Abstract
During and after transendothelial migration, neutrophils undergo a number of phenotypic changes resulting from encounters with endothelium-derived factors. This report uses an in vitro model with human umbilical vein endothelial cells and isolated human neutrophils to examine the effects of two locally derived cytokines, granulocyte (G)-macrophage (M) colony-stimulating factor (GM-CSF) and G-CSF, on oncostatin M (OSM) expression. Neutrophils contacting activated HUVEC expressed and released increased amounts of oncostatin M (OSM), a proinflammatory cytokine known to induce polymorphonuclear neutrophil adhesion and chemotaxis. Neutrophil transendothelial migration resulted in threefold higher OSM expression and protein levels compared with nontransmigrated cells. Addition of anti-GM-CSF neutralizing antibody reduced OSM expression level but anti-G-CSF was without effect. GM-CSF but not G-CSF protein addition to cultures of isolated neutrophils resulted in a significant increase in OSM protein secretion. However, inhibition of β2 integrins by neutralizing antibody significantly reduced GM-CSF-induced OSM production indicating this phenomenon is adhesion dependent. Thus cytokine-stimulated endothelial cells can produce sufficient quantities of GM-CSF to influence in an adhesion-dependent manner, the phenotypic characteristics of neutrophils resulting in the latter's transmigration. Both transmigration and adhesion phenomenon lead to increased production of OSM by neutrophils that then play a major role in inflammatory response.
Keywords: neutrophils, endothelial cells, adhesion, transmigration
neutrophils are the first-line defenders arriving at sites of acute inflammation. Their recruitment requires them to adhere to the luminal surface of the vascular endothelium and to migrate through the endothelial cell (EC) monolayer. The activation of endothelium by various proinflammatory stimuli including cytokines such as interleukin-1 (IL-1) and tumor necrosis factor (TNF) plays a pivotal role in EC-neutrophil cell interactions. This response may occur via the expression of adhesion molecules on the EC surface as well as through augmentation of chemotactic agents (35). Soluble factors released by activated ECs include cytokines (e.g, IL-6, IL-15) and chemokines (e.g, IL-8) that modulate neutrophil functions (39).
Granulocyte (G)-macrophage (M)-colony-stimulating factor (GM-CSF) and G-CSF produced by ECs play an important role in regulating blood-vessel function. GM-CSF, a member of the hematopoietic growth factor family, is produced and released by monocytes, fibroblasts, vascular smooth muscle cells, and ECs in response to IL-1 and TNF (37). G-CSF is produced by endothelium, macrophages, and a number of other immune cells to stimulate the growth of neutrophil granulocyte precursors (41). Recombinant and purified G-CSF and GM-CSF not only serve as growth and differentiation factors for neutrophil precursors, they stimulate mature neutrophils and induce functional changes in phagocytosis (39), oxidative burst (36), degranulation (44), adhesion (6), transmigration (43), and apoptosis (1). In the present investigation we analyzed the possible contributions of endothelium-derived G-CSF and GM-CSF, growth factors with known potential to stimulate mature neutrophils (6, 7) but poorly investigated in the context of transendothelial migration. Our interest was whether local expression of these growth factors influences the level of transmigration, the survival of transmigrated neutrophils, or the gene expression in neutrophils.
We chose oncostatin M (OSM) as one measure of possible influence of these factors on gene expression. Goren et al. (14) have recently observed neutrophil-dependent accumulation of OSM in the early stages of dermal wound healing, and Cross et al. (8) found that neutrophils obtained from arthritic joint fluid synthesize OSM. OSM is a pleotropic cytokine belonging to the IL-6 family that includes IL-6, IL-11, ciliary neurotrophic factor (CNTF), cardiotrophin-1 (CT), novel neurotrophin-1/B cell-stimulating factor-3 (NNT-1/BSF-3), and leukemia inhibitory factor (LIF) (30, 32, 38, 45). Recent evidence indicates that OSM is distinct among the IL-6 family members in activating chemokine secretion by fibroblasts (23, 24, 25), and ECs respond to OSM by increasing synthesis of P-selectin, an adhesion molecule involved in the localization of leukocytes during inflammation (19, 27). Since circulating neutrophils are known to contain OSM (15) and can be activated by GM-CSF to synthesize OSM (8), their early arrival in the cascade of inflammation may release sufficient OSM to augment the inflammatory response.
