Abstract
The multifunctional cytoprotective protein peroxiredoxin 6 (Prdx6) maintains cellular homeostasis and membrane integrity by regulating expression of intracellular reactive oxygen species (ROS) and phospholipid turnover. Using cells derived from targeted inactivation of Prdx6 gene or its depletion by RNA interference or aging, we showed that Prdx6 deficiency in cells evoked unfolded protein response (UPR), evidenced by increased expression or activation of proapoptotic factors, CHOP, ATF4, PERK, IRE-α and eIF2-α and by increased caspases 3 and 12 processing. Those cells displayed enhanced and sustained expression of endoplasmic reticulum (ER) stress-related chaperon proteins, Bip/glucose-regulated protein 78, calnexin, and calreticulin. Under cellular stress induced by hypoxia (1% O2 or CoCl2 treatment) or tunicamycin, Prdx6-deficient cells exhibited aberrant activation of ER stress-responsive genes/protein with higher expression of ROS, and died with apoptosis. Wild-type cells exposed to tunicamycin or hypoxia remained relatively insensitive with lower expression of ROS and ER-responsive genes than did Prdx6-deficient cells, but upregulation of ER stress responsive proteins or chaperones mimicked the UPR response of Prdx6-deficient or aging cells. Expression of Prdx6 blocked ER stress-induced deleterious signaling by optimizing physiologically aberrant expression of ER stress responsive genes/proteins in Prdx6-deficient cells or cells facing stressors, and rescued the cells from apoptosis. These findings demonstrate that impaired homeostasis and progression of pathogenesis in Prdx6-deficient lens epithelial cells or in aging cells should be blocked by a supply of Prdx6. The results provide a new molecular basis for understanding the etiology of several age-associated degenerative disorders, and potentially for developing antioxidant Prdx6-based therapeutics.
Keywords: peroxiredoxin 6, antioxidant, unfolded protein response, reactive oxygen species, endoplasmic reticulum
oxidative stress has an impact on biological processes involved in cell survival and aging as well as on pathogenesis of diseases ranging from cataractogenesis to age-related neurodegenerative conditions (3, 9, 14, 19, 39, 75). To counteract oxidative stress, hosts have evolved antioxidant defense systems that include superoxide dismutases (SODs), catalase (Cat), glutathione peroxidase (Gpx), and antioxidant peroxiredoxins (Prdxs). The physiological role of antioxidants depends on their levels of expression and activity in cells. A decrease in expression of antioxidants leads to uncontrolled overproduction of intracellular reactive oxygen species (ROS), resulting in dysfunction of cellular organelles including those in the endoplasmic reticulum (ER) (63, 85). The normally functioning ER provides a unique and balanced oxidizing microenvironment for protein folding (28, 50, 70). Newly synthesized proteins gain their native conformation in the ER, where an efficient quality control system allows only correctly folded molecules to exit for their allotted destinations (23, 30). Thus, the unfolded protein response (UPR) is an adaptive signaling pathway evolved to prevent the accumulation of misfolded protein in the ER lumen. When cellular homeostasis fails in the increased redox environment generated by reduced expression of antioxidants, spontaneous accumulation of proteins eventually leads to ER stress-induced cell death (70). Furthermore, diverse cellular stresses, as may be caused by chemotherapeutic drugs, radiation, hypoxia, or cellular stress, can initiate or accelerate both oxidative and ER stresses, causing greater damage in cells (53, 62, 78). Prdx6 acts to alter gene expression by optimizing ROS expression, which culminates in either apoptosis or cell recovery (9, 14, 18, 38, 75). Thus maintenance of nearly constant levels of cellular Prdx6 expression is crucial to maintenance of the physiological levels of ROS necessary to regulate cell survival signaling. Cells deficient in the Prdx6 gene are known to display higher expression of ROS with impaired homeostasis and spontaneous apoptosis (14, 75), but the intracellular mechanism(s) by which Prdx6 deficiency relays adverse signaling and causes cells to be more sensitive to oxidative stress (14) is not known.
Prdx6 is a member of the selenium-independent peroxidase family that has GSH peroxidase as well as acidic Ca2+-independent phospholipase A2 (PLA2) activities. Prdx6 has been found essential for maintaining cellular homeostasis (14, 16, 52, 72). We reported earlier that an extrinsic supply of Prdx6 provides cytoprotection against various stressors and delays the progression of cataractogenesis (14, 16, 35, 56). Although Prdx6 is classified as a peroxiredoxin based on homology of structure, its properties differ from those of other mammalian peroxidase family members, and the sequence associated with the protective activity of Prdx6 is not present in other peroxiredoxins (52). All six mammalian isoforms of Prdxs are relatively expressed at high levels and are differentially localized in cytoplasm, mitochondria, ER, nucleus, and peroxisomes (12, 33, 79), protecting them from various stressors. On the basis of the number of conserved catalytic cysteines (peroxidatic cysteine), they are generally divided into two groups, 1-Cys and 2-Cys Prdxs. Prdx6 is the only member of the family that has non-selenium peroxidase and Ca2+-independent PLA2 activities (8, 36, 52, 54). In cells, Prdx6 participates in oxidative defense by suppressing intracellular enzyme inactivation and membrane phospholipid peroxidation, and by eliminating excess ROS (13, 20, 38, 75).
UPR is primarily an adaptive response aiming to restore ER homeostasis and protect cells from stress. With prolonged stress, the ER stress receptors can initiate proapoptotic pathways, leading to cell death (69). CHOP/Gadd153 was the first molecule found to mediate ER stress-induced apoptosis (61). Empirically, different cell types have been observed to respond differently to oxidative stress inducers such as ischemia (47). Because Prdx6-deficient cells are vulnerable to oxidative stress, and hypoxia activates UPR (32, 46, 85), the subsequent abnormal signaling can undermine cellular survival when threshold levels of cellular defense are inadequate. In the current study, we found that lens epithelial cells (LECs) isolated from Prdx6-deficient mice (14) displayed increased expression of CHOP and Bip, leading to the prediction that Prdx6 depletion may be associated with ER stress and that deficiency of Prdx6 is the initiator of UPR/ER stress in these cells. In addition, our investigations revealed the induction of all three arms of UPR—PERK, ATF6, and IRE1-α— and their downstream targets in these cells, in which aberrant expression levels were further modulated by hypoxia (1% O2 or CoCl2, a hypoxia mimic) (1). These data underscore the role of Prdx6 in maintaining ER stress homeostasis by regulating cellular ROS at normal physiological levels. However, perturbation of ER homeostasis may occur under various conditions. Given the role of Prdx6 in maintaining signaling pathways, its deficiency may influence cell signaling, including both the UPR-mediated survival response and the apoptotic cell death response to ER stress.
Using Prdx6-deficient cells coupled with Prdx6 overexpression and aging cells, we found that loss of Prdx6 led to initiation of UPR/ER stress signaling as evidenced by selective upregulation of UPR/ER stress-associated proteins such as Bip, CHOP, ATF4, and so on. These proteins were further increased in Prdx6−/− cells exposed to oxidative/ER stressors, leading to apoptosis. In contrast, delivery of Prdx6 prevented overmodulation of the proteins and cell death. We also found that Prdx6-deficient cells had enhanced sensitivity to oxidative stress from ROS accumulation, and these cells displayed altered expression of antioxidants and chaperon proteins and mRNA. The data presented here provide evidence that Prdx6 deficiency induces/initiates UPR/ER stress-induced abnormality and cell death, at least in lens cells. We propose that Prdx6 is necessary for maintaining a homeostatic regulatory system and allowing cells to maintain an intracellular microenvironment favorable for cellular function.
MATERIALS AND METHODS
Generation and validation of LECs isolated from lenses of Prdx6−/− and Prdx6+/+ mice.
All animal experiments followed the recommendations set forth in the “Statement for the Use of Animals in Ophthalmic and Visual Research” by the Association for Research in Vision and Ophthalmology. Animal studies were approved by the University of Nebraska Medical Center (UNMC). LECs isolated from Prdx6-targeted mutants (Prdx6−/−) and wild-type (Prdx6+/+) mice were generated and maintained in Dulbecco's modified Eagle's medium (DMEM) with 10% fetal bovine serum (FBS), as described earlier (14). Prdx6−/− 129/Sv mice were generated at Harvard Medical School (Boston, MA) under the supervision of Dr. David R. Beier. For the present study, we used Prdx6−/− mutant mice of pure 129 background, and, as controls, wild-type 129/Sv inbred mice of the same sex and age (Prdx6+/+). All animals were maintained under specific pathogen-free conditions in an animal facility. LECs were isolated from mice of identical age, and Western analysis was carried out to confirm the presence of αA-crystallin, a specific marker of LECs. Cells from 3–5 passages were used for the experiments.
Isolation of LECs from human subjects.
Eye lenses isolated from human eyes aged 18, 23, 24, 63, 64, and 74 yr were obtained from the Lions Eye Bank, UNMC. LECs were generated as described earlier with some modification (59). Briefly, clear lenses were washed with DMEM medium containing penicillin-streptomycin (100 μg/ml) and amphotericin B (25 μg/ml). Capsules were spread by forceps with cell layers upwards on the surface of plastic culture petri dishes. Complete DMEM containing 15% FBS serum was added. The growth of explants culture was monitored routinely. For subcultivation, monolayer of culture was incubated with trypsin (GIBCO), and the dissociated cells were split as described earlier (59, 68). LECs obtained from 2 to 3 passages were used for the experiments.
Cell culture and generation of hypoxic stress.
LECs were cultured in 96-well plates or 100-mm petri dishes according to the requirements of the experiment. For each assay, cells were first cultured in DMEM containing 10% FBS for 24 h. Then the medium was replaced with DMEM containing either 0.1% bovine serum albumin (BSA) or 1% FBS. For hypoxic treatment, cells were either exposed to 1% oxygen (O2) in a hypoxic chamber (24) or treated with different concentrations of cobalt chloride (CoCl2) (37, 84). Tunicamycin, a known ER stress inducer (27, 86), was used as a positive control.
Construction of Prdx6 antisense.
Human LEC cDNA library was used to isolate Prdx6 cDNA having a full-length open reading frame. A full-length Prdx6 antisense construct was made by subcloning Prdx6 cDNA into a pcDNA3.1/NT-GFP-TOPO vector in reverse orientation. Plasmid was amplified following TOP 10 bacterial cell transformation as described earlier (17).
