Abstract
Recent studies have shown that overexpression of the transmembrane protein Zrt- and Irt-like protein 14 (Zip14) stimulates the cellular uptake of zinc and nontransferrin-bound iron (NTBI). Here, we directly tested the hypothesis that Zip14 transports free zinc, iron, and other metal ions by using the Xenopus laevis oocyte heterologous expression system, and use of this approach also allowed us to characterize the functional properties of Zip14. Expression of mouse Zip14 in RNA-injected oocytes stimulated the uptake of 55Fe in the presence of l-ascorbate but not nitrilotriacetic acid, indicating that Zip14 is an iron transporter specific for ferrous ion (Fe2+) over ferric ion (Fe3+). Zip14-mediated 55Fe2+ uptake was saturable (K0.5 ≈ 2 μM), temperature-dependent (apparent activation energy, Ea = 15 kcal/mol), pH-sensitive, Ca2+-dependent, and inhibited by Co2+, Mn2+, and Zn2+. HCO3− stimulated 55Fe2+ transport. These properties are in close agreement with those of NTBI uptake in the perfused rat liver and in isolated hepatocytes reported in the literature. Zip14 also mediated the uptake of 109Cd2+, 54Mn2+, and 65Zn2+ but not 64Cu (I or II). 65Zn2+ uptake also was saturable (K0.5 ≈ 2 μM) but, notably, the metal-ion inhibition profile and Ca2+ dependence of Zn2+ transport differed from those of Fe2+ transport, and we propose a model to account for these observations. Our data reveal that Zip14 is a complex, broad-scope metal-ion transporter. Whereas zinc appears to be a preferred substrate under normal conditions, we found that Zip14 is capable of mediating cellular uptake of NTBI characteristic of iron-overload conditions.
Keywords: cadmium transport, hereditary hemochromatosis, iron, homeostasis iron transport, Xenopus laevis oocyte, SLC39A14, thalassemia, zinc transport
iron-overload conditions (e.g., thalassemia, hereditary hemochromatosis) are characterized by the appearance in plasma of nontransferrin-bound iron (NTBI) and result in cardiomyopathy, diabetes, hepatic cancer, and cirrhosis. Identification of the routes of cellular NTBI uptake will therefore provide novel targets for therapeutics.
Zrt- and Irt-like protein-14 (Zip14) is a member of a large family of mammalian metal-ion transporters, the SLC39 gene family (6, 11, 12, 22, 26). Zip14 (synonyms SLC39A14, KIAA0062) is strongly expressed in the intestine (25, 35) but its subcellular localization there is not yet clear. Notably, Zip14 is abundantly expressed in the liver, heart, and pancreas (25, 35, 42), the major sites of organ damage in iron overload. Our previous data identified Zip14 as a candidate route for NTBI uptake since overexpression of Zip14 in human embryonic kidney (HEK)293, SF9, or HeLa cell lines stimulated NTBI uptake (14, 25), whereas small interfering RNA (siRNA) suppression of endogenous Zip14 in AML12 mouse hepatocytes decreased NTBI uptake (25).
We have expressed mouse Zip14 in RNA-injected Xenopus oocytes, an efficient heterologous expression system ideal for direct assays of membrane transport and tolerant of broad manipulation of experimental conditions. We used radiotracer assays to test the hypothesis that Zip14 transports free iron and to examine the functional properties and metal-ion substrate profile of Zip14.
MATERIALS AND METHODS
Reagents.
Restriction enzymes were obtained from New England Biolabs (Ipswich, MA). All other reagents were obtained from Sigma-Aldrich (St. Louis, MO) or Research Products International (Prospect, IL) unless otherwise indicated.
Expression of mouse Zip14 and human DMT1 in Xenopus oocytes.
We performed laparotomy and ovariectomy on adult female Xenopus laevis frogs (Nasco, Fort Atkinson, WI) under 3-aminoethylbenzoate methanesulfonate anesthesia (0.1% in 1:1 water/ice, by immersion) following a protocol approved by the University of Cincinnati Institutional Animal Care and Use Committee. Ovarian tissue was isolated and treated with collagenase A (Roche Diagnostics, Indianapolis, IN), and oocytes were isolated and stored at 17°C in modified Barths' medium as previously described (28). We expressed in oocytes the Zip14 short isoform transcript, the product of the mouse SLC39A14 gene, GenBank sequence accession number BC021530, in pCMVSport6 as previously described (25, 27, 47) under the SP6 promoter. We linearized the pCMVSport6-Zip14 construct using HpaI and synthesized RNA in vitro using the mMESSAGE mMACHINE/SP6 RNA polymerase transcription kit (Applied Biosystems/Ambion, Austin, TX) according to the manufacturers' protocols. Human divalent metal-ion transporter-1 (DMT1) isoform 1A/IRE(+) RNA was prepared as described (30, 41). Defolliculate stage V-VI oocytes were injected with 50 ng of RNA and incubated for 3–5 days (Zip14) or 6 days (DMT1) before being used in functional assays.
We also expressed in oocytes enhanced green fluorescence protein (EGFP) fusion proteins of Zip14 and DMT1. To construct NH2-terminal-EGFP-Zip14 (EGFP-Zip14) in pCMVSport6, we used forward (5′-CTGCCGCCCCTCACTAGTGCCACCTCC-3′) and reverse (5′-GGAGGTGGCACTAGTGAGGGGCGGCAG-3′) primers to amplify the EGFP sequence from pEGFP-N1 (Clontech, Mountain View, CA) flanked by SpeI restriction sites and ligated the EGFP sequence into the NH2-terminal region of pCMVSport6-Zip14 at an SpeI restriction site we created by site-directed mutagenesis. The NH2-terminal-EGFP-Zip14 sequence was then cut out and ligated into the pOX(+) oocyte expression vector (30) using KpnI and NotI. COOH-terminal-EGFP-tagged DMT1 (DMT1-EGFP), a gift of Dr. Elizabeta Nemeth and Dr. Bo Qiao (David Geffen-UCLA School of Medicine), was generated by subcloning human DMT1 isoform 1A/IRE(+) cDNA (30) into pEGFP-N3 upstream of the EGFP coding sequence, deleting the intervening 74 nucleotides by site-directed mutagenesis, and subcloning the DMT1-EGFP cDNA back into pOX(+). EGFP-tagged constructs in pOX(+) were linearized using SnaBI and RNA synthesized as before. Oocytes were injected with 50 ng of RNA and incubated 4 (EGFP-Zip14) or 6 days (DMT1-EGFP) before being used for confocal microscopy or immunoblotting of oocyte membrane fractions.
Analysis of EGFP-Zip14 and DMT1-EGFP fusion-protein expression in oocytes.
We imaged EGFP-Zip14 and DMT1-EGFP protein expression in the oocyte by using the Zeiss LSM 7 DUO confocal laser-scanning microscope (excitation at 488 nm) fitted with the EC Plan-Neofluar ×10/0.3 and LD C-Apochromat ×40/1.1 W Korr objectives to measure emission in the band 500–531 nm at a pinhole setting of 9.9 μm.
Western blot analysis of membrane fractions from oocytes expressing EGFP-Zip14 or DMT1-EGFP.
We separated the total membrane fraction from homogenates of oocytes (≈20 of each) expressing EGFP-Zip14 or DMT1-EGFP by sucrose-density fractionation as described (5), except that we added protease inhibitor cocktail set I (EMD4Biosciences, Gibbstown, NJ) to all solutions. We used the Pierce BCA protein assay kit (Thermo Scientific, Rockford, IL) to estimate protein concentration. Oocyte membrane fractions containing ∼3 μg of total protein were mixed with Laemmli buffer (1× final concentration), heated for 30 min at 37°C, and electrophoretically separated on a sodium dodecylsulfate (SDS)-polyacrylamide gel (7.5% acrylamide). Proteins from the gel were transferred to an Optitran BA-S 85 nitrocellulose blotting membrane (Whatman, Piscataway, NJ). The blot was incubated for 1 h in blocking solution [5% nonfat dry milk in Tris-buffered saline (TBS), pH 7.4, containing 0.01% Tween 20 (TBST)], followed by a 1-h incubation with a 1:5,000 dilution of anti-GFP MAb-2 mouse antibody (Thermo Scientific, Rockford, IL). The blot was washed in TBST and then incubated for 40 min with a 1:5,000 dilution of ZyMax goat anti-mouse IgG horseradish peroxidase conjugate (Invitrogen, Carlsbad, CA). After washing in TBST and TBS was completed, immunoreactivity was visualized by using SuperSignal West Pico enhanced chemiluminescent substrate (Thermo Scientific) and X-ray film. We performed reversible Ponceau staining (40) of the blot to obtain an index of protein loading in each lane. Signal intensities of the immunoreactive bands and of Ponceau staining were quantified by densitometry by using GENETOOLS software (SynGene, Frederick, MD).
