Abstract
The large-conductance voltage- and Ca2+-activated K+ (BK) channel is expressed in many smooth muscle types, but its role in human detrusor smooth muscle (DSM) is unclear. With a multidisciplinary approach spanning channel molecules, single-channel activity, freshly isolated human DSM cells, intact DSM preparations, and the BK channel specific inhibitor iberiotoxin, we elucidated human DSM BK channel function and regulation. Native human DSM tissues were obtained during open surgeries from patients with no preoperative history of overactive bladder. RT-PCR experiments on single human DSM cells showed mRNA expression of BK channel α-, β1-, and β4-subunits. Western blot and immunocytochemistry confirmed BK channel α, β1, and β4 protein expression. Native human BK channel properties were described using the perforated whole cell configuration of the patch-clamp technique. In freshly isolated human DSM cells, BK channel blockade with iberiotoxin inhibited a significant portion of the total voltage step-induced whole cell K+ current. From single BK channel recordings, human BK channel conductance was calculated to be 136 pS. Voltage-dependent iberiotoxin- and ryanodine-sensitive transient BK currents were identified in human DSM cells. In current-clamp mode, iberiotoxin inhibited the hyperpolarizing membrane potential transients and depolarized the cell resting membrane potential. Isometric DSM tension recordings revealed that BK channels principally control the contractions of isolated human DSM strips. Collectively, our results indicate that BK channels are fundamental regulators of DSM excitability and contractility and may represent new targets for pharmacological or genetic control of urinary bladder function in humans.
Keywords: urinary bladder, patch-clamp, calcium-dependent potassium channels
detrusor smooth muscle (DSM), the primary muscle forming the bladder wall, exhibits spontaneous and nerve-evoked action potentials that trigger Ca2+-dependent phasic contractions (1, 3, 6, 9, 15, 16, 20, 30, 31). In guinea pigs, Ca2+ entry through L-type voltage-gated Ca2+ (CaV) channels is responsible for the upstroke of the action potential, which activates the DSM phasic contractions, whereas the repolarization phase of the DSM action potential is mediated predominantly by the activity of the large-conductance voltage- and Ca2+-activated K+ (BK) channels (16). Further studies by our group and others suggest that the BK channel is the major K+ channel that regulates DSM cell excitability and contractility in various animal species (6, 10, 16, 17, 20, 30, 32). Pharmacological inhibition of DSM BK channels increases the action potential duration and frequency, as well as the spontaneous phasic and tonic contractions in animal models (17, 30). However, fundamental information is lacking regarding the presence and functional role of the BK channel in human DSM.
The BK channels are composed of a pore-forming α-subunit and auxiliary β-subunits that form tetramers (5, 10, 29, 30). Although the α-subunit is encoded by a single gene, a number of splice variants with differential regulatory mechanisms have been reported (8). The coassembly of four different regulatory β (β1–4)-subunits further determines the tissue-specific functions of the BK channel (5, 31). The smooth muscle-specific β1-subunit increases the apparent Ca2+ and voltage sensitivity of BK channels and is particularly enriched in mouse DSM (30). A recent study on mouse and rat DSM reveals that, in addition to the smooth muscle-specific β1-subunit, the neuronal β4-subunit is also involved in assembling the BK channel (10). However, the BK channel molecular structure in human DSM is unknown.
Ca2+ is an important regulator of DSM contractility and BK channels (29, 30, 32). The BK channels are activated by membrane depolarization and intracellular Ca2+ and are blocked with high affinity by the scorpion venom iberiotoxin (35). As the only member of the voltage-gated K+ channel family activated by both voltage and Ca2+, the BK channel is uniquely suited to serve as a Ca2+/voltage signal integrator in the modulation of membrane excitability (29). In guinea pig DSM, BK channels are under the local control of so-called “Ca2+ sparks” caused by Ca2+ release from the ryanodine receptors (RyRs) of the sarcoplasmic reticulum (SR), adjacent to the plasma membrane (18, 32). The Ca2+ sparks trigger spontaneous transient outward currents (STOCs) through the BK channel (also known as “transient BK currents,” TBKC), which contribute to the resting membrane potential (18, 20, 32). However, the existence of Ca2+ sparks and related TBKC has never been documented in human DSM; thus, whether BK channel data can be translated from animal models to human DSM is unclear. Species differences in human and animal DSM ion channel expression, action potential shapes, and patterns of contractility are well documented (1, 3, 15). Because the BK channel is one of the most important determinants in shaping the DSM action potential repolarization phase, the reported species differences are likely due to diverse BK channel properties. Therefore, evidence from human DSM tissues is needed to ascertain the role of BK channels in DSM function.
As outlined above, the presence of the BK channel in human DSM has not been well documented, and the role of BK channels in human DSM function is not well described (7, 12, 15, 33, 36). Therefore, we sought to define the BK channel molecular structure and its physiological role at a cellular and tissue level in native human DSM. We applied a multidisciplinary approach of molecular biological and electrophysiological techniques on freshly isolated single human DSM cells, isometric tension recordings of isolated human DSM strips, and pharmacological protocols using the BK channel specific inhibitor iberiotoxin.
MATERIALS AND METHODS
Human DSM tissue collection.
Human DSM tissue specimens were obtained from routine open bladder surgeries in accordance with the approved institutional review board protocol, HR 16918. These studies were reviewed and approved by the Medical University of South Carolina institutional review board. All specimens were obtained from patients with no preoperative history of overactive bladder (OAB). We included 53 patients, 37 males and 16 females, from 32 to 87 yr of age (mean=65.2 ± 1.6 yr). For the molecular biology experiments, a small piece of DSM tissue was separated at the operating room and stored in RNAlater solution for immediate RNA extraction.
Fresh DSM single cell isolation.
Small DSM strips were cut from the mucosa-free DSM specimens and placed in dissection solution (2 ml) supplemented with 1 mg/ml BSA, 1 mg/ml papain (Worthington, Lakewood, NJ), and 1 mg/ml dithioerythritol and incubated for 30 min at 37°C. Next, DSM strips were transferred to dissection solution (2 ml) containing 1 mg/ml BSA, 1 mg/ml collagenase (type II; Sigma), and 100 μM CaCl2 for 7–14 min at 37°C. After the incubation, DSM strips were washed with fresh BSA containing dissection solution. Individual cells were released from the tissue by passing the enzyme-treated strips through a Pasteur pipette.
RT-PCR.
