate secondary antibodies mentioned above. Samples were examined with a Nikon microscope equipped with fluorescence and digital imaging with a cooled CCD camera. Three random fields from each coverslip were photographed using equal exposure times under UV filter to get cell counts of total cells (stained by DAPI) and appropriate filter for the counterstained fluorophore to count the numbers of cells positive for each marker. For immunocytochemistry for nNOS, ChAT, and VIP, only cells that showed staining in the neuronal processes were scored as positive. Total numbers of cells and cells positive for each marker on every coverslip were counted by an investigator blinded to the treatment groups. The average counts for each group were calculated for all the experiments, and statistical analysis was performed.
Whole Cell Patch-Clamp Recording
Patch-clamp recordings were performed on cells that were cultured for 10 days. The electrodes were pulled from borosilicate glass capillaries. For current-clamp recordings, cells were recorded in oxygenated external solution at room temperature, containing 119 mM NaCl, 2.5 mM KCl, 1.3 mM MgSO4, 2.5 mM CaCl2, 1 mM Na2HPO4, 26.2 mM NaHCO3, and 11 mM glucose and perfused at a rate of 2 ml/min. The internal electrode solution for current-clamp recordings consisted of 120 mM potassium gluconate, 40 mM HEPES, 5 mM MgCl2, 0.3 mM Mg-GTP, 2 mM Na-ATP, with pH adjusted to 7.2 with KOH. For voltage-clamp recordings, the external solution consisted of 130 mM NaCl, 2 mM MgCl2, 3 mM KCl, 1 mM CaCl2, 10 mM HEPES-Na, and 10 mM glucose.
For Na+-current recordings, the internal solution consisted of 120 mM CsF, 10 mM HEPES, 11 mM EGTA, 1 mM CaCl2, 1 mM MgCl2, 10 mM TEA-Cl, and 11 mM CsOH, and pH was adjusted to 7.4 using CsOH. For K+-current recording, the internal solution consisted of 140 mM KCl, 10 mM HEPES, 2 mM MgCl2, 11 mM EGTA, 1.2 mM Mg-ATP. Tetrodotoxin (TTX 0.5–1.0 μM) was added to the external solution to block the Na+ currents. For afterhyperpolarization (AHP) recording, the internal solution consisted of 140 mM KCl, 10 mM HEPES, 2 mM MgCl2, 11 mM EGTA, 1.2 mM Mg-ATP. The pH of all external and internal solutions was adjusted to 7.4 and 7.2, respectively. The osmolality of all solutions was adjusted to ∼290–300 mosmol/kg. All reagents and drugs used in electrophysiology were purchased from Sigma (St. Louis, MO) or Tocris (Ellisville, MO). The resistance of the pipette was around ∼2–3 (MΩ) in the bath solution. Following gigaohm seal, whole cell recording mode was established by breaking the membrane with rapid negative pressure. Most recordings were made in single whole cell patch-clamp recordings using an AxoPatch 200B amplifier and DigiData 1200 A/D converter. In some cases, paired cells were recorded simultaneously using two recording pipettes with Axo700B and DigiData 1440A A/D converter. The series resistance of pipettes was less than 10 MΩ and compensated for 70–80% using built-in offset compensation circulatory of the Axon amplifier. Data were collected using pClamp 10.
Statistical Analysis
Data were analyzed using Clampfit 10.2, and statistical analysis was performed with StatVeiw and Microsoft Excel using two-tailed t-test. Significance was deemed when the P value was found to be <0.05.