Media for functional assays in oocytes.
Oocytes were superfused or incubated at room temperature (22–25°C), unless otherwise indicated, in a standard transport medium containing 98 mM NaCl, 1 mM KCl, 2 mM CaCl2, and 1 mM MgCl2, and buffered using 0–5 mM 2-(N-morpholino)ethanesulfonic acid and 0–5 mM N′,N′-diethylpiperazine (GFS Chemicals, Columbus, OH) to obtain pH 7.5 or as otherwise indicated. Media were supplemented with 1 mM l-ascorbic acid in all experiments with Fe2+ (except as indicated in Fig. 3C) and with other metals as indicated; media were supplemented with 1 mM l-histidine in experiments with copper.
Fig. 3.
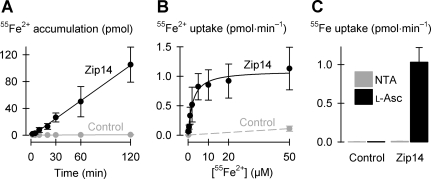
Zip14 mediates cellular uptake of free iron. A: time course of uptake of 2 μM 55Fe2+ (in the presence of 1 mM l-ascorbic acid) in control oocytes and oocytes expressing Zip14 (n = 8–11 per group). Linear regression of the data for Zip14 (black symbols and lines) yielded a slope of 0.89 ± 0.02 pmol/min and y-intercept at −1.6 ± 0.9 pmol (adjusted r2 = 1.0; P < 0.001). For control (gray symbols and lines), the regression had slope 0.005 ± 0.0002 pmol/min and y-intercept 0.02 ± 0.01 pmol (adjusted r2 = 0.99; P < 0.001). B: 55Fe2+ saturation kinetics in the range 0.2–50 μM Fe2+ in the presence of 1 mM l-ascorbic acid (n = 14–16). Data for Zip14 (black symbols and lines) were fit by Eq. 1 yielding parameters VmaxFe = 1.1 ± 0.1 pmol/min, nHFe = 1.1 ± 0.2, K0.5Fe = 2.3 ± 0.5 μM (adjusted r2 = 0.97, P < 0.001). 55Fe2+ uptake was measured at 0.2 and 50 μM Fe2+ in control oocytes (gray symbols and lines) and joined by a linear fit. C: uptake of 2 μM 55Fe from media containing 1 mM nitrilotriacetic acid (NTA) (in place of l-ascorbic acid, l-Asc) or 1 mM l-ascorbic acid in control oocytes and oocytes expressing Zip14 (n = 11–13). Two-way ANOVA revealed an interaction (P < 0.001); within NTA, Zip14 did not differ from control (unadjusted P = 0.90).
We prepared ion-substituted media as follows: 1) a bicarbonate-containing medium was prepared by substituting 30 mM NaCl with 30 mM NaHCO3 in transport medium equilibrated for 15 min with 5.6% CO2-94.4% N2 and adjusting final pH immediately after gassing; 2) a low-Cl− medium was prepared by substituting NaCl in standard transport medium (103 mM Cl−) with sodium isethionate (5 mM Cl−); and 3) low-Ca2+ or Ca2+-free media were prepared by equimolar replacement of CaCl2 with additional MgCl2.
Radiotracer transport assays in oocytes.
We used 55Fe (added as FeCl3) at final specific activity of 0.3–1.6 GBq/mg, 45Ca (added as CaCl2) at final specific activity of 130–620 MBq/mg, and 54Mn (added as MnCl2) at final specific activity of 500–550 MBq/mg, each obtained from Perkin-Elmer Life Science Products (Boston, MA); 109Cd (added as CdCl2) at final specific of activity 57–170 MBq/mg and 65Zn (added as ZnCl2) at final specific activity at 180–420 MBq/mg, obtained from the National Laboratory (Oak Ridge, TN); and 64Cu (added as CuCl2) at 1.4–4.9 GBq/mg, obtained from Washington University-St. Louis (St. Louis, MO).
We determined the time course of 2 μM 55Fe2+ accumulation between 2 min and 2 h (see Fig. 3A). In subsequent experiments, radiotracer metal-ion uptake was measured over 10 min (with the exception of metal-ion uptake in the presence of bicarbonate, measured over 2 min). We terminated radiotracer uptake by rapidly washing the oocytes three times in ice-cold pH 7.5 transport medium containing 1 mM l-ascorbic acid. Oocytes were then solubilized using 5% (wt/vol) SDS and radiotracer content assayed by liquid scintillation counting using Scintisafe-30% liquid scintillation cocktail (Fisher Scientific, Pittsburgh, PA).
Concentration-dependence data were fit by a modified Hill function (Eq. 1) for which VS is the velocity (uptake) of substrate S (55Fe2+ or 65Zn2+), VmaxS is the derived maximum velocity, S is the concentration of substrate S, K0.5S the substrate concentration at which velocity was half-maximal, and nH is the Hill coefficient for S.
| (1) |
Uptakes obtained over the range of temperatures ∼15–30°C were fit by an integrated Arrhenius function (Eq. 2), for which Ea is the apparent activation energy, A is the y-intercept, R is the universal gas constant (1.987 cal·mol−1·K), T is the absolute temperature, and V is the velocity (55Fe2+ or 65Zn2+ uptake).
| (2) |
55Fe2+ uptakes obtained over a range of extracellular Ca2+ concentrations of 0.15–6.0 mM were fit by a one-site ligand-binding function (Eq. 3) for which VFe is the velocity (55Fe2+ uptake), VmaxFe is the derived maximum velocity, [Ca2+] is the extracellular Ca2+ concentration, and apparent KdCa is the [Ca2+] at which 55Fe2+ uptake velocity was half-maximal.
| (3) |
Statistical and regression analysis.
Statistical and regression analyses were performed using SigmaPlot version 11 (Systat Software) with critical significance level α = 0.05. Radiotracer uptake data were presented as mean and standard deviation (SD) for n independent observations and analyzed using one-way or two-way ANOVA followed by pairwise multiple comparisons using the Holm-Šidák test. Data were fit by a linear function or by Eqs. 1–3 by using the least-squares method of linear or nonlinear regression followed by F-tests of the significance of the fit to the model; SE is the standard error of the estimate, and P is the significance of the fit. Where appropriate, fit parameters were compared using Student's t-test.
RESULTS
EGFP-Zip14 expression in Xenopus oocytes.
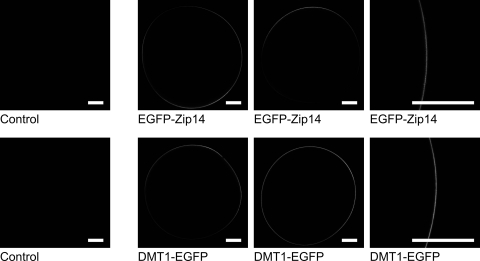
We used confocal laser-scanning microscopy to image the expression of an NH2-terminal EGFP-fusion protein of murine Zip14 (EGFP-Zip14) in RNA-injected oocytes (Fig. 1). We observed strong fluorescence throughout most of the plasma membrane but no detectable intracellular fluorescence. We also examined the expression of a COOH-terminal EGFP-fusion protein of human DMT1 isoform 1A/IRE(+) (DMT1-EGFP). DMT1-EGFP was expressed throughout the plasma membrane, consistent with the pattern of anti-DMT1 immunofluorescence (same isoform) described previously (30). We detected no fluorescence in control oocytes. Whereas the expression pattern of EGFP-Zip14 was similar to that of DMT1-EGFP, fluorescence was more intense in oocytes expressing DMT1-EGFP; however, since EGFP fluorescence varies depending on the microenvironment and steric mobility, fluorescence signals for the two fusion proteins are not directly comparable.
Fig. 1.