Mucosa-free whole DSM tissue or freshly isolated DSM cells were used for RT-PCR experiments as previously described (9, 10). Briefly, freshly isolated DSM single cells were left to settle at the bottom of a chamber for 5 min and then washed out several times before individual selection by suction into a glass pipette. Single DSM cells were then expelled into a 1.5-ml centrifuge tube with RNAlater (Qiagen, Hilden, Germany) and then pelleted at 1,000 g for 3 min. Total RNA was isolated from whole DSM tissue kept in RNAlater, as well as from freshly isolated DSM cells using the RNeasy Mini Kit (Qiagen). Extracted RNA was reverse-transcribed into cDNA using Moloney murine leukemia virus reverse transcriptase (Promega) and oligo(dT) primers. All specific primers for BKα-, BKβ1-, BKβ2-, BKβ3-, and BKβ4-subunits were designed based on Genbank human sequences. Specific primer pair sequences are as follows: β-actin, sense: CTCCATCCTGGCCTCGCTGT, antisense: GCTGTCACCTTCACCGTTCC (268 base pairs); BK channel α-subunit, sense: GGAACTCACCCAACACC, antisense: TGACAGGATAACGCACAT (455 base pairs); BK channel β1-subunit, sense: TGCCACCTGATTGAGACC, antisense: TGCGGAGAAGCAGTAGAAG (258 base pairs); BK channel β2-subunit, sense: CAGGACATCCACCATAA, antisense: AGAAAGTTTCCAGCAGT (376 base pairs); BK channel β3-subunit, sense: GATTATCCCTCCAGTCCC, antisense: AAGGCTTTAGAATGGTTGTT (268 base pairs); and BK channel β4-subunit, sense: CATTTGTGGTGGGCGTTCT, antisense: ACAGGTTCCGCAGGTGG (168 base pairs). The cDNA production was PCR amplified using GoTaq Green Master Mix (Promega) and specific primers for BK channel subunits. The PCR annealing temperature for each primer pair was optimized using a mastercycler gradient thermocycler (Eppendorf, Hamburg, Germany). Thirty cycles were used for detection from tissue samples, and 35 cycles were used for isolated DSM single cells.
Western blot.
For Western blot, the fresh mucosa-free DSM specimen was cut and used for membrane protein extraction as previously described (9, 10). Briefly, the blots were incubated with the affinity-purified rabbit polyclonal antibodies anti-KCa1.1 (BKCa) (1:200), anti-sloβ1 (KCNMB1) (1:200), and anti-sloβ4 (KCNMB4) (1:100) (Alomone Labs, Jerusalem, Israel) overnight at 4°C. Next, the membrane was washed with TBS with Tween 20 four times and incubated with goat anti-rabbit IgG conjugated with horseradish peroxidase (diluted to 1:2,500) in the blocking buffer for 1 h at room temperature. Bound antibodies were detected by the enhanced chemiluminescence substrate kit (Amersham, Piscataway, NJ) according to the manufacturer's instructions.
Immunocytochemistry.
For immunocytochemical detection of individual BK channel subunits, freshly isolated human DSM cells were dropped on the glass coverslip for settling 1.5 h at room temperature and then processed as previously described (9, 10). The following primary antibodies were used: rabbit polyclonal anti-KCa1.1 (BKCa), anti-sloβ1 (KCNMB1), and anti-sloβ4 (KCNMB4) (1:100) (Alomone Laboratories). DSM cells were labeled with secondary antibody [Cy3-conjugated anti-rabbit IgG, at 1:200, PBS/3% normal donkey serum/0.01% Triton X-100 (Jackson ImmunoResearch, West Grove, PA)]. After being labeled, cells were washed with PBS and incubated with phalloidin for 2 h in the dark, cells were then washed two more times and incubated with 4′,6-diamidino-2-phenylindole for 15 min and washed again, and then mounted onto slides with DABCO.
Electrophysiological (patch-clamp) recordings.
All electrophysiological recordings were made with the amphotericin-perforated, whole cell configuration of the patch-clamp technique (14, 19). Whole cell currents were recorded using an Axopatch 200B amplifier, Digidata 1440A, and pCLAMP version 10.2 software (Molecular Devices, Union City, CA). An eight-pole Bessel filter 900CT/9L8L (Frequency Devices) was used to filter the recorded signals. Patch-clamp pipettes were made from borosilicate glass (Sutter Instruments, Novato, CA), coated with dental wax to reduce capacitance, and polished with a Micro Forge MF-830 fire polisher (Narishige Group, Tokyo, Japan) to give a final tip resistance of ∼4–6 MΩ. Single BK channel currents were recorded in whole cell mode as previously described (20, 32). All patch-clamp experiments were conducted at room temperature (22–23°C).
Studies on DSM contractility.
Mucosa- and urothelium-free DSM tissue were cut into strips (5–10 mm long; 2–4 mm wide), which were placed between clips in thermostatically controlled tissue baths at 37°C. Approximately 1.0 g of force was applied to stretch the DSM strips, which were left for at least 45 min to acclimate and develop stable spontaneous contractions. Nerve-evoked contractions were induced by electrical field stimulation (EFS).
Solutions and drugs.
The Ca2+-free dissection solution, Ca2+-containing physiological salt solution, and patch-clamp solutions have been described previously (6, 9, 20). Dithioerythritol, iberiotoxin, and tetrodotoxin were purchased from Sigma; ryanodine (9,21-dehydroryanodine) was from Calbiochem; BSA, thapsigargin, and amphotericin-B were from Thermo Fisher Scientific.
Data analysis and statistics.
MiniAnalysis, GraphPad Prism 4.03, and Clampfit 10.2 software were used for analyzing data, which are summarized as means ± SE (n=no. of DSM strips or cells; N=no. of patients). Data were compared using paired Student's t-test. A P value <0.05 was considered statistically significant.
RESULTS
Detection of BK channel α-, β1-, and β4-subunits in whole tissue and freshly isolated human DSM cells by RT-PCR, Western blot, and immunocytochemistry.