Western blot analysis and antibodies.
Cytoplasmic and nuclear extracts or total cell lysates were prepared in ice-cold radio immunoprecipitation assay (RIPA) lysis buffer, as described previously (15, 17). Equal amounts of protein samples were loaded onto a 10% SDS gel, blotted onto polyvinylidene fluoride membrane (PerkinElmer, Waltham, MA), and immunostained with primary antibodies at the appropriate dilutions. The antibodies were Prdx6 monoclonal antibody (Lab Frontier, Seoul, Korea), pIRE1-α (sc-20790, Santa Cruz Biotechnology), ATF4 (ab50546, Abcam, Cambridge, MA), ATF6-α (sc-22799, Santa Cruz Biotechnology), tropomyocin (Tmp 1α and 1β, sc-28543, Santa Cruz Biotechnology), Bip (no. 3183, Cell Signaling Technology), calnexin (SPA-860, Stressgen), CHOP (sc-7351, Santa Cruz Biotechnology), peIF2-α (sc-11386, Santa Cruz Biotechnology), pPERK (sc-32577, Santa Cruz Biotechnology), caspase 3 (no. 9665, Cell Signaling), and caspase 12 (no. 2202, Cell Signaling, ab8117, Abcam; sc-5627, Santa Cruz Biotechnology). Membranes were incubated with horseradish peroxidase-conjugated secondary antibodies. Specific protein bands were visualized by incubating the membrane with luminal reagent (Santa Cruz Biotechnology), and the images were recorded with FUJIFILM-LAS-4000 luminescent image analyzer (FUJIFILM Medical Systems). To ascertain comparative expression and equal loading of the protein samples, the membrane stained earlier was stripped and reprobed with β-actin antibody (Sigma).
Expression and purification of TAT-HA-Prdx6 fusion protein.
Construction and purification of Prdx6 linked to TAT were performed as described previously (38). Briefly, a full-length cDNA of Prdx6 was isolated from human LEC cDNA library and cloned into TAT-HA-Prdx6 prokaryotic expression vector. Recombinant protein was purified using Ni2+-nitrilotriacetic acid Sepharose column. Escherichia coli BL21 (DE3) was transformed with pTAT-HA-Prdx6, and the cells were harvested in binding buffer and sonicated. Immediately after centrifugation, supernatant containing TAT-HA-Prdx6 was loaded onto a 2.5-ml column. The fusion protein was washed, eluted with an elution buffer, and dialyzed. The purified protein was either used directly for protein transduction or aliquoted and stored frozen in 10% glycerol at −80°C for later use. A batch of recombinant protein TAT-HA-Prdx6 was passed through a Detoxi-Gel endotoxin-removing gel column (no. 20344; Pierce, Rockford, IL) to exclude endotoxin contamination, if any. In a parallel experiment, this preparation was used to compare protective efficacy of Prdx6-linked to TAT-HA that was not purified through the column.
Quantitative real-time PCR.
Quantitative real-time PCR was performed using LightCycler 480II as described earlier (38, 72). Prdxs and β-actin primers were purchased from Roche Applied Sciences. Gpx1 and catalase-specific primers were used as described earlier (72). The comparative Cp method was used to calculate relative fold expression levels using LightCycler 480 software (release 1.5.0 SP3). The Cps of target genes were normalized to the levels of β-actin as an endogenous control in each group. Primers were as follows: β-actin forward: 5′-CTAAGGCCAACCGTGAAAAG-3′ and reverse: 5′-ACCAGAGGCATACAGGGACA-3′; Prdx1 forward: 5′-GTGAGACCTGTGGCTCGAC-3′ and reverse: 5′-TGTCCATCTGGCATAACAGC-3′; Prdx2 forward: 5′-GACGAGCATGGGGAAGTCT-3′ and reverse: 5′-TCCTTGCTGTCATCCACATT-3′; Prdx3 forward: 5′-GTGCCTCTTGCGTGCTCT-3′ and reverse: 5′-ACTTGCATGACGAGCAACC-3′; Prdx4 forward: 5′-TGACAAGCATGGAGAAGTCTG-3′ and reverse: 5′-CAGCTGGATCTGGGATTATTG-3′; Prdx5 forward: 5′-GATTGAAGAGTGGGGTCGAG-3′ and reverse: 5′-TCTGTCGCCTTCCCAAAG-3′; Prdx6 forward: 5′-TTTCAATAGACAGTGTTGAGGATCA-3′ and reverse: 5′- CGTGGGTGTTTCACCATTG-3′.
Assay of intracellular ROS level.
Intracellular ROS level was measured by use of fluorescent dye dichlorofluorescin diacetate (H2-DCF-DA), a nonpolar compound that is converted into a polar derivative (dichlorofluorescein) by cellular esterase after incorporation into cells (14, 38). Cells were cultured in 96-well plates for 24 h with DMEM having 10% FBS. Cells were then either exposed to 1% O2 or treated with CoCl2 and tunicamycin at various doses for different time intervals. The medium was replaced with Hanks' solution containing 10 μM H2-DCF-DA dye, and cells were incubated. Following 30 min of incubation at room temperature, intracellular fluorescence was detected with excitation at 485 nm and emission at 530 nm as measured by a Spectra Max Gemini EM (Molecular Devices, Sunnyvale, CA).
Cell viability assay.
A colorimetric MTS assay (Promega, Madison, MI) was performed as described earlier (14, 38). This assay of cellular proliferation/viability uses 3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2 to 4-(sulfophenyl)2H-tetrazolium salt. When added to medium containing viable cells, MTS is reduced to a water-soluble formazan salt. The A490 nm value was measured after 4 h with an ELISA reader. Results were normalized with absorbance of the untreated control(s).
Cell apoptosis assay.
Apoptosis was measured by flow cytometry to quantify the levels of oxidative stress-induced apoptotic cells. The annexin V binding assay was performed with the annexin V-FITC/PI Apoptosis Detection Kit I (BD Biosciences), following the company's protocol. Briefly, Prdx6+/+ and Prdx6−/− cells were seeded in 100-mm plates and were subjected to stressors or chemical chaperone [4-phenylbutyrate (4-PBA)] for variable periods. After an incubation period, cells were washed twice with ice-cold phosphate-buffered saline solution (PBS). Finally, 105 cells were resuspended in 100 μl of binding buffer. The cell suspension (1 × 105/100 μl) was then incubated with 5 μl of annexin V-FITC and 5 μl of propidium iodide (PI) for 15 min at room temperature in the dark. A volume of 500 μl was maintained by adding 400 μl of binding buffer to each mixture, and samples were analyzed by flow cytometry within 1 h (UNMC core facilities). All experiments were carried out in triplicate.
Statistical methods.
Data are means ± SD of the indicated number of experiments. Data were analyzed by Dunnett's multiple conversion tests or Student's t-test when appropriate. P < 0.05 was defined as indicating a statistically significant difference.
RESULTS
Cells deficient in Prdx6 had enhanced expression of ER stress response genes, were sensitive to oxidative stress, and showed abnormal phenotypes with spontaneous apoptosis.
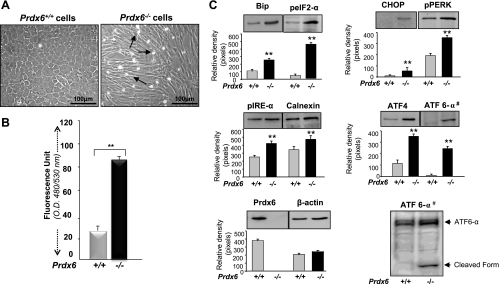
ER stress and oxidative stress are closely linked, and oxidative stress is known to disrupt protein folding (50). Prdx6 plays a vital role in maintaining cellular survival signaling by optimizing ROS expression at physiological levels, as shown by the finding that LECs and lenses isolated from mice in which Prdx6 has been genetically deleted (Prdx6−/−) are susceptible to oxidative stress-induced deleterious signaling (14, 38–40, 72–74, 76). To examine whether Prdx6 deficiency in cells evokes ER stress, we used LECs isolated from Prdx6-mutant (Prdx6−/−) and wild-type (Prdx6+/+) mice and cultured the cells in DMEM containing 10% FBS. Cells were analyzed at different time intervals and photomicrographed. As reported earlier, 48 h after culture, Prdx6−/− cells displayed significant abnormal morphological changes with elevated expression of ROS as determined by the ROS-responsive fluorescence probe 2′,7′-dichlorofluorescein (Fig. 1B). The cells became elongated and fiberlike, were packed irregularly, formed cellular aggregates, and detached more frequently. They also underwent spontaneous cell death (Fig. 1A). These morphological changes in Prdx6−/− LECs were similar to those observed in transdifferentiation in anterior subcapsular cataracts and posterior capsule opacifications (45) after cataract surgery.
Fig. 1.
A: photomicrograph of Prdx6−/− cells cultured in vitro showing phenotypic abnormalities. Lens epithelial cells (LECs) were isolated from peroxiredoxin 6 (Prdx6)-targeted mutants (Prdx6−/−) and wild-type (Prdx6+/+) mice. Cells were cultured in complete DMEM medium (GIBCO) overnight. The medium was replaced with DMEM containing 0.1% BSA. Significant morphological alterations were observed in Prdx6−/− cells; they became elongated and fiberlike, formed cellular aggregates, packed irregularly, and showed apoptosis. Arrows indicate dead cells. B: involvement of oxidative stress in Prdx6−/− cells, showing elevated levels of reactive oxygen species (ROS). ROS-responsive fluorescence probe 2′,7′-dichlorofluorescein (H2-DCF-DA) assay was conducted to monitor the intracellular ROS level. Prdx6+/+ and Prdx6−/− cells were cultured in 96-well plates in DMEM + 10% FBS. The next day, the medium was replaced with HBSS containing H2-DCF-DA dye and fluorescence intensity was measured. Histogram values are means ± SD of three independent experiments. OD, optical density. **Statistically significant difference (P < 0.001 vs. control). C: Western analysis of Prdx6−/− and Prdx6+/+ cells showing expression or activation of Bip, CHOP, calnexin, peIF2-α, pPERK, pIRE-α, ATF4, and ATF6-α (#, nuclear extract) in Prdx6−/− and Prdx6+/+ cells. Cells were cultured in 60-mm plates for 48 h, cell lysates were prepared, proteins were resolved on 10% SDS-PAGE, and Western analysis was done. Membranes were stripped/restripped and immunostained with different antibodies. In each experiment, β-actin was used as an internal marker. Protein bands were quantified using a densitometer, and levels were normalized to corresponding β-actin levels; histograms are shown below the protein bands. Data represent means ± SD of three independent experiments. **P < 0.001 vs. control.