In the present study, utilizing an in vitro model where neutrophils transmigrated across an IL-1β-stimulated endothelial monolayer, we investigated the roles of possible mediators normally produced by cytokine-activated ECs. Our results indicate endothelial production of GM-CSF influences production of OSM in an adhesion-dependent manner.
MATERIALS AND METHODS
Neutrophil isolation.
Human peripheral neutrophils were isolated from heparinized (10 U/ml) venous blood that was sedimented in 6% dextran (250,000 mol wt; Spectrum Gardena, CA) and centrifuged over a gradient of 6.07% Ficoll (400,000 mol wt) and 10% Hypaque (Sanofi Withrop Pharmaceuticals, New York, NY) at room temperature (35). Neutrophil purity (H&E) and viability (trypan blue exclusion) were >95% with <2% monocytes and lymphocytes combined. Neutrophils were kept at room temperature in Dulbecco's phosphate-buffered saline (PBS) (without calcium and magnesium, Invitrogen, Carlsbad, CA) for up to 2 h before being used. Human subjects donating blood gave their informed consent. All experimental work for this paper was carried out at Baylor College of Medicine in the Department of Pediatrics, Section of Leukocyte Biology and was performed under the human protocol H-11736 approved by the Baylor College of Medicine IRB.
Endothelial cell culture.
Human umbilical vein endothelial cell (HUVEC) were isolated from 5–10 human umbilical veins by collagenase perfusion as described previously (35). Pooled cells were seeded in T75 flasks (pretreated with 0.2% gelatin; Difco, Detroit, MI) and grown in M199 medium (Invitrogen) supplemented with 10% fetal bovine serum and 10% bovine calf serum (Hyclone Labs; Logan, UT), penicillin (50 U/ml)-streptomycin (50 μg/ml), Fungizone (2.5 μg/ml), 10 mM HEPES (Invitrogen), 1% heparin, and 50 μg/ml endothelial cell growth supplement (Collaborative Biomedical Products; Bedford, MA). Five days after seeding was completed, cells were trypsinized and passaged at confluence onto gelatin-coated 24-mm transwell inserts (3-μm pore size). Confluent monolayers were allowed to mature (4–5 days) before using them in the transendothelial migration experiments (4).
Neutrophil transendothelial migration.
Primary HUVEC dissociated using trypsin/EDTA were seeded at confluence onto gelatin-coated 3.0-μm pore polycarbonate transwell filters (Corning, Corning, NY), which were placed in a matching six-well plates and cultured for 4 days. Immediately before the assays, HUVEC monolayers were stimulated with IL-1β (10 U/ml) for 4 h at 37°C. After stimulation, the inserts were washed twice with HBSS, then transferred to six-well plates coated with a thin layer of 1% agarose, and incubated in minimal essential medium (Invitrogen) with 0.5% human serum albumin (Sigma-Aldrich). The agarose facilitated removal of transmigrated neutrophils for further analysis and minimized additional activation by the plastic wells. Freshly isolated polymorphonuclear neutrophils (PMN) from healthy volunteers were placed on top of the insert at a ratio of 4:1 (PMN:endothelial) and incubated at 37°C for 1 or 4 h. Nontransmigrated neutrophils were then collected from the top of the insert, and transmigrated neutrophils were collected from the bottom of the insert. The residual red cells in the PMN were lysed using FACS Lysing Solution (Becton Dickenson, San Jose, CA). For control, neutrophils were incubated on the inert agarose surface under the same conditions used for transwells. All samples were plated in triplicates, and transmigration was assessed by plotting the number of transmigrated cells in the lower compartment. In some experiments, blocking antibodies were added to both upper and lower chambers before addition of neutrophils. G-CSF (0.5 μg/ml), GM-CSF (0.5 μg/ml), and control IgG (0.5μg/ml) were purchased from R&D Systems (Minneapolis, MN). TS1/18 monoclonal antibody (Anti-CD18), which recognize the β2 subunit of lymphocyte function-associated antigen-1 and macrophage-1 antigen (MAC-1), was produced from clones purchased from ATCC (Manassas, VA) and was used at 3 μg/ml (35).
