Abstract
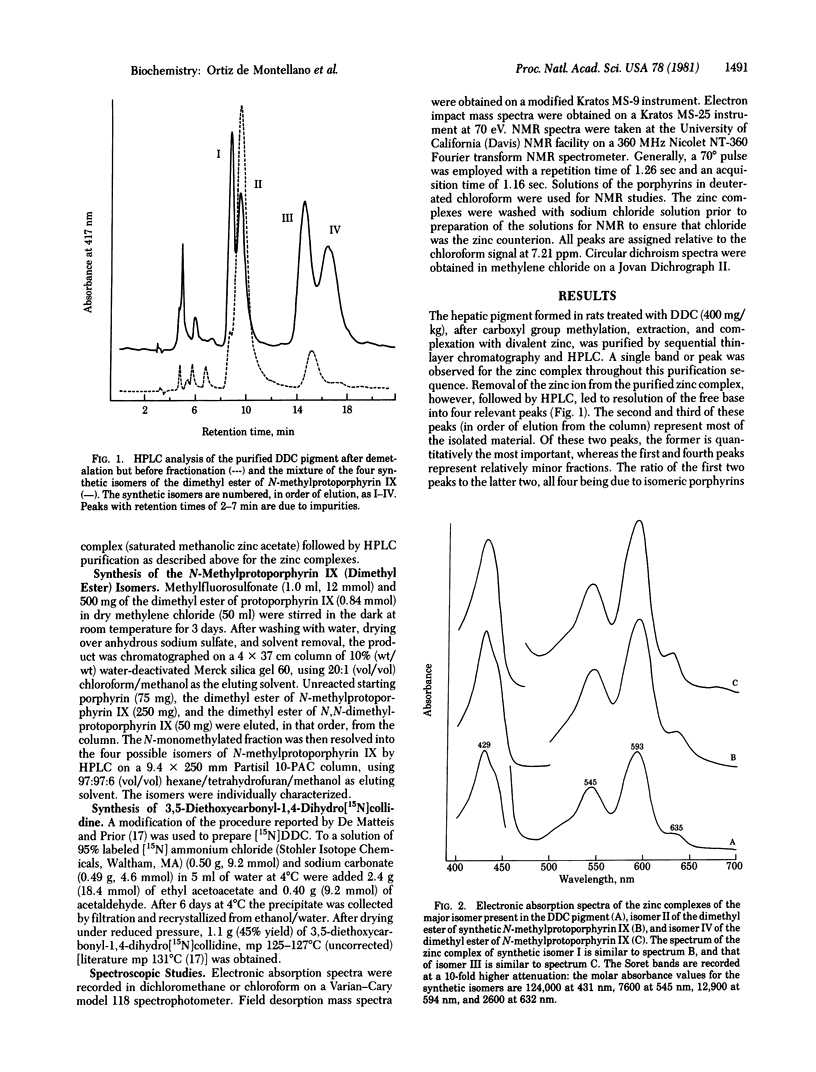
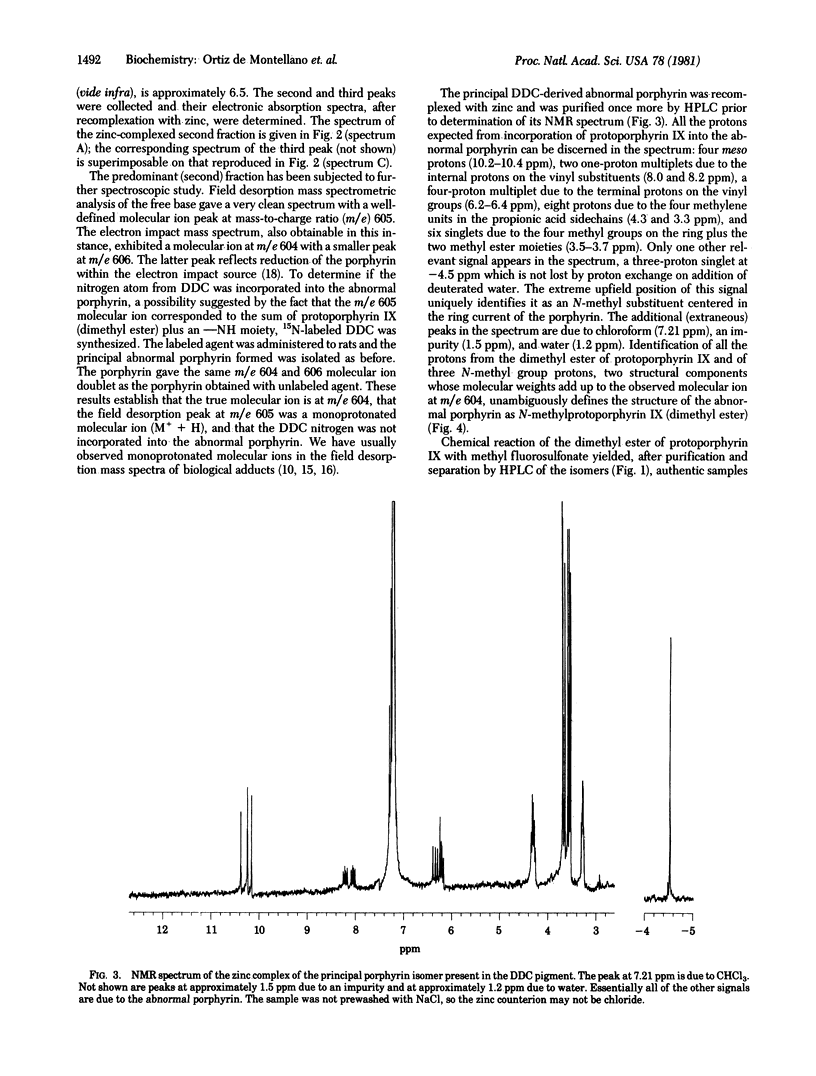
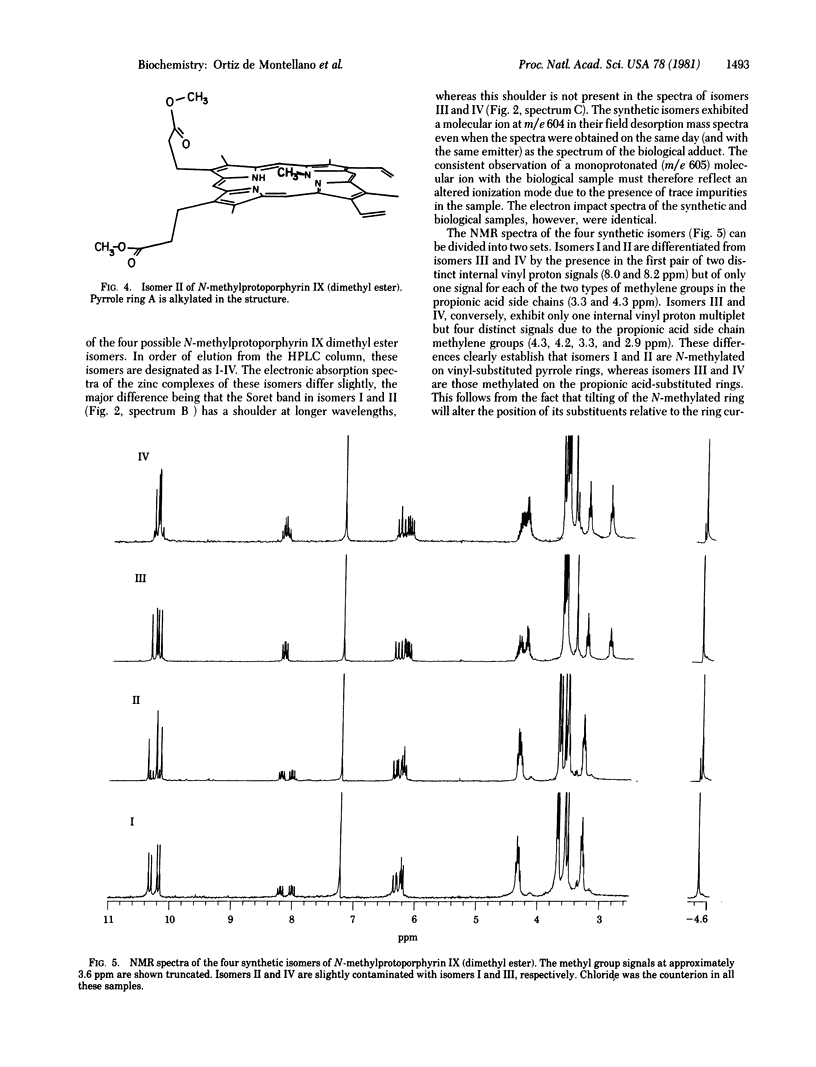
The hepatic pigment accumulated in 3,5-diethoxycarbonyl-1,4-dihydrocollidine-treated rats, which has been reported to inhibit ferrochelatase, has been isolated and purified. The pigment has been resolved into one major, one minor, and two trace components, all of which appear to be isomeric porphyrins. The major fraction has been unambiguously identified by spectroscopic methods as the isomer of N-methylprotoporphyrin IX (isolated as the dimethyl ester) in which vinyl-substituted pyrrole ring A is methylated. The minor product appears to be an isomer of the same porphyrin with the N-methyl group on propionic acid-substituted ring C, and the trace components have the same high-pressure liquid chromatography retention times as the other two possible isomers of the porphyrin. The four isomers of N-methylprotoporphyrin IX have been chemically synthesized, independently characterized, and used to confirm the structures of the biologically products.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- DE MATTEIS F., PRIOR B. E. Experimental hepatic porphyria caused by feeding 3,5-diethoxycarbonyl-1,4-dihydro-2,4,6-trimethylpyridine. Comparison with sedormid porphyria. Biochem J. 1962 Apr;83:1–8. doi: 10.1042/bj0830001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Matteis F., Abbritti G., Gibbs A. H. Decreased liver activity of porphyrin-metal chelatase in hepatic porphyria caused by 3,5-diethoxycarbonyl-1,4-dihydrocollidine. Studies in rats and mice. Biochem J. 1973 Jul;134(3):717–727. doi: 10.1042/bj1340717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Matteis F., Gibbs A. H. Drug-induced conversion