Oxidative stress delays recovery of unfolded proteins and initiates the ER stress response (UPR) (48, 49). ER stress pathways involve three major distinct stress sensor proteins, PERK, pIRE1-α, and ATF6; Bip is the master regulator of those sensor proteins. Therefore, we first monitored the expression levels of Bip in Prdx6−/− cells and found that the cells had increased Bip expression. Dissociation of Bip from PERK leads to the autophosphorylation of PERK, which in turn phosphorylates eIF2-α and activates ATF4 translation. ATF4 increases the expression of proapoptotic factor CHOP (43, 61, 66, 67, 87). Investigating the expression levels of ER stress-related proteins in Prdx6−/− cells, we found that, indeed, Prdx6-depleted cells carried increased expression of phosphorylated PERK and eIF2-α, and that CHOP and ATF4 protein levels were also higher (Fig. 1C), indicating the prevalence of UPR/ER stress response in Prdx6-deficient cells. Furthermore, the dissociation of Bip from IRE1-α permits the activation of that protein, and the activated IRE1-α is a proapoptotic factor. On the other side, the dissociation of Bip from ATF6 permits the translocation of ATF6 into the Golgi compartment of intramembrane proteolysis. To test whether these two factors are also elevated in Prdx6−/− cells, we extracted cytosolic and nuclear extracts from Prdx6-depleted cells. Western analysis demonstrated increased levels of pIRE1-α and ATF6 expression in cytosolic and nuclear fraction of Prdx6−/− cells, respectively. Notably, ATF6-α was cleaved, giving rise to 37-kDa protein band (Fig. 1C; ATF6-α #) in Prdx6−/− cells, but not in Prdx6+/+ cells. Taken together, Western analysis and the relative densitometry of protein bands as shown in Fig. 1C revealed that Prdx6 depletion activated ER stress signaling in LECs.
Prdx6 deficiency influenced the expression of other members of the Prdx family (Prdxs 1–5) and antioxidant/chaperone protein.
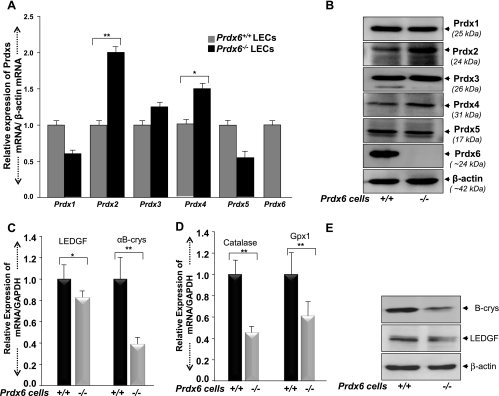
Our previous report and present data indicate that Prdx6−/− cells are under oxidative pressure (redox state), that such cells are unable to maintain homeostasis, and that they undergo apoptosis (14, 16, 38, 39, 52, 54, 72). In the present study, Prdx6-deficient cells harbored activated ER stress signaling with phosphorylation of eIF2-α, a global translational inhibition (Fig. 1C). Notably, the cells were obviously damaged during oxidative stress, behaving like aging cells with low antioxidant levels. Other enzymes appeared unable to maintain LEC homeostasis. We hypothesized that levels of other enzymes may be altered in Prdx6−/− cells. By using real-time PCR or Western analysis, we monitored the levels of mRNA and protein expression of known major cytoprotective molecules: Prdxs 1–5, Cat, Gpx1, chaperone protein αB-crystallin, and survival transcriptional protein LEDGF, using probes specific to corresponding genes/proteins (14, 16, 38–40, 72). We found that the mRNA and protein expression levels of Prdx 1 and 5 were decreased. In contrast, Prdx 2 was significantly increased, and expression of Prdx 3 and 4 was also increased (Fig. 2, A and B). Furthermore, these cells displayed a reduction in expression of Cat and Gpx-1 accompanied by diminished expression of αB-crystallin and LEDGF (Fig. 2, C–E). The increased expression of Prdx 2, 3, and 4 (Fig. 2, A and B) was not able to attenuate the adverse reaction in Prdx6−/− cells. We propose that Prdx6−/− cells represent a model that can be used to elucidate the signaling pathways involved in aging cells, because aging is related to a decline in expression of protective proteins.
Fig. 2.
A: quantitative real-time PCR showing differential expression of Prdx1–6 mRNA in Prdx6+/+ and Prdx6−/− LECs. Total RNA was isolated and transcribed into cDNA. Real-time PCR was performed using specific primers as described in materials and methods. mRNA expression of each Prdx was adjusted to the mRNA copies of β-actin. B: Western analysis showing expression of Prdxs 1–6 protein in Prdx6+/+ and Prdx6−/− cells. β-Actin bands show equal loading. C: quantitative real-time PCR showing reduced levels of LEDGF and αB-crystallin in Prdx6−/− cells in comparison to Prdx6+/+ LECs. D: real-time PCR showing the reduced levels of catalase and glutathione peroxidase in Prdx6−/− cells. E: Western blot showing reduced expression of LEDGF and αB-crystallin (B-crys) protein in Prdx6−/− cells. β-Actin was used to analyze equal loading. *P < 0.05, **P < 0.001, statistically significant difference.
Prdx6 knockdown initiated ER response similar to that in Prdx6-deficient cells.
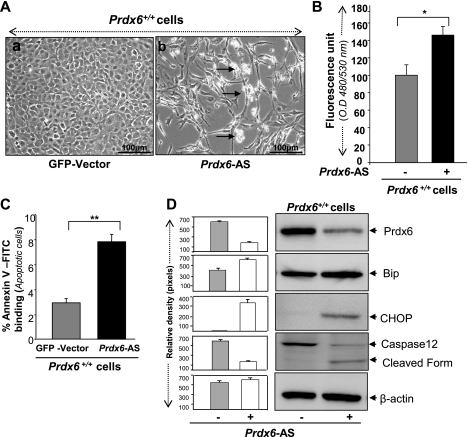
Prdx6−/− cells are under chronic oxidative stress that may not provide the underlying injurious signaling that occurs during acute oxidative stress. Therefore, we depleted Prdx6 by transfecting cells with antisense specific to Prdx6 (17). The transfection caused elevation of ROS expression and significant cell death (Fig. 3, A and B). Next, to examine the type of cell death triggered by knockdown of Prdx6, we analyzed the transfected cells for annexin V-FITC/PI assay (Fig. 3C, black bar). Results indicated that reduced expression of Prdx6-induced apoptosis in LECs. In parallel experiments, cell extracts were prepared and subjected to Western analysis to examine whether ER stress genes were upregulated. Of these genes, CHOP and Bip/glucose-regulated protein 78 (GRP/78) were often used as UPR/ER stress markers. As expected, Prdx6 knockdown strikingly induced the expression of CHOP and Bip, and the cells showed activated expression of caspase 12 (Fig. 3D, right lane and histogram).
Fig. 3.
A: photomicrograph of Prdx6+/+ cells following transfection with either green fluorescent protein (GFP)-vector (a) or Prdx6-antisense (Prdx6-AS) (b). Prdx6+/+ cells exhibit morphological changes and cell death (white rounded cells, indicated by arrows) similar to Prdx6−/− cells, showing the importance of Prdx6 in maintaining cellular homeostasis. B: intracellular ROS level increased in Prdx6+/+ cells transfected with Prdx6-AS. Cells were transfected with either Prdx6-AS or GFP-vector, and ROS levels were measured using H2-DCF-DA dye. *P < 0.05. C: annexin V-FITC/propidium iodide (PI) binding assay showing apoptotic cell death in cells transfected with Prdx6-AS. Cells transfected with either GFP-vector or Prdx6-AS were subjected to apoptotic cell death assay using annexin V-FITC/PI staining followed by flow cytometric analysis. Representative histogram shows % annexin V-FITC/PI staining of cells (gray and black bars). **P < 0.001. D: Western analysis showing reduced level of Prdx6 and increased expression of endoplasmic reticulum (ER) stress-response proteins in Prdx6+/+ cells following transfection of Prdx6-AS. This result shows that Prdx6 downregulation or depletion initiates ER stress. Histograms represent means ± SD of three independent Western blot experiments.
Aging cells displayed UPR.
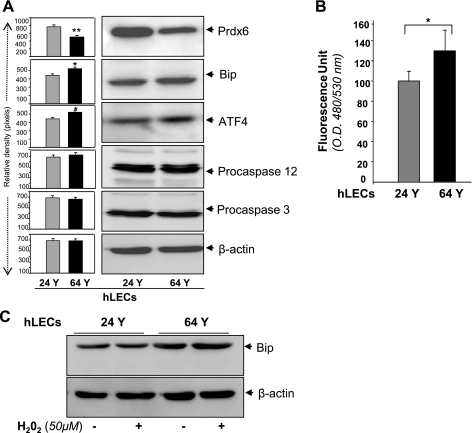
The ability to engender resistance to stress under adverse conditions is a fundamental cellular defense. The resistance capability of cells is evidently decreased during aging due to reduced expression of defense molecules (65). Earlier research revealed a decline of Prdx6 mRNA and protein in aging mouse LECs. In the present study we sought to extend that finding by determining whether reduced expression of Prdx6 in aging cells contributes to baseline UPR/ER stress, making these cells susceptible to oxidative stress. We cultured LECs isolated from human subjects aged 24 and 64 yr. Using Western analysis, we examined expression of Prdx6, Bip, and ATF4 and found that, indeed, ER stress-associated genes or their products were activated (but not dramatically) in the aged cells than in the younger ones (Fig. 4A). The aged cells also had elevated ROS expression (Fig. 4B). In addition, among cells facing oxidative stress from H2O2, the aged cells were more sensitive to ER stress and had increased expression of the UPR marker Bip, a key indicator of ER stress (Fig. 4C). Collectively, the results revealed that loss of Prdx6 was a major event in initiation/activation of ER stress signaling as observed in Prdx6-deficient cells. Since such cells characteristically behave similarly to aged cells, in later experiments, Prdx6−/− cells were utilized as a model system for aging cells.
Fig. 4.