Quantitative polymerase chain reaction analysis of OSM.
Total RNA was isolated from transmigrated, nontransmigrated, and control neutrophils using TRIzol reagent following the manufacturer's instructions (Invitrogen). RT reactions were performed as previously described (13). The cDNA was analyzed immediately or stored. Primers and probes for human OSM, 18S and GAPDH were acquired from Applied Biosystems (Assays-on-demand, Foster City, CA). These predesigned and preoptimized TaqMan gene expression human sequence-based assays are provided in 20× format and used according to maufacturer's instructions. The preformulated assay consists of two unlabeled PCR primers (900 nM each final concentration) and a dye-labeled TaqMan MGB probe (250 nM final concentration). Real-time TaqMan PCR systems for OSM was multiplexed with 18S or GAPDH (internal standard). One microliter of each cDNA sample was analyzed. All assays were run in triplicates in a 96-well format plate. Real-time fluorescent detection of PCR products was performed on ABI 7500 (PE Applied Biosystems, Foster City, CA) using the following thermocycling conditions: 1 cycle of 50°C for 2 min and 95°C for 10 min; 40 cycles of 95°C for 15 s and 60°C for 1 min. Data were analyzed by the Sequence Detection Systems (SDS) Software (PE Applied Biosystems). Subsequent analysis was performed on the data output from the SDS software using Microsoft Excel. Relative RNA expression was determined using the formula Rel ExP = 2−(ΔΔCt), where, for example, ΔΔCt = (CT OSM-CT 18S). The ratio for OSM/18S in transmigrated and nontransmigrated neutrophil samples was then normalized to ratio seen in control neutrophils seeded on agarose surface and expressed as the means ± SE.
ELISA.
G-CSF, GM-CSF, IL-8, and OSM protein levels were quantified in cell-free supernatants by using commercial enzyme-linked immunosorbent assays (ELISA) kit (Quantikine; R&D Systems) following the manufacturer's instructions.
Adhesion study.
Forty-eight-well nontissue culture plates were coated with fibrinogen (Sigma-Alrich) by adding 0.5 ml of fibrinogen solution (0.1 mg/ml saline) for at least 2 h while incubating at 37°C. After the coating was completed, the wells were washed three times with PBS. Neutrophil suspensions of 0.5 ml (8 × 106 cells/ml) were added in each well in the presence or absence of recombinant GM-CSF (R&D Systems) in combination of ± anti-CD antibody used at 3 μg/ml. In some experiments, control IgG (0.5 μg/ml) was used. The cells were incubated at 37°C for 45 min at which time point neutrophils were then collected for RNA analysis. The residual red cells in the PMN were lysed using FACS Lysing Solution (Becton Dickenson). For comparison, neutrophils were incubated on inert agarose surface. All samples were plated in triplicates.
Data are expressed as means ± SE. Statistical differences were analyzed using a Student's t-test, and P values <0.05 were considered to indicate statistical significance.
RESULTS
Neutrophils express OSM after contact with activated ECs.
We studied whether G-CSF and GM-CSF derived from ECs can affect OSM expression. First, kinetic studies using an in vitro model of neutrophil trafficking performed as described in materials and methods revealed that transendothelial migration of neutrophils through IL-β-stimulated monolayers was >90% complete at the end of 1 h incubation. Thus we chose this time to assess the effect of transendothelial migration on PMN endogenous OSM expression level.
