Abstract
Activated neutrophils interacting with the vessel wall can alter vascular permeability to macromolecules such as albumin via release of various secretion products that induce changes in the endothelial monolayer. In the current work we used cremaster microvessels of anesthetized mice to show that, in addition to this paracrine mechanism, leukocyte ligation of endothelial ICAM-1 directly activates endothelial cell (EC) signaling, altering EC permeability to albumin [i.e., solute permeability (Ps)]. We show that antibody cross-linking of surface ICAM-1 in intact microvessels is sufficient to increase Ps even in the absence of interacting leukocytes. Unstimulated arterioles do not support leukocyte-EC interactions, but despite this, antibody ligation of ICAM-1 in these vessels induced a twofold increase in Ps. Similarly, in venules that were depleted of interacting neutrophils, Ps was decreased to below resting levels and was restored by ligation of ICAM-1. Use of function-blocking antibodies to separately block leukocyte rolling or adhesion under unstimulated or TNF-α-activated conditions established that both rolling and adhered leukocytes contribute to Ps regulation in situ. Both rolling and adhesion activated EC-dependent signaling mechanisms that increased Ps. ICAM-1 ligation with primary antibody alone or primary followed by secondary antibodies showed that regulation of Ps is directly dependent on the degree of ICAM-1 clustering. Under physiological versus inflamed conditions, respectively, this ICAM-1 clustering-dependent regulation of Ps switches from PKC dependent and Src independent to Src dependent and PKC independent. This study thus identifies a new mechanism by which antiadhesion treatment may constitute a potential therapy for tissue edema.
Keywords: leukocyte-endothelial signaling, adhesion molecules, microvessel barrier function
inflammatory disorders are characterized by changes in vascular permeability that encompass both accommodation of leukocyte transmigration (23), and regulation of solute and water exchange (7, 29). The ability of leukocytes to alter vascular permeability to water and key macromolecules such as albumin has come into focus recently and has been elegantly summarized in recent reviews (10, 28). Thus, leukocyte-dependent alterations in barrier function can occur in a paracrine fashion, i.e., result from release of materials by leukocytes upon activation. For example, neutrophil secretion products such as heparin-binding protein (CAP37) (16) and neutrophil elastase (18) can induce changes in permeability. Similarly, other leukocyte-derived products such as leukotrienes (4, 32), reactive oxygen species (52), and TNF-α (12, 13, 17, 39) affect vascular permeability under various conditions. However, in addition to release of paracrine products from activated leukocytes, there is emerging evidence indicating that leukocyte-endothelial cell (EC) interactions can alter vessel permeability by directly inducing EC-dependent changes. One key molecule that has been implicated in this process is ICAM-1 (39).
ICAM-1 is a transmembrane glycoprotein with five extracellular IgG-like domains and a short cytoplasmic tail. While the extracellular domains of ICAM-1 directly bind β2-integrins on leukocytes, thus physically mediating both leukocyte adhesion in venules (41, 50) and leukocyte rolling in arterioles (42), the intracellular tail of ICAM-1 is associated with cytoskeletal proteins (2, 45) and plays an important role in signal transduction leading to leukocyte transmigration (41, 45, 50). Leukocyte engagement of surface ICAM-1 leads to EC cytoskeletal and junctional reorganization (48), which is mediated by key players such as myosin light chain kinase and Src family kinases (11, 48), Rho family of monomeric GTPases (19), and junctional VE-cadherin (1, 47). Thus, activation of these EC signaling pathways downstream of ICAM-1 engagement by leukocytes implies that there is a direct role for leukocyte adhesive interactions with ECs in regulation of the barrier function of the vascular wall. Indeed, we have recently confirmed this by demonstrating that the TNF-α-induced increase in microvascular permeability to albumin [i.e., solute permeability (Ps)] is ICAM-1 dependent and is contingent on ICAM-1 interactions with CD18 in both venules and arterioles (39). Moreover, in an earlier study (42) we showed that, in arterioles, the interactions between ICAM-1 and CD18 were directly involved in mediating leukocyte rolling, thus suggesting that leukocyte rolling, in addition to leukocyte adhesion, might also contribute to Ps regulation. These studies also emphasize the ability of the arteriolar microcirculation to respond to proinflammatory stimuli, thus contributing, in concert with the venular microcirculation, to the regulation of tissue homeostasis.
Engagement of β2-integrins also triggers intracellular signaling in leukocytes, leading to release of paracrine products that alter vascular permeability. For example, ligation of lymphocyte function–associated antigen 1 (LFA-1) induces neutrophil degranulation and secretion of heparin-binding protein (15, 16), which can alter vascular permeability by binding to endothelial ligands (16). In our current study, however, we provide evidence that ligation of endothelial ICAM-1 by interacting leukocytes directly modulates vascular permeability independently of agents released from circulating leukocytes. We thus extend the idea that endothelium actively participates in regulation of vessel barrier function upon leukocyte ligation of adhesion molecules on the EC surface.1
To do this, we measured Ps in intact blood-perfused microvessels where, before Ps measurements, leukocyte-EC interactions could be altered and quantified in the same vessels. We were able to compare Ps in unstimulated and TNF-α-activated arterioles and venules and identify the contribution of both leukocyte rolling and adhesion to Ps regulation. We show that differences in the degree of ICAM-1 cross-linking lead to activation of different downstream signaling pathways, and that in unstimulated and activated tissue, respectively, PKC- and Src-dependent signaling mechanisms are involved downstream of ICAM-1 in the regulation of vessel permeability to albumin. Importantly, we provide evidence that ligation of endothelial ICAM-1 by interacting leukocytes directly modulates vascular permeability independently of agents released from circulating leukocytes, as we show that antibody ligation of ICAM-1 in the absence of circulating leukocytes and their secretion products is sufficient to induce changes in Ps.
MATERIALS AND METHODS
Animal preparation.
All procedures were approved by the Institutional Review Board of the University of Rochester. Male wild-type (WT) (C57BL6J; Jackson Laboratories), or TNF-α receptor 1 knockout mice (TNFR1 KO) (Tnfrsf1atm1Imx/J; Jackson Laboratories, or a gift from G. Pryhuber, University of Rochester) aged 12–15 wk old were initially anesthetized with pentobarbital sodium (65 mg/kg ip) and maintained throughout the experiment as described elsewhere (25, 43). The cremaster muscle was exteriorized and gently pinned over a quartz pedestal for visualization by confocal intravital microscopy (25, 35). During preparation and observation, the tissue was continuously superfused with warmed physiological salt solution (PSS) with the following composition (in mM): 131.9 NaCl, 4.7 KCl, 2.0 CaCl2, 1.2 MgSO4, 18 NaHCO3, pH 7.4 at 36°C, and equilibrated with gas containing 0% O2, 5% CO2, and 95% N2 to maintain tissue Po2 <15 torr (34). A total of 116 mice, weight ranging from 22–29 g, were used for this study, with groups divided as indicated in figures and figure legends. Upon completion of the protocol, the animal was euthanized by anesthetic overdose.
Confocal intravital microscopy.
Observations were made using an Olympus BX61WI microscope through an Olympus PlanF1 immersion objective (×10, 0.65 numerical aperture), allowing spatial resolution of 1.8 μm. All observed microvessels ranged from 30 to 80 μm in diameter. Leukocytes interacting with ECs were observed using brightfield images that were acquired via a charge-coupled device (CCD) camera (Dade MTI CD72, DageMTI, Michigan City, IN). To measure Ps, fluorescence images were acquired by illuminating the tissue with a 50 mW argon laser and imaging with a Nipkow disk confocal head (CSU 10, Yokogawa Yokogawa Electric, Tokyo, Japan) attached to an intensified CCD camera (XR Mega 10, Stanford Photonics, Palo Alto, CA): laser power and camera gain settings were unchanged throughout all the experiments, as previously described (26, 35, 43). All images were recorded using a DVD recorder (SONY DVO100MD) at 30 frames per second for offline analysis.