A: enhanced expression of ER stress-responsive proteins in primary LECs obtained from aging human subjects. Western analysis is a representative of 24- and 64-yr-old subjects. Cell lysate was prepared from LECs isolated from lenses of young (18 or 24 yr old) and aging (63, 64, or 74 yr old) human subjects and analyzed by Western analysis. Protein blots were quantified using a densitometer, and levels were normalized to corresponding β-actin levels; histograms are shown beside the protein bands. B: ROS expression in aging and younger cells. Data represent means ± SD of three independent experiments. hLECs, human LECs. C: increased expression of Bip in cells after H2O2 exposure. Cells were treated with 50 μM H2O2 for 24 h, and cell lysate was prepared. A marked increase in the expression of Bip was observed in aged cells after H2O2 exposure (top). Results are derived from three independent experiments. *P <0.05, **P < 0.001.
Prdx6−/− cells were sensitive to hypoxia-induced cytotoxicity and displayed increased ROS expression.
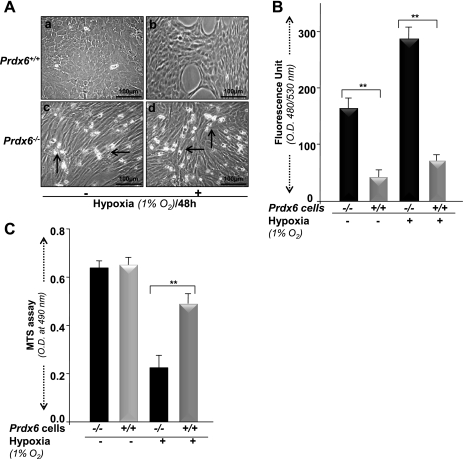
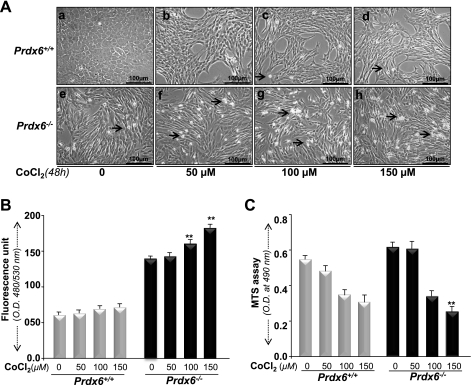
Prdx6 is a multifunctional protein that protects cells against oxidative stress by optimizing ROS (14, 38, 54, 72). Hypoxia increases intracellular ROS production in a variety of cells (7, 26, 46, 55, 69, 77). Mitochondria have been proposed as a primary source of ROS production during hypoxic stress. Prdx6 has been found to be translocated into mitochondria during ischemia, suggesting that Prdx6 may eliminate hypoxia-induced ROS-mediated cell injury (12, 14, 38, 72) and thereby maintain survival signaling (14, 38). We tested the ability of Prdx6 to mount cytoprotection against hypoxic stress. Exposure of cells to hypoxia (1% O2 for 48 h) or to variable concentrations of the hypoxia mimic CoCl2 significantly increased cell injury in Prdx6-deficient cells, as shown in photomicrographs (Fig. 5Ad vs. Ab and Fig. 6Ab vs. Af, Ac vs. Ag, and Ad vs. Ah). These cells displayed an abundance of ROS expression when measured by H2-DCF-DA fluorescence dye (Fig. 5B, black bar, and Fig. 6B, black bar), in contrast to Prdx6+/+ cells. However, photomicrographs showed cell death in Prdx6−/− cells without hypoxia, because these cells are always in redox state (14, 38, 72). In contrast, Prdx6+/+ cells showed some growth inhibition.
Fig. 5.
A: Prdx6+/+ and Prdx6−/− cells with or without hypoxia stress showing morphological changes (photomicrograph) and cell death (rounded white cells, indicated by arrows). Cells were cultured in 60-mm plates and exposed to 1% O2 with a hypoxic chamber. Prdx6−/− cells were highly sensitive to hypoxic stress (d). In contrast, Prdx6+/+ cells were resistant to hypoxia stress (b), demonstrating the protective role of Prdx6. B: measurement of intracellular redox state of Prdx6−/− and Prdx6+/+ cells after hypoxia. Cells were exposed to 1% oxygen, and ROS level was measured with H2-DCF-DA dye. ROS were further elevated in Prdx6−/− cells after hypoxia exposure (B, gray bar vs. black bar). C: effect of hypoxia on viability of Prdx6−/− and Prdx6+/+ cells. Cells were cultured in 48-well plates containing DMEM supplemented with 10% FBS; 24 h later, cells were exposed to 1% O2 for 48 h and cell viability was estimated using colorimetric MTS assay. Gray and black bars denote relative cell viability of Prdx6−/− and Prdx6+/+ cells with or without hypoxia following normalization with absorbance. Data represent means ± SD of three independent experiments. **P < 0.001, statistically significant difference.
Fig. 6.
A: photomicrograph of Prdx6−/− and Prdx6+/+ cells with or without CoCl2 treatment. Cells were treated with different concentrations of CoCl2 (50, 100, 150 μM) for 48 h. No significant cell death occurred in Prdx6+/+ cells (top: b, c, d). In contrast, significant cell death was observed in Prdx6−/− cells (bottom: f, g, h). Arrows indicate dead cells (rounded white cells). B: H2-DCF-DA assay showing increased ROS in Prdx6−/− cells after CoCl2 treatment. Dye was added to the cells with or without CoCl2 treatment, and fluorescence intensity was measured. C: MTS assay showing higher susceptibility of Prdx6−/− cells to CoCl2 treatment. Prdx6−/− or Prdx6+/+ cells were exposed to various concentrations of CoCl2, and MTS assay was conducted after 48 h. A significant decrease in survival of Prdx6−/− cells was observed compared with Prdx6+/+ cells, suggesting that Prdx6 is essential to protect cells from hypoxia-induced damage. Results were normalized according to the absorbance of the untreated control at termination of experiments. Data represent means ± SD of three independent experiments. **P < 0.001, statistically significant difference.
Next we evaluated the protective efficacy of Prdx6. Prdx6+/+ and Prdx6−/− cells were exposed to hypoxia (1% O2) or treated with increasing concentrations of CoCl2 and were analyzed for cell viability by MTS assay and apoptosis assay (annexin V-FITC/PI binding assay). The Prdx6+/+ cells were significantly viable and survived well even with exposure to hypoxic stressors (1% O2: Fig. 5C, gray bar, or hypoxia mimic, CoCl2; Fig. 6C, gray bars), demonstrating the ability of Prdx6 to protect cells against hypoxia-induced apoptosis (Fig. 7A, B, and C, gray bars).
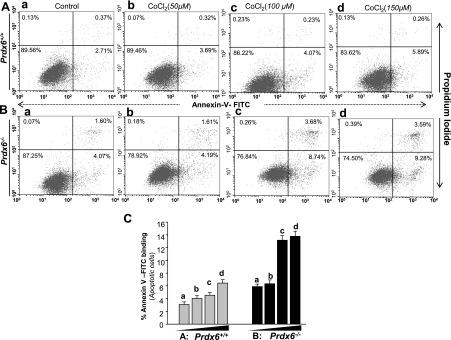
Fig. 7.
Prdx6−/− cells are more susceptible to CoCl2 (hypoxia mimic)-induced apoptosis. Cells were treated with different concentrations of CoCl2 (a, untreated; b, 50 μM; c, 100 μM; d, 150 μM). After 48 h of treatment, apoptosis was evaluated using annexin V-FITC/PI staining followed by flow cytometry. A and B: representative plots showing annexin V-FITC/PI staining of Prdx6+/+ and Prdx6−/− cells. The proportion of late or apoptotic cells is shown. C: histogram showing results of three independent experiments. A significant increase of apoptotic cells is observed (compare gray vs. black bars). Data represent means ± SD of three independent experiments.
Hypoxia-exposed Prdx6−/− cells showed enhanced ER stress.
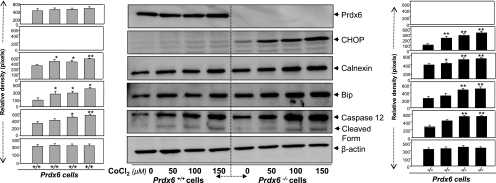
ER stress is triggered by a variety of stressors including hypoxia (81). Prdx6−/− cells display ER stress signaling and are vulnerable to stress-induced cell death. To examine whether hypoxia would stimulate ER stress signaling in Prdx6-deficient cells, we first exposed the cells to variable concentrations of CoCl2, a hypoxia mimic (10), and measured the expression and activation of caspase 12 (no. 2202, Cell Signaling) and the induction of CHOP, known to be involved in ER stress-induced apoptosis. Compared with Prdx6+/+ cells, Prdx6−/− cells exposed to hypoxia were found to be more vulnerable to cell death and underwent apoptosis (Figs. 5 and 6) and showed increased expression of CHOP and caspase 12 (and cleaved form of caspase 12) (Fig. 8, right, and black bars). However, Prdx6-deficient cells harbored relatively higher amounts and activated caspase 12. Densitometry analysis of protein bands also showed elevation of Bip and calnexin (Fig. 8), but these chaperone proteins were not able to suppress cell death, suggesting that ER stress occurs downstream of oxidative stress.
Fig. 8.
Western analysis of ER stress-responsive proteins in Prdx6+/+ and Prdx6−/− cells after CoCl2 treatment. Cells were treated with different concentrations of CoCl2 (50, 100, 150 μM). Following 48 h of treatment, cell lysate was prepared and Western analysis was performed. An increased expression pattern of Bip, calnexin, CHOP, and caspase 12 was observed in Prdx6−/− cells (right) in comparison to Prdx6+/+ (left). Protein bands were quantified and histograms are shown at left or right of each Western blot. Data represent means ± SD of three independent experiments. *P < 0.05 and **P < 0.001.