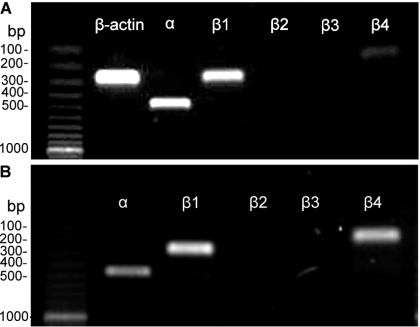
To detect mRNA expression of BK channel subunits, RT-PCR experiments were performed on human DSM whole tissue. BK channel subunit-specific primers were used to determine the expression of all known α, β1-, β2-, β3-, and β4-subunits (see materials and methods). mRNA message expression for α, β1, and β4 was detected in DSM whole tissue (Fig. 1A). Whereas the expression of mRNA messages for the pore-forming α- and the smooth muscle-specific β1-subunits was expected, detection of the neuronal-specific β4-subunit was an interesting finding consistent with our recent report on rat and mouse DSM (10). The presence of various cells within the DSM layer, such as neurons, fibroblasts, vascular smooth muscle, and endothelial cells, may result in detection of BK channel subunits expressed in non-DSM cell types. To address this, we performed single-cell RT-PCR experiments on freshly isolated DSM cells (10), an approach that eliminates any contamination from other cell types. mRNA message expression for α, β1, and β4 was also detected in freshly isolated DSM cells (Fig. 1B). A lack of genomic DNA contamination was also confirmed by designing the primers across the exon junctions for α, β1-, β2-, and β3-subunits. Negative control experiments for PCR were performed in the absence of the reverse transcriptase enzyme (−RT) to avoid genomic DNA contamination. All RT-PCR products from the intact DSM tissue and isolated DSM cells were purified using the GenElute PCR Clean-Up Kit (Sigma) and sequenced directly at the University of South Carolina Environmental Genomics Core Facility for identity confirmation. These results demonstrate that freshly isolated human DSM cells directly express mRNA for α-, β1-, and β4-subunits (Fig. 1B).
Fig. 1.
Detection of mRNA expression of large-conductance voltage- and Ca2+-activated K+ (BK) channel α-, β1-, β2-, β3-, and β4-subunits in human detrusor smooth muscle (DSM) whole tissue (A) and freshly isolated cells (B). The expected product sizes were 455 bp for the α-subunit, 258 bp for the β1-subunit, 376 bp for the β2-subunit, 268 bp for the β3-subunit, 168 bp for the β4-subunit, and 268 bp for the housekeeping gene β-actin. Only α-, β1-, and β4-subunits but not β2 and β3 were detected. Results were verified in 12 DSM preparations obtained from 4 patients.
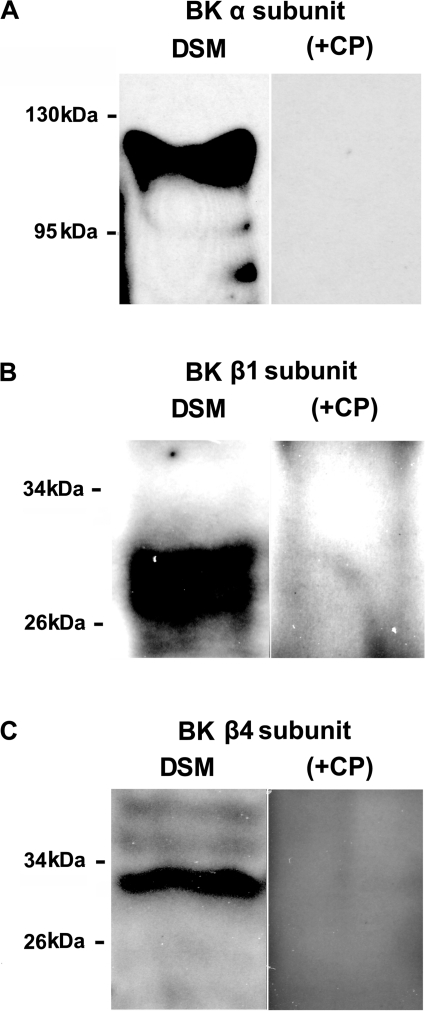
To confirm the presence of BK channel α, β1, and β4 proteins in human DSM tissue, we used a Western blot technique with specific antibodies (Fig. 2). Preabsorption of the primary antibody with its antigenic competing peptide indicated the specificity of the antibodies for their intended epitope. The Western blot experiments confirmed the presence of BK channel α, β1, and β4 proteins in human DSM tissue.
Fig. 2.
Western blot detection of BK channel subunit protein expression in human DSM tissues. BK channel proteins for the α-subunit (A), β1-subunit (B), and β4-subunit (C) were detected. The immunoreactive band was eliminated by a competing peptide (+CP). The results were verified in 3 separate Western blot reactions using proteins isolated from 3 patients.
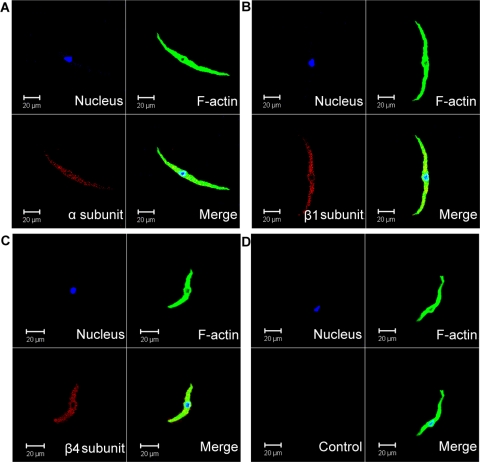
Immunocytochemical labeling confirmed the specific expression of BK channel α-, β1-, and β4-subunit proteins in freshly isolated human DSM cells. Specific antibodies for BK channel α-, β1-, and β4-subunits indicated the expression of these channel subunits in single DSM cells (Fig. 3). Control treatments were performed as follows: 1) omission of the primary antibody confirmed the specificity of the secondary antibody and 2) absorption of the primary antibody by a competing peptide confirmed the specificity of the primary antibody (Fig. 3). The immunocytochemical experiments confirmed that human DSM cells express BK channel α, β1, and β4 proteins.
Fig. 3.
Immunocytochemical detection of BK channel α-, β1-, and β4-subunits in freshly isolated human DSM cells using specific antibodies. Red staining (bottom left) indicates detection of the α-subunit (A), β1-subunit (B), and β4-subunit (C). Cell's nuclei are shown in blue (top left); F-actin is shown in green (top right). The merged images are also shown (bottom right) and illustrate overlap of nucleus, F-actin, and the expected BK channel subunit. In control experiments (D), the primary antibody was omitted, and cells were incubated only with the secondary antibody (Control). Images at ×63 were acquired with a Zeiss LSM 510 META confocal microscope. The slides for each group were imaged with the same laser power, gain settings, and pinhole for the controls and antibody treatment. Results were verified in 9 DSM cells freshly isolated from 3 patients.
BK channel controls the excitability of freshly isolated human DSM cells (patch-clamp recordings).