Imaging of enhanced green fluorescent protein (EGFP)-Zrt, Irt-like protein 14 (Zip14), and divalent metal-ion transporter 1 (DMT1)-EGFP expression in Xenopus oocytes. Confocal laser-scanning microscopy of control oocytes and oocytes expressing EGFP-Zip14 or DMT1-EGFP. Representative images are presented in which the optical slice (7.3 μm at ×10 magnification or 0.6 μm at ×40) approximately bisects the oocyte. Scale bars (white) indicate 0.2 mm.
For a semiquantitative comparison of expression levels, we used Western blot analysis (anti-GFP) of membrane fractions isolated from oocytes expressing EGFP-Zip14 or DMT1-EGFP (Fig. 2). We observed for DMT1-EGFP two strong bands at ≈80 and ≈110 kDa. The band at ≈110 kDa likely represents glycosylated DMT1 (3, 30). For EGFP-Zip14, we observed three strong bands, at ≈40, ≈80, and ≈170 kDa. The band at ≈40 kDa may represent a Zip14 monomer, precursor, or degradation product, whereas the band at ≈170 kDa likely represents an oligomer. We performed densitometric analysis of immunoreactive bands and used as an index of gel loading the densities of reversible Ponceau staining (40) of the blot (not shown). After normalizing by the amount of protein loaded (which was ∼25% more for DMT1-EGFP than for Zip14-EGFP), we found that the ratio of protein expression between DMT1-EGFP and Zip14-EGFP was 1.7. We considered the possibility that the 40-kDa Zip14-EGFP band represents a nonfunctional peptide, in which case the ratio of protein expression is 2.9; however, the 40-kDa band is immunoreactive with anti-Zip14 antibody, and we do not expect any synthesis of free EGFP in the oocyte system, so we suspect the 40-kDa band represents a degradation product. In either event, the two proteins are expressed in oocytes on the same order, DMT1-EGFP modestly higher in this preparation (see discussion for a comparison of their functional activities).
Fig. 2.
Western blot analysis of membrane fractions from Xenopus oocytes expressing EGFP-Zip14 and DMT1-EGFP by using anti-green fluorescent protein (GFP) antibody. Each lane (numbered at top) was loaded with membrane fractions (∼3 μg protein per lane) isolated from the following: lane 1, control oocytes; lanes 2 and 3, oocytes expressing DMT1-EGFP; lanes 4 and 5, DMT1; lanes 6 and 7, EGFP-Zip14; lanes 8 and 9, Zip14. Intensities of the immunoreactive bands in each lane of the Western blot were normalized by quantity of protein loaded in each lane determined by densitometric analysis of reversible Ponceau staining (40) of the blot (not shown).
Zip14 mediates cellular uptake of free iron.
We expressed murine Zip14 in RNA-injected oocytes and used radiotracer assays to characterize its functional properties. In the presence of l-ascorbic acid, expression of Zip14 stimulated up to 150-fold the uptake of 2 μM 55Fe2+ compared with that in control oocytes (Fig. 3). 55Fe2+ accumulation was linear from 2 min up to at least 2 h (Fig. 3A). Subsequent transport experiments were conducted over 10 min, within the linear phase of 55Fe2+ uptake (except see Fig. 6A). Zip14-mediated 55Fe2+ uptake was saturable (Fig. 3B); the Fe2+ concentration at which 55Fe2+ uptake was half-maximal (K0.5Fe) was 2.3 ± 0.5 μM. The Hill coefficient (nHFe) for Fe2+ was ≈1, indicating a lack of cooperativity. Whereas Zip14 readily transported Fe2+ in the presence of ascorbate, the uptake of 55Fe added as FeCl3 in the presence of the Fe(III) chelator nitrilotriacetic acid (NTA) and in the absence of an exogenous reducing agent did not differ between control oocytes and oocytes expressing Zip14 (Fig. 3C). Therefore, Zip14 transports ferrous ion (Fe2+) and not ferric ion (Fe3+).
Fig. 6.
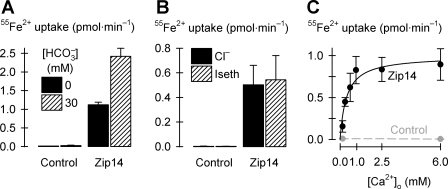
Ion dependence of Zip14-mediated 55Fe2+ transport. A: effect of bicarbonate (HCO3−) on Zip14-mediated uptake of 2 μM 55Fe2+ (n = 10–11) measured over 2 min to minimize pH changes with time; two-way ANOVA revealed an interaction (P < 0.001). B: effect of Cl− replacement with isethionate (Iseth) on Zip14-mediated uptake of 2 μM 55Fe2+ (n = 12–15); two-way ANOVA revealed no effect of Cl− replacement (P = 0.58) and no interaction (P = 0.58). C: uptake of 2 μM 55Fe2+ as a function of extracellular calcium concentration ([Ca2+]o) in oocytes expressing Zip14 (black, n = 9–11); data were fit by Eq. 3 to obtain VmaxFe = 1.0 ± 0.1 pmol/min, KdCa = 0.39 ± 0.12 mM (adjusted r2 = 0.90, P = 0.002). Data for control oocytes (gray, n = 10) were joined by a linear fit.
Properties of Zip14-mediated Fe2+ transport.
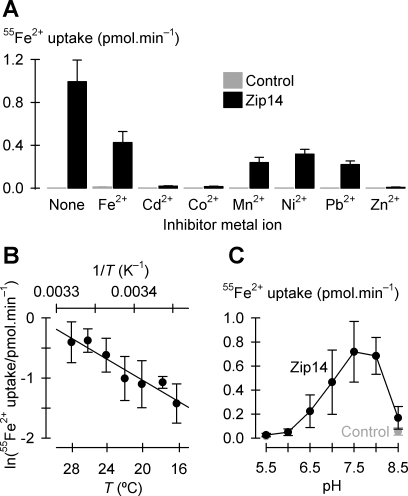
Zip14-mediated 55Fe2+ transport was inhibited by 10-fold excess concentrations of Cd2+, Co2+, Mn2+, Ni2+, Pb2+, or Zn2+ (Fig. 4A). Whereas a 10-fold excess of unlabeled Fe2+ inhibited 55Fe2+ uptake less potently (by 57 ± 7% SE) than we expected (≈90%) for a simple homogeneous system (44), Cd2+, Co2+, and Zn2+ afforded complete inhibition of 55Fe2+ transport.
Fig. 4.
Properties of Zip14-mediated Fe2+ transport. A: metal-ion inhibition profile of Zip14-mediated 55Fe2+ transport. Uptake of 2 μM 55Fe2+ in the absence (None) or presence of a range of candidate inhibitor metal ions each at 20 μM, in the presence of 1 mM l-ascorbic acid, in control oocytes (gray bars) and oocytes expressing Zip14 (black bars) (n = 10–14). Within Zip14, all metals inhibited 55Fe2+ uptake (P < 0.001). B: temperature (T) dependence of Zip14-mediated uptake of 2 μM 55Fe2+ (n = 9–13). Data were fit by Eq. 2 to obtain activation energy (Ea) = 15.2 ± 2.0 kcal/mol, ln(A) = 25.0 ± 3.5 (adjusted r2 = 0.90, P < 0.001). For clarity, control data are not displayed. C: uptake of 2 μM 55Fe2+ as a function of extracellular pH in oocytes expressing Zip14 (black symbols and line, n = 27–30). Within Zip14, uptakes at each pH differed from one another (unadjusted P < 0.007) except pH 6.5 cf. pH 8.5 (unadjusted P = 0.20), pH 7.5 cf. pH 8.0 (unadjusted P = 0.44), and pH 6.0 cf. pH 5.5 (unadjusted P = 0.54). For this experiment, uptakes in control oocytes (gray symbols and line) were tested only at pH 5.5 and 8.5 (n = 31–32). Zip14 did not differ from control at pH 5.5 (unadjusted P = 0.77) but did at pH 8.5 (unadjusted P = 0.004).
We found that Zip14-mediated 55Fe2+ transport was temperature dependent in the tested range 16–28°C (Fig. 4B). The Arrhenius plot was linear over this temperature range and the apparent activation energy (Ea) was 15 ± 2 kcal/mol. An alternative index of temperature dependence, the factor by which activity increased with every 10-degree increase in T (Q10), was 2.4 ± 0.3 (obtained from the fit parameters of a 3-parameter single-exponential growth function; adjusted r2 = 0.91, P = 0.003). Zip14-mediated 55Fe2+ transport was pH sensitive (Fig. 4C). Zip14 was active over a narrow pH range, the optimal pH was 7.5, and Zip14-mediated 55Fe2+ transport was abolished at pH 6.0 and below.