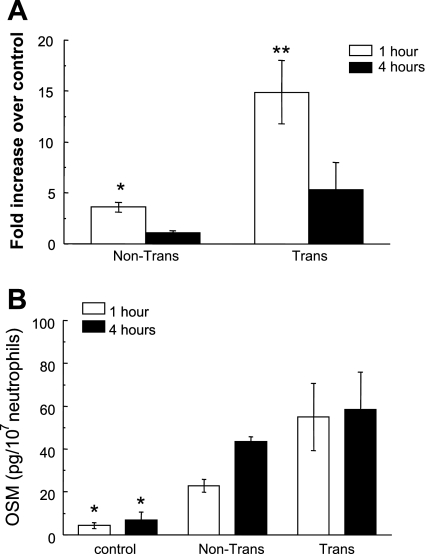
Neutrophils contacting IL-1β-stimulated HUVEC monolayers for 1 h exhibited increased expression of OSM (Fig. 1A). This was true for neutrophils removed from the upper surface of the monolayer or those that had transmigrated into the lower compartment of the transwell chamber, though transmigrated cells contained higher levels of mRNA than nontransmigrated cells. At 4 h after contacting the HUVEC monolayer, the levels of OSM mRNA were significantly lower than at the 1-h time both for transmigrated and nontransmigrated neutrophils. To confirm synthesis of OSM protein, culture supernatants were analyzed by ELISA, revealing significant elevations of OSM at both 1 and 4 h after contact with activated HUVEC (Fig. 1B). Supernatants collected from activated ECs in the absence of added neutrophils were without detectible OSM (data not shown).
Fig. 1.
Effects of transendothelial transmigration on oncostatin M (OSM) production in neutrophils. A: real-time PCR analysis of OSM mRNA levels in transmigrated and nontransmigrated neutrophils 1 or 4 h after addition of neutrophils to IL-1-stimulated human umbilical vein endothelial cells (HUVEC). Data are presented as fold change over naïve neutrophils ± SE (*P < 0.05 compared with naïve neutrophils; **P < 0.01 compared with nontransmigated, naïve neutrophils or neutrophils at 4 h; n = 4). B: OSM protein levels after neutrophil transendothelial migration. The cell-free supernatant was collected and analyzed by ELISA for OSM from control (naïve) neutrophils incubated under nonadhesive conditions, nontransmigrated neutrophils, and transmigrated cells at 1 and 4 h after addition of neutrophils to the monolayers of IL-1-stimulated HUVEC (*P < 0.01 compared with all supernatants from the transmigration chamber; n = 4).
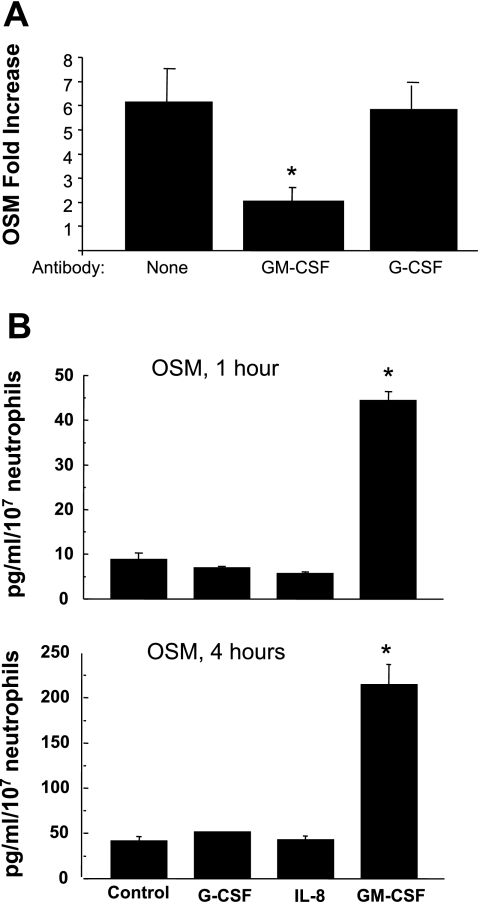
The contribution of G-CSF, GM-CSF, and IL-8 to this increased expression of OSM was evaluated by addition of blocking antibodies to the culture coincident with the addition of neutrophils. Anti-GM-CSF was effective in reducing the expression of OSM (Fig. 2A), whereas anti-G-CSF and anti-IL-8 (data not shown) failed to reduce expression. In a separate experiment, the addition of recombinant GM-CSF to cultures of isolated neutrophils resulted in a significant increase in OSM protein secretion after 1 or 4 h (Fig. 2B). However, recombinant G-CSF or IL-8 had no effect on OSM protein levels.