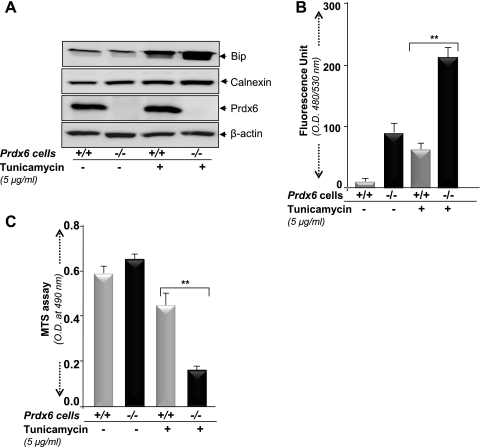
In experiments examining the sensitivity of Prdx6−/− cells to ER stress, Prdx6−/− cells were exposed to the known stress inducer tunicamycin. The cells revealed elevated levels of Bip and calnexin (Fig. 9A), showed increased intracellular ROS (Fig. 9B), and less viability (Fig. 9C) and underwent apoptosis (data not shown). Taken together, results demonstrated that loss of Prdx6 in cells activated ER stress, and such cells had enhanced sensitivity to ER stress-induced cytotoxicity.
Fig. 9.
A: Prdx6−/− LECs are more susceptible to ER stress. Prdx6+/+ and Prdx6−/− cells were treated with tunicamycin (5 μg/ml) and cell lysate was prepared after 48 h of treatment for Western analysis. B: H2-DCF-DA dye assay indicating a significant increase in intracellular ROS level in Prdx6−/− cells after tunicamycin treatment. **P < 0.001. C: MTS assay demonstrating that Prdx6−/− cells are more susceptible to tunicamycin treatment. Significant cell death was observed in Prdx6−/− cells in comparison to Prdx6+/+ cells (gray bar vs. black bar). An equal number of cells were considered to normalize data. **P < 0.001, statistically significant differences.
Prdx6 delivery optimized hypoxia-induced overstimulation of ER stress signaling in Prdx6−/− cells.
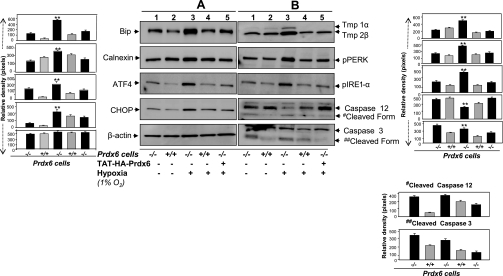
Earlier studies (14, 16, 38) found that Prdx6-depleted cells bear an abundance of ROS and show phenotypic changes with spontaneous apoptosis. Treatment with MnSOD mimetic or Prdx6 supply attenuates these adverse cellular events (14, 16, 38). But it was unknown whether a supply of Prdx6 would attenuate the basal prevalence of ER stress in Prdx6−/− cells or the stimulation of ER stress in these cells during stress. Hypoxic stress is associated with increased production of free radicals. Also, accumulation of unfolded proteins triggers ER stress and is considered a part of the cellular response to hypoxia (32). We used TAT-linked Prdx6 to supply Prdx6 to deficient cells facing hypoxic stress (38) and measured expression levels of ER stress-associated genes or gene products as well as intracellular level of TAT-linked Prdx6 (data not shown). Western analysis showed that, in Prdx6−/− cells exposed to 1% O2, the level of ER stress-related genes/products PERK, ATF4, eIF2-α, Bip, and CHOP along with caspase 12 (sc-5627, Santa Cruz Biotechnology) and 3 (no. 9665, Cell Signaling) were overstimulated significantly (Fig. 10, A and B) compared with the levels in Prdx6+/+ cells. We also examined levels of tropomyosin protein (Tmp 1α and 2β) expression, suggested to be unregulated during adverse signaling. Indeed, expression of these proteins was increased. The cells that received an extrinsic supply of Prdx6 showed inhibition of activated ER stress-related genes under hypoxic conditions, as evidenced by reduced expression of their protein level (Fig. 10, A and B, lane 3 vs. lane 5 and gray bars vs. black bars). Interestingly, the expression of cleaved caspases (activated forms) was reduced, suggesting that Prdx6 attenuates the processing of caspases (Fig. 10, caspase 12 cleaved form or caspase 3 cleaved form, and histogram). No effect was observed on β-actin used as control, indicating the role of Prdx6 deficiency-mediated activation of ER stress.
Fig. 10.
A and B: Western blot analysis of protein extracts from Prdx6−/− and Prdx6+/+ cells showing upregulation of ER-stress related proteins following hypoxia and its normalization by Prdx6 delivery. Prdx6−/− cells were supplied with TAT-HA-Prdx6 before hypoxia stress (1% O2) to evaluate its ability to prevent hypoxia-induced overexpression of ER stress-related proteins (40). Cell lysates were prepared after 48 h of stress using RIPA buffer, and Western analysis was performed. Notably, extrinsic supply of Prdx6 to Prdx6−/− cells could restore hypoxia-induced overmodulation of ER stress-related proteins (compare lane 3 vs. lane 5 and lane 3 vs. lane 4). Expression levels (protein bands) were quantified and values are presented as histograms. Data represent the mean ± S.D. of three independent experiments. **P < 0.001, statistically significant differences vs. control.
Sodium 4-PBA, a chemical chaperone, blocked apoptosis in Prdx6−/− LECs.
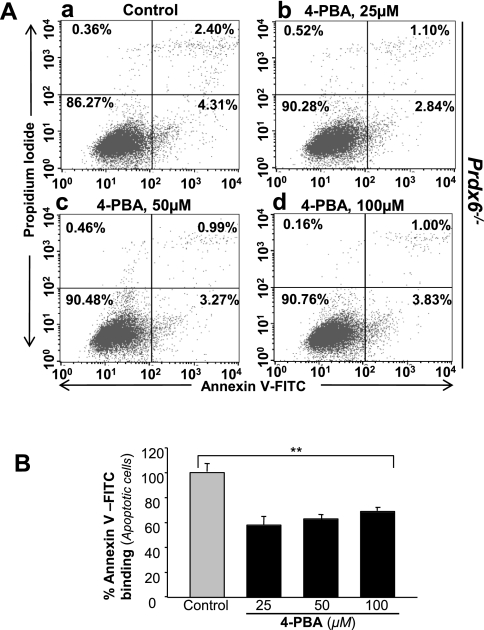
Recently, several researchers have reported that sodium 4-PBA acts as a chemical chaperone to reverse the mislocalization or aggregation of proteins in ER and therefore to inhibit UPR/ER stress-induced apoptosis (22, 25, 83). Cells deficient in Prdx6 are more susceptible to internal and external stresses leading to apoptosis (38, 72). To determine whether apoptosis in Prdx6-deficient cells is due to ER stress, we used 4-PBA, an inhibitor of ER stress-induced apoptosis. Cells were cultured with increasing amounts of 4-PBA, and after 16 h, cells were analyzed for annexin V-FITC/PI binding. The results showed that 4-PBA delivery attenuated apoptosis in Prdx6−/− cells (Fig. 11, gray vs. black bars), demonstrating prevalence of ER stress-induced apoptotic signaling in these cells. However, none of the 4-PBA concentrations used in the experiment provided 100% protection, indicating the possible involvement of some other type of ER-stress-induced apoptotic signaling that is not attenuated by the addition of 4-PBA.
Fig. 11.
A supply of sodium phenylbutyrate (4-PBA) inhibits apoptosis in Prdx6−/− cells cultured in vitro. Cells were cultured in DMEM supplemented with increasing concentrations of 4-PBA (25, 50, and 100 μM); 16 h later, cells were analyzed for apoptosis using annexin V-FITC/PI staining following company's protocol (Apoptosis Detection Kit, BD Biosciences). A: representative plots displaying annexin V-FITC/PI staining of Prdx6−/− cells treated (b, 25 μM; c, 50 μM; d, 100 μM) or untreated (a) with 4-PBA. B: results showing inhibition of apoptosis in Prdx6−/− cells in presence of 4-PBA (black bars). Experiments were done three times and values are represented as means ± SD. **P < 0.001, statistically significant differences.
DISCUSSION
An imbalance between cellular antioxidant defense system(s) and ROS-driven oxidative stress has been implicated in pathogenesis of cancer and degenerative disorders such as Alzheimer's and Parkinson's diseases and cataractogenesis (13, 14, 16, 38, 40, 71). In cells, Prdx6 participates in oxidative defense by eliminating excess ROS and thereby optimizing them at cellular physiological levels to maintain cell survival signaling (13, 14, 16, 38, 40, 72, 74, 76). In our earlier studies, we have shown that those Prdx6-deficient cells undergo spontaneous apoptosis and are more susceptible to oxidative stress (14, 38). Recent accumulating evidence reveals that oxidative stress is closely linked to UPR and activates UPR/ER stress (32, 46, 50, 82, 85). In the present study, by using Prdx6-deficient cells as a model system, we have unveiled death signaling that causes abnormalities and apoptosis (13, 16, 38, 40, 72). Our study suggests a model in which deficiency or loss of the antioxidant Prdx6 causes initiation of UPR, and this process becomes overstimulated in response to stressors. Figure 1B shows that Prdx6-deficient cells contain higher than normal levels of ROS and undergo spontaneous apoptosis. These results were consistent with previous published reports (14, 38). These cells express activated and upregulated ER stress genes/gene products such as Bip/GRP/78 and calnexin, markers of ER stress (Fig. 1C). Bip and calnexin are ER-resident chaperon proteins that play major roles in maintaining ER quality control. Under resting or unstressed conditions, Bip binds to the luminal domain of all three ER stress receptors and inhibits activation of PERK, IRE1-α, and ATF6 (4, 42). During UPR/ER stress, these molecules dissociate from Bip and become activated. Thus UPR activation is correlated with Bip dissociation with these receptors. In our Western analysis experiment, we found upregulation and activation of ATF6 and pPERK, suggesting that Prdx6−/− cells are under ER stress (Fig. 1C).
Overproduction of ROS induced by various internal or external stresses is injurious to cells and tissues. To cope with cellular stressors, cells have evolved defense systems that include chaperones like αB-crystallin survival factor and a variety of antioxidants such as Cat, GPxs, and Prdxs. By examining the expression levels of other antioxidants and molecules involved in protecting cells from stressors in Prdx6−/− cells, we found that the mRNA and protein expression of Prdx2 and Prdx4 were significantly upregulated. Other antioxidants, including αB-crystallin and the survival factor LEDGF, were downregulated (Fig. 2, C and E). On the basis of that finding and because other Prdxs and protective molecules did not counteract the changes in Prdx6−/− LECs, we think that Prdx6 plays a pivotal role in blocking ER stress, at least in LECs. Wang et al. (72) and Manevich and Fisher (52), in work with Prdx6−/− mice, reported that endogenously produced mouse Prdx6 functions in vivo as an antioxidant enzyme, and its function is not redundant with that of other Prdxs and antioxidant enzymes. Prdx6 has both GSH peroxidase and PLA2 activities (52), and recently we found that Prdx6 maintains Ca2+ homeostasis (13, 16). These features make Prdx6 different from other antioxidants.