Drug application.
To induce local inflammation, recombinant mouse TNF-α (0.5 μg TNF-α in 0.25 ml saline, Sigma-Aldrich) was injected intrascrotally 3 h before the start of the surgical preparation. Observation of the selected microvessels was made between 4 and 5 h after the TNF-α injection. Blocking antibodies (P-selectin, RB40.34, 30 μg in 100 μl PBS, BD Pharmingen; CD18, GAME-46, BD Pharmingen, 100 μg in 100 μl PBS and rat IgG control isotype R3–34, BD Pharmingen, 100 μg in 100 μl PBS), as well as ICAM-1 ligating antibody (YN/1.7.4, 100 μg in 100 μl PBS and rat IgG2b control isotype, 100 μg in 100 μl PBS, Ebioscience) were administered intravenously via a second catheter inserted into the jugular vein. Data were collected 30 min after antibody administration. PKC blocker bisindolylmaleimid l (BIM, 1 μM in 0.01% DMSO, Calbiochem) or Src blocker PP2 (2 μM in 0.01% DMSO, Calbiochem) was added for 10 min to the superfusion solution which was applied onto the tissue.
Permeability measurements.
Albumin permeability of intact cremaster muscle microvessels was measured using the approach previously published from our laboratory and detailed in references (35, 39). Briefly, fluorescence intensity from the selected microvessel and surrounding tissue was measured and used to calculate the solute flux (Js) per unit surface area (S) and concentration gradient (ΔC) using the relation: Ps = Js/SΔC = 1/ΔI0(dIf/dt)i(D/4), where ΔI0 is the fluorescence intensity of the test solute filling the vessel, (dIf/dt) is the initial change in fluorescence intensity in the measured conditions as labeled BSA moves across the vessel wall, and D is the microvessel diameter (20). The vessel surface area and volume that act as a source of BSA-488 were corrected from that of a cylindrical blood vessel to the relevant confocal slice (15 μm depth for the ×10 objective) as described earlier (35). For permeability measurements, a microvessel upstream of the targeted vessels was cannulated with a micropipette and perfused with 10 mg/ml BSA in PSS in which 10% was BSA conjugated with Alexa 488, as detailed previously (35). In each preparation, the targeted vessel (arteriole or venule) was visualized and recorded continuously for 3–6 s (baseline) before the BSA-488 perfusion, during 1 min perfusion, and 3–6 s after the perfusion was stopped and blood flow was reestablished. Recorded sequences were analyzed with NIH Image software (version 6.1; National Institutes of Health, Bethesda, MD). A region of interest (ROI) was identified and the total fluorescence intensity was measured for the ROI starting with baseline and throughout the minute of perfusion with BSA-488. To control for local variations in pressure and flow in the selected regions of intact network, the intensity in the vessel itself was also quantified. Measurements in vessels exhibiting changes in the fluorescence intensity during the measured period were discarded.
Leukocyte-EC interactions.
The methods to quantify leukocyte-EC interactions in-situ have been previously established in our laboratory (26, 41, 42). Briefly, rolling leukocytes were defined as any leukocytes observed translating along the vessel wall in continuous contact with the endothelium. The number of rolling leukocytes (rolling flux) on the vessel wall was calculated by counting leukocytes rolling past a line perpendicular to the vessel axis per 40-s time interval. All leukocytes that remained stationary or did not exceed a displacement of >8 μm (one leukocyte diameter) during 30 s were considered adhered. To deplete neutrophils, mice were injected with anti-GR-1 antibody (Ab), RGB-8C5 (200 μg/mouse, ip) as described elsewhere (36) 12 h before observations. This decreased the circulating neutrophil population by ∼95%, as confirmed by flow cytometry (data not shown).
In situ immunofluorescence labeling of ICAM-1.
To quantify the relative expression of ICAM-1 on the EC surface, we utilized an approach that we developed previously (26, 42, 43) to immunofluorescently label surface molecules in intact blood-perfused microvessels. Briefly, microvessels were locally cannulated with glass micropipettes and perfused with anti-ICAM-1 antibody (YN/1.7.4 conjugated to Alexa 488, BioLegend, 30 μg/ml) for 15 min. At the completion of the perfusion, blood flow was reestablished in the targeted microvascular region and the inflow arterioles were checked for tone and blood flow. To account for localized variability in the optical properties of the tissue, a second cannulation was used to perfuse the target vessels with fluorescent standard solution (0.05 mg/ml FITC-dextran in saline, 150 kDa molecular mass, Sigma-Aldrich) after the completion of acquisition protocols (26, 43); intensities are expressed relatively to the intensity of the standard solution. The linearity of fluorochrome intensity with concentration in our system was confirmed as previously described (26, 42, 43).
Statistical analysis.
Statistical tests were performed using Graphpad Prism (version 4.0) to undertake t-tests, ANOVA, linear regression, or correlation analyses as appropriate. Populations were considered to be significantly different when P < 0.05.
RESULTS
Initiation of leukocyte rolling in arterioles results in increased Ps.
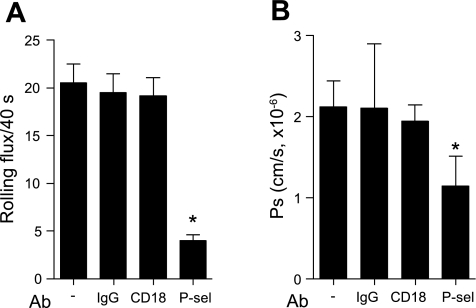
Leukocyte rolling is absent in control arterioles but is induced by TNF-α treatment (27, 42). TNF-α treatment also leads to an in increase in Ps in arterioles (39), and a significant upregulation in the surface expression of ICAM-1 in these vessels (43). Thus, here we hypothesized that ICAM-1 ligation by rolling leukocytes in arterioles is directly involved in the observed Ps increase. To test this, we used CD18 and P-selectin function-blocking Abs (GAME-46 and RB40.34, 100 μg iv), which we have shown previously (42) to attenuate leukocyte rolling in TNF-α-treated arterioles. As expected, blockade of either CD18 or P-selectin significantly decreased the number of rolling leukocytes in these arterioles (9.9 ± 0.9 and 7.1 ± 0.6 cells/40 s, respectively) compared with untreated vessels (16.2 ± 1.2 cells/40 s, Fig. 1A). In the presence of either of these blocking Abs, Ps in arterioles was also decreased (Fig. 1B) and was not significantly different from basal (unstimulated) levels (1.3 ± 0.2 × 10−6 cm/s, CD18 and 1.2 ± 0.1 × 10−6 cm/s, P-selectin), providing strong evidence that the increased Ps in TNF-α-treated arterioles was due to leukocyte rolling interactions. In combination, blockade of both CD18 and P-selectin produced a further small, but not significant, decrease in the number of rolling leukocytes and the Ps, compared with blockade of P-selectin alone.
Fig. 1.
Initiation of leukocyte rolling in arterioles results in increased solute permeability (Ps). To induce leukocyte rolling in arterioles, mice were treated with TNF-α (500 ng intrascrotally) 4 h before observations. Rat IgG control isotype (R3–34, 100 μg), anti-P-selectin (RB40.34, 30 μg), anti-CD18 (GAME-46, 100 μg), or a combination of anti-P-selectin and CD18 blocking antibodies (Abs) was injected intravenously; leukocyte rolling (A) was quantified 30 min following Ab injection, and 20 min later, Ps (B) was measured in the same vessels. Blockade of CD18 and P-selectin (separately or together) significantly reduced leukocyte rolling in arterioles and significantly reduced Ps. *Significantly different from IgG control group (P < 0.05). For all groups the measurements were made in n = 10–16 arterioles from 3–5 mice.
Leukocyte rolling interactions in unstimulated venules contribute to Ps regulation.