of liver haem into modified porphyrins. Evidence for two classes of products. Biochem J. 1980 Apr 1;187(1):285–288. doi: 10.1042/bj1870285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Matteis F., Gibbs A. H., Tephly T. R. Inhibition of protohaem ferro-lyase in experimental porphyria. Isolation and partial characterization of a modified porphyrin inhibitor. Biochem J. 1980 Apr 15;188(1):145–152. doi: 10.1042/bj1880145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson A. H., Dearden G. R. N-methylporphyrins. Ann N Y Acad Sci. 1973;206:151–176. doi: 10.1111/j.1749-6632.1973.tb43210.x. [DOI] [PubMed] [Google Scholar]
- ONISAWA J., LABBE R. F. Effects of diethyl-1, 4-dihydro-2, 4,6-trimethylpyridine-3,5-dicarboxylate on the metabolism of porphyrins and iron. J Biol Chem. 1963 Feb;238:724–727. [PubMed] [Google Scholar]
- Ortiz de Montellano P. R., Kunze K. L., Mico B. A. Destruction of cytochrome P-450 by olefins: N-alkylation of prosthetic heme. Mol Pharmacol. 1980 Nov;18(3):602–605. [PubMed] [Google Scholar]
- Ortiz de Montellano P. R., Kunze K. L. Self-catalyzed inactivation of hepatic cytochrome P-450 by ethynyl substrates. J Biol Chem. 1980 Jun 25;255(12):5578–5585. [PubMed] [Google Scholar]
- Ortiz de Montellano P. R., Mico B. A. Destruction of cytochrome P-450 by allylisopropylacetamide is a suicidal process. Arch Biochem Biophys. 1981 Jan;206(1):43–50. doi: 10.1016/0003-9861(81)90063-1. [DOI] [PubMed] [Google Scholar]
- Ortiz de Montellano P. R., Mico B. A. Destruction of cytochrome P-450 by ethylene and other olefins. Mol Pharmacol. 1980 Jul;18(1):128–135. [PubMed] [Google Scholar]
- Ortiz de Montellano P. R., Mico B. A., Yost G. S. Suicidal inactivation of cytochrome P-450. Formation of a heme-substrate covalent adduct. Biochem Biophys Res Commun. 1978 Jul 14;83(1):132–137. doi: 10.1016/0006-291x(78)90407-2. [DOI] [PubMed] [Google Scholar]