Moreover, antioxidants also play a pivotal in maintaining a reductive cytosolic environment through both catalytic and nonenzymatic processes (56). In particular, antioxidants are likely to regulate intracellular ROS in a localized fashion. The role of Prdx6 in maintaining cellular survival signaling pathways and its expression in mitochondria (12) indicate that the expression level may influence both the UPR-mediated survival and apoptotic cell death responses to ER stress. We posit that deficiency or reduced expression of Prdx6 may initiate ER stress due to cellular redox-environment, and that ER stress can be overstimulated during hypoxia or oxidative stresses, leading to apoptotic signaling. Prdx6-antisense experiments, which revealed upregulated expression of ER stress-associated proteins involved in ER stress signaling, support the notion that Prdx6 deficiency is one cause of initiation of ER stress (Fig. 3C). Oxidative stress causes ER dysfunction (30). Our data revealed a decline in Prdx6 expression in aging cells (Fig. 4A). These cells showed an increase in Bip with higher expression of ATF4 and caspases (Fig. 4A), suggesting that aged cells bear activated ER stress signaling that is associated with reduced expression of Prdx6. The ER provides a unique and balanced oxidizing microenvironment for protein folding (28, 50, 70). However, after dissociation from luminal surface of ER, Bip/GRP78 activates three ER bound proteins: 1) type 1 ER transmembrane protein kinase (IRE1) (4, 11); 2) activating transcriptional factor 6 (ATF6); and 3) PKR-like ER kinase (PERK) (43, 66, 67). Procaspase 12 is an ER-associated proximal effector of apoptosis (64). Activated caspase 12 further activates caspase 9, which in turn activates caspase 3, leading to apoptosis (51, 80). Free ATF6 translocates to nucleus to activate transcription of many unfolded protein-responsive genes including Bip/GRP78 and CHOP. PERK, on the other hand, is a kinase that phosphorylates a subunit of the translation initiation factor (eIF2-α). Phosphorylated eIF2-α activates a transcriptional factor, ATF4, and the upregulation of ATF4 activates the transcription of Bip/GRP78 and CHOP. The elevation of CHOP results in a downregulation of Bcl2, activation of caspases and, finally, apoptosis (57). Thus, Bip/GRP78, ATF4, CHOP, and caspase 12 are the key enzymes during ER stress, and the signaling cascade is activated in Prdx6-deficient cells (Figs. 1, 2, 3, 5, and 6). Recent studies have indicated a close link between ER stress and caspase 12 expression and activation. Indeed, constitutive expression and activation of this protein was observed in Prdx6−/− cells. Furthermore, the observation that caspase 12 processing occurs during ER stress induced apoptosis has supported the idea that caspase 12 could be the initiator caspase in ER stress-mediated apoptosis. However, the phenotype of mouse embryonic fibroblasts (MEF) cells isolated from caspase 12-deficient mice concerning this notion is moderate as the absence of caspase 12 in ER stress-induced apoptosis (60), arguing the involvement of other mechanisms. Although our data suggest that Prdx6−/− cells bear higher expression and activation of caspase 12, it may be dispensable for the execution of cell death prompted by ER stress, and that other molecular mechanisms may be involved (34, 41, 44).
Diverse cellular stresses, such as chemotherapeutic drugs, radiation, and hypoxia, can initiate or accelerate both oxidative and ER stresses, the damaging effects of which are more pronounced in cells with reduced expression of antioxidants. Because hypoxia is considered a triggering stimulus for redox disturbances in cellular microenvironment and is known to induce ER stress (1), we exposed LECs to 1% O2 or CoCl2, a hypoxia-mimicking agent. Our data showed that the hypoxic condition further activated the ER stress response genes in Prdx6−/− cells (Fig. 8), and the cells displayed a dramatic increase in ROS level, had reduced viability, and underwent apoptosis (Figs. 6, A–C, and Fig. 7). To validate this finding, we used 1% O2 to evoke hypoxic stress in cultured cells supplemented with or without Prdx6. We found that a supply of Prdx6 reversed abnormal ER stress signaling during hypoxia, indicating that Prdx6 depletion may be associated with ER stress-mediated apoptosis. Furthermore, CHOP/Gadd153 was the first molecule observed to mediate ER stress-induced apoptosis (61), and was found to be accelerated in Prdx6-deficient cells (Figs. 8 and 10).
Our data also showed elevated expression of calnexin in Prdx6−/− cells. Calnexin is an ER-resident molecular chaperone that plays an essential role in the correct folding of membrane proteins. eIF2 is a regulatory protein involved in polypeptide chain initiation. Kaufman and colleagues (6) reported that eIF2-α phosphorylation levels increase in mammalian cells that are undergoing apoptosis. We found that cells deficient in Prdx6 showed elevated levels of peIF2-α. Because phosphorylation of eIF2-α is mediated by four distinct protein kinases—heme-regulated inhibitor kinase (HRI), protein kinase RNA (PKR), PKR-like ER kinase (PERK), and general control non-derepressible-2 (GCN2)—we sought to identify the kinase responsible for eIF2-α phosphorylation. We found that the level of pPERK was high in Prdx6-depleted cells.
Empirically, it has been observed that different cell types respond differently to ischemia (47). Particular cell types or aging cells (redox state) with reduced antioxidant may be more susceptible to hypoxia-induced injury, while cells repeatedly exposed to hypoxia may adapt and gain resistance against hypoxic stress (5, 58). Upregulation of hypoxia-inducible factor (HIF)-dependent proteins such as heme oxygenase-1 (HO-1) and Glut-1 has been found and has been shown to be protective. Thus expression level of hypoxia may potentially modify cell survival, but the effect may be related to cell type (47). The lens, which is excluded from circulation, is supposed to be in a hypoxic environment (2, 21, 29, 31). However, recently, mild hypoxia was shown to elevate the defense system of cells, enabling such cells to adapt to hypoxic stress. We believe that the lens has naturally adapted to minimize damage caused by the external and internal environments and has higher expression of several enzymes, including Cat, SOD, Gpx, and Prdx6. We have reported that Prdx6 is relatively enriched in the lens (39). These vitally encoded proteins may well form potential targets for treatment of age-associated degenerative disorders.
In summary, the study presented here describes, for the first time, the finding that Prdx6 deficiency activates ER responses in mammalian cells. Using aging cells and Prdx6-antisense experiments, we have demonstrated that loss or lack of Prdx6 results in ER stress with increased sensitivity to cell injury from hypoxia or oxidative stress. Raising the expression level of Prdx6 in cells by extrinsic supply was found to attenuate deleterious ER stress signaling by normalizing ER stress responses and ROS expression. The results extend our understanding of a plausible mechanism in cells with Prdx6 deficiency and cells facing stress, and add evidence of the important role of Prdx6 and its potential use in treatment.
GRANTS
Grants provided by the National Eye Institute, National Institutes of Health (EY-13394 and EY-17613; to D. P. Singh) and Research to Prevent Blindness are gratefully acknowledged. Grant support by American Health Assistance Foundation (to N. Fatma) is gratefully acknowledged.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
REFERENCES
- 1. Azfer A, Niu J, Rogers LM, Adamski FM, Kolattukudy PE. Activation of endoplasmic reticulum stress response during the development of ischemic heart disease. Am J Physiol Heart Circ Physiol 291: H1411–H1420, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Barbazetto IA, Liang J, Chang S, Zheng L, Spector A, Dillon JP. Oxygen tension in the rabbit lens and vitreous before and after vitrectomy. Exp Eye Res 78: 917–924, 2004 [DOI] [PubMed] [Google Scholar]
- 3. Beckman KB, Ames BN. The free radical theory of aging matures. Physiol Rev 78: 547–581, 1998 [DOI] [PubMed] [Google Scholar]
- 4. Bertolotti A, Zhang Y, Hendershot LM, Harding HP, Ron D. Dynamic interaction of BiP and ER stress transducers in the unfolded-protein response. Nat Cell Biol 2: 326–332, 2000 [DOI] [PubMed] [Google Scholar]
- 5. Bolli R. Cardioprotective function of inducible nitric oxide synthase and role of nitric oxide in myocardial ischemia and preconditioning: an overview of a decade of research. J Mol Cell Cardiol 33: 1897–1918, 2001 [DOI] [PubMed] [Google Scholar]
- 6. Boyce M, Bryant KF, Jousse C, Long K, Harding HP, Scheuner D, Kaufman RJ, Ma D, Coen DM, Ron D, Yuan J. A selective inhibitor of eIF2alpha dephosphorylation protects cells from ER stress. Science 307: 935–939, 2005 [DOI] [PubMed] [Google Scholar]
- 7. Chandel NS, Maltepe E, Goldwasser E, Mathieu CE, Simon MC, Schumacker PT. Mitochondrial reactive oxygen species trigger hypoxia-induced transcription. Proc Natl Acad Sci USA 95: 11715–11720, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Chen JW, Dodia C, Feinstein SI, Jain MK, Fisher AB. 1-Cys peroxiredoxin, a bifunctional enzyme with glutathione peroxidase and phospholipase A2 activities. J Biol Chem 275: 28421–28427, 2000 [DOI] [PubMed] [Google Scholar]