Comparison of iron-transport activities mediated by Zip14 and DMT1.
We compared 55Fe2+ transport in oocytes expressing Zip14 and DMT1. DMT1 is known to be maximally stimulated at low pH (30). The 55Fe2+ transport activity at pH 5.5 in oocytes expressing DMT1 was 1.6-fold (±0.5-fold SE) the 55Fe2+ transport activity at pH 7.5 in oocytes expressing Zip14 (Fig. 5). We verified that the 55Fe2+ transport activities of the EGFP-fusion proteins of Zip14 and DMT1 were similar to those of their nontagged counterparts (data not shown). Given the slightly higher protein levels for DMT1 compared with Zip14 (Fig. 2), these data indicate that Zip14 and DMT1 mediate similar 55Fe2+ fluxes per functional unit, i.e., the turnover rates of the transport cycle are similar.
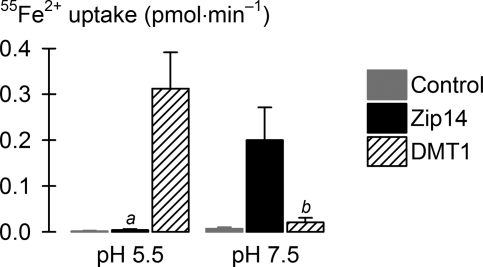
Fig. 5.
Comparison of the iron-transport activities of mouse Zip14 and human DMT1. Uptake of 2 μM 55Fe2+ was measured at pH 5.5 and 7.5 in control oocytes (gray bars) and oocytes expressing mouse Zip14 (black bars) or human DMT1 isoform 1A/IRE(+) (hatched bars) (n = 9–12). ANOVA, P < 0.001; aUnadjusted P = 0.90 cf. control at pH 5.5; bunadjusted P = 0.52 cf. control at pH 7.5; DMT1 at pH 5.5 differed from Zip14 at pH 7.5 (unadjusted P < 0.001).
Ion dependence of Zip14-mediated Fe2+ transport.
When we applied voltage-clamp protocols as described previously (30, 31, 41) in studies of DMT1, we did not observe any metal-ion-evoked currents or presteady-state currents in oocytes expressing Zip14 (data not shown). We provisionally conclude that Zip14 is not rheogenic (i.e., net charge movement is zero) and considered that Zip14-mediated Fe2+ uptake may be associated with an anion influx or countertransport of (i.e., exchange with) (a) cation(s). Since HCO3− stimulated zinc uptake via human Zip2 expressed in K562 erythroleukemia cells (13), we tested the effects of HCO3− on Zip14 activity. We found that addition of 30 mM HCO3− to the medium stimulated by 130% ± 6% (SE) the uptake of 2 μM 55Fe2+ in oocytes expressing Zip14 (Fig. 6A). In a second preparation, we found that excess Zn2+ completely inhibited Zip14-mediated 55Fe2+ transport both in the absence and presence of HCO3− (data not shown, P < 0.001); in a third preparation, excess Mn2+ inhibited Zip14-mediated 55Fe2+ transport in the absence of HCO3− (by 40% ± 11%, SE) to a similar degree to that in the presence of HCO3− (by 65% ± 5%, SE) (data not shown; P = 0.06 for absence cf. presence of HCO3−). Cl− replacement with the organic anion isethionate had no effect on Zip14-mediated 55Fe2+ uptake (Fig. 6B). These data may be interpreted in one of two ways, such that Zip14-mediated iron-transport activity 1) is stimulated by extracellular HCO3− but is not associated with an anion flux; or 2) is associated with a nonspecific anion influx and that HCO3− is a preferred anion. In any event, Zip14 does not appear to be an obligatory HCO3− cotransporter (although nominally HCO3−-free media will contain micromolar amounts of HCO3− arising from atmospheric CO2 alone).
Zip14-mediated 55Fe2+ transport was dependent on the extracellular Ca2+ concentration (Fig. 6C). 55Fe2+ transport was half-maximal at Ca2+ concentration of 0.4 ± 0.1 mM (i.e., apparent KdCa). In separate experiments (not shown), we found that the effect of raising the Ca2+ concentration from 0.3 to 2.0 mM was to increase nearly fivefold the ImaxFe for 55Fe2+ transport (P = 0.018, by Student's t-test) without effect on K0.5Fe (P = 0.96) (data were fit by Eq. 1); i.e., increasing Ca2+ accelerated iron transport without altering the affinity of Zip14 for Fe2+.
Metal-ion substrate profile of Zip14.
The ZIP (SLC39) family of transporters is known primarily for its role in zinc homeostasis (22), but some ZIP transporters are capable of also transporting other metal ions. We therefore examined the substrate profile of Zip14 by direct measurement of radiotracer uptake. As well as stimulating the uptake of 55Fe2+, expression of Zip14 in oocytes increased the uptake of 2 μM 109Cd2+, 54Mn2+, and 65Zn2+ (Fig. 7A); of these, the flux of 55Fe2+ was the greatest and 54Mn2+ the lowest. Zip14 expression did not alter the rate of 64Cu2+ uptake either in the presence or absence of l-ascorbic acid compared with control oocytes (Fig. 7B). Therefore, Zip14 is capable of transporting Fe2+, Cd2+, Mn2+, and Zn2+ but not Cu+ or Cu2+.
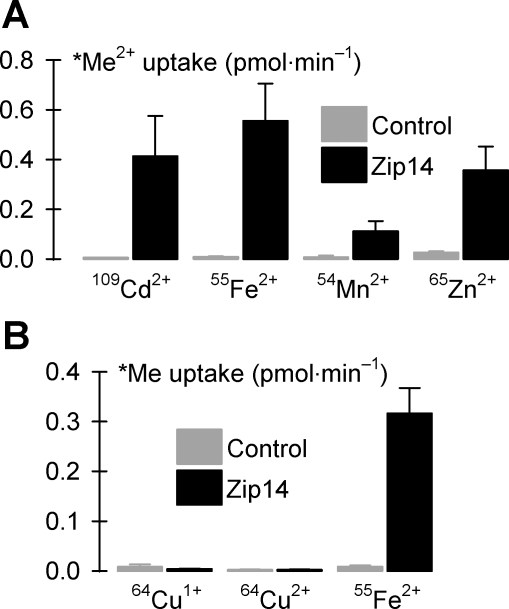
Fig. 7.
Metal-ion substrate profile of Zip14. A: uptake of radionuclide metal ions (*Me2+, each at 2 μM in the presence of 1 mM l-ascorbic acid) in control oocytes and oocytes expressing Zip14 (n = 17–27). Two-way ANOVA revealed an interaction (P < 0.001); for all metals, Zip14 differed from control (unadjusted P < 0.001); within Zip14, all metals differed from one another (109Cd2+ vs. 65Zn2+, unadjusted P = 0.039; all other pairwise comparisons, unadjusted P < 0.001). B: uptake of 2 μM 64Cu was measured in the presence of 1 mM l-histidine and in the presence (64Cu1+) or absence (64Cu2+) of 1 mM l-ascorbic acid and compared with uptake of 2 μM 55Fe2+ in the presence of 1 mM l-ascorbic acid in control oocytes and oocytes expressing Zip14 (n = 13–15). Two-way ANOVA revealed an interaction (P < 0.001); Zip14 did not differ from control for 64Cu1+ (P = 1.0) or 64Cu2+ (P = 0.56) but differed for 55Fe2+ (P < 0.001).
Properties of Zip14-mediated Zn2+ transport.
The uptake of 65Zn2+ in oocytes expressing Zip14 was saturable (Fig. 8A); the Zn2+ concentration at which 65Zn2+ uptake was half-maximal (K0.5Zn) was 1.9 ± 0.6 μM and was not significantly different from the K0.5Fe we obtained for 55Fe2+ transport (Fig. 3B) (P = 0.66, by Student's t-test). Zip14-mediated uptake of 2 μM 65Zn2+ was strongly inhibited by 10-fold excess unlabeled Zn2+ (by 88% ± 3% SE) and more weakly by Cd2+ (56% ± 3%) but was only marginally inhibited by Fe2+ (14% ± 5%) and not by any other metal ion tested (Fig. 8B). Therefore, the metal-ion inhibition profile for 65Zn2+ transport differed from that for 55Fe2+ transport.