RESULTS
LM-MP Coculture Significantly Increases Neurogenic Differentiation of CNS-NSC, Compared with Noncocultured Control
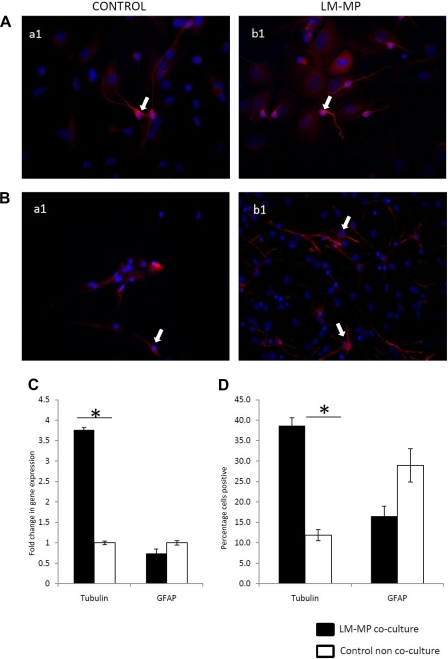
Immunocytochemical analysis shows that CNS-NSC differentiated in the presence of LM-MP (Test) gave rise to significantly more neurons compared with Control noncocultured cells (Test: 38.67 ± 1.9, Control: 11.83 ± 1.35, P < 0.001; Fig. 1, A and D). This observation was additionally confirmed by qRT-PCR analysis, where we observed a significant increase in the expression of the neuronal marker βIII-tubulin (Test: 3.76 ± 0.05, Control: 1.0 ± 0.04, P < 0.001; Fig. 1C). LM-MP cocultured cells also produced fewer glial cells on immunocytochemistry although this difference did not reach statistical significance (Test: 16.53 ± 2.41, Control: 28.97 ± 4.05, P = 0.057; Fig. 1B, D) and on qRT-PCR analysis (Test: 0.74 ± 0.11, Control: 1.0 ± 0.05, P = 0.09; Fig. 1C). In addition, immunofluorescence staining of differentiated CNS-NSC with the appropriate isotype control shows no background staining for the secondary antibodies used, thereby showing the specificity of the used primary antibodies (Fig. 2).
Fig. 1.
Representative images (under ×40 magnification) of noncocultured control neural stem cells (NSC) (a1) and cocultured cells with longitudinal muscle containing myenteric plexus (LM-MP) (b1) stained with nuclear stain DAPI (blue) and antibody specific to βIII-tubulin (red) (A), and glial fibrillary acidic protein (GFAP) (red) (B). Arrows indicate cells found positive for the tested marker. C: percentage of control and LM-MP cultured cells positive for βIII-tubulin and GFAP on immunocytochemistry. D: fold change of gene expression of tubulin and GFAP in LM-MP cocultured cells compared with control noncocultured cells. Data are expressed as means ± SE. *Differences between these 2 groups are significant (P < 0.01, unpaired t-test).
Fig. 2.
Representative images (under ×40 magnification) of isotype controls using mouse IgG (A) and rabbit IgG (B) counterstained for their respective anti-mouse and anti-rabbit secondary antibodies.
LM-MP Coculture Significantly Increases Specific Neurotransmitter- and Neuropeptide-Expressing Neurons from Differentiating CNS-NSC, Compared with Noncocultured Control
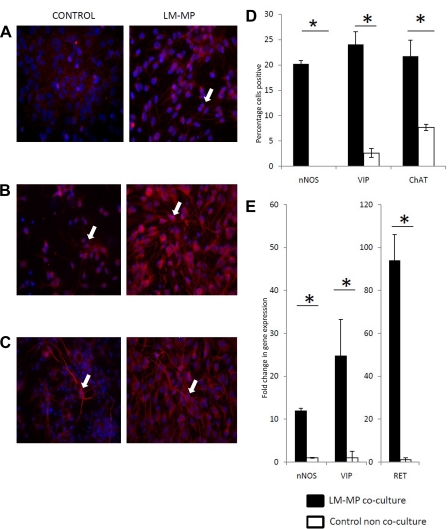
Immunocytochemistry with specific antibodies against nNOS, ChAT, and VIP showed a significant increase in the percentage of neurons positive for these neurotransmitters and neuropeptides (nNOS: Test: 20.23 ± 0.66, Control: 0 ± 0, P = 0.001; ChAT: Test: 21.73 ± 3.18, Control: 7.66 ± 0.61, P = 0.012; VIP: Test: 24.07 ± 2.52, Control: 2.62 ± 0.87, P = 0.001; Fig. 3, A–D). Immunocytochemistry of LM-MP cocultured and noncocultured cells with antibody specific to nNOS showed that, although LM-MP cocultured cells were stained in their neuronal processes (and were scored positive), noncocultured cells stained consistent with a sarcolemmal pattern (7) and not in the processes, and hence were not scored as positive. With qRT-PCR analysis, we observe a significant difference in the expression of genes important for the development and function of the ENS: RET (Test: 93.9 ± 12.10, Control: 1.0 ± 0.11, P = 0.001; Fig. 4A); nNOS (Test: 11.91 ± 0.65, Control: 1.0 ± 0.1, P < 0.001), ChAT (transcript detected in LM-MP coculture and undetected in noncocultured), VIP (Test: 24.76 ± 8.48, Control: 1.0 ± 1.53, P = 0.005) (Fig. 3E).