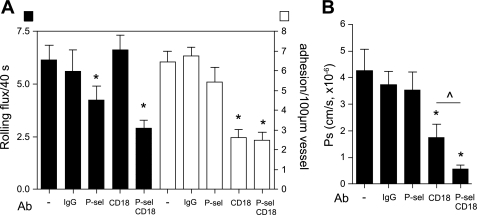
The majority of interacting leukocytes in unstimulated venules exhibit rolling interactions (42). To test the contribution of leukocyte rolling to Ps regulation in unstimulated venules, we used P-selectin function-blocking Ab (RB40.34, 100 μg iv) to block leukocyte rolling, and then measured Ps in the same vessels. We found that the significant reduction in leukocyte rolling that follows blockade of P-selectin (>4-fold decrease, Fig. 2) was accompanied by a significant decrease in Ps in these vessels (from 2.2 ± 0.46 × 10−6 cm/s, untreated, to 1.1 ± 0.4 × 10−6 cm/s, P-selectin block, Fig. 2B). Interestingly, this venular Ps in the absence of leukocyte rolling was not significantly different from Ps in unstimulated arterioles [1.0 ± 0.1 × 10−6 cm/s; (39)] which also lack leukocyte-EC interactions (42). Our finding that basal Ps is similar in both vessel types in the absence of interacting leukocytes suggests that leukocyte rolling in unstimulated venules could account for the differences in Ps between unstimulated venules and unstimulated arterioles that we showed previously (39). As expected from earlier work (39), adhesion-blocking anti-CD18 Ab had no significant effect on both leukocyte rolling and Ps in unstimulated venules (Fig. 2).
Fig. 2.
Leukocyte rolling contributes to Ps regulation in venules. Rat IgG control isotype (R3–34, 100 μg), anti-P-selectin (RB40.34, 30 μg), and anti-CD18 (GAME-46, 100 μg) blocking Abs were injected intravenously, and leukocyte rolling in unstimulated venules was quantified 30 min following Ab injection. Ps in the same vessels was measured 20 min later. Blockade of P-selectin, which mediates most of the rolling at this time point, but not CD18, significantly reduced leukocyte rolling (A) and Ps (B). *Significantly different from IgG control group (P < 0.05). For all groups the measurements were made in n = 10–13 venules from 3–5 mice.
Leukocyte adhesion in venules is required for the TNF-α-induced increase in Ps.
TNF-α treatment produces a dramatic increase in leukocyte adhesion in cremaster venules (43) as well as an increase in Ps (39). To test the hypothesis that leukocyte adhesion is required for the TNF-α-induced increase in venular Ps, we measured Ps in TNF-α-activated venules that were pretreated with CD18 blocking Ab. Confirming our earlier findings (39), blockade of CD18 significantly decreased the number of adhered leukocytes from 6.5 ± 0.5 leukocytes/100 μm to 2.9 ± 0.4 leukocytes/100 μm (Fig. 3), but it had no significant effect on the number of rolling leukocytes under these conditions (Fig. 3). Note that the number of rolling leukocytes in TNF-α-activated venules (Fig. 3A) is significantly lower compared with unstimulated venules (Fig. 2A), as the vessel wall under inflamed conditions is occupied primarily by adhered leukocytes (38). The typical TNF-α-induced increase in Ps was abolished following CD18 block (Fig. 3B), with Ps decreased to the level found in unstimulated venules, directly supporting the hypothesis that leukocyte adhesion to endothelial ICAM-1 via β2-integrins is essential for the TNF-α-induced increase in Ps. Note that because we show elsewhere in this study that anti-ICAM-1 antibodies induce signaling in ECs that leads to changes in Ps, we could not use this approach to support the data in Fig. 3. However, in previous work (39) we showed that genetic deletion of ICAM-1 (ICAM-1 KO mice) yielded similar findings to those of Fig. 3. We also asked whether the remaining leukocyte rolling in TNF-α-activated venules also contributed to Ps regulation. Blockade of P-selectin alone, while significantly decreasing leukocyte rolling as expected (from 6.2 ± 0.7 leukocytes/40 s, in untreated venules, to 4.2 ± 0.7, with P-selectin block, Fig. 3), did not significantly alter leukocyte adhesion (6.5 ± 0.5 leukocytes/100 μm, untreated venules, vs. 5.5 ± 1.0, P-selectin, Fig. 3). Ps in these vessels remained unchanged with P-selectin Ab, consistent with our hypothesis that leukocyte adhesion is essential for the TNF-α-induced, ICAM-1-mediated increase in venular Ps. Furthermore, we found that blockade of both CD18 and P-selectin together significantly attenuated both leukocyte adhesion and leukocyte rolling in the same vessel (Fig. 3A), effectively eliminating ∼90% of all leukocyte interactions and leading to a further decrease in Ps (from 4.3 ± 0.8 × 10−6 cm/s, TNF-α alone, to 0.6 ± 0.2 × 10−6 cm/s, TNF-α + CD18 + P-selectin Abs) compared with that achieved by CD18 block alone (1.8 ± 0.5 × 10−6 cm/s, Fig. 3B). Ps values under these conditions were again similar to Ps in unstimulated arterioles where neither rolling nor adhesion occurs [Fig. 4; (42)]. Thus, taken together, these data show that both rolling and adhered leukocytes contribute to Ps regulation in venules; however, leukocyte adhesion is required for the TNF-α-induced increase in Ps.
Fig. 3.
Leukocyte adhesion in venules is required for the TNF-α-induced increase in Ps. To induce inflammation, mice were treated with TNF-α (500 ng intrascrotally) 4 h before observations. Either rat IgG control isotype (R3–34, 100 μg), anti-P-selectin (RB40.34, 30 μg), anti-CD18 (GAME-46, 100 μg), or a combination of anti-P-selectin and CD18 blocking Abs was injected intravenously. Leukocyte rolling (A, black bars) and adhesion (A, white bars) were quantified in activated venules 30 min after Ab injection. Ps (B) in the same vessels was measured 20 min later. As expected, leukocyte rolling and adhesion were significantly decreased by anti-P-selectin and anti-CD18 Abs, respectively. Ab block of adhesion with CD18 significantly decreased Ps to levels observed in unstimulated venules (Fig. 2); blockade of the residual rolling in these vessels using P-selectin Ab in combination with anti-CD18 Ab decreased Ps further to a level that is expected for basal Ps in ICAM-1 knockout mice (in Ref. 39). *Significantly different from IgG control group (P < 0.05). ^Significantly different from each other (P < 0.05). For all groups the measurements were made in n = 10–16 venules from 4–6 mice.
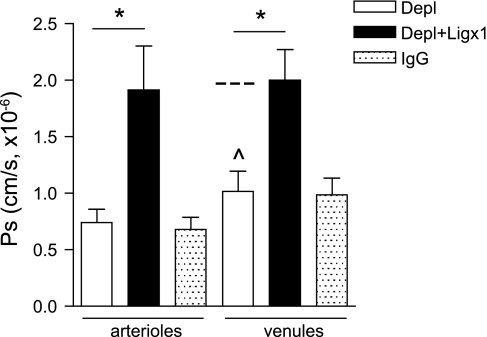
Fig. 4.
Ligation of ICAM-1 by rolling neutrophils regulates Ps in unstimulated microvessels. To test the role of rolling neutrophils in Ps regulation, circulating neutrophils were depleted (Depl) using anti-GR-1 Ab (RGB-8C5, 200 μg/mouse ip injection 12 h before microcirculatory measurements), and Ps was measured in unstimulated arterioles and venules in the absence of neutrophils. In separate experiments in neutrophil-depleted animals, Ps was measured in unstimulated arterioles and venules following ligation (Ligx1) of ICAM-1 with an anti-ICAM-1 mAb (YN/1.7.4, 50 μg/ml iv). Ps measurements were performed 20 min post-Ab ligation. Neutrophil depletion had no effect on baseline Ps in unstimulated arterioles (as expected, because unstimulated arterioles do not support leukocyte rolling interactions); however, Ps in venules was significantly decreased (below basal level as indicated by dashed line and presented in Fig. 2B). Ligation with ICAM-1 Ab significantly increased Ps in both vessel types: to a value not significantly different from Ps measured in activated arterioles (Fig. 1) and unactivated venules (Fig. 2), both of which characteristically support rolling interactions. *Significantly different from each other (P < 0.05). ^Significantly different from Ps value in unstimulated venules (Fig. 2B). For all groups the measurements were made in n = 7–10 vessels from 2–4 mice.