Fig. 8.
Properties of 65Zn2+ transport. A: 65Zn2+ saturation kinetics in the range 0.1–10 μM Zn2+ (n = 10–11). Data for Zip14 (black symbols and line) were fit by Eq. 1 yielding parameters VmaxZn = 1.8 ± 0.2 pmol/min, nHZn = 0.9 ± 0.1, K0.5Zn = 1.9 ± 0.6 μM (adjusted r2 = 0.99, P < 0.001). 65Zn2+ uptake was measured at 0.1, 1.0, and 10 μM Zn2+ in control oocytes (gray symbols and line) and fit by linear regression. B: metal-ion inhibition profile of Zip14-mediated 65Zn2+ transport. Uptake of 2 μM 65Zn2+ in the absence (None) or presence of a range of candidate inhibitor metal ions each at 20 μM, in the presence of 1 mM l-ascorbic acid, in control oocytes (gray bars), and oocytes expressing Zip14 (black bars) (n = 10–14). Within Zip14, each metal ion inhibited 65Zn2+ uptake (aunadjusted P < 0.001, cunadjusted P = 0.003) except b,dnot significant (bunadjusted P = 0.87, dunadjusted P = 0.13). C: temperature dependence of Zip14-mediated uptake of 2 μM 65Zn2+ (n = 10–11). Data were fit by Eq. 2 to obtain Ea = 13.9 ± 1.8 kcal/mol, ln(A) = 24.0 ± 3.1 (adjusted r2 = 0.92, P = 0.002). For clarity, control data are not displayed. D: uptake of 2 μM 65Zn2+ as a function of extracellular pH in oocytes expressing Zip14 (black symbols and line, n = 9–13). Within Zip14, uptakes did not differ from one another within the pH ranges marked by the bars above the graph (not significant, unadjusted P > 0.006) except for pH 6.5 cf. pH 7.5 (unadjusted P < 0.001); all other pairwise comparisons, unadjusted P < 0.001. Uptakes in control oocytes (gray symbols and line) were tested only at pH 5.5 and 8.5 (n = 11–13). Zip14 did not differ from control at pH 5.5 (unadjusted P = 0.17) but did at pH 8.5 (unadjusted P < 0.001). E: uptake of 2 μM 65Zn2+ in the presence of 2 mM Ca2+ (black bars) or its absence (hatched bars) in control oocytes and oocytes expressing Zip14 (n = 32–35). Two-way ANOVA revealed a lack of interaction (P = 0.46).
We found that Zip14-mediated 65Zn2+ transport was temperature dependent in the tested range 15–30°C (Fig. 8C). The Arrhenius plot was linear over this temperature range; the apparent Ea was 14 ± 2 kcal/mol and was not significantly different from that obtained for 55Fe2+ transport (Fig. 4B) (P = 0.65, by Student's t-test). HCO3− stimulated 65Zn2+ transport in oocytes expressing Zip14 (data not shown, P < 0.001) in the same manner as it did for 55Fe2+ transport (Fig. 6A). Zip14-mediated 65Zn2+ transport was pH sensitive (Fig. 8D). The optimal pH for 65Zn2+ transport was 7.5 (as for 55Fe2+ transport, Fig. 4C); however, 65Zn2+ transport activity was observed over a broader pH range than was 55Fe2+ transport, and 65Zn2+ transport activity persisted at pH 6.0 or lower. Zip14-mediated transport of 2 μM 65Zn2+ was not affected by replacement of extracellular Ca2+ by Mg2+ (Fig. 8E). Therefore, whereas Zip14 transported Fe2+ and Zn2+ with identical K0.5 and temperature-dependence parameters, the properties of Zip14-mediated 65Zn2+ transport differed from those of 55Fe2+ transport with respect to the metal-ion inhibition profile, pH dependence, and Ca2+ dependence.
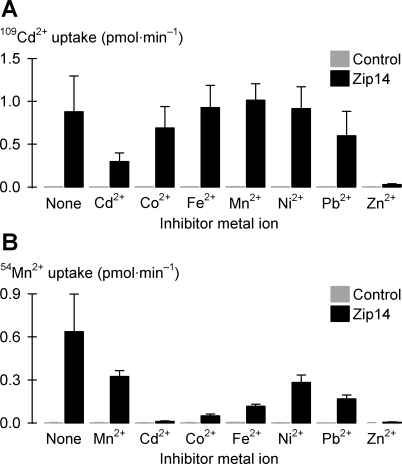
Metal-ion inhibition profiles for Zip14-mediated Cd2+ and Mn2+ transport.
Uptake of 2 μM 109Cd2+ was inhibited by 10-fold excess unlabeled Cd2+ or Zn2+ but not by any other metal ion tested (Fig. 9A). In contrast, the uptake of 2 μM 54Mn2+ was inhibited by a broad range of divalent metal ions at 10-fold excess, including Cd2+, Co2+, Fe2+, Ni2+, Pb2+, and Zn2+ (Fig. 9B). Therefore the metal-ion inhibition profile for Zip14-mediated Cd2+ transport resembles that for Zn2+ transport (Fig. 8B), whereas the metal-ion inhibition profile for Zip14-mediated Mn2+ transport resembles that for Fe2+ transport (Fig. 4A). Likewise, the pH ranges of Zip14-mediated Cd2+ and Mn2+ transport activities resembled those of Zn2+ and Fe2+ transport activities, respectively (data not shown). HCO3− stimulated Zip14-mediated 109Cd2+ transport (interaction P = 0.040) and 54Mn2+ transport (interaction P < 0.001) (data not shown) in the same manner as it did for 55Fe2+ transport (Fig. 6A); however, whereas HCO3− markedly stimulated transport of Fe2+ and Mn2+, HCO3− only modestly stimulated transport of Zn2+ and Cd2+.
Fig. 9.
Metal-ion inhibition profiles of Zip14-mediated Cd and Mn transport. A: uptake of 2 μM 109Cd2+ in the absence (None) or presence of a range of candidate inhibitor metal ions each at 20 μM, in the presence of 1 mM l-ascorbic acid, in control oocytes (gray bars), and oocytes expressing Zip14 (black bars) (n = 8–15). Within Zip14, Cd2+ and Zn2+ inhibited 109Cd2+ uptake (unadjusted P < 0.001); all other comparisons cf. “None” were not significant (unadjusted P > 0.031). B: uptake of 2 μM 54Mn2+ in the absence (None) or presence of a range of candidate inhibitor metal ions each at 20 μM, in the presence of 1 mM l-ascorbic acid, in control oocytes (gray bars), and oocytes expressing Zip14 (black bars) (n = 10–15). Within Zip14, each metal ion inhibited 54Mn2+ uptake (unadjusted P < 0.001).
Zip14-mediated Ca2+ transport and the effects of divalent metal ions.
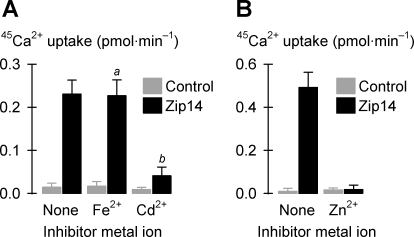
Since we found Zip14-mediated 55Fe2+ transport to be Ca2+ dependent, we examined whether Zip14 could mediate the influx of 45Ca2+ (at physiological concentrations) either in the presence or absence of divalent metal ions. To reduce background activity, we included in the media 100 μM niflumic acid since we found (in experiments not shown) that doing so reduced by 86% ± 20% (SE) the endogenous uptake of 150 μM 45Ca2+ in control oocytes. Expression of Zip14 stimulated the uptake of 45Ca2+ at 150 or 300 μM in the absence of divalent transition metal ions (Fig. 10, A and B). Notably, the 45Ca2+ fluxes at these concentrations were lower than the fluxes we had obtained for 55Fe2+ or 65Zn2+ transport at ≈1/100th the concentration (Figs. 3 and 8). 45Ca2+ transport was inhibited by lower concentrations of unlabeled Cd2+ (30 μM) or Zn2+ (15 μM) but not by Fe2+ (30 μM) (Fig. 10, A and B).
Fig. 10.