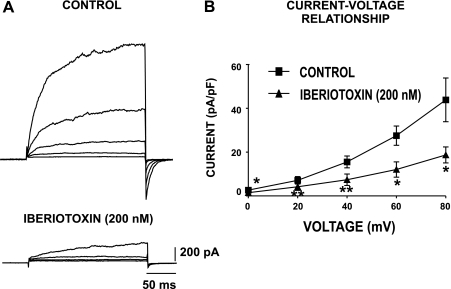
Our previous electrophysiological studies with experimental animals indicate that the voltage step-induced whole cell currents consist of an iberiotoxin-sensitive BK current component (20, 32). Under conventional patch-clamp conditions, iberiotoxin also inhibits a large portion of the whole cell outward current in cultured human DSM cells (36). Here, for the first time, we examined the whole cell outward K+ current in freshly isolated human DSM cells under perforated patch-clamp conditions, which preserve the native physiological environment of the cell and channel regulatory mechanisms. The average human DSM cell capacitance was 22.4 ± 1.0 pF (n=137 cells; N=51 patients). Perforated DSM cells were held at a holding potential (Vh) of −70 mV, and brief voltage-step depolarization steps (200 ms) from 0 to +80 mV in 20-mV intervals were applied. As shown in Fig. 4, the cells responded with gradual increases in the outward currents with each depolarizing voltage step (n=13; N=10). Application of the BK channel selective inhibitor iberiotoxin (200 nM) statistically significantly suppressed the total whole cell outward current (n=8; N=8; P < 0.05; Fig. 4B). This indicates that the BK channel carries the majority of the outward current in perforated human DSM cells; therefore, it is the major determinant of human DSM cell excitability under physiological conditions.
Fig. 4.
Whole cell voltage-dependent and iberiotoxin-sensitive outward currents in freshly isolated human DSM cells. Human DSM cells were held at −70 mV and then stepped from 0 to +80 mV for 200 ms in 20-mV increments. A: original recordings of whole cell outward currents from a human DSM cell under control conditions and 10 min after application of 200 nM iberiotoxin, a highly specific BK channel inhibitor. The whole cell outward currents were significantly inhibited by iberiotoxin. B: current-voltage relationship of the whole cell outward current in the presence and absence of 200 nM iberiotoxin. Values are means ± SE (n=8 DSM cells; N=8 patients; *P < 0.05; **P < 0.01).
TBKC, also known as STOCs, have been previously reported in guinea pig and rat DSM cells by our group and others (13, 18, 20, 21, 32). In DSM from animal species, TBKC are activated by localized Ca2+ releases from the SR, known as Ca2+ sparks, and contribute to the cell excitability and contractility (18, 20, 32). However, TBKC in human DSM have never been reported. Therefore, we tested freshly isolated human DSM cells for TBKC and further examined the voltage and Ca2+ regulation of those electrical events. Using a whole cell perforated patch-clamp technique, we provide the first electrophysiological evidence for the presence of TBKC in freshly isolated human DSM cells (Figs. 5 and 6). We examined the voltage dependence of TBKC amplitude and frequency at various voltages in the range from −40 to +40 mV. Membrane depolarization caused a statistically significant increase in TBKC amplitude, frequency, and decay time (Table 1).
Fig. 5.
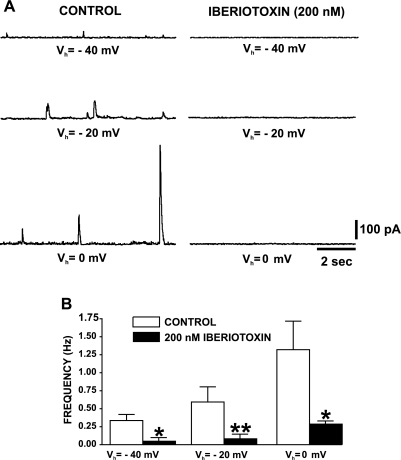
The BK channel specific inhibitor iberiotoxin inhibits transient BK currents (TBKC) in freshly isolated human DSM cells. A: original recordings of TBKC from a human DSM cell held at −40, −20, and 0 mV before (control) and 10 min after application of 200 nM iberiotoxin. B: summary data showing iberiotoxin inhibitory effect on TBKC frequency in human DSM cells. Values are means ± SE (n=5; N=3; *P < 0.05; **P < 0.01). Vh, holding potential.
Fig. 6.
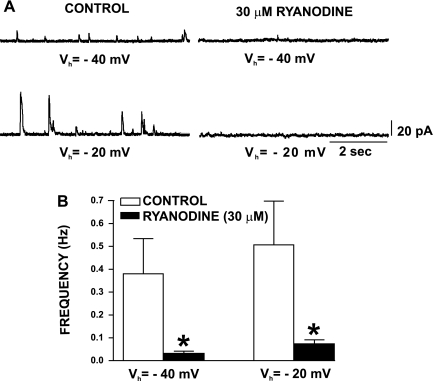
Ryanodine inhibits TBKC in freshly isolated human DSM cells. A: original recordings of TBKC from human DSM cells held at −40 or −20 mV before (control) and 20–30 min after application of 30 μM ryanodine. B: summary data showing ryanodine inhibitory effect on TBKC frequency in human DSM cells. Values are means ± SE (n=4; N=4; *P < 0.05).
Table 1.
Summary data for the TBKC parameters in freshly isolated human DSM cells
| Vh, mV | Amplitude, pA | Frequency, Hz | Rise Time, ms | Decay Time, ms | n | N |
|---|---|---|---|---|---|---|
| −40 | 5.7±2.2 | 0.22±0.1 | 26.4±10.8 | 10.5±3.8 | 12 | 9 |
| −20 | 11.2±2.9 | 0.29±0.1 | 31.2±8.6 | 28.9±11.3 | 12 | 9 |
| 0 | 18.4±5.1* | 0.6±0.1* | 40.2±8.3 | 33.7±14.1 | 11 | 9 |
| +20 | 29.9±8.4** | 0.86±0.2** | 48.7±8.5 | 49.3±17.7* | 11 | 9 |
| +40 | 32.1±9.0** | 1.78±0.5** | 36.6±3.6 | 53.7±0.4* | 7 | 7 |
Values are means ± SE for transient large-conductance voltage- and Ca2+-activated potassium (BK) current (TBKC) parameters measured at holding voltages (Vh) from −40 to +40 mV. n, No. of individual detrusor smooth muscle (DSM) cells; N, no. of patients. Rise time, time from baseline to peak; decay time, time from peak to baseline.
P < 0.05 and
P < 0.005 vs. means at Vh=−40 mV.
In experimental animal DSM cells, TBKC are eliminated by BK channel inhibition with iberiotoxin or tetraethylammonium or by blocking the RyR with ryanodine, whereas caffeine, an RyR agonist, has an initial stimulatory effect on TBKC (18, 20, 21, 32). With iberiotoxin (200 nM), we confirmed that the TBKC in human DSM cells are indeed mediated by BK channels. Blocking the BK channels with 200 nM iberiotoxin suppressed TBKC amplitude and frequency in single human DSM cells at all recording voltages (n=5; N=3; P < 0.05; Fig. 5).