Antibody ligation of ICAM-1 in the absence of interacting neutrophils is sufficient to alter Ps in unstimulated microvessels.
In Figs. 1 and 2 we demonstrated that blockade of leukocyte rolling in TNF-α-activated arterioles, and in unstimulated venules, was accompanied by a decrease in Ps. Based on our previous findings (39) where we showed that surface ICAM-1 is essential for Ps regulation in both arterioles and venules, we hypothesized that the observed decrease in Ps was due to insufficient engagement of ICAM-1 in the absence of rolling leukocytes. To test this, we depleted circulating neutrophils using anti-GR-1 Ab (RGB-8C5, 200 μg/mouse, ip) injected 12 h before the experimental protocols, and measured Ps in unstimulated arterioles and venules, as well as in microvessels following ICAM-1 ligation. Neutrophil depletion (∼95%) was confirmed by flow cytometry [using a combination of anti-GR-1 and anti-F4/80 Abs, as described elsewhere (5)]. We have previously established (40) that ∼60% of all interacting leukocytes in cremaster microvessels are neutrophils; after neutrophil depletion the total number of interacting leukocytes was reduced by ∼60%, confirming that circulating neutrophils had been depleted. As expected, neutrophil depletion had no effect on Ps in unstimulated arterioles, since under these conditions arterioles do not support intrinsic leukocyte-EC interactions (42). In contrast, Ps in venules in the absence of rolling neutrophils was significantly decreased (1 ± 0.2 × 10−6 cm/s, Fig. 4), compared with untreated venules (2.2 ± 0.46 × 10−6 cm/s, Fig. 2). These findings are consistent with data in Fig. 2A, where a decrease in Ps in venules was observed following blockade of leukocyte rolling using P-selectin blocking Ab. Importantly, and further supporting our hypothesis, Ab ligation of ICAM-1 in neutrophil-depleted venules restored Ps to normal basal levels (2 ± 0.3 × 10−6 cm/s, Fig. 4). These findings strongly suggest that direct engagement of ICAM-1 by rolling leukocytes contributes to Ps regulation. Consistent with this conclusion, ligation of ICAM-1 in neutrophil-depleted unstimulated arterioles also significantly increased Ps (1.9 ± 0.4 × 10−6 cm/s, Fig. 4) compared with both untreated arterioles and arterioles that were ligated with a nonspecific IgG. This observation was also consistent with the increase in Ps that was observed in arterioles upon initiation of leukocyte rolling by treatment with TNF-α (Fig. 1). Together, these observations confirm that indeed, ligation of ICAM-1 by rolling leukocytes contributes to Ps regulation. These data also indicate that neutrophils, as the dominant leukocyte subpopulation in microvessels, are primarily responsible for the EC-dependent, ICAM-1-mediated changes in Ps, independently of their secretion products.
The degree of ICAM-1 cross-linking is directly linked to increased Ps.
While Ab ligation of ICAM-1 in unstimulated arterioles significantly increases Ps (Ref. 39 and Fig. 4) thus mimicking the effect on Ps produced in arterioles by leukocyte rolling, it had no significant effect in unstimulated venules, which intrinsically support leukocyte rolling (26, 43). To test whether further cross-linking of ICAM-1 has the capacity to increase Ps to a level typical of TNF-α treatment, we used anti-ICAM-1 primary Ab followed by a secondary Ab and measured the effect of this additional cross-linking on Ps. This approach has been shown to induce ICAM-1 clustering on endothelial cells (49, 51). Additional cross-linking of ICAM-1 with the secondary Ab did not produce a further increase in arteriolar Ps; however, it significantly increased Ps in venules from 2.1 ± 0.5 × 10−6 cm/s (Cont, Fig. 5) to 3.2 ± 0.5 × 10−6 cm/s (Ligx2, Fig. 5). As the majority of rolling leukocytes in unstimulated venules become firmly adhered upon TNF-α stimulation (38), these results suggest that while ligation of ICAM-1 with primary Ab alone mimics the ICAM-1 engagement typical of leukocyte rolling, the formation of larger clusters of ICAM-1, using the secondary Ab, mimics leukocyte adhesion. These findings also suggest that ICAM-1 clustering by adhered leukocytes is required for the TNF-α-mediated increase in Ps in venules. In addition, we confirmed that under these conditions, Ps regulation was specific for ICAM-1, as ligation of VCAM-1 in unstimulated microvessels had no significant effect on Ps (Fig. 5). VCAM-1 was chosen as the cross-linking control because, like ICAM-1, it is a member of the Ig superfamily and is also involved in leukocyte-EC interactions via ligation by interacting leukocytes. Also, again similarly to ICAM-1, VCAM-1 expression can be detected in both resting arterioles and venules, and it is upregulated by treatment with proinflammatory cytokines such as TNF-α (42).
Fig. 5.
The degree of ICAM-1 ligation differentially affects Ps. Unstimulated arterioles and venules (Cont) were intraluminally perfused with either mouse anti-ICAM-1 or mouse anti-VCAM-1 mAbs (50 μg/ml iv) and rat anti-mouse secondary Ab (50 μg/ml intraluminally) in sequence (Ligx2). Ps was measured in selected microvessels 20 min post-Ab ligation. Exposure to both the primary and the secondary Abs against ICAM-1 but not VCAM-1 significantly increased Ps, suggesting that ICAM-1 but not VCAM-1 clustering is involved in Ps regulation. *Significantly different from IgG control group (P < 0.05). For all groups the measurements were made in n = 7–10 vessels from 4–5 mice.
ICAM-1 expression and ICAM-1-dependent signaling are essential for Ps regulation.
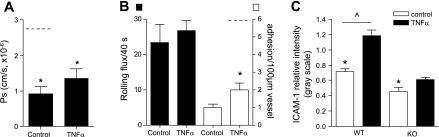
TNF-α treatment results in a significant increase in Ps in both arterioles and venules (39). TNF-α receptor 1 (TNFR1, also called p55) is known to play a key role in mediating changes in vascular permeability in liver (12) and lungs (3). Thus, we asked whether TNF-α induces an increase in Ps in cremaster microvessels by acting on TNFR1. To do so, we measured Ps in either unstimulated or TNF-α-activated cremaster venules in TNFR1 KO mice. We found that, unlike in WT mice, TNF-α treatment in TNFR1 KO mice failed to increase Ps (1.4 ± 0.3 × 10−6 cm/s, Fig. 6A) compared with unstimulated venules (0.93 ± 0.7 × 10−6 cm/s, Fig. 6A), confirming that indeed TNFR1 plays an important role in TNF-α-mediated changes in Ps. Intriguingly, Ps values in TNFR1 KO venules under both control and TNF-α-activated conditions were significantly lower than those measured in unstimulated venules in WT mice (Fig. 6A) but were not different from Ps measured in control arterioles (∼1.0 × 10−6 cm/s).
Fig. 6.