Zip14-dependent Ca2+ uptake and the effects of divalent metal ions. A: uptake of 150 μM 45Ca2+ in the absence (None) or presence of 30 μM Fe2+ or 30 μM Cd2+ in control oocytes (gray bars) and oocytes expressing Zip14 (black bars, n = 9–12) in media containing 1 mM l-ascorbic acid and 100 μM niflumic acid. Two-way ANOVA revealed an interaction (P < 0.001); within Zip14, anot significant (unadjusted P = 0.68) and bP < 0.001 cf. “None”. B: uptake of 300 μM 45Ca2+ in the absence (None) or presence of 15 μM Zn2+ in control oocytes (gray bars) and oocytes expressing Zip14 (black bars, n = 12–13) in media containing 100 μM niflumic acid. Two-way ANOVA revealed an interaction (P < 0.001).
DISCUSSION
Functional properties of Zip14 support a role in the cellular uptake of nontransferrin-bound iron.
We have examined the functional properties of mouse Zip14 expressed in RNA-injected Xenopus oocytes and found that Zip14 is capable of transporting free iron. Zip14 is specific for ferrous ion (Fe2+), whereas ferric ion (Fe3+) is not transported. It is generally considered that most NTBI is present in plasma as Fe(III)citrate; however, a significant portion of the iron at the plasma membrane is expected to be reduced since 1) plasma l-ascorbic acid concentrations are typically ≈70 μM, 2) mammalian hepatocytes (34) and other cell types (19, 20) express surface ferrireductases, and 3) citrate is capable of forming chelates of both Fe(II) and Fe(III).
Zip14-mediated 55Fe2+ uptake was saturable (K0.5Fe ≈ 2 μM), temperature-dependent (apparent activation energy, Ea = 15 kcal/mol), Ca2+ dependent (KdCa = 0.4 mM), and inhibited by Co2+, Mn2+, and Zn2+. HCO3− stimulated 55Fe2+ transport. These data agree well with those obtained from measurements of NTBI uptake in other preparations. For example, NTBI uptake in the perfused rat liver was temperature dependent (Ea = 14 kcal/mol), Ca2+ dependent (KdCa = 0.6 mM), and inhibited by Co2+, Mn2+, and Zn2 (46). The K0.5Fe obtained in the study just cited was 16 μM, higher than the K0.5Fe we obtained in oocytes expressing mouse Zip14; however, higher K0.5 estimates are expected in perfusion studies since binding by other membrane proteins cannot easily be controlled. In a second study, iron uptake in isolated rat hepatocytes was mediated by a high-affinity transport system (K0.5Fe = 1.3 μM) that was Ca2+ dependent (KdCa of 0.6–0.75 mM); however, inhibition by other divalent metal ions was not observed in that preparation (2).
Substantial increases in plasma NTBI are characteristic of iron-overload disorders (e.g., thalassemia, hereditary hemochromatosis) (1, 8, 16, 18, 37). Consistent with the functional properties of Zip14, a role for Zip14 in NTBI uptake in vivo is supported by the tissue distribution of Zip14 and its subcellular localization. Zip14 is a plasma-membrane protein (25) that is abundantly expressed in the liver, heart, and pancreas (35, 42), the three organs that preferentially accumulate iron during iron overload.
Zip14 is a complex, broad-scope metal-ion transporter.
Zip14 also mediated the uptake of 109Cd2+, 54Mn2+, and 65Zn2+ but not 64Cu (I or II). Zip14-mediated 65Zn2+ uptake in oocytes also was saturable (K0.5Zn ≈2 μM) and displayed kinetic properties reminiscent of zinc uptake in cultured rat hepatocytes (38). Notably, the properties of Zn2+ transport in oocytes expressing Zip14 differed from those of Fe2+ transport. 109Cd2+ and 65Zn2+ transport escaped inhibition by all metals except those two (Fe2+ afforded only weak inhibition of 65Zn2+ transport), whereas 55Fe2+ and 54Mn2+ transport was inhibited by every divalent metal ion tested. This observation reminds us of the importance of determining the substrate profile of transporters by directly measuring transport of each candidate substrate instead of relying on inferences from the inhibition profile for just one radiolabeled test substrate. At least one group has relied on the latter approach and interpreted the lack of inhibition of Zip14-mediated 109Cd2+ uptake by Fe2+ in viral-infected mouse fetal fibroblasts (15) to contradict our earlier observation that Zip14 could transport iron (25). Given the likelihood that Zip14 shares basic mechanistic properties in common with other members of the mammalian SLC39 family of Zip transporters, the substrate profiles of transporters within the family may not be fully represented at present. For example, the conclusions that mouse Zip1, Zip2, and human ZIP4 and ZIP5 are zinc-specific transporters based on the lack of inhibition by other metals (9, 10, 45) may need to be reexamined.
The pattern of incomplete mutual inhibition observed here between the divalent metal ions tested is not consistent with a single saturable transport process (44). Whereas Zip14-mediated 55Fe2+ transport was completely inhibited by excess Zn2+, we found that Fe2+ only marginally inhibited 65Zn2+ transport. We found that 55Fe2+ transport was Ca2+ dependent, whereas 65Zn2+ transport was not, and that Cd2+ and Zn2+, but not Fe2+, inhibited the modest 45Ca2+ fluxes observed in oocytes expressing Zip14. A model that can explain these observations is one in which there exist two metal-ion translocation pathways within Zip14. Fe2+ transport via the first of these translocation pathways is dependent on (i.e., functionally coupled with) Ca2+ transport via the second. Mutual inhibition of Fe2+ and Mn2+ transport activities indicates that these metals share the first translocation pathway. Zn2+ and Cd2+ however are capable of coordinating with both pathways, thereby completely inhibiting Fe2+ transport both by competing for the first translocation pathway and by inhibiting the Ca2+ transport otherwise supporting Fe2+ transport. The additional ionic species that account(s) for the apparent net neutral transport activity of Zip14 are (is) not presently understood.
We provisionally conclude that Zip14 is not rheogenic (i.e., net charge movement is zero) based on the lack of any metal-ion-evoked currents in oocytes expressing Zip14. While the ratio of protein expression of DMT1-EGFP relative to Zip14-EGFP (see results and Fig. 2) was 1.7 (or 2.9 if the ≈40-kDa band for Zip14-EGFP is excluded), the ratio of their functional activities (Fig. 5) was 1.6. Therefore, the two proteins are equally active (1.7/1.6 ≈ 1) or, at most, Zip14-EGFP is 1.8 times (2.9/1.6) as active per functional unit (assuming that each operates as a monomer). Whereas DMT1 exhibits large Fe2+-evoked currents (30), Zip14 does not. Fluxes of up to 2.5 pmol/min observed in nonclamped Zip14-expressing oocytes in this study should correspond to a pure divalent metal-ion current of −8 nA (converted using the Faraday) over an order of magnitude greater than the noise in the voltage clamp (≈0.5 nA root mean square), and much larger currents would be expected under voltage clamp at −70 mV. In a recent study (24), investigators reconstituted a bacterial homolog ZIPB into liposomes and observed a nonsaturable, voltage-dependent channel-like activity for ZIPB. However, bacterial ZIPB shares with mouse Zip14 only 15% identity at the amino acid level (pairwise alignment using VectorNTI software, Invitrogen, Carlsbad, CA), and ZIPB differs from Zip14 with respect to nearly every property tested, including its pH dependence, temperature dependence, saturation kinetics, substrate profile, and anion dependence (24), providing no basis for anticipating that ZIPB and Zip14 share a common mechanism. Moreover, currents observed for ZIPB were specific for zinc and were obtained with millimolar zinc concentrations and in the absence of most physiological ions (24).
Two murine models of the acute-phase response have demonstrated the inducibility of Zip14 in the liver (27). IL-6, IL-1β, and nitric oxide are among the mediators of Zip14 expression in hepatocytes and lead to Zip14-dependent zinc accumulation (23, 27). ChIP assays have shown AP-1 association with the Zip14 promoter and Zip14 hnRNA, indicative of transcription, is increased by nitric oxide (23). Under such conditions there is an increase in Zip14 associated with the plasma membrane. These responses have been interpreted as physiological homeostatic responses to stresses and infections. How such responses are factored into pathophysiological outcomes, such as iron-overload disorders, warrants investigation.
pH dependence of Zip14-mediated Fe2+ transport and its implications for cell-specific iron and zinc transport.