- Ortiz de Montellano P. R., Yost G. S., Mico B. A., Dinizo S. E., Correia M. A., Kumbara H. Destruction of cytochrome P-450 by 2-isopropyl-4-pentenamide and methyl 2-isopropyl-4-pentenoate: mass spectrometric characterization of prosthetic heme adducts and nonparticipation of epoxide metabolites. Arch Biochem Biophys. 1979 Oct 15;197(2):524–533. doi: 10.1016/0003-9861(79)90276-5. [DOI] [PubMed] [Google Scholar]
- Tephly T. R., Gibbs A. H., De Matteis F. Studies on the mechanism of experimental porphyria produced by 3,5-diethoxycarbonyl-1,4-dihydrocollidine. Role of a porphyrin-like inhibitor of protohaem ferro-lyase. Biochem J. 1979 Apr 15;180(1):241–244. doi: 10.1042/bj1800241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tephly T. R., Hasegawa E., Baron J. Effect of drugs on heme synthesis in the liver. Metabolism. 1971 Feb;20(2):200–214. doi: 10.1016/0026-0495(71)90092-8. [DOI] [PubMed] [Google Scholar]
- Wada O., Yano Y., Urata G., Nakao K. Behavior of hepatic microsomal cytochromes after treatment of mice with drugs known to disturb porphyrin metabolism in liver. Biochem Pharmacol. 1968 Apr;17(4):595–603. doi: 10.1016/0006-2952(68)90275-x. [DOI] [PubMed] [Google Scholar]
- Waterfield M. D., Del Favero A., Gray C. H. Effect of 1,4-dihydro-3,5-dicarbethoxycollidine on hepatic microsomal haem, cytochrome b5 and cytochrome P450 in rabbits and mice. Biochim Biophys Acta. 1969 Jul 30;184(2):470–473. doi: 10.1016/0304-4165(69)90054-3. [DOI] [PubMed] [Google Scholar]
- de Matteis F. Disturbances of liver porphyrin metabolism caused by drugs. Pharmacol Rev. 1967 Dec;19(4):523–557. [PubMed] [Google Scholar]