To determine the role of the Ca2+ release from the SR (Ca2+ sparks) in triggering TBKC in freshly isolated human DSM cells, we applied ryanodine (30 μM), which locks the RyR in an open state, depleting SR Ca2+. Figure 6 shows original recordings and summary data of TBKC in freshly isolated human DSM before and after application of 30 μM ryanodine, which reduced TBKC amplitude and frequency at the recorded voltages (n=4; N=4; P < 0.05; Fig. 6). These data suggest that, in human DSM cells, TBKC are activated by Ca2+ release from the RyR.
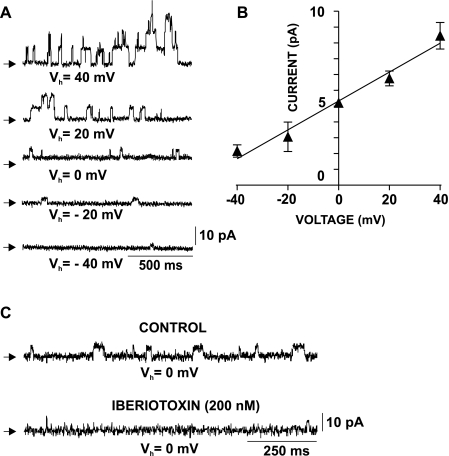
Using the perforated mode of the whole cell patch-clamp technique, we conducted the first direct recordings of single BK channel currents in freshly isolated human DSM cells (Fig. 7). To record single BK currents, all known Ca2+ sources for BK channel activation were eliminated by inhibiting the CaV channels (1 μM nifedipine), and the Ca2+ release from the SR (30 μM ryanodine and 100 nM thapsigargin). To estimate the voltage dependence of the single BK current amplitude, we recorded single BK channel openings at various voltages from Vh=−40 to +40 mV (n=11; N=10; Fig. 7). Single BK channel current amplitude increased toward the positive voltages (n=11; N=10; P < 0.05; Fig. 7A). The single BK channel conductance in freshly isolated human DSM cells, determined by the slope of the current-voltage relationship, was 135.6 pS (n=11; N=10; P < 0.05; Fig. 7B). The single BK channel currents were inhibited by 200 nM iberiotoxin (n=5; N=5; P < 0.05; Fig. 7C). Single-channel conductance value, voltage dependence, and iberiotoxin sensitivity support BK channel-mediated single-channel currents in freshly isolated human DSM cells.
Fig. 7.
Single BK channel recordings in freshly isolated human DSM cells. A: original recordings showing a series of single BK channel openings at Vh from −40 to +40 mV in the whole cell configuration. The single BK channel amplitude was voltage dependent; increasing Vh toward positive voltages increased the single channel amplitude. Arrows indicate the closed channel state. B: current-voltage relationship for the single BK channel amplitude in freshly isolated human DSM cells. Values are means ± SE (n=11; N=10). C: original recordings showing the inhibitory effect of iberiotoxin on single BK channel currents at Vh=0 mV. Shown are current traces before (control) and 10 min after application of 200 nM iberiotoxin (n=5; N=5; P < 0.05). Ryanodine (30 μM), thapsigargin (100 nM), and nifedipine (1 μM) were present throughout the experiments to eliminate all Ca2+ sources for BK channel activation, hence TBKC.
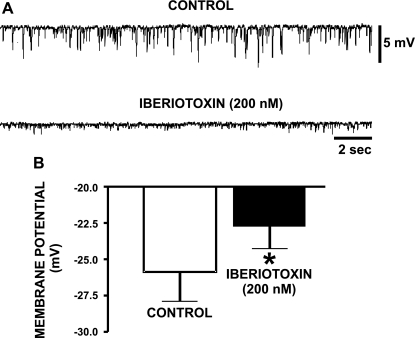
We hypothesized that the BK channels contribute to regulating the resting membrane potential in human DSM cells. In current-clamp mode, the resting membrane potential of freshly isolated human DSM cells was −28.6 ± 3.0 mV (n=16; N=14). Some cells exhibited spontaneous transient hyperpolarizations (STHs) (n=4; N=4; Fig. 8A), which are likely triggered by SR Ca2+ sparks and subsequent TBKC. To determine the role of the BK channel in resting membrane potential maintenance in human DSM cells, we tested iberiotoxin on the resting membrane potential measured in current-clamp mode. Blocking the BK channel with 200 nM iberiotoxin depolarized the DSM cell resting membrane potential by 3.1 ± 1.4 mV (n=12; N=9; P < 0.05; Fig. 8B) and inhibited STHs (n=3; N=3; Fig. 8A). These findings suggest that the BK channel controls the resting membrane potential in human DSM cells.
Fig. 8.
Inhibition of the BK channels by iberiotoxin eliminates the spontaneous transient hyperpolarizations and causes membrane potential depolarization in freshly isolated human DSM cells. A: original current-clamp recordings of resting membrane potential in a freshly isolated human DSM cell. Visible are STHs of the resting membrane potential, which were inhibited by 200 nM iberiotoxin. B: inhibition of the BK channel with iberiotoxin (200 nM) caused a statistically significant depolarization of the cell membrane potential in human DSM cells. Values are means ± SE (n=12; N=9; *P < 0.05).
BK channel controls the contractility of isolated human DSM strips.
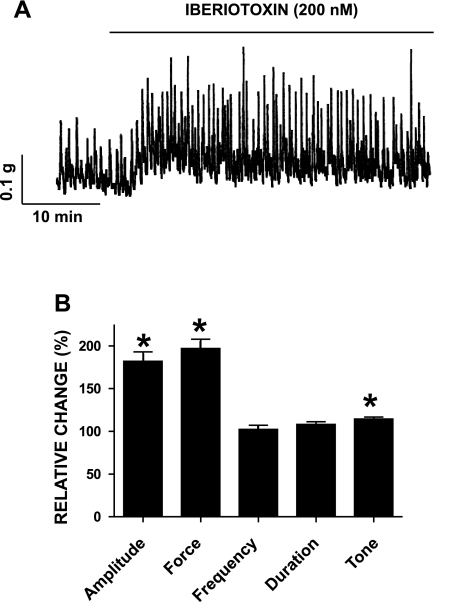
We investigated the contribution of the BK channel to the spontaneous phasic and tonic contractions in freshly isolated human DSM strips with iberiotoxin. After application of 1 μM tetrodotoxin, to eliminate possible neurotransmitter release, strips were allowed to stabilize for at least 15 min before iberiotoxin application (200 nM). The phasic contraction amplitude, muscle force integral, and tone were significantly increased with iberiotoxin (n=12; N=7; P < 0.05; Fig. 9). We then examined the contribution of the BK channels in the nerve-evoked contractions of human DSM strips by stimulating the DSM nerves with EFS (2–50 Hz). BK channel blockade with iberiotoxin caused a statistically significant increase in the EFS-induced contraction amplitude at all stimulation frequencies except for the two highest frequencies of 40 and 50 Hz (n=9; N=7; P < 0.05; data not shown). This finding indicates that the BK channel is essential for the modulation of the contractions in human DSM.