Fig. 3.
Representative images (under ×40 magnification) of noncocultured control NSC and cocultured cells with LM-MP stained with nuclear stain DAPI (blue) and antibody specific to neuronal nitric oxide synthase (nNOS) (red) (A), vasoactive intestinal peptide (VIP) (red) (B), and choline acetyltransferase (ChAT) (red) (C). Arrows indicate cells found positive for the tested marker. D: percentage of control and LM-MP cultured cells positive for nNOS, VIP, and ChAT on immunocytochemistry. E: fold change of gene expression of nNOS, VIP, and RET in LM-MP cocultured cells compared with control noncocultured cells. Data are expressed as means ± S.E. *Differences between these 2 groups are significant (P < 0.01, unpaired t-test).
Fig. 4.
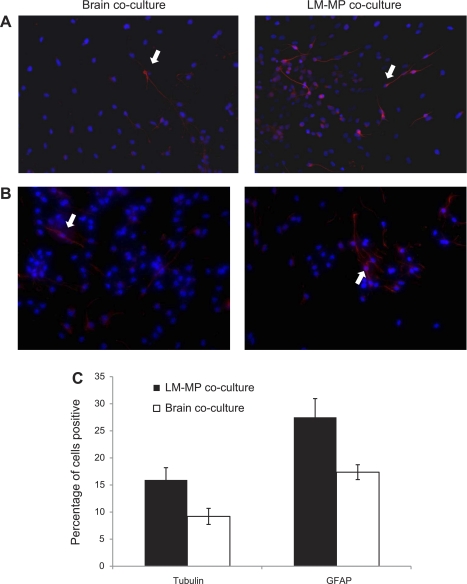
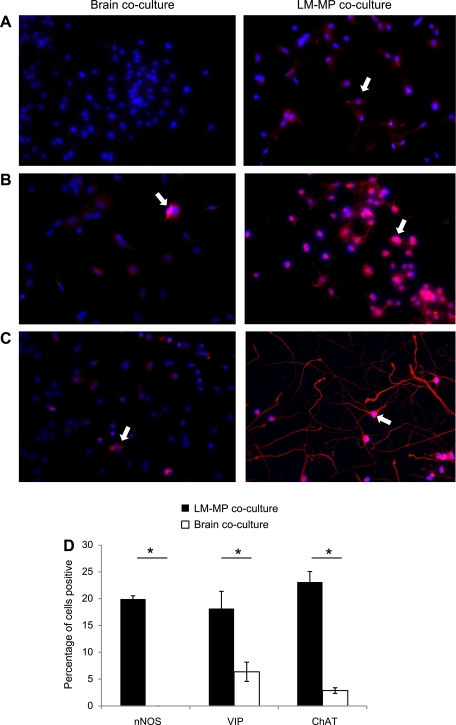
Representative images (under ×40 magnification) of brain cocultured NSC and LM-MP cocultured NSC stained with nuclear stain DAPI (blue) and antibody specific to βIII-tubulin (red) (A) and GFAP (red) (B). Arrows indicate cells found positive for the tested marker. C: percentage of brain and LM-MP cocultured cells positive for βIII-tubulin and GFAP on immunocytochemistry. Data are expressed as means ± SE.
LM-MP Coculture Promotes Neurogenesis and Gliogenesis and Significantly Increases Specific Neurotransmitter- and Neuropeptide-Expressing Neurons from Differentiating CNS-NSC, Compared with Brain Cocultured Control
Immunocytochemical analysis shows that CNS-NSC differentiated in the presence of LM-MP gave rise to more neurons (LM-MP: 15.93 ± 2.26, Brain: 9.2 ± 1.48, P = 0.07; Fig. 4, A and C) and glial cells (LM-MP: 27.50 ± 3.45, Brain: 17.37 ± 1.37, P = 0.053; Fig. 4, B and C) compared with brain cocultured cells. However, these differences in numbers of neurons formed do not reach statistical significance, whereas differences in the numbers of glial cells do present a strong trend between LM-MP and brain cocultured cells.
Immunocytochemistry with specific antibodies against nNOS, ChAT, and VIP showed a significant increase in the percentage of neurons positive for these neurotransmitters and neuropeptide in LM-MP cocultured cells compared with brain cocultured cells (nNOS: LM-MP: 19.93 ± 0.6, Brain: 0 ± 0, P = 0.001; ChAT: LM-MP: 18.17 ± 2.9, Brain: 6.37 ± 1.79, P = 0.032; VIP: LM-MP: 23.10 ± 1.96, Brain: 2.87 ± 0.52, P = 0.001; Fig. 5, A–D).