- 9. Chong ZZ, Li F, Maiese K. Oxidative stress in the brain: novel cellular targets that govern survival during neurodegenerative disease. Prog Neurobiol 75: 207–246, 2005 [DOI] [PubMed] [Google Scholar]
- 10. Corley KM, Taylor CJ, Lilly B. Hypoxia-inducible factor 1alpha modulates adhesion, migration, and FAK phosphorylation in vascular smooth muscle cells. J Cell Biochem 96: 971–985, 2005 [DOI] [PubMed] [Google Scholar]
- 11. DeGracia DJ, Kumar R, Owen CR, Krause GS, White BC. Molecular pathways of protein synthesis inhibition during brain reperfusion: implications for neuronal survival or death. J Cereb Blood Flow Metab 22: 127–141, 2002 [DOI] [PubMed] [Google Scholar]
- 12. Eismann T, Huber N, Shin T, Kuboki S, Galloway E, Wyder M, Edwards MJ, Greis KD, Shertzer HG, Fisher AB, Lentsch AB. Peroxiredoxin-6 protects against mitochondrial dysfunction and liver injury during ischemia-reperfusion in mice. Am J Physiol Gastrointest Liver Physiol 296: G266–G274, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Fatma N, Kubo E, Sen M, Agarwal N, Thoreson WB, Camras CB, Singh DP. Peroxiredoxin 6 delivery attenuates TNF-alpha-and glutamate-induced retinal ganglion cell death by limiting ROS levels and maintaining Ca2+ homeostasis. Brain Res 1233: 63–78, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Fatma N, Kubo E, Sharma P, Beier DR, Singh DP. Impaired homeostasis and phenotypic abnormalities in Prdx6−/− mice lens epithelial cells by reactive oxygen species: increased expression and activation of TGFbeta. Cell Death Differ 12: 734–750, 2005 [DOI] [PubMed] [Google Scholar]
- 15. Fatma N, Kubo E, Takamura Y, Ishihara K, Garcia C, Beebe DC, Singh DP. Loss of NF-kappaB control and repression of Prdx6 gene transcription by reactive oxygen species-driven SMAD3-mediated transforming growth factor beta signaling. J Biol Chem 284: 22758–22772, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Fatma N, Kubo E, Toris CB, Stamer WD, Camras CB, Singh DP. PRDX6 attenuates oxidative stress- and TGFbeta-induced abnormalities of human trabecular meshwork cells. Free Radic Res 43: 783–795, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Fatma N, Singh DP, Shinohara T, Chylack LT., Jr Transcriptional regulation of the antioxidant protein 2 gene, a thiol-specific antioxidant, by lens epithelium-derived growth factor to protect cells from oxidative stress. J Biol Chem 276: 48899–48907, 2001 [DOI] [PubMed] [Google Scholar]
- 18. Finkel T, Holbrook NJ. Oxidants, oxidative stress and the biology of ageing. Nature 408: 239–247, 2000 [DOI] [PubMed] [Google Scholar]
- 19. Fisher AB, Beers MF. Letter to the editor: Hyperoxia and acute lung injury. Am J Physiol Lung Cell Mol Physiol 295: L1066, 2008. Author reply L1067, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Fisher AB, Dodia C, Feinstein SI, Ho YS. Altered lung phospholipid metabolism in mice with targeted deletion of lysosomal-type phospholipase A2. J Lipid Res 46: 1248–1256, 2005 [DOI] [PubMed] [Google Scholar]
- 21. Fitch CL, Swedberg SH, Livesey JC. Measurement and manipulation of the partial pressure of oxygen in the rat anterior chamber. Curr Eye Res 20: 121–126, 2000 [PubMed] [Google Scholar]
- 22. Gardian G, Browne SE, Choi DK, Klivenyi P, Gregorio J, Kubilus JK, Ryu H, Langley B, Ratan RR, Ferrante RJ, Beal MF. Neuroprotective effects of phenylbutyrate in the N171–82Q transgenic mouse model of Huntington's disease. J Biol Chem 280: 556–563, 2005 [DOI] [PubMed] [Google Scholar]
- 23. Gething MJ, Sambrook J. Protein folding in the cell. Nature 355: 33–45, 1992 [DOI] [PubMed] [Google Scholar]
- 24. Giordano FJ. Oxygen, oxidative stress, hypoxia, and heart failure. J Clin Invest 115: 500–508, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Gong B, Zhang LY, Lam DS, Pang CP, Yam GH. Sodium 4-phenylbutyrate ameliorates the effects of cataract-causing mutant gammaD-crystallin in cultured cells. Mol Vis 16: 997–1003, 2010 [PMC free article] [PubMed] [Google Scholar]
- 26. Guzy RD, Hoyos B, Robin E, Chen H, Liu L, Mansfield KD, Simon MC, Hammerling U, Schumacker PT. Mitochondrial complex III is required for hypoxia-induced ROS production and cellular oxygen sensing. Cell Metab 1: 401–408, 2005 [DOI] [PubMed] [Google Scholar]
- 27. Han C, Nam MK, Park HJ, Seong YM, Kang S, Rhim H. Tunicamycin-induced ER stress upregulates the expression of mitochondrial HtrA2 and promotes apoptosis through the cytosolic release of HtrA2. J Microbiol Biotechnol 18: 1197–1202, 2008 [PubMed] [Google Scholar]
- 28. Harding HP, Zhang Y, Zeng H, Novoa I, Lu PD, Calfon M, Sadri N, Yun C, Popko B, Paules R, Stojdl DF, Bell JC, Hettmann T, Leiden JM, Ron D. An integrated stress response regulates amino acid metabolism and resistance to oxidative stress. Mol Cell 11: 619–633, 2003 [DOI] [PubMed] [Google Scholar]
- 29. Helbig H, Hinz JP, Kellner U, Foerster MH. Oxygen in the anterior chamber of the human eye. Ger J Ophthalmol 2: 161–164, 1993 [PubMed] [Google Scholar]
- 30. Helenius A, Marquardt T, Braakman I. The endoplasmic reticulum as a protein-folding compartment. Trends Cell Biol 2: 227–231, 1992 [DOI] [PubMed] [Google Scholar]
- 31. Holekamp NM, Shui YB, Beebe DC. Vitrectomy surgery increases oxygen exposure to the lens: a possible mechanism for nuclear cataract formation. Am J Ophthalmol 139: 302–310, 2005 [DOI] [PubMed] [Google Scholar]
- 32. Hotokezaka Y, van Leyen K, Lo EH, Beatrix B, Katayama I, Jin G, Nakamura T. alphaNAC depletion as an initiator of ER stress-induced apoptosis in hypoxia. Cell Death Differ 16: 1505–1514, 2009 [DOI] [PubMed] [Google Scholar]
- 33. Immenschuh S, Baumgart-Vogt E. Peroxiredoxins, oxidative stress, and cell proliferation. Antioxid Redox Signal 7: 768–777, 2005 [DOI] [PubMed] [Google Scholar]
- 34. Kalai M, Lamkanfi M, Denecker G, Boogmans M, Lippens S, Meeus A, Declercq W, Vandenabeele P. Regulation of the expression and processing of caspase-12. J Cell Biol 162: 457–467, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Kang JH, Shin I, Han JS. Changes of phospholipase D activity in TNF-alpha and anti-Fas/Apo1 monoclonal antibody induced apoptosis in HL-60 and A20 cells. Exp Mol Med 30: 21–27, 1998 [DOI] [PubMed] [Google Scholar]
- 36. Kang SW, Baines IC, Rhee SG. Characterization of a mammalian peroxiredoxin that contains one conserved cysteine. J Biol Chem 273: 6303–6311, 1998 [DOI] [PubMed] [Google Scholar]
- 37. Kim KS, Sengupta S, Berk M, Kwak YG, Escobar PF, Belinson J, Mok SC, Xu Y. Hypoxia enhances lysophosphatidic acid responsiveness in ovarian cancer cells and lysophosphatidic acid induces ovarian tumor metastasis in vivo. Cancer Res 66: 7983–7990, 2006 [DOI] [PubMed] [Google Scholar]
- 38. Kubo E, Fatma N, Akagi Y, Beier DR, Singh SP, Singh DP. TAT-mediated PRDX6 protein transduction protects against eye lens epithelial cell death and delays lens opacity. Am J Physiol Cell Physiol 294: C842–C855, 2008 [DOI] [PubMed] [Google Scholar]
- 39. Kubo E, Miyazawa T, Fatma N, Akagi Y, Singh DP. Development- and age-associated expression pattern of peroxiredoxin 6, and its regulation in murine ocular lens. Mech Ageing Dev 127: 249–256, 2006 [DOI] [PubMed] [Google Scholar]
- 40. Kubo E, Singh DP, Fatma N, Akagi Y. TAT-mediated peroxiredoxin 5 and 6 protein transduction protects against high-glucose-induced cytotoxicity in retinal pericytes. Life Sci 84: 857–864, 2009 [DOI] [PubMed] [Google Scholar]
- 41. Lamkanfi M, Kalai M, Vandenabeele P. Caspase-12: an overview. Cell Death Differ 11: 365–368, 2004 [DOI] [PubMed] [Google Scholar]
- 42. Li J, Ni M, Lee B, Barron E, Hinton DR, Lee AS. The unfolded protein response regulator GRP78/BiP is required for endoplasmic reticulum integrity and stress-induced autophagy in mammalian cells. Cell Death Differ 15: 1460–1471, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Liu CY, Schroder M, Kaufman RJ. Ligand-independent dimerization activates the stress response kinases IRE1 and PERK in the lumen of the endoplasmic reticulum. J Biol Chem 275: 24881–24885, 2000 [DOI] [PubMed] [Google Scholar]
- 44. Liu H, Baliga R. Endoplasmic reticulum stress-associated caspase 12 mediates cisplatin-induced LLC-PK1 cell apoptosis. J Am Soc Nephrol 16: 1985–1992, 2005 [DOI] [PubMed] [Google Scholar]
- 45. Liu J, Hales AM, Chamberlain CG, McAvoy JW. Induction of cataract-like changes in rat lens epithelial explants by transforming growth factor beta. Invest Ophthalmol Vis Sci 35: 388–401, 1994 [PubMed] [Google Scholar]
- 46. Liu L, Ning X, Sun L, Zhang H, Shi Y, Guo C, Han S, Liu J, Sun S, Han Z, Wu K, Fan D. Hypoxia-inducible factor-1 alpha contributes to hypoxia-induced chemoresistance in gastric cancer. Cancer Sci 99: 121–128, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Loor G, Schumacker PT. Role of hypoxia-inducible factor in cell survival during myocardial ischemia-reperfusion. Cell Death Differ 15: 686–690, 2008 [DOI] [PubMed] [Google Scholar]