Fig. 9.
Inhibition of the BK channels by iberiotoxin caused an increase in spontaneous phasic contraction amplitude, muscle force integral, and tone of isolated human DSM strips. A: original recordings of spontaneous phasic contractions followed by application of 200 nM iberiotoxin. B: summary data showing a statistically significant increase in human DSM spontaneous phasic contraction amplitude, muscle force integral, and muscle tone. Values are means ± SE (n=12; N=7; *P < 0.05). Spontaneous contractions were taken to be 100%.
DISCUSSION
This study is the first systematic identification and characterization of the BK channel in native human DSM. Using a multilevel approach spanning BK channel molecules, single-channel activity, freshly isolated human DSM cells, and intact DSM tissue preparations, we have elucidated key aspects of human DSM BK channel function under normal physiological conditions. Of note, our work was performed with freshly isolated native human DSM cells (not cultured cells). Our studies reveal that the BK channel is a physiologically relevant regulator of human DSM excitability and contractility.
Using the perforated patch-clamp technique, we investigated the properties of the human DSM BK current in freshly isolated human DSM cells. In fact, to our knowledge, the perforated patch-clamp mode has never been applied to native human DSM cells to study BK channel activity. The advantage of the perforated mode (compared with the conventional mode) of the patch-clamp technique is that it maintains the native cell environment, preserving innate Ca2+ signaling mechanisms. Blocking the BK current with iberiotoxin inhibited ∼60% of the total voltage step-induced K+ current (recorded at +80 mV) in freshly isolated human DSM cells (Fig. 4). Based on our original single BK channel recordings, we calculated the single BK channel conductance in native human DSM cells to be 136 pS (Fig. 7), which is consistent with the reported 122 pS in guinea pig DSM (16). In guinea pig, rat, and mouse DSM, the BK channel contributes to setting the resting membrane potential (6, 16, 20, 34). In current-clamp mode, we observed that blocking the BK channel with iberiotoxin statistically significantly depolarized the membrane of human DSM cells (Fig. 8). Iberiotoxin also inhibited the STHs, which have an additional contribution in setting the resting membrane potential (Fig. 8). The importance of the BK channel in controlling the cell membrane potential derives from its dual regulatory role, one direct role on the resting membrane potential and another one via the STHs. Thus our data clearly suggest that BK channels control the cell membrane potential in human DSM.
We obtained evidence in human DSM that BK channels are regulated by differential Ca2+ signals. Here, we report for the first time the existence of ryanodine-sensitive, voltage-dependent TBKC in native human DSM cells under physiological conditions using the perforated patch-clamp technique (Figs. 5 and 6 and Table 1). TBKC in human DSM cells were inhibited by iberiotoxin (Fig. 5), suggesting that they are mediated by the BK channels. Human DSM TBKC were also inhibited by ryanodine (Fig. 6), suggesting that human DSM TBKC are controlled by the RyRs and Ca2+ spark activity. These TBKC determined the observed iberiotoxin-sensitive STHs in current-clamp mode (Fig. 8), which significantly contribute to setting the cell membrane potential in human DSM.
The current pharmacological treatment of OAB is limited to antimuscarinics, which have multiple adverse effects (2); thus, alternative therapies are urgently needed. Our studies with isolated human DSM strips revealed that blocking the BK channel with iberiotoxin significantly increased the spontaneous phasic contraction amplitude, muscle force integral, and tone (Fig. 9), consistent with earlier findings in animal DSM (17, 30, 35). Furthermore, our results showed that iberiotoxin significantly increased the amplitude of the EFS-induced contractions, indicating that BK channels also oppose DSM contractility in response to excitatory neurotransmitters. Based on these findings, genetic mutations causing BK channel malfunction would be expected to cause DSM overactivity in humans. Indeed, genetic deletion of the BK channel regulatory β1- or pore-forming α-subunits leads to DSM overactivity in mouse models (6, 28, 30). In rats, overexpression of the BK channel α-subunit using gene transfer techniques eliminated DSM overactivity caused by partial bladder outlet obstruction (PBOO) (11). In rabbits, PBOO-induced DSM overactivity is associated with decreased BK channel expression (7), and investigators suggest that this mechanism may also be involved in the etiology of DSM overactivity in patients with benign prostatic hyperplasia (7). The ability of BK channel gene transfer to ameliorate experimental DSM overactivity is consistent with the opposing phenomenon documented by our group and others on mice lacking BK channel subunits (6, 28, 30, 34). BK channel gene transfer is now in phase I clinical trials for treatment of erectile dysfunction, and a similar trial is planned for the treatment of OAB (26, 27). Increasing BK channel expression can reduce DSM overactivity. Moreover, BK channel openers cause DSM hyperpolarization and relaxation (23, 24, 33, 37).
To identify the molecular fingerprints of the human DSM BK channel, we applied a combined molecular biology approach. The single-cell RT-PCR experiments showed mRNA expression of the pore-forming α- and regulatory β1- and β4-subunits directly in DSM cells and ruled out the expression of the β2- and β3-subunits (Fig. 1). Using Western blot and immunocytochemistry, we confirmed the expression of α, β1, and β4 BK channel proteins in human DSM (Figs. 2 and 3). Thus we have shown that the human DSM BK channel has a unique composition involving α-, β1-, and β4-subunits, making it distinct from the BK channels expressed in other tissues (5). BK channels have not been reported to exist in heterotetrameric complexes of mixed regulatory β-subunits where more than one type of β-subunits coexist. Therefore, there should be two populations of BK channels in human DSM, one composed of α/β1- and another one of α/β4-subunits. These data are consistent with recent reports on mouse and rat DSM BK channel composition (10, 22).