Fig. 5.
Representative images (under ×40 magnification) of brain cocultured NSC and LM-MP cocultured NSC stained with nuclear stain DAPI (blue) and antibody specific to nNOS (red) (A), VIP (red) (B), and ChAT (red) (C). Arrows indicate cells found positive for the tested marker. D: percentage of brain and LM-MP cocultured cells positive for nNOS, VIP, and ChAT on immunocytochemistry. Data are expressed as mean ± SE. *Differences between these 2 groups are significant (P < 0.01, unpaired t-test).
LM-MP Coculture Promotes an “Enteric-Like” Behavior of Differentiated Neurons
LM-MP cocultured and noncocultured cells display neuronal electrical behavior.
Both LM-MP cocultured and noncocultured cells displayed the presence of Na+ and K+ current on the tenth day of coculture. The resting potential of differentiated cells ranged from −30 to −50 mV without any DC bias current injection and varied from batch to batch. The differentiated cells of noncoculture had resting potential of −43.8 ± 4.5 mV (n = 9) and input resistance of 2,057 ± 925 MΩ. In some experiments, paired cells (n = 4 pairs) were recorded simultaneously to investigate whether there are any synaptic or direct connection between two adjunct cells as illustrated in Fig. 6A. Depolarizing current injections elicited trains of action potentials with little frequency accommodation. In the absence of current injections, there is spontaneous firing of action potentials in cells under both conditions. Because spontaneous firings of action potentials were completely abolished with a small (−10 pA)-bias current injection to hyperpolarize the membrane potential (from around −55 mV to −65 mV), the action potentials are likely triggered by intrinsic membrane potential fluctuation rather than driven by synaptic activity (Fig. 6B). Both spontaneous and depolarizing current-elicited action potentials were completely blocked by TTX (0.5 μM, Fig. 6C), indicating that they are mediated by TTX-sensitive voltage-gated Na+ channels.
Fig. 6.
Basic electrophysiological characterization of NSC neurons without coculture. A: illustration of dual patch-clamp recordings from a pair of differentiated NSCs. Scale bar represents 10 μm. B: under current-clamp mode, cell excitability was assessed by a family of hyperpolarizing and depolarizing current injections. At resting potential (Cell 11a −45 mV and Cell 11b −40 mV), trains of action potentials were elicited by depolarizing current injections. Both cells displayed spontaneous firing of action potentials following the evoked events. Both cells were manually held to more negative potential to −55 to −60 mV with −10 pA bias current injections, and all spontaneous firing of action potentials were abolished. These 2 adjacent cells had no direct synaptic or gap junction connection as firing of action potentials in one cell did not influence activity on the other. Trans of action potentials elicited by depolarizing current injections were completely blocked by tetrodotoxin (TTX, 0.5 μM).
Coculture with LM-MP decreases cell excitability and reduces voltage-gated Na+ currents.
Electrophysiological differences between LM-MP cocultured cells and noncocultured cells were first studied under current-clamp mode. There was a significant lowering of resting membrane potential (−36 ± 4 mV in Test compared with −44 ± 5 mV in Control; n = 9 for each group, P < 0.05). Compared with noncocultured NSC, cells cocultured with LM-MP show a decrease in cell excitability, as few action potentials were elicited by depolarizing current injection (Fig. 7, A and B). In voltage-clamp mode and under condition of blocking K+ currents, a series of depolarizing voltage steps elicited a family of Na+ currents from a holding potential of −100 mV in both noncocultured and LM-MP cocultured cells (Fig. 7C). Peak Na+ current amplitudes in LM-MP cocultured cells (−224 ± 102 pA, n = 5) were significantly lower than noncocultured cells (−799 ± 291 pA, n = 4, P < 0.01: Fig. 7D).
Fig. 7.
Cells cocultured with LM-MP decrease in cell excitability compared with noncocultured cells. Cell excitability was assessed by a series of depolarizing current injections under current-clamp mode in a noncocultured cell (A) and cells cocultured with LM-MP (B). C: in voltage-clamp mode, a representative family of voltage-gated Na+ currents was elicited by a series of voltage steps with a 10-mV increment from a holding potential of −100 mV. D: averaged peak Na+ current amplitudes were elicited by a depolarizing voltage step in cocultured and noncocultured cells at a holding potential of −100 mV (n = 5 per group). **Differences between these 2 groups are very significant (P < 0.01, unpaired t-test).