ICAM-1 expression and ICAM-1-dependent signaling are essential for Ps regulation. Ps (A) and leukocyte rolling (black bars) and adhesion (white bars) (B) were quantified in unstimulated (control) and TNF-α-treated venules in TNF-α receptor 1 (TNFR1) knockout (KO) mice. Both unstimulated and TNF-α-activated venules were significantly less permeable than unstimulated venules in wild-type (WT) mice (dashed line and Fig. 2B). Similarly, the increase in adhesion that was observed in TNF-α-treated venules in TNFR1 KO mice (B) was significantly less than that in WT mice (dashed line and Fig. 3A). *Significantly different from WT venules (P < 0.05). C: the relative expression of ICAM-1 unstimulated (control) and TNF-α-activated venules was quantified in TNFR1 KO mice and compared with WT mice. Microvessels were locally cannulated and perfused with anti-ICAM-1 (YN/1.7.4 conjugated to Alexa 488, 30 μg/ml) for 15 min. The data are presented relative to the fluorescent standard solution (0.05 mg/ml FITC-dextran, 150 kDa molecular mass) to account for tissue variability. TNFR1 KO mice have significantly lower levels of ICAM-1 under both unstimulated and activated conditions. *,^Significantly different from each other (P < 0.05). For all groups the measurements were made in n = 9–15 venules from 3–5 mice.
As we showed in Figs. 1 and 4, rolling leukocytes can contribute to Ps regulation by direct ligation of ICAM-1. Either ICAM-1 density on the EC surface or the number of rolling leukocytes, or both, might affect this signal transduction; thus we hypothesized that the lower Ps in venules of TNFR1 KO mice (compared with venules of WT mice) could be due either to lower baseline leukocyte rolling in these animals or to lower baseline expression of ICAM-1. We measured leukocyte rolling in control venules in TNFR1 KO mice and found no difference from that in WT (Fig. 6B). Furthermore, the total and differential leukocyte counts in TNFR1 KO mice were not different from those of WT mice (data not shown). TNF-α treatment, as expected, had no effect on the number of rolling leukocytes in TNFR1 KO venules (26.8 ± 2.9 leukocytes/40 s with TNF-α vs. 23.4 ± 5.2 in unstimulated conditions, Fig. 6B) and did not induce the significant increase in leukocyte adhesion that occurs in WT venules. Thus it appears unlikely that leukocyte rolling per se in TNFR1 KO mice is responsible for the lower Ps in venules of these mice. As the engagement of ICAM-1 by leukocytes is dependent on the availability of ICAM-1 on the EC surface, expression levels of this adhesion molecule might also affect Ps; we thus measured the expression of ICAM-1 in control and TNF-α-activated venules in TNFR1 KO mice. The basal expression of ICAM-1 in control venules in TNFR1 KO was significantly lower than in WT mice (0.45 ± 0.05 vs. 0.72 ± 0.04, relative intensity gray scale units, Fig. 6C). These findings suggest that the lower Ps in TNFR1 KO compared with WT venules is due to decreased ligation of ICAM-1 by rolling leukocytes as a result of the lower availability of surface ICAM-1. The number of rolling leukocytes per se is not affected by the decreased expression of ICAM-1, as ICAM-1 does not directly mediate leukocyte rolling in venules, but only contributes to rolling kinetics (41). As expected, TNF-α treatment failed to significantly increase the expression of ICAM-1 in venules in TNFR1 KO mice (0.6 ± 0.04, relative intensity gray scale units), compared with venules of WT mice (1.2 ± 0.07, relative intensity gray scale units, Fig. 6C). This explains why TNF-α treatment failed to induce leukocyte adhesion in these mice, and it also suggests that TNF-α treatment induces an increase in ICAM-1 expression in WT mice by acting via TNFR1. Importantly, these data again confirm the role for ICAM-1-mediated signaling in Ps regulation.
To explore in more detail how ICAM-1 triggers signaling that alters Ps, we asked whether the cytosolic tail of ICAM-1 plays a role in regulation of Ps changes in situ. To do this, we used a cell-permeable mouse ICAM-1 tail peptide, as previously described (41), which inhibits ICAM-1-mediated signaling (50) and hence alters VE-cadherin rearrangement and decreases leukocyte transmigration in WT mice in situ (41). As expected (41, 50), treatment with the ICAM-1 tail peptide had no effect on leukocyte rolling or adhesion; however, the ICAM-1 tail peptide, but not the control peptide, significantly decreased Ps in both unstimulated arterioles (0.5 ± 0.6 × 10−6 cm/s) and venules (0.63 ± 0.9 × 10−6 cm/s) compared with Ps in untreated vessels. These data thus confirm that the outside-in signal transduction in ECs that is mediated directly by ICAM-1 regulates Ps.
ICAM-1 clustering is essential for switching from PKC-dependent to Src-dependent regulation of Ps.
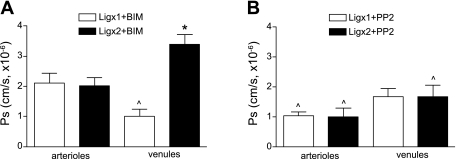
We previously showed (39) that ICAM-1-mediated Ps regulation under resting conditions was PKC dependent, whereas the TNF-α-induced increase in Ps was Src dependent and PKC independent. Here we extend these findings to show that cross-linking of ICAM-1 in the absence of a TNF-α stimulus is sufficient to switch the dominant signaling pathway from PKC (as seen in resting tissue) to Src dependent (as seen in activated tissue). We showed in the earlier study (39) that, in unstimulated arterioles, ligation of ICAM-1 with primary Ab produced an increase in Ps, similar in magnitude to that induced by TNF-α treatment. Here we show that this increase in Ps is dependent on Src-mediated signaling and is not dependent on PKC. The Src blocker, PP2 (2 μM, 10 min in superfusate), ablated the ligation-induced increase in Ps to 1.0 ± 0.1 × 10−6 cm/s (Fig. 7B), in contrast to the PKC blocker, BIM (1 μM, 10 min in superfusate), which had no significant effect on the ICAM-1-ligation-dependent increase in Ps (2.1 ± 0.3, × 10−6 cm/s, Ab ligation + BIM, Fig. 7A). Additional cross-linking of ICAM-1 with a secondary Ab produced no additional increase in Ps in arterioles compared with the ligation with primary Ab alone (Fig. 7A) and was, as predicted, blocked by PP2 treatment (Fig. 7B). In venules, Ps regulation following ligation with primary Ab was dependent on PKC-mediated signaling and not Src, because in the presence of the PKC blocker BIM, but not with the Src blocker PP2, ligation-dependent Ps in these vessels was significantly decreased (1.1 ± 0.2 × 10−6 cm/s, Fig. 7A, vs. 1.8 ± 0.4 × 10−6 cm/s, Fig. 7B, respectively). This was consistent with the PKC-dependent regulation in unstimulated venules that we have reported previously (39) and which is also dependent on the presence of rolling leukocytes (Fig. 2). Furthermore, and consistent with the Src-dependent Ps regulation in TNF-α-treated venules that we reported earlier (39), the increase in venular Ps that was induced by ligation with both primary followed by the secondary Abs (3.2 ± 0.5 × 10−6 cm/s, Fig. 5) was blocked by the Src inhibitor PP2 (1.1 ± 0.2 × 10−6 cm/s, Fig. 7B). PKC block had no effect on Ps under these conditions (3.4 ± 0.3 × 10−6 cm/s, Fig. 7A). Thus, ICAM-1 cross-linking with primary followed by secondary Abs switched the venule to an inflammatory mode, as is also achieved for Ps with TNF-α (39). These data indicate that Ps changes induced by the maximal ICAM-1 clustering achievable for each vessel type are Src dependent, whereas Ps changes induced by nonmaximal ICAM-1 clustering are PKC dependent. Thus, the degree of ICAM-1 cross-linking contributes to determination of which downstream signaling pathway is dominant, and it serves as a cue for the microvessels to switch to the inflammatory state.
Fig. 7.