The smooth muscle specific BK channel β1-subunit has a key physiological role in bladder function (30), but the physiological role of the β4-subunit in human DSM is less clear. Our results show that all human DSM cells responded to iberiotoxin, indicating a predominant expression of the α/β1 BK channel complex. The β4-subunit is known to make the BK channel insensitive to iberiotoxin and charybdotoxin (25); therefore, the α/β4 BK channel complex may have a secondary role in human DSM. A significant portion of the whole cell outward current remains in the presence of iberiotoxin (Fig. 4). This residual current is likely mediated by the iberiotoxin-insensitive α/β4 BK channel complex in combination with other voltage-gated K+ channels. In future experiments, the remaining current observed in the presence of iberiotoxin could be probed with a small molecule BK channel blocker (paxilline) to investigate the involvement of the α/β4 BK channel complex. The BK channel β4-subunit decreases channel voltage sensitivity and slows activation kinetics (5). Studies on dentate granule cells using BK channel β4−/− knockout mice had increased action potential frequency (4). Therefore, it is reasonable to speculate that the β4-subunit may have a similar regulatory role in human DSM. Recent studies in a rat model of overactive DSM due to PBOO show that β4-subunit expression is decreased in a manner dependent on the severity of the PBOO (22). Under pathological conditions of PBOO when β4-subunit expression decreases (22), DSM exhibits increased phasic contractions that probably are associated with an increased action potential frequency.
From our data based on native human DSM, we draw several important conclusions: 1) human DSM expresses BK channel α-, β1-, and β4-subunits; 2) the BK current is the main component of the total outward K+ current and controls the cell membrane potential in isolated human DSM cells; 3) the human DSM BK channel is regulated by differential Ca2+ signals, including a ryanodine-sensitive mechanism; and 4) BK channels control human DSM contractility. Thus, our results revealed that the BK channels are fundamental regulators of DSM excitation-contraction coupling and may represent important targets for pharmacological or genetic control of urinary bladder function in humans. Direct or indirect modulation of the BK channel by targeting its regulatory mechanisms has the potential for controlling OAB syndrome with few adverse effects.
GRANTS
This study was supported by National Institute of Diabetes and Digestive and Kidney Diseases Grants DK-084284 and DK-083687 to G. V. Petkov.
DISCLOSURES
No conflicts of interest are declared by the authors.
ACKNOWLEDGMENTS
We thank Sean Brown, Lei Liu, Serge Afeli, and Dr. Xiangli Cui for their help with some of the experiments; Medical University of South Carolina (MUSC) Urology staff surgeons: Drs. Thomas Keane, Harry Clarke, Stephen Savage, Ross Rames, and Jonathan Picard, as well as the MUSC Urology Residents: Drs. Ahmed M. El-Zawahry, Avi C. Weiss, Gary W. Bong, Kelly Doyle, Matthew McIntyre, Matt Eskridge, Jonathan N. Hamilton, Robin Bhavsar, Timothy R. Yoost, and Vinh Q. Trang for help with human tissue collection; and Drs. Jennifer G. Schnellmann and Shankar Parajuli, Serge Afeli, and Rupal Pandey for the critical evaluation of the manuscript.
REFERENCES
- 1. Andersson KE, Arner A. Urinary bladder contraction and relaxation: physiology and pathophysiology. Physiol Rev 84: 935–986, 2004 [DOI] [PubMed] [Google Scholar]
- 2. Andersson KE, Wein AJ. Pharmacology of the lower urinary tract: basis for current and future treatments of urinary incontinence. Pharmacol Rev 56: 581–631, 2004 [DOI] [PubMed] [Google Scholar]
- 3. Brading AF. Spontaneous activity of lower urinary tract smooth muscles: correlation between ion channels and tissue function. J Physiol 570: 13–22, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Brenner R, Chen QH, Vilaythong A, Toney GM, Noebels JL, Aldrich RW. BK channel beta4 subunit reduces dentate gyrus excitability and protects against temporal lobe seizures. Nat Neurosci 8: 1752–1759, 2005 [DOI] [PubMed] [Google Scholar]
- 5. Brenner R, Jegla TJ, Wickenden A, Liu Y, Aldrich RW. Cloning and functional characterization of novel large conductance calcium-activated potassium channel beta subunits, hKCNMB3 and hKCNMB4. J Biol Chem 275: 6453–6461, 2000 [DOI] [PubMed] [Google Scholar]
- 6. Brown SM, Bentcheva-Petkova LM, Liu L, Hristov KL, Chen M, Kellett WF, Meredith AL, Aldrich RW, Nelson MT, Petkov GV. Beta-adrenergic relaxation of mouse urinary bladder smooth muscle in the absence of large-conductance Ca2+-activated K+ channel. Am J Physiol Renal Physiol 295: F1149–F1157, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Chang S, Gomes CM, Hypolite JA, Marx J, Alanzi J, Zderic SA, Malkowicz B, Wein AJ, Chacko S. Detrusor overactivity is associated with downregulation of large-conductance calcium- and voltage-activated potassium channel protein. Am J Physiol Renal Physiol 298: F1416–F1423, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Chen L, Tian L, MacDonald SH, McClafferty H, Hammond MS, Huibant JM, Ruth P, Knaus HG, Shipston MJ. Functionally diverse complement of large conductance calcium- and voltage-activated potassium channel (BK) alpha-subunits generated from a single site of splicing. J Biol Chem 280: 33599–33609, 2005 [DOI] [PubMed] [Google Scholar]
- 9. Chen M, Kellett WF, Petkov GV. Voltage-gated K(+) channels sensitive to stromatoxin-1 regulate myogenic and neurogenic contractions of rat urinary bladder smooth muscle. Am J Physiol Regul Integr Comp Physiol 299: R177–R184, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Chen M, Petkov GV. Identification of large conductance calcium activated potassium channel accessory beta4 subunit in rat and mouse bladder smooth muscle. J Urol 182: 374–381, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Christ GJ, Day NS, Day M, Santizo C, Zhao W, Sclafani T, Zinman J, Hsieh K, Venkateswarlu K, Valcic M, Melman A. Bladder injection of “naked” hSlo/pcDNA3 ameliorates detrusor hyperactivity in obstructed rats in vivo. Am J Physiol Regul Integr Comp Physiol 281: R1699–R1709, 2001 [DOI] [PubMed] [Google Scholar]
- 12. Darblade B, Behr-Roussel D, Oger S, Hieble JP, Lebret T, Gorny D, Benoit G, Alexandre L, Giuliano F. Effects of potassium channel modulators on human detrusor smooth muscle myogenic phasic contractile activity: potential therapeutic targets for overactive bladder. Urology 68: 442–448, 2006 [DOI] [PubMed] [Google Scholar]