LM-MP coculture augments the A-type K± currents.
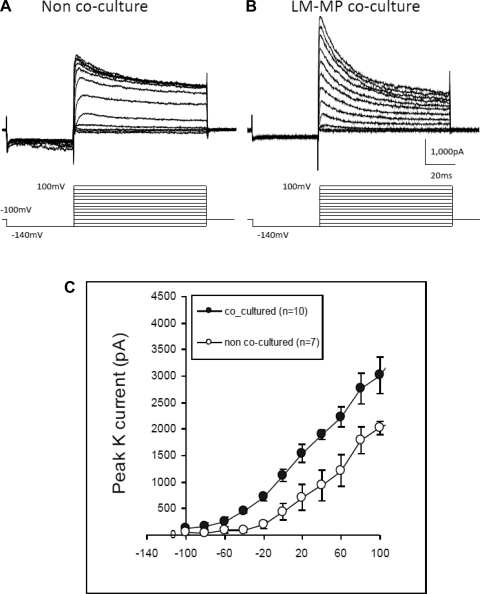
Voltage-gated K+ currents were recorded while completely blocking the Na+ current. When holding potential at −100 mV, K+ currents were induced by a two-voltage-step protocol with the first negative pulse to −140 mV for 40 ms to completely remove inactivated state of A-current and the second pulse subsequently to depolarize the cell to various levels in 10-mV increments for 80 ms to activate the A-type and delayed-rectifier K+ currents (Fig. 8, A and B). The current-voltage plot of the peak K+ current amplitude shows that coculture with LM-MP significantly increases the A-current compared with noncoculture (Fig. 8C, n = 5 each group; P < 0.01).
Fig. 8.
Cells cocultured with LM-MP register increased A-current compared with noncocultured cells. A and B: representative traces of A-current and delayed rectifying K+ currents elicited by a family of voltage steps under voltage-clamp mode at a holding potential of −100 mV. A 2-voltage-step protocol was used with the first step hyperpolarizing the cell to −140 mV for 50 ms and then depolarized to −70 mV with 10-voltage increment. C: averaged peak amplitudes of the A-current elicited by the 2-voltage-step protocol (n = 5 per group). K+ current amplitude differences between these 2 groups are significant (P < 0.01, unpaired t-test).
LM-MP coculture enhances late AHP.
In current-clamp mode, applying a short (50 ms) depolarizing current pulse (10 pA) evoked a single action potential followed by an early AHP. The early AHP was similar between noncocultured and LM-MP cocultured cells (Fig. 9A). However, a large, long-lasting late AHP was observed on repolarization only in LM-MP cocultured cells (Fig. 9A). The membrane potential fell below the precurrent injection level and was measured as −9.9 ± 0.9 mV (n = 5) in LM-MP cocultured cells, whereas the AHP was 0.9 ± 1.3 mV (n = 5, P < 0.01) in the noncoculture cells (Fig. 9B). To verify the late AHP, we tested its sensitivity to the blocker of the small-conductance Ca2+-activated K+ (SK) channels, apamin. Figure 9C shows that amplitudes of AHP were almost completely blocked (from 9.8 ± 1.2 mV reduced to 0.3 ± 1.4 mV; n = 3, P < 0.05, one-tail paired t-test) by the application of apamin (30 nM).
Fig. 9.
Cells cocultured with LM-MP show increased apamin-sensitive late afterhyperpolarizations (AHP) compared with noncocultured cells. A: applying a short (50 ms) and large depolarizing current pulse (10 pA) evokes a single action potential followed by an early fast AHP. A long-lasting late AHP was observed on the repolarization. B: mean slow AHP is −9.9 ± 0.9 mV (n = 5) in respect with the precurrent injection level in cells cocultured with LM-MP, whereas the AHP was 0.9 ± 1.3 mV (n = 5, P < 0.01) in the noncocultured cells. C: mean amplitudes of AHP were almost blocked by apamin (30 nM). The AHP was reduced from 9.8 ± 1.2 mV in control to 0.3 ± 1.4 mV in apamin (n = 3, P < 0.05, 1-tail paired t-test). D: fold change of gene expression of Ca2+-dependent K+ channel genes (KCNN) in LM-MP cocultured cells compared with control noncocultured cells. Data are expressed as means ± SE. Differences between these 2 groups are significant (P < 0.01, unpaired t-test).