- 48. Malhotra JD, Kaufman RJ. The endoplasmic reticulum and the unfolded protein response. Semin Cell Dev Biol 18: 716–731, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Malhotra JD, Kaufman RJ. Endoplasmic reticulum stress and oxidative stress: a vicious cycle or a double-edged sword? Antioxid Redox Signal 9: 2277–2293, 2007 [DOI] [PubMed] [Google Scholar]
- 50. Malhotra JD, Miao H, Zhang K, Wolfson A, Pennathur S, Pipe SW, Kaufman RJ. Antioxidants reduce endoplasmic reticulum stress and improve protein secretion. Proc Natl Acad Sci USA 105: 18525–18530, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Mandic A, Hansson J, Linder S, Shoshan MC. Cisplatin induces endoplasmic reticulum stress and nucleus-independent apoptotic signaling. J Biol Chem 278: 9100–9106, 2003 [DOI] [PubMed] [Google Scholar]
- 52. Manevich Y, Fisher AB. Peroxiredoxin 6, a 1-Cys peroxiredoxin, functions in antioxidant defense and lung phospholipid metabolism. Free Radic Biol Med 38: 1422–1432, 2005 [DOI] [PubMed] [Google Scholar]
- 53. Manevich Y, Shuvaeva T, Dodia C, Kazi A, Feinstein SI, Fisher AB. Binding of peroxiredoxin 6 to substrate determines differential phospholipid hydroperoxide peroxidase and phospholipase A(2) activities. Arch Biochem Biophys 485: 139–149, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Manevich Y, Sweitzer T, Pak JH, Feinstein SI, Muzykantov V, Fisher AB. 1-Cys peroxiredoxin overexpression protects cells against phospholipid peroxidation-mediated membrane damage. Proc Natl Acad Sci USA 99: 11599–11604, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Mansfield KD, Guzy RD, Pan Y, Young RM, Cash TP, Schumacker PT, Simon MC. Mitochondrial dysfunction resulting from loss of cytochrome c impairs cellular oxygen sensing and hypoxic HIF-alpha activation. Cell Metab 1: 393–399, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Mates JM, Perez-Gomez C, Nunez de Castro I. Antioxidant enzymes and human diseases. Clin Biochem 32: 595–603, 1999 [DOI] [PubMed] [Google Scholar]
- 57. McCullough KD, Martindale JL, Klotz LO, Aw TY, Holbrook NJ. Gadd153 sensitizes cells to endoplasmic reticulum stress by down-regulating Bcl2 and perturbing the cellular redox state. Mol Cell Biol 21: 1249–1259, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Murry CE, Jennings RB, Reimer KA. Preconditioning with ischemia: a delay of lethal cell injury in ischemic myocardium. Circulation 74: 1124–1136, 1986 [DOI] [PubMed] [Google Scholar]
- 59. Nagineni CN, Bhat SP. Alpha B-crystallin is expressed in kidney epithelial cell lines and not in fibroblasts. FEBS Lett 249: 89–94, 1989 [DOI] [PubMed] [Google Scholar]
- 60. Nakagawa T, Zhu H, Morishima N, Li E, Xu J, Yankner BA, Yuan J. Caspase-12 mediates endoplasmic-reticulum-specific apoptosis and cytotoxicity by amyloid-beta. Nature 403: 98–103, 2000 [DOI] [PubMed] [Google Scholar]
- 61. Oyadomari S, Mori M. Roles of CHOP/GADD153 in endoplasmic reticulum stress. Cell Death Differ 11: 381–389, 2004 [DOI] [PubMed] [Google Scholar]
- 62. Park BJ, Lim YS, Lee HJ, Eum WS, Park J, Han KH, Choi SY, Lee KS. Anti-oxidative effects of Phellinus linteus and red ginseng extracts on oxidative stress-induced DNA damage. BMB Rep 42: 500–505, 2009 [DOI] [PubMed] [Google Scholar]
- 63. Patenaude A, Ven Murthy MR, Mirault ME. Mitochondrial thioredoxin system: effects of TrxR2 overexpression on redox balance, cell growth, and apoptosis. J Biol Chem 279: 27302–27314, 2004 [DOI] [PubMed] [Google Scholar]
- 64. Rao RV, Peel A, Logvinova A, del Rio G, Hermel E, Yokota T, Goldsmith PC, Ellerby LM, Ellerby HM, Bredesen DE. Coupling endoplasmic reticulum stress to the cell death program: role of the ER chaperone GRP78. FEBS Lett 514: 122–128, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Salminen A, Kaarniranta K. ER stress and hormetic regulation of the aging process. Ageing Res Rev 9: 211–217, 2010 [DOI] [PubMed] [Google Scholar]
- 66. Shen J, Chen X, Hendershot L, Prywes R. ER stress regulation of ATF6 localization by dissociation of BiP/GRP78 binding and unmasking of Golgi localization signals. Dev Cell 3: 99–111, 2002 [DOI] [PubMed] [Google Scholar]
- 67. Shi Y, Vattem KM, Sood R, An J, Liang J, Stramm L, Wek RC. Identification and characterization of pancreatic eukaryotic initiation factor 2 alpha-subunit kinase, PEK, involved in translational control. Mol Cell Biol 18: 7499–7509, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Singh DP, Ohguro N, Chylack LT, Jr, Shinohara T. Lens epithelium-derived growth factor: increased resistance to thermal and oxidative stresses. Invest Ophthalmol Vis Sci 40: 1444–1451, 1999 [PubMed] [Google Scholar]
- 69. Szegezdi E, Fitzgerald U, Samali A. Caspase-12 and ER-stress-mediated apoptosis: the story so far. Ann NY Acad Sci 1010: 186–194, 2003 [DOI] [PubMed] [Google Scholar]
- 70. Tu BP, Weissman JS. Oxidative protein folding in eukaryotes: mechanisms and consequences. J Cell Biol 164: 341–346, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Valko M, Leibfritz D, Moncol J, Cronin MT, Mazur M, Telser J. Free radicals and antioxidants in normal physiological functions and human disease. Int J Biochem Cell Biol 39: 44–84, 2007 [DOI] [PubMed] [Google Scholar]
- 72. Wang X, Phelan SA, Forsman-Semb K, Taylor EF, Petros C, Brown A, Lerner CP, Paigen B. Mice with targeted mutation of peroxiredoxin 6 develop normally but are susceptible to oxidative stress. J Biol Chem 278: 25179–25190, 2003 [DOI] [PubMed] [Google Scholar]
- 73. Wang X, Phelan SA, Petros C, Taylor EF, Ledinski G, Jurgens G, Forsman-Semb K, Paigen B. Peroxiredoxin 6 deficiency and atherosclerosis susceptibility in mice: significance of genetic background for assessing atherosclerosis. Atherosclerosis 177: 61–70, 2004 [DOI] [PubMed] [Google Scholar]
- 74. Wang Y, Feinstein SI, Manevich Y, Ho YS, Fisher AB. Peroxiredoxin 6 gene-targeted mice show increased lung injury with paraquat-induced oxidative stress. Antioxid Redox Signal 8: 229–237, 2006 [DOI] [PubMed] [Google Scholar]
- 75. Wang Y, Lu Q, Sheldon FS, Ho YS, Phelan SA, Beers MF, Fisher AB. [Antioxidative role of peroxiredoxin 6 in acute lung injury]. Zhonghua Er Ke Za Zhi 46: 739–744, 2008 [PubMed] [Google Scholar]
- 76. Wang Y, Phelan SA, Manevich Y, Feinstein SI, Fisher AB. Transgenic mice overexpressing peroxiredoxin 6 show increased resistance to lung injury in hyperoxia. Am J Respir Cell Mol Biol 34: 481–486, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Wu W, Platoshyn O, Firth AL, Yuan JX. Hypoxia divergently regulates production of reactive oxygen species in human pulmonary and coronary artery smooth muscle cells. Am J Physiol Lung Cell Mol Physiol 293: L952–L959, 2007 [DOI] [PubMed] [Google Scholar]
- 78. Wu Y, Feinstein SI, Manevich Y, Chowdhury I, Pak JH, Kazi A, Dodia C, Speicher DW, Fisher AB. Mitogen-activated protein kinase-mediated phosphorylation of peroxiredoxin 6 regulates its phospholipase A(2) activity. Biochem J 419: 669–679, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Wu YZ, Manevich Y, Baldwin JL, Dodia C, Yu K, Feinstein SI, Fisher AB. Interaction of surfactant protein A with peroxiredoxin 6 regulates phospholipase A2 activity. J Biol Chem 281: 7515–7525, 2006 [DOI] [PubMed] [Google Scholar]
- 80. Xie Q, Khaoustov VI, Chung CC, Sohn J, Krishnan B, Lewis DE, Yoffe B. Effect of tauroursodeoxycholic acid on endoplasmic reticulum stress-induced caspase-12 activation. Hepatology 36: 592–601, 2002 [DOI] [PubMed] [Google Scholar]
- 81. Xu C, Bailly-Maitre B, Reed JC. Endoplasmic reticulum stress: cell life and death decisions. J Clin Invest 115: 2656–2664, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Xue X, Piao JH, Nakajima A, Sakon-Komazawa S, Kojima Y, Mori K, Yagita H, Okumura K, Harding H, Nakano H. Tumor necrosis factor alpha (TNFalpha) induces the unfolded protein response (UPR) in a reactive oxygen species (ROS)-dependent fashion, and the UPR counteracts ROS accumulation by TNFalpha. J Biol Chem 280: 33917–33925, 2005 [DOI] [PubMed] [Google Scholar]
- 83. Yam GH, Gaplovska-Kysela K, Zuber C, Roth J. Sodium 4-phenylbutyrate acts as a chemical chaperone on misfolded myocilin to rescue cells from endoplasmic reticulum stress and apoptosis. Invest Ophthalmol Vis Sci 48: 1683–1690, 2007 [DOI] [PubMed] [Google Scholar]
- 84. Yang WW, Shu B, Zhu Y, Yang HT. E2F6 inhibits cobalt chloride-mimetic hypoxia-induced apoptosis through E2F1. Mol Biol Cell 19: 3691–3700, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Yokouchi M, Hiramatsu N, Hayakawa K, Okamura M, Du S, Kasai A, Takano Y, Shitamura A, Shimada T, Yao J, Kitamura M. Involvement of selective reactive oxygen species upstream of proapoptotic branches of unfolded protein response. J Biol Chem 283: 4252–4260, 2008 [DOI] [PubMed] [Google Scholar]
- 86. Yoshida H, Oku M, Suzuki M, Mori K. pXBP1(U) encoded in XBP1 pre-mRNA negatively regulates unfolded protein response activator pXBP1(S) in mammalian ER stress response. J Cell Biol 172: 565–575, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Zinszner H, Kuroda M, Wang X, Batchvarova N, Lightfoot RT, Remotti H, Stevens JL, Ron D. CHOP is implicated in programmed cell death in response to impaired function of the endoplasmic reticulum. Genes Dev 12: 982–995, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]