ICAM-1 clustering is essential for switching from PKC-dependent to Src-dependent regulation of Ps. Unstimulated arterioles and venules were intraluminally perfused with either a mouse anti-ICAM-1 mAb (50 μg/ml iv) alone (Ligx1) or followed by rat anti-mouse secondary Ab (50 μg/ml intraluminally) (Ligx2). Ten to twenty minutes later, the tissue was superfused with either PKC inhibitor bisindolylmaleimid l (BIM; in 0.01% DMSO, 1 μM for 10 min) (A) or Src inhibitor PP2 (in 0.01% DMSO, 2 μM for 10 min) (B), and Ps was measured in selected microvessels. In arterioles, leukocyte rolling in activated conditions is mimicked by Ligx1, and this Ligx1-dependent increase in Ps is not PKC dependent (A) but is Src dependent (B). In venules, rolling in unstimulated conditions is mimicked by Ligx1, and Ps is decreased below normal by PKC block (A) and is independent of Src (B). Adhesion in activated venules is mimicked by Ligx2, and the increased Ps seen with this ligation is independent of PKC (A) and significantly decreased by Src block (B). Thus, Ps changes induced by the maximal ICAM-1 clustering achievable for each vessel type are Src dependent, whereas Ps changes induced by nonmaximal ICAM-1 clustering are PKC dependent. ^Significantly different from measurements obtained in the absence of the inhibitors (P < 0.05), as shown in Fig. 5 and previously (39). *Significantly different from Ligx1. For all groups, the measurements were made in n = 7–10 venules from 3–5 mice.
DISCUSSION
In this study we show that leukocyte engagement of EC surface receptors directly activates EC signaling, altering EC permeability. We conclude this from our findings that first, unstimulated arterioles do not support leukocyte-EC interactions; however, ICAM-1 cross-linking in these vessels induced an increase in solute permeability (Ps) (Ref. 39 and Fig. 5); and second, that while depleting the unstimulated venules of interacting neutrophils results in decreased Ps (below basal levels), Ab engagement of ICAM-1 was sufficient to restore Ps to expected basal levels (Fig. 4). Thus, in addition to the established paracrine regulation of Ps by circulating leukocytes, direct engagement of ICAM-1 in microvessels activates a separate, ICAM-1-dependent, mechanism in ECs that regulates Ps. Our findings also suggest that both leukocyte rolling and adhesive interactions with the endothelial surface receptor are able to initiate EC signaling leading to alterations in Ps, thus directly contributing to Ps regulation in both unstimulated and inflamed microvessels. Our study further indicates that these signaling pathways differ under physiological vs. inflamed conditions and are directly dependent on the degree of ICAM-1 clustering.
Our results confirm and extend earlier studies that show that permeability is regulated in arterioles under both physiological and inflamed conditions (14, 21, 22, 35, 39). In addition to changes in the expression of surface adhesion molecules and the regulation of leukocyte trafficking in arterioles during inflammation, the arteriolar microcirculation thus also contributes to protein exchange and fluid homeostasis, although clearly, given its characteristically higher permeability and larger surface area, the venular microcirculation will quantitatively dominate regulation of these processes in intact tissues. We speculate that in the arteriolar microcirculation, regulation of permeability will also facilitate delivery of regulatory macromolecules (either directly or via their carriage by albumin) to their target cells outside the vascular compartment.
In arterioles, the interactions between β2-integrins and ICAM-1 can sustain leukocyte rolling (42). Our current data (Figs. 1 and 3) show that in these vessels, ligation of surface ICAM-1 by rolling leukocytes triggers ICAM-1-dependent signaling (via activation of Src kinase, Fig. 7) leading to increased Ps. In unstimulated venules, leukocyte rolling also contributes to Ps regulation (Fig. 2); however, in contrast to arterioles, where initiation of leukocyte rolling in activated vessels results in increased Ps, in venules, intrinsic leukocyte rolling helps maintain basal Ps in the unstimulated (physiological) condition. Our data show that in venules, in the absence of leukocyte interactions [either by Ab blockade of rolling interactions (Fig. 2) or by depletion of neutrophils (Fig. 4)], Ps is significantly decreased to levels not different from those seen in unstimulated arterioles, where no leukocyte interactions with EC are typically observed (43). We conclude from this that leukocyte-EC interactions are a more important contributor to regulation of Ps than are the established differences in structure and EC morphology between arterioles and venules.
Our data indicate that, in venules, leukocyte rolling contributes to Ps regulation by transiently ligating ICAM-1. We showed previously that leukocyte rolling in venules is not directly mediated by ICAM-1; thus blockade of ICAM-1 does not significantly decrease the number of rolling leukocytes (42). However, there is evidence that ICAM-1 is indeed being ligated by the rolling leukocyte in venules, as it takes part in stabilizing the rolling interactions, and in the absence of ICAM-1, leukocyte rolling velocity is increased (41). In the current work we further show that the degree of ICAM-1 cross-linking is reflected in its ability to signal changes in Ps. Ligation of ICAM-1 by rolling leukocytes, or ligation with primary Ab alone, could increase Ps; however, to achieve an increase in Ps comparable to that induced by TNF-α (and the consequent presence of adhered leukocytes), a higher degree of ICAM-1 clustering (cross-linking with both primary and secondary antibodies) was needed. This suggests that, similarly to the different degree of Ab-induced clustering, engagement of ICAM-1 by rolling vs. adhered leukocytes might also result in a different degree of ICAM-1 clustering, with greater ICAM-1 clustering being induced by leukocyte adhesion. As apparent expression density of ICAM-1 in resting and inflamed arterioles and venules is highly heterogeneous (43), it will be of great importance in future studies to determine the degree of ICAM-1 clustering that is needed to induce changes in Ps under these various conditions.
As described above, our data show that the interactions between β2-integrins and ICAM-1 directly trigger alterations in barrier function. ICAM-1 is known to bind both CD11b (Mac-1) and CD11a (LFA-1) on leukocytes (3, 8). Mac-1 (9) and LFA-1 (37) bind ICAM-1 at different extracellular domains. Moreover, it is established that LFA-1 and Mac-1 have different roles in mediating leukocyte-EC interactions (31); thus it is possible that binding of each of these integrins to ICAM-1 will have different clustering effects, leading to differential effects on Ps. This raises the important question as to which of the β2-integrins, LFA-1 or Mac-1 (or both), is primarily responsible for these changes in Ps.
There is evidence that ICAM-1 valence and its distribution on EC-surface is important for ICAM-1 signal transduction. For example, ICAM-1 dimers, compared with monomers, have been shown to more efficiently bind LFA-1 (24, 30). Similarly, ICAM-1 dimers, but not monomers, activate Src in neutrophils (33). Leukocyte adhesion to endothelium induces formation of ICAM-1 clusters (44) and assembly of cytoskeletal machinery (46), suggesting that when clustered, ICAM-1 might trigger either different, or at the least a higher magnitude, of signaling. Confirming this idea, we show that cross-linking of ICAM-1 (using primary followed by secondary Abs, Fig. 5) indeed increased Ps in unstimulated venules similarly to the increase in Ps induced by leukocyte adhesion following TNF-α treatment (Fig. 3). Note that Ps in TNF-α-treated venules was still significantly higher than that induced by ICAM-1 clustering alone, in the absence of adhesion of activated leukocytes, consistent with the expectation that secretion products from activated leukocytes are also involved in this process.
Overexpression of ICAM-1 in monolayers results in increased permeability (6), strongly arguing that ICAM-1 plays an important role in Ps regulation. However, we show that in in situ microvessels, ICAM-1 expression density alone is insufficient to mediate increases in Ps; this requires ligation of ICAM-1 by rolling leukocytes. The expression of ICAM-1 in unstimulated venules is at least twofold higher compared with unstimulated arterioles (43); however, in the absence of leukocyte rolling, Ps in unstimulated venules (Fig. 2) was not significantly different from that measured in unstimulated arterioles (39). Note that basally expressed ICAM-1 also contributes to basal Ps regulation independently of leukocytes, via a PKC-dependent signaling mechanism, as in the absence of ICAM-1 (in ICAM-1 KO mice) Ps in both arterioles and venules is significantly lower than basal Ps in WT mice (39).