LM-MP coculture significantly increases the expression of specific apamin-sensitive and -insensitive channel genes.
qRT-PCR analysis of the expression of KCNN channel genes showed a significant increase in the expression of apamin-sensitive channel KCNN1 (P = 0.026) and apamin-resistant channel KCNN4 (P = 0.021) in Test cells cocultured with LM-MP, compared with Control cells. We find that the expression of KCNN3 shows a tendency to be higher in LM-MP cocultured cells (P = 0.058), but no difference was seen in the expression of KCNN2 between Test and Control cells (Fig. 9D).
DISCUSSION
Our results show that LM-MP coculture induces significant tissue-specific effects on the differentiation of CNS-NSC, compared with control conditions. First, LM-MP induces differentiating CNS-NSC to assume an enteric-like phenotype as assessed by neurotransmitter and neuropeptide expression. Second, LM-MP induced the differentiated NSC to have a reduction in Na+ currents, enhancement in A-type K+ currents, and the presence of late AHP. Thus we have successfully demonstrated the utility of an in vitro model to induce neural stem cells of non-gut origin to assume an enteric-like phenotype. This paper thus adds to the considerable body of literature that attests to the remarkable plasticity of CNS-derived NSC (9). Our findings suggest that it is possible for derivatives of NSC from a different source, such as the CNS, to become functional enteric-like neurons in the presence of putative gut-derived factors and helps explain the gain of function when transplanted into the stomach of nNOS knockout mice (21), namely their ability to migrate along chick neural crest pathways and differentiate into a phenotype similar to neural crest cell derivatives (9) and their ability to make functional connections with human colonic smooth muscle cells (21).
When CNS-derived NSC are differentiated in the presence of the gut microenvironment, the derived cells have a molecular profile significantly different from those cells differentiated in standard differentiation conditions. Overall there was increased expression of panneuronal markers, suggesting that LM-MP induces neurogenesis in the overall differentiation process. More importantly, however, markers for the expression of the principal ENS neurotransmitters (or the enzymes responsible for their synthesis) such as acetylcholine, VIP, and NO (15, 30), were significantly increased in the presence of LM-MP compared with both noncocultured as well as brain cocultured controls. This indicates the presence of specific gut-derived factors that facilitate the development of the observed phenotype of differentiated neurons. In addition, LM-MP also induced an increase in expression of RET compared with noncocultured controls, which is important for the development of a normal ENS (1). We hypothesize that LM-MP first induces the expression of RET, which then allows its ligand, glial-derived neurotrophic factor (GDNF), putatively secreted by the muscle (25), to induce differentiation of the other neurotransmitter markers. Further experiments, using overexpression of RET and blockers of GDNF-RET signaling, are required to answer this question.
Enteric motor reflexes and programmed activity requires precisely timed sequential activation of inhibitory and excitatory neurons (15, 30). Compared with neurons from other organs, enteric neurons have three outstanding and interrelated physiological features that facilitate this function: 1) reciprocal connections with each other; 2) a slow excitatory postsynaptic potential (EPSP) resulting from high-speed firing in other sensory neurons; and 3) a large AHP potential, defined as a current that follows a burst or train of action potentials (27), present in some neurons at the soma. Slow EPSP depolarize the cell body, generate action potentials, and reduce the AHP. Conversely, the AHP limits the firing rate and reduces transmission of slow EPSP (3). Such a system allows the generation of a coordinated motor response such as peristalsis (11, 12). Neurons from organs, such as brain and heart, also express medium and slow AHP that are known to be apamin sensitive, whereas neurons from ENS are known to be apamin resistant (29).
The results of the electrophysiological experiments showed that neurons in both noncoculture and LM-MP coculture are able to generate Na+ current, suggesting that cells can become electrically mature under both conditions. This is not surprising because the noncoculture conditions represent the default differentiation paradigm for NSC. The ability of CNS-NSC to generate TTX-sensitive action potentials (Figs. 6 and 7) and the presence of voltage-gated Na+ and K+ currents (Figs. 7B and 8) are in agreement with most reported electrophysiological studies of CNS-NSC (10, 16).
In enteric neurons, inhibitory signal substances decrease neuronal excitability by increasing K+ currents (14). Compared with noncoculture, we found that, although the LM-MP cocultured cells expressed a lower (less negative) resting membrane potential, they nevertheless exhibit a trend toward reduced cell excitability, presumably resulting from an increase in K+ currents.