- 13. Ganitkevich VY, Isenberg G. Depolarization-mediated intracellular calcium transients in isolated smooth-muscle cells of guinea-pig urinary-bladder. J Physiol (Lond) 435: 187–205, 1991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Hamill OP, Marty A, Neher E, Sakmann B, Sigworth FJ. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch 391: 85–100, 1981 [DOI] [PubMed] [Google Scholar]
- 15. Hashitani H, Brading AF. Electrical properties of detrusor smooth muscles from the pig and human urinary bladder. Br J Pharmacol 140: 146–158, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Heppner TJ, Bonev AD, Nelson MT. Ca(2+)-activated K+ channels regulate action potential repolarization in urinary bladder smooth muscle. Am J Physiol Cell Physiol 273: C110–C117, 1997 [DOI] [PubMed] [Google Scholar]
- 17. Herrera GM, Heppner TJ, Nelson MT. Regulation of urinary bladder smooth muscle contractions by ryanodine receptors and BK and SK channels. Am J Physiol Regul Integr Comp Physiol 279: R60–R68, 2000 [DOI] [PubMed] [Google Scholar]
- 18. Herrera GM, Heppner TJ, Nelson MT. Voltage dependence of the coupling of Ca2+ sparks to BKCa channels in urinary bladder smooth muscle. Am J Physiol Cell Physiol 280: C481–C490, 2001 [DOI] [PubMed] [Google Scholar]
- 19. Horn R, Marty A. Muscarinic activation of ionic currents measured by a new whole-cell recording method. J Gen Physiol 92: 145–159, 1988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Hristov KL, Cui X, Brown SM, Liu L, Kellett WF, Petkov GV. Stimulation of beta3-adrenoceptors relaxes rat urinary bladder smooth muscle via activation of the large-conductance Ca2+-activated K+ channels. Am J Physiol Cell Physiol 295: C1344–C1353, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Imaizumi Y, Henmi S, Uyama Y, Atsuki K, Torii Y, Ohizumi Y, Watanabe M. Characteristics of Ca2+ release for activation of K+ current and contractile system in some smooth muscles. Am J Physiol Cell Physiol 270: C772–C782, 1996 [DOI] [PubMed] [Google Scholar]
- 22. Kita M, Yunoki T, Takimoto K, Miyazato M, Kita K, de Groat WC, Kakizaki H, Yoshimura N. Effects of bladder outlet obstruction on properties of Ca2+-activated K+ channels in rat bladder. Am J Physiol Regul Integr Comp Physiol 298: R1310–R1319, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Layne JJ, Nausch B, Olesen SP, Nelson MT. BK channel activation by NS11021 decreases excitability and contractility of urinary bladder smooth muscle. Am J Physiol Regul Integr Comp Physiol 298: R378–R384, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Malysz J, Buckner SA, Daza AV, Milicic I, Perez-Medrano A, Gopalakrishnan M. Functional characterization of large conductance calcium-activated K+ channel openers in bladder and vascular smooth muscle. Naunyn-Schmiedeberg's Arch Pharmacol 369: 481–489, 2004 [DOI] [PubMed] [Google Scholar]
- 25. Meera P, Wallner M, Toro L. A neuronal beta subunit (KCNMB4) makes the large conductance, voltage- and Ca2+-activated K+ channel resistant to charybdotoxin and iberiotoxin. Proc Natl Acad Sci USA 97: 5562–5567, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Melman A, Bar-Chama N, McCullough A, Davies K, Christ G. Plasmid-based gene transfer for treatment of erectile dysfunction and overactive bladder: results of a phase I trial. Isr Med Assoc J 9: 143–146, 2007 [PubMed] [Google Scholar]
- 27. Melman A, Biggs G, Davies K, Zhao W, Tar MT, Christ GJ. Gene transfer with a vector expressing Maxi-K from a smooth muscle-specific promoter restores erectile function in the aging rat. Gene Ther 15: 364–370, 2008 [DOI] [PubMed] [Google Scholar]
- 28. Meredith AL, Thorneloe KS, Werner ME, Nelson MT, Aldrich RW. Overactive bladder and incontinence in the absence of the BK large conductance Ca2+-activated K+ channel. J Biol Chem 279: 36746–36752, 2004 [DOI] [PubMed] [Google Scholar]
- 29. Petkov GV. Ion channels. In: Pharmacology: Principles and Practice, edited by Hacker M, Messer W, Bachmann K. New York, NY: Elsevier, 2009, chapt. 16, p. 385–425 [Google Scholar]
- 30. Petkov GV, Bonev AD, Heppner TJ, Brenner R, Aldrich RW, Nelson MT. Beta1-subunit of the Ca2+-activated K+ channel regulates contractile activity of mouse urinary bladder smooth muscle. J Physiol 537: 443–452, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Petkov GV, Heppner TJ, Bonev AD, Herrera GM, Nelson MT. Low levels of KATP channel activation decrease excitability and contractility of urinary bladder. Am J Physiol Regul Integr Comp Physiol 280: R1427–R1433, 2001 [DOI] [PubMed] [Google Scholar]
- 32. Petkov GV, Nelson MT. Differential regulation of Ca2+-activated K+ channels by beta-adrenoceptors in guinea pig urinary bladder smooth muscle. Am J Physiol Cell Physiol 288: C1255–C1263, 2005 [DOI] [PubMed] [Google Scholar]
- 33. Siemer C, Bushfield M, Newgreen D, Grissmer S. Effects of NS1608 on MaxiK channels in smooth muscle cells from urinary bladder. J Membr Biol 173: 57–66, 2000 [DOI] [PubMed] [Google Scholar]
- 34. Sprossmann F, Pankert P, Sausbier U, Wirth A, Zhou XB, Madlung J, Zhao H, Bucurenciu I, Jakob A, Lamkemeyer T, Neuhuber W, Offermanns S, Shipston MJ, Korth M, Nordheim A, Ruth P, Sausbier M. Inducible knockout mutagenesis reveals compensatory mechanisms elicited by constitutive BK channel deficiency in overactive murine bladder. FASEB J 276: 1680–1697, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Suarez-Kurtz G, Garcia ML, Kaczorowski GJ. Effects of charybdotoxin and iberiotoxin on the spontaneous motility and tonus of different guinea pig smooth muscle tissues. J Pharmacol Exp Ther 259: 439–443, 1991 [PubMed] [Google Scholar]
- 36. Takemoto J, Masumiya H, Nunoki K, Sato T, Nakagawa H, Ikeda Y, Arai Y, Yanagisawa T. Potentiation of potassium currents by beta-adrenoceptor agonists in human urinary bladder smooth muscle cells: a possible electrical mechanism of relaxation. Pharmacology 81: 251–258, 2008 [DOI] [PubMed] [Google Scholar]
- 37. Turner SC, Carroll WA, White TK, Gopalakrishnan M, Coghlan MJ, Shieh CC, Zhang XF, Parihar AS, Buckner SA, Milicic I, Sullivan JP. The discovery of a new class of large-conductance Ca2+-activated K+ channel opener targeted for overactive bladder: synthesis and structure-activity relationships of 2-amino-4-azaindoles. Bioorg Med Chem Lett 13: 2003–2007, 2003 [DOI] [PubMed] [Google Scholar]