We recently identified two distinct ICAM-1-mediated signaling pathways, PKC-dependent in unstimulated vessels, and Src-dependent in TNF-α-activated microvessels (39). Based on the current data, we hypothesize that ligation of ICAM-1 by rolling leukocytes leads to dimerization of ICAM-1, which turns on a PKC-dependent pathway, versus leukocyte adhesion, which leads to ICAM-1 clustering and a resultant switch to Src-dependent signaling: this hypothesis remains to be tested directly in future studies. As evident from data presented in Fig. 7, ICAM-1 clustering is indeed essential to switch from PKC-dependent to Src-dependent regulation of Ps.
In summary, in this study we demonstrate clearly that leukocyte engagement of ECs via ICAM-1 can lead to alteration in vessel permeability independently of effects on permeability produced by leukocyte secretion products. We also show that both leukocyte rolling and leukocyte adhesion play a role in this Ps regulation, presumably by inducing different degrees of ICAM-1 clustering. In turn, these events regulate Ps changes via different EC signaling mechanisms.
GRANTS
This work was supported by National Heart, Lung, and Blood Institute Grants HL-75186 and HL-18208.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
ACKNOWLEDGMENTS
We thank Dr. V. H. Huxley and Dr. K. Fujiwara for critical reading of the manuscript.
Footnotes
This article is the topic of an Editorial Focus by Marcie R. Williams and Francis W. Luscinskas (49a).
REFERENCES
- 1. Allingham MJ, van Buul JD, Burridge K. ICAM-1-mediated, Src- and Pyk2-dependent vascular endothelial cadherin tyrosine phosphorylation is required for leukocyte transendothelial migration. J Immunol 179: 4053–4064, 2007 [DOI] [PubMed] [Google Scholar]
- 2. Amos C, Romero IA, Schultze C, Rousell J, Pearson JD, Greenwood J, Adamson P. Cross-linking of brain endothelial intercellular adhesion molecule (ICAM)-1 induces association of ICAM-1 with detergent-insoluble cytoskeletal fraction. Arterioscler Thromb Vasc Biol 21: 810–816, 2001 [DOI] [PubMed] [Google Scholar]
- 3. Basit A, Reutershan J, Morris MA, Solga M, Rose CE, Jr, Ley K. ICAM-1 and LFA-1 play critical roles in LPS-induced neutrophil recruitment into the alveolar space. Am J Physiol Lung Cell Mol Physiol 291: L200–L207, 2006 [DOI] [PubMed] [Google Scholar]
- 4. Bjork J, Hedqvist P, Arfors KE. Increase in vascular permeability induced by leukotriene B4 and the role of polymorphonuclear leukocytes. Inflammation 6: 189–200, 1982 [DOI] [PubMed] [Google Scholar]
- 5. Chiang EY, Hidalgo A, Chang J, Frenette PS. Imaging receptor microdomains on leukocyte subsets in live mice. Nat Methods 4: 219–222, 2007 [DOI] [PubMed] [Google Scholar]
- 6. Clark PR, Manes TD, Pober JS, Kluger MS. Increased ICAM-1 expression causes endothelial cell leakiness, cytoskeletal reorganization and junctional alterations. J Invest Dermatol 127: 762–774, 2007 [DOI] [PubMed] [Google Scholar]
- 7. Czabanka M, Peter C, Martin E, Walther A. Microcirculatory endothelial dysfunction during endotoxemia–insights into pathophysiology, pathologic mechanisms and clinical relevance. Curr Vasc Pharmacol 5: 266–275, 2007 [DOI] [PubMed] [Google Scholar]
- 8. Diamond MS, Staunton DE, de Fougerolles AR, Stacker SA, Garcia-Aguilar J, Hibbs ML, Springer TA. ICAM-1 (CD54): a counter-receptor for Mac-1 (CD11b/CD18). J Cell Biol 111: 3129–3139, 1990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Diamond MS, Staunton DE, Marlin SD, Springer TA. Binding of the integrin Mac-1 (CD11b/CD18) to the third immunoglobulin-like domain of ICAM-1 (CD54) and its regulation by glycosylation. Cell 65: 961–971, 1991 [DOI] [PubMed] [Google Scholar]
- 10. DiStasi MR, Ley K. Opening the flood-gates: how neutrophil-endothelial interactions regulate permeability. Trends Immunol 30: 547–556, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Etienne-Manneville S, Manneville JB, Adamson P, Wilbourn B, Greenwood J, Couraud PO. ICAM-1-coupled cytoskeletal rearrangements and transendothelial lymphocyte migration involve intracellular calcium signaling in brain endothelial cell lines. J Immunol 165: 3375–3383, 2000 [DOI] [PubMed] [Google Scholar]
- 12. Ferrero E, Zocchi MR, Magni E, Panzeri MC, Curnis F, Rugarli C, Ferrero ME, Corti A. Roles of tumor necrosis factor p55 and p75 receptors in TNF-α-induced vascular permeability. Am J Physiol Cell Physiol 281: C1173–C1179, 2001 [DOI] [PubMed] [Google Scholar]
- 13. Friedl J, Puhlmann M, Bartlett DL, Libutti SK, Turner EN, Gnant MF, Alexander HR. Induction of permeability across endothelial cell monolayers by tumor necrosis factor (TNF) occurs via a tissue factor-dependent mechanism: relationship between the procoagulant and permeability effects of TNF. Blood 100: 1334–1339, 2002 [PubMed] [Google Scholar]
- 14. Gaudreault N, Perrin RM, Guo M, Clanton CP, Wu MH, Yuan SY. Counter regulatory effects of PKCbetaII and PKCdelta on coronary endothelial permeability. Arterioscler Thromb Vasc Biol 28: 1527–1533, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Gautam N, Herwald H, Hedqvist P, Lindbom L. Signaling via beta(2) integrins triggers neutrophil-dependent alteration in endothelial barrier function. J Exp Med 191: 1829–1839, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Gautam N, Olofsson AM, Herwald H, Iversen LF, Lundgren-Akerlund E, Hedqvist P, Arfors KE, Flodgaard H, Lindbom L. Heparin-binding protein (HBP/CAP37): a missing link in neutrophil-evoked alteration of vascular permeability. Nat Med 7: 1123–1127, 2001 [DOI] [PubMed] [Google Scholar]
- 17. He P, Wang J, Zeng M. Leukocyte adhesion and microvessel permeability. Am J Physiol Heart Circ Physiol 278: H1686–H1694, 2000 [DOI] [PubMed] [Google Scholar]
- 18. Hermant B, Bibert S, Concord E, Dublet B, Weidenhaupt M, Vernet T, Gulino-Debrac D. Identification of proteases involved in the proteolysis of vascular endothelium cadherin during neutrophil transmigration. J Biol Chem 278: 14002–14012, 2003 [DOI] [PubMed] [Google Scholar]
- 19. Hippenstiel S, Tannert-Otto S, Vollrath N, Krull M, Just I, Aktories K, von Eichel-Streiber C, Suttorp N. Glucosylation of small GTP-binding Rho proteins disrupts endothelial barrier function. Am J Physiol Lung Cell Mol Physiol 272: L38–L43, 1997 [DOI] [PubMed] [Google Scholar]
- 20. Huxley VH, Curry FE. Differential actions of albumin and plasma on capillary solute permeability. Am J Physiol Heart Circ Physiol 260: H1645–H1654, 1991 [DOI] [PubMed] [Google Scholar]
- 21. Huxley VH, Wang JJ, Sarelius IH. Adaptation of coronary microvascular exchange in arterioles and venules to exercise training and a role for sex in determining permeability responses. Am J Physiol Heart Circ Physiol 293: H1196–H1205, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Huxley VH, Williams DA, Meyer DJ, Jr, Laughlin MH. Altered basal and adenosine-mediated protein flux from coronary arterioles isolated from exercise-trained pigs. Acta Physiol Scand 160: 315–325, 1997 [DOI] [PubMed] [Google Scholar]
- 23. Johnson-Leger C, Aurrand-Lions M, Imhof BA. The parting of the endothelium: miracle, or simply a junctional affair? J Cell Sci 113: 921–933, 2000 [DOI] [PubMed] [Google Scholar]