We also found the presence of a late AHP in the LM-MP cocultured cells but not in the noncocultured cells. This is consistent with the increased expression of the AHP-producing channel genes KCNN1 and 4. KCNN1, 2, and 3 mediate the apamin-sensitive small-conductance Ca-activated K+ current, whereas KCNN4 mediates intermediate-conductance Ca-activated K+ currents (27, 29). A pronounced AHP has been suggested to be one of the characteristics of the enteric neurons, which determines the firing patterns of enteric neurons (23, 24). However, the AHP of the enteric neurons is known to be apamin resistant (29), whereas the LM-MP-induced AHP in CNS-NSC is apamin sensitive. These results suggest that the differentiation factors secreted by the LM-MP guide the cells to mimic an ENS-like behavior but not necessarily via the exact same molecular machinery. Thus, even though they are not identical to enteric neurons, neurons derived from CNS-NSC under the influence of gut tissue may well be capable of contributing to gut function upon transplantation, as observed in previous experiments (20). Further experiments will be needed to confirm this potential.
We hypothesize that the LM-MP secretes putative factor(s) that cause NSC to differentiate into an enteric-like phenotype. We do not yet know whether this is achieved by a crosstalk between the tissue and the differentiating NSC or whether these factors are produced constitutively by the LM-MP. To answer this question, we are using the same assay to help identify these putative factor(s). This model offers a faster and more efficient method to prescreen stem cell potential to assume or mimic an enteric phenotype in vitro. Although this assay provides an efficient platform to test for enteric potential, it is not an alternative for in vivo transplantation studies to test for gut motility and rescue of function in disease models. However, we believe that this model will help and optimize pretransplant manipulation of stem cells for therapeutic use in such studies.
GRANTS
This study was supported by grants from the NIDDK (1R01DK080920) and Stanford Digestive Disease Center (P30 DK56339) grants to P. Pasricha and the National Institute of Mental Health (R01 MH078194) to X. Xie.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
REFERENCES
- 1. Arighi E, Borrello MG, Sariola H. RET tyrosine kinase signaling in development and cancer. Cytokine Growth Factor Rev 16: 441–467, 2005 [DOI] [PubMed] [Google Scholar]
- 2. Belknap WM. Hirschsprung's Disease. Curr Treat Options Gastroenterol 6: 247–256, 2003 [DOI] [PubMed] [Google Scholar]
- 3. Bertrand PP, Thomas EA. Multiple levels of sensory integration in the intrinsic sensory neurons of the enteric nervous system. Clin Exp Pharmacol Physiol 31: 745–755, 2004 [DOI] [PubMed] [Google Scholar]
- 4. Brannvall K, Corell M, Forsberg-Nilsson K, Fex Svenningsen A. Environmental cues from CNS, PNS, and ENS cells regulate CNS progenitor differentiation. Neuroreport 19: 1283–1289, 2008 [DOI] [PubMed] [Google Scholar]
- 5. Chelimsky G, Chelimsky TC. Evaluation and treatment of autonomic disorders of the gastrointestinal tract. Semin Neurol 23: 453–458, 2003 [DOI] [PubMed] [Google Scholar]
- 6. Cho S, Wood A, Bowlby MR. Brain slices as models for neurodegenerative disease and screening platforms to identify novel therapeutics. Curr Neuropharmacol 5: 19–33, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Crosbie RH, Barresi R, Campbell KP. Loss of sarcolemma nNOS in sarcoglycan-deficient muscle. FASEB J 16: 1786–1791, 2002 [DOI] [PubMed] [Google Scholar]
- 8. De Giorgio R, Camilleri M. Human enteric neuropathies: morphology and molecular pathology. Neurogastroenterol Motil 16: 515–531, 2004 [DOI] [PubMed] [Google Scholar]
- 9. Gage FH. Mammalian neural stem cells. Science 287: 1433–1438, 2000 [DOI] [PubMed] [Google Scholar]