Our data indicated that Zip14 transports iron within a narrow pH range (pH ≥ 6.5) and optimally at pH ≈ 7.5. We therefore expect that wherever Zip14 is expressed on plasma membranes, such as is observed in hepatocytes (25), this transporter should be capable of mediating cellular uptake of NTBI characteristic of iron-overload conditions. The observation that zinc potently inhibits iron uptake suggests that Zip14 expressed at the plasma membrane should primarily serve zinc transport under normal conditions.
Zip14 may also participate in transferrin-associated iron uptake in hepatocytes (47) by mobilizing iron from early endosomes to cytoplasm. Suppression of Zip14 expression by siRNA resulted in the inhibition of iron assimilation in HepG2 cells by ∼50% (47). Iron is liberated from the transferrin-transferrin receptor complex after only relatively modest endosomal acidification (50% dissociation at pH ≈ 6.5; Ref. 36), and we expect Zip14 to be functional, albeit suboptimally, at pH 6.5. In contrast, DMT1, which is H+ coupled and maximally stimulated at low pH (Fig. 5) (30, 31), may serve as the predominant route of iron mobilization from late endosomes and lysosomes, and DMT1 is essential for erythroid iron assimilation (17).
Our previous study indicated that, in mice, Zip14 is most highly expressed in the small intestine among the tissues tested (25). Although its plasma-membrane localization and apical/basolateral distribution in intestine is yet to be established, Zip14 could play a role in zinc and iron acquisition in the neonate before the onset of expression of intestinal Na+/H+ exchangers (4) around weaning. Thereafter, the maturation of the acidic microclimate (≈pH 6.0) at the mammalian intestinal brush border (32, 33) should be expected to suppress Zip14 iron-transport activity (see Figs. 4C and 5). In contrast, DMT1, the principal mechanism by which nonheme iron is taken up at the intestinal brush border (reviewed in Refs. 21 and 29), is required to maintain iron homeostasis in the adult (17) but is not essential for iron acquisition in the suckling mammal (43). Since Zip14-mediated Zn2+ transport was active over a broader pH range (see Fig. 8D) than was Fe2+ transport, Zip14 may also contribute to intestinal Zn2+ transport in the adult; however, its activity is not sufficient to compensate for loss of ZIP4 in acrodermatitis enteropathica (10). This zinc malabsorption disorder is corrected with oral zinc therapy, demonstrating the ability of other transporters to supply essential amounts of zinc during diminished ZIP4 transport activity, and zinc absorption appears to be served by a multiplicity of transporters (7, 22).
In conclusion, our study reveals that Zip14 is a complex metal-ion transporter whose broad substrate profile includes Cd2+, Fe2+, Mn2+, and Zn2+. Whereas Zip14 may serve to transport Fe2+ out of transferrin-containing endosomes in hepatocytes (47), we speculate that Zn2+ would be the predominant physiological substrate of Zip14 wherever it is expressed at the plasma membrane. Zip14 efficiently transported Cd2+, so Zip14 should be considered a candidate mechanism of cellular uptake in cadmium exposure. Our observation of incomplete mutual inhibition for Zip14 raises the possibility that additional iron transporters may be hiding in the ZIP (SLC39) family of metal-ion transporters.
GRANTS
This study was supported by Grants R01 DK-080047 (to B. Mackenzie), R01 DK-080706 (to M. D. Knutson), R01 DK031127 (to R. J. Cousins), and P30 DK-078392 (Digestive Health Center, Cincinnati Children's Hospital and University of Cincinnati) from the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) and by the University of Cincinnati (to B. Mackenzie). The production of copper-64 at Washington University-St. Louis School of Medicine is supported by Grant R24 CA086307 from the National Cancer Institute (NCI). The content of this study is solely the responsibility of the authors and does not necessarily represent the official views of the NCI, NIDDK, or the National Institutes of Health.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
ACKNOWLEDGMENTS
We thank Sarah R. Anthony, Jesse M. Ewald, and Yossief Haileab (University of Cincinnati) for help in the laboratory, and Drs. Elizabeta Nemeth and Bo Qiao (David Geffen-UCLA School of Medicine) for graciously providing the EGFP-tagged DMT1.
This work was presented in part at Experimental Biology, April 18–22, 2009 at New Orleans, LA (39).
Present address of N. Zhao: Dept. of Cell and Developmental Biology, Oregon Health & Science Univ., Portland, OR 97239. Present address of J. P. Liuzzi: Dept. of Dietetics and Nutrition, Robert Stempel School of Public Health and Social Work, Florida International Univ., Miami, FL 33199.
REFERENCES
- 1. Andrews NC. Disorders of iron metabolism. N Engl J Med 341: 1986–1995, 1999 [DOI] [PubMed] [Google Scholar]
- 2. Barisani D, Berg CL, Wessling-Resnick M, Gollan JL. Evidence for a low Km transporter for nontransferrin-bound iron in isolated rat hepatocytes. Am J Physiol Gastrointest Liver Physiol 269: G570–G576, 1995 [DOI] [PubMed] [Google Scholar]
- 3. Canonne-Hergaux F, Zhang AS, Ponka P, Gros P. Characterization of the iron transporter DMT1 (NRAMP2/DCT1) in red blood cells of normal and anemic mk/mk mice. Blood 98: 3823–3830, 2001 [DOI] [PubMed] [Google Scholar]
- 4. Collins JF, Xu H, Kiela PR, Zeng J, Ghishan FK. Functional and molecular characterization of NHE3 expression during ontogeny in rat jejunal epithelium. Am J Physiol Cell Physiol 273: C1937–C1946, 1997 [DOI] [PubMed] [Google Scholar]
- 5. Colman A. Translation of eukaryotic messenger RNA in Xenopus oocytes. In: Transcription and Translation, edited by Hames BD, Higgins SJ. Oxford: IRL, p 271–302, 1984 [Google Scholar]
- 6. Cousins RJ, Liuzzi JP, Lichten LA. Mammalian zinc transport, trafficking, and signals. J Biol Chem 281: 24085–24089, 2006 [DOI] [PubMed] [Google Scholar]
- 7. Cragg RA, Christie GR, Phillips SR, Russi RM, Kury S, Mathers JC, Taylor PM, Ford D. A novel zinc-regulated human zinc transporter, hZTL1, is localized to the enterocyte apical membrane. J Biol Chem 277: 22789–22797, 2002 [DOI] [PubMed] [Google Scholar]
- 8. de Valk B, Addicks MA, Gosriwatana I, Lu S, Hider RC, Marx JJ. Non-transferrin-bound iron is present in serum of hereditary haemochromatosis heterozygotes. Eur J Clin Invest 30: 248–251, 2000 [DOI] [PubMed] [Google Scholar]
- 9. Dufner-Beattie J, Langmade SJ, Wang F, Eide D, Andrews GK. Structure, function, and regulation of a subfamily of mouse zinc transporter genes. J Biol Chem 278: 50142–50150, 2003 [DOI] [PubMed] [Google Scholar]
- 10. Dufner-Beattie J, Wang F, Kuo YM, Gitschier J, Eide D, Andrews GK. The acrodermatitis enteropathica gene ZIP4 encodes a tissue-specific, zinc-regulated zinc transporter in mice. J Biol Chem 278: 33474–33481, 2003 [DOI] [PubMed] [Google Scholar]
- 11. Eide DJ. The SLC39 family of metal ion transporters. Pflügers Arch 447: 796–800, 2004 [DOI] [PubMed] [Google Scholar]
- 12. Eng BH, Guerinot ML, Eide D, Saier MH. Sequence analyses and phylogenetic characterization of the ZIP family of metal ion transport proteins. J Membrane Biol 166: 1–7, 1998 [DOI] [PubMed] [Google Scholar]
- 13. Gaither LA, Eide DJ. Functional expression of the human hZIP2 zinc transporter. J Biol Chem 275: 5560–5564, 2000 [DOI] [PubMed] [Google Scholar]