- 24. Jun CD, Shimaoka M, Carman CV, Takagi J, Springer TA. Dimerization and the effectiveness of ICAM-1 in mediating LFA-1-dependent adhesion. Proc Natl Acad Sci USA 98: 6830–6835, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kim MB, Sarelius IH. Distributions of wall shear stress in venular convergences of mouse cremaster muscle. Microcirculation 10: 167–178, 2003 [DOI] [PubMed] [Google Scholar]
- 26. Kim MB, Sarelius IH. Role of shear forces and adhesion molecule distribution on P-selectin-mediated leukocyte rolling in postcapillary venules. Am J Physiol Heart Circ Physiol 287: H2705–H2711, 2004 [DOI] [PubMed] [Google Scholar]
- 27. Kunkel EJ, Jung U, Ley K. TNF-α induces selectin-mediated leukocyte rolling in mouse cremaster muscle arterioles. Am J Physiol Heart Circ Physiol 272: H1391–H1400, 1997 [DOI] [PubMed] [Google Scholar]
- 28. Lindbom L. Regulation of vascular permeability by neutrophils in acute inflammation. Chem Immunol Allergy 83: 146–166, 2003 [DOI] [PubMed] [Google Scholar]
- 29. Mehta D, Malik AB. Signaling mechanisms regulating endothelial permeability. Physiol Rev 86: 279–367, 2006 [DOI] [PubMed] [Google Scholar]
- 30. Miller J, Knorr R, Ferrone M, Houdei R, Carron CP, Dustin ML. Intercellular adhesion molecule-1 dimerization and its consequences for adhesion mediated by lymphocyte function associated-1. J Exp Med 182: 1231–1241, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Phillipson M, Heit B, Colarusso P, Liu L, Ballantyne CM, Kubes P. Intraluminal crawling of neutrophils to emigration sites: a molecularly distinct process from adhesion in the recruitment cascade. J Exp Med 203: 2569–2575, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Rosengren S, Olofsson AM, von Andrian UH, Lundgren-Akerlund E, Arfors KE. Leukotriene B4-induced neutrophil-mediated endothelial leakage in vitro and in vivo. J Appl Physiol 71: 1322–1330, 1991 [DOI] [PubMed] [Google Scholar]
- 33. Sarantos MR, Zhang H, Schaff UY, Dixit N, Hayenga HN, Lowell CA, Simon SI. Transmigration of neutrophils across inflamed endothelium is signaled through LFA-1 and Src family kinase. J Immunol 181: 8660–8669, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Sarelius IH. Cell and oxygen flow in arterioles controlling capillary perfusion. Am J Physiol Heart Circ Physiol 265: H1682–H1687, 1993 [DOI] [PubMed] [Google Scholar]
- 35. Sarelius IH, Kuebel JM, Wang J, Huxley VH. Macromolecule permeability of in situ and excised rodent skeletal muscle arterioles and venules. Am J Physiol Heart Circ Physiol 290: H474–H480, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Soehnlein O, Zernecke A, Eriksson EE, Rothfuchs AG, Pham CT, Herwald H, Bidzhekov K, Rottenberg ME, Weber C, Lindbom L. Neutrophil secretion products pave the way for inflammatory monocytes. Blood 112: 1461–1471, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Stanley P, Hogg N. The I domain of integrin LFA-1 interacts with ICAM-1 domain 1 at residue Glu-34 but not Gln-73. J Biol Chem 273: 3358–3362, 1998 [DOI] [PubMed] [Google Scholar]
- 38. Sumagin R, Lamkin-Kennard KA, Sarelius IH. A separate role for ICAM-1 and fluid shear in regulating leukocyte interactions with straight regions of venular wall and venular convergences. Microcirculation 16: 508–520, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Sumagin R, Lomakina E, Sarelius IH. Leukocyte-endothelial cell interactions are linked to vascular permeability via ICAM-1-mediated signaling. Am J Physiol Heart Circ Physiol 295: H969–H977, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Sumagin R, Prizant H, Lomakina E, Waugh RE, Sarelius IH. LFA-1 and Mac-1 define characteristically different intralumenal crawling and emigration patterns for monocytes and neutrophils in situ. J Immunol 185: 7057–7066, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Sumagin R, Sarelius IH. Intercellular adhesion molecule-1 enrichment near tricellular endothelial junctions is preferentially associated with leukocyte transmigration and signals for reorganization of these junctions to accommodate leukocyte passage. J Immunol 184: 5242–5252, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Sumagin R, Sarelius IH. A role for ICAM-1 in maintenance of leukocyte-endothelial cell rolling interactions in inflamed arterioles. Am J Physiol Heart Circ Physiol 293: H2786–H2798, 2007 [DOI] [PubMed] [Google Scholar]
- 43. Sumagin R, Sarelius IH. TNF-α activation of arterioles and venules alters distribution and levels of ICAM-1 and affects leukocyte-endothelial cell interactions. Am J Physiol Heart Circ Physiol 291: H2116–H2125, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Tilghman RW, Hoover RL. The Src-cortactin pathway is required for clustering of E-selectin and ICAM-1 in endothelial cells. FASEB J 16: 1257–1259, 2002 [DOI] [PubMed] [Google Scholar]
- 45. Van Buul JD, Allingham MJ, Samson T, Meller J, Boulter E, Garcia-Mata R, Burridge K. RhoG regulates endothelial apical cup assembly downstream from ICAM1 engagement and is involved in leukocyte trans-endothelial migration. J Cell Biol 178: 1279–1293, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Van Buul JD, van Rijssel J, van Alphen FP, Hoogenboezem M, Tol S, Hoeben KA, van Marle J, Mul EP, Hordijk PL. Inside-out regulation of ICAM-1 dynamics in TNF-alpha-activated endothelium. PLoS One 5: e11336, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Venkiteswaran K, Xiao K, Summers S, Calkins CC, Vincent PA, Pumiglia K, Kowalczyk AP. Regulation of endothelial barrier function and growth by VE-cadherin, plakoglobin, and beta-catenin. Am J Physiol Cell Physiol 283: C811–C821, 2002 [DOI] [PubMed] [Google Scholar]
- 48. Wang Q, Doerschuk CM. The signaling pathways induced by neutrophil-endothelial cell adhesion. Antioxid Redox Signal 4: 39–47, 2002 [DOI] [PubMed] [Google Scholar]
- 49. Wang Q, Pfeiffer GR, 2nd, Stevens T, Doerschuk CM. Lung microvascular and arterial endothelial cells differ in their responses to intercellular adhesion molecule-1 ligation. Am J Respir Crit Care Med 166: 872–877, 2002. [DOI] [PubMed] [Google Scholar]
- 49a. Williams MR, Luscinskas FW. Leukocyte rolling and adhesion via ICAM-1 signals to endothelial permeability. Focus on “Leukocyte rolling and adhesion both contribute to regulation of microvascular permeability to albumin via ligation of ICAM-1” (July 20, 2011). doi:10.1152/ajpcell.00250.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Yang L, Froio RM, Sciuto TE, Dvorak AM, Alon R, Luscinskas FW. ICAM-1 regulates neutrophil adhesion and transcellular migration of TNF-alpha-activated vascular endothelium under flow. Blood 106: 584–592, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Yang L, Kowalski JR, Yacono P, Bajmoczi M, Shaw SK, Froio RM, Golan DE, Thomas SM, Luscinskas FW. Endothelial cell cortactin coordinates intercellular adhesion molecule-1 clustering and actin cytoskeleton remodeling during polymorphonuclear leukocyte adhesion and transmigration. J Immunol 177: 6440–6449, 2006 [DOI] [PubMed] [Google Scholar]
- 52. Zhu L, He P. fMLP-stimulated release of reactive oxygen species from adherent leukocytes increases microvessel permeability. Am J Physiol Heart Circ Physiol 290: H365–H372, 2006 [DOI] [PubMed] [Google Scholar]