- 10. Goffredo D, Conti L, Di Febo F, Biella G, Tosoni A, Vago G, Biunno I, Moiana A, Bolognini D, Toselli M, Cattaneo E. Setting the conditions for efficient, robust and reproducible generation of functionally active neurons from adult subventricular zone-derived neural stem cells. Cell Death Differ 15: 1847–1856, 2008 [DOI] [PubMed] [Google Scholar]
- 11. Grundy D, Al-Chaer ED, Aziz Q, Collins SM, Ke M, Tache Y, Wood JD. Fundamentals of neurogastroenterology: basic science. Gastroenterology 130: 1391–1411, 2006 [DOI] [PubMed] [Google Scholar]
- 12. Grundy D, Schemann M. Enteric nervous system. Curr Opin Gastroenterol 22: 102–110, 2006 [DOI] [PubMed] [Google Scholar]
- 13. Hirano I. Pathophysiology of achalasia. Curr Gastroenterol Rep 1: 198–202, 1999 [DOI] [PubMed] [Google Scholar]
- 14.Johnson LR. Physiology of the Gastrointestinal Tract. New York: Raven, 1987 [Google Scholar]
- 15. Kunze WA, Furness JB. The enteric nervous system and regulation of intestinal motility. Annu Rev Physiol 61: 117–142, 1999 [DOI] [PubMed] [Google Scholar]
- 16. Lim CG, Kim SS, Suh-Kim H, Lee YD, Ahn SC. Characterization of ionic currents in human neural stem cells. Korean J Physiol Pharmacol 12: 131–135, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Mashimo H, Kjellin A, Goyal RK. Gastric stasis in neuronal nitric oxide synthase-deficient knockout mice. Gastroenterology 119: 766–773, 2000 [DOI] [PubMed] [Google Scholar]
- 18. McCallum RW, Brown RL. Diabetic and nondiabetic gastroparesis. Curr Treat Options Gastroenterol 1: 1–7, 1998 [DOI] [PubMed] [Google Scholar]
- 19. Mearin F, Mourelle M, Guarner F, Salas A, Riveros-Moreno V, Moncada S, Malagelada JR. Patients with achalasia lack nitric oxide synthase in the gastro-oesophageal junction. Eur J Clin Invest 23: 724–728, 1993 [DOI] [PubMed] [Google Scholar]
- 20. Micci MA, Kahrig KM, Simmons RS, Sarna SK, Espejo-Navarro MR, Pasricha PJ. Neural stem cell transplantation in the stomach rescues gastric function in neuronal nitric oxide synthase-deficient mice. Gastroenterology 129: 1817–1824, 2005 [DOI] [PubMed] [Google Scholar]
- 21. Micci MA, Pasricha PJ. Neural stem cells for the treatment of disorders of the enteric nervous system: strategies and challenges. Dev Dyn 236: 33–43, 2007 [DOI] [PubMed] [Google Scholar]
- 22. Micci MA, Pattillo MT, Kahrig KM, Pasricha PJ. Caspase inhibition increases survival of neural stem cells in the gastrointestinal tract. Neurogastroenterol Motil 17: 557–564, 2005 [DOI] [PubMed] [Google Scholar]
- 23. Neylon CB, Fowler CJ, Furness JB. Regulation of the slow afterhyperpolarization in enteric neurons by protein kinase A. Auton Neurosci 126: 258–263, 2006 [DOI] [PubMed] [Google Scholar]
- 24. Nurgali K, Stebbing MJ, Furness JB. Correlation of electrophysiological and morphological characteristics of enteric neurons in the mouse colon. J Comp Neurol 468: 112–124, 2004 [DOI] [PubMed] [Google Scholar]
- 25. Peters RJ, Osinski MA, Hongo JA, Bennett GL, Okragly AJ, Haak-Frendscho M, Epstein ML. GDNF is abundant in the adult rat gut. J Auton Nerv Syst 70: 115–122, 1998 [DOI] [PubMed] [Google Scholar]
- 26. Sivarao DV, Mashimo HL, Thatte HS, Goyal RK. Lower esophageal sphincter is achalasic in nNOS(−/−) and hypotensive in W/W(v) mutant mice. Gastroenterology 121: 34–42, 2001 [DOI] [PubMed] [Google Scholar]
- 27. Stocker M. Ca(2+)-activated K+ channels: molecular determinants and function of the SK family. Nat Rev Neurosci 5: 758–770, 2004 [DOI] [PubMed] [Google Scholar]
- 28. Tzounopoulos T, Kim Y, Oertel D, Trussell LO. Cell-specific, spike timing-dependent plasticities in the dorsal cochlear nucleus. Nat Neurosci 7: 719–725, 2004 [DOI] [PubMed] [Google Scholar]
- 29. Vogalis F, Harvey JR, Furness JB. TEA- and apamin-resistant K(Ca) channels in guinea-pig myenteric neurons: slow AHP channels. J Physiol 538: 421–433, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Wood JD, Alpers DH, Andrews PL. Fundamentals of neurogastroenterology. Gut 45, Suppl 2: II6–II16, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]