- 14. Gao J, Zhao N, Knutson MD, Enns CA. The hereditary hemochromatosis protein, HFE, inhibits iron uptake via down-regulation of Zip14 in HepG2 cells. J Biol Chem 283: 21462–21468, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Girijashanker K, He L, Soleimani M, Reed JM, Li H, Liu Z, Wang B, Dalton TP, Nebert DW. Slc39a14 gene encodes ZIP14, a metal/bicarbonate symporter: similarities to the ZIP8 transporter. Mol Pharmacol 73: 1413–1423, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Grootveld M, Bell JD, Halliwell B, Aruoma OI, Bomford A, Sadler PJ. Non-transferrin-bound iron in plasma or serum from patients with idiopathic hemochromatosis. Characterization by high performance liquid chromatography and nuclear magnetic resonance spectroscopy. J Biol Chem 264: 4417–4422, 1989 [PubMed] [Google Scholar]
- 17. Gunshin H, Fujiwara Y, Custodio ÁO, Direnzo C, Robine S, Andrews NC. Slc11a2 is required for intestinal iron absorption and erythropoiesis but dispensable in placenta and liver. J Clin Invest 115: 1258–1266, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Hershko C, Graham G, Bates GW, Rachmilewitz EA. Non-specific serum iron in thalassaemia: an abnormal serum iron fraction of potential toxicity. Br J Haematol 40: 255–263, 1978 [DOI] [PubMed] [Google Scholar]
- 19. Inman RS, Coughlan MM, Wessling-Resnick M. Extracellular ferrireductase activity of K562 cells is coupled to transferrin-independent iron transport. Biochemistry 33: 11850–11857, 1994 [DOI] [PubMed] [Google Scholar]
- 20. Jordan I, Kaplan J. The mammalian transferrin-independent iron transport system may involve a surface ferrireductase activity. Biochem J 302: 875–879, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Knutson MD. Iron-sensing proteins that regulate hepcidin and enteric iron absorption. Ann Rev Nutr 30: 149–171, 2010 [DOI] [PubMed] [Google Scholar]
- 22. Lichten LA, Cousins RJ. Mammalian zinc transporters: nutritional and physiologic regulation. Ann Rev Nutr 29: 153–176, 2009 [DOI] [PubMed] [Google Scholar]
- 23. Lichten LA, Liuzzi JP, Cousins RJ. Interleukin-1β contributes via nitric oxide to the upregulation and functional activity of the zinc transporter Zip14 (Slc39a14) in murine hepatocytes. Am J Physiol Gastrointest Liver Physiol 296: G860–G867, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Lin W, Chai J, Love J, Fu D. Selective electrodiffusion of zinc ions in a Zrt-, Irt-like protein, ZIPB. J Biol Chem 285: 39013–39020, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Liuzzi JP, Aydemir F, Nam H, Knutson MD, Cousins RJ. Zip14 (Slc39a14) mediates non-transferrin-bound iron uptake into cells. Proc Natl Acad Sci USA 103: 13612–13617, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Liuzzi JP, Cousins RJ. Mammalian zinc transporters. Ann Rev Nutr 24: 151–172, 2004 [DOI] [PubMed] [Google Scholar]
- 27. Liuzzi JP, Lichten LA, Rivera S, Blanchard RK, Aydemir TB, Knutson MD, Ganz T, Cousins RJ. Interleukin-6 regulates the zinc transporter Zip14 in liver and contributes to the hypozincemia of the acute-phase response. Proc Natl Acad Sci USA 102: 6843–6848, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Mackenzie B. Selected techniques in membrane transport. In: Biomembrane Transport, edited by Van W, inkle LJ. San Diego, CA: Academic, 1999, p. 327–342 [Google Scholar]
- 29. Mackenzie B, Garrick MD., II Iron Imports. Iron uptake at the apical membrane in the intestine. Am J Physiol Gastrointest Liver Physiol 289: G981–G986, 2005 [DOI] [PubMed] [Google Scholar]
- 30. Mackenzie B, Takanaga H, Hubert N, Rolfs A, Hediger MA. Functional properties of multiple isoforms of human divalent metal-ion transporter 1 (DMT1). Biochem J 403: 59–69, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Mackenzie B, Ujwal ML, Chang MH, Romero MF, Hediger MA. Divalent metal-ion transporter DMT1 mediates both H+-coupled Fe2+ transport and uncoupled fluxes. Pflügers Arch 451: 544–558, 2006 [DOI] [PubMed] [Google Scholar]
- 32. McEwan GTA, Daniel H, Fett C, Burgess MN, Lucas ML. The effect of Escherichia coli STa enterotoxin and other secretagogues on mucosal surface pH of rat small intestine in vivo. Proc R Soc Lond B Biol Sci 234: 219–237, 1988 [DOI] [PubMed] [Google Scholar]
- 33. McEwan GTA, Lucas ML, Mathan VI. A combined TDDA-PVC pH and reference electrode for use in the upper small intestine. J Med Eng Tech 14: 16–20, 1990 [DOI] [PubMed] [Google Scholar]
- 34. Moridani MY, O'Brien PJ. Iron complexes of deferiprone and dietary plant catechols as cytoprotective superoxide radical scavengers. Biochem Pharmacol 62: 1579–1585, 2001 [DOI] [PubMed] [Google Scholar]
- 35. Nomura N, Nagase T, Miyajima N, Sazuka T, Tanaka A, Sato S, Seki N, Kawarabayasi Y, Ishikawa K, Tabata S. Prediction of the coding sequences of unidentified human genes. II. The coding sequences of 40 new genes (KIAA0041-KIAA0080) deduced by analysis of cDNA clones from human cell line KG-1. DNA Res 1: 223–229, 1994 [DOI] [PubMed] [Google Scholar]
- 36. Núñez MT, Gaete V, Watkins JA, Glass J. Mobilization of iron from endocytic vesicles: the effects of acidification and reduction. J Biol Chem 265: 6688–6692, 1990 [PubMed] [Google Scholar]
- 37. Olivieri NF. Transfusional iron overload. In: Molecular and Cellular Iron Transport, edited by Templeton DM. New York: Marcel-Dekker, 2002, p. 725–747 [Google Scholar]
- 38. Pattison SE, Cousins RJ. Kinetics of zinc uptake and exchange by primary cultures of rat hepatocytes. Am J Physiol Endocrinol Metab 250: E677–E685, 1986 [DOI] [PubMed] [Google Scholar]
- 39. Pinilla Tenas JJ, Sparkman BK, Illing AC, Liuzzi JP, Cousins RJ, Knutson MD, Mackenzie B. Properties of the zinc transporter ZIP14 suggest a role in cellular uptake of nontransferrin-bound iron (NTBI) characteristic of iron-overload conditions (Abstract). FASEB J 23: 975.1, 2009 [Google Scholar]
- 40. Romero-Calvo I, Ocón B, Martínez-Moya P, Suárez MD, Zarzuelo A, Martínez-Augustin O, Sánchez de Medina F. Reversible Ponceau staining as a loading control alternative to actin in Western blots. Anal Biochem 401: 318–320, 2010 [DOI] [PubMed] [Google Scholar]
- 41. Shawki A, Mackenzie B. Interaction of calcium with the human divalent metal-ion transporter-1. Biochem Biophys Res Commun 393: 471–475, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Taylor KM, Morgan HE, Johnson A, Nicholson RI. Structure-function analysis of a novel member of the LIV-1 subfamily of zinc transporters, ZIP14. FEBS Lett 579: 427–432, 2005 [DOI] [PubMed] [Google Scholar]
- 43. Thompson K, Molina RM, Donaghey T, Brain JD, Wessling-Resnick M. Iron absorption by Belgrade rat pups during lactation. Am J Physiol Gastrointest Liver Physiol 293: G640–G644, 2007 [DOI] [PubMed] [Google Scholar]
- 44. Van Winkle LJ. Transport kinetics. In: Biomembrane Transport, edited by Van W, inkle LJ. San Diego, CA: Academic, 1999, p. 65–131 [Google Scholar]
- 45. Wang F, Kim BE, Petris MJ, Eide DJ. The mammalian Zip5 protein is a zinc transporter that localizes to the basolateral surface of polarized cells. J Biol Chem 279: 51433–51441, 2004 [DOI] [PubMed] [Google Scholar]
- 46. Wright TL, Brissot P, Ma WL, Weisiger RA. Characterization of non-transferrin-bound iron clearance by rat liver. J Biol Chem 261: 10909–10914, 1986 [PubMed] [Google Scholar]
- 47. Zhao N, Gao J, Enns CA, Knutson MD. ZRT/IRT-like protein 14 (ZIP14) promotes the cellular assimilation of iron from transferrin. J Biol Chem 285: 32141–32150, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]