Fig. 2.
Polymorphonuclear neutrophil (PMN) OSM expression is dependent on granulocyte (G) macrophage (M)-colony-stimulating factor (GM-CSF) but not G-CSF. A: effects of blocking endogenous G-CSF and GM-CSF on OSM expression in neutrophils. Isolated neutrophils were incubated on monolayers of IL-1-stimulated HUVEC for 1 h with and without blocking antibody against GM-CSF or G-CSF. The neutrophils were collected and mRNA levels for OSM were determined by quantitative real-time PCR. Data are presented as fold change in transmigrated over nontransmigrated neutrophils ± SE. *P < 0.01 compared with absence of blocking antibody or presence of anti-G-CSF antibody; n = 6. B: OSM release by PMNs is dependent on GM-CSF but not G-CSF or IL-8. Isolated neutrophils were incubated in the presence of recombinant G-CSF, IL-8, or GM-CSF for either 1 or 4 h. Culture supernatant was collected and analyzed for OSM by ELISA. *P < 0.01 compared with all other groups.
Role of adhesive interactions in enhanced PMN OSM expression.
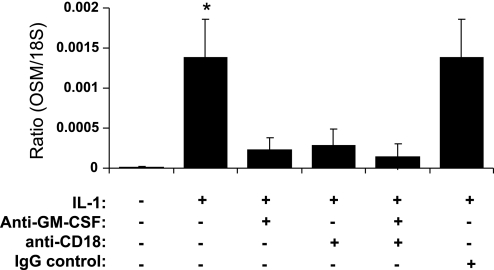
We next investigated whether conditioned media from IL-1-activated HUVEC was independently capable of inducing OSM expression in neutrophils. There was no change in OSM message levels after exposure of neutrophils to media from ECs activated with IL-1 for 4 h (data not shown). Therefore, soluble factors, mainly GM-CSF as we have already demonstrated, appear to be incapable of independently mediating increased OSM expression in neutrophils. In fact, assessment of GM-CSF quantities in the conditioned media of HUVEC previously activated with IL-1 for 4 h then coincubated with PMNs for 1 h revealed 21 ± 0.7 pg·ml·10−7 cells (data not shown). This measurement is consistent with other reports (7, 37) and recognized in our report as sufficient to promote enhanced neutrophil chemotaxis. We therefore postulated that GM-CSF-mediated increase in PMN OSM expression could be adhesion dependent. To confirm this, a neutralizing antibody against β2 integrin (CD18) was added to the transwell study in the presence and/or absence of anti-GM-CSF neutralizing antibody. Our results revealed elevated OSM message level in neutrophils after contact with EC that is attenuated following addition of anti-GM-CSF Ab consistent with previous observations (Fig. 3). Preincubation of neutrophils with anti-CD18 Ab for 15 min followed by their addition to HUVEC monolayer significantly reduced PMN OSM levels compared with neutrophils in contact with IL-1-activated EC. Addition of both anti-GM-CSF and anti-CD18 seemed to give similar results as to addition of each independently. A control IgG isotype was ineffective in altering OSM levels and gave similar results to what is seen in absence of any antibody.
Fig. 3.
Effects of blocking β2-integrin (CD18) on OSM production in neutrophils contacting EC. Neutrophils were preincubated with or without anti-CD18 or IgG isotype for 15 min at which time they were added to IL-1-activated EC present in the upper chamber of transmigration assay in the presence or absence of anti-GM-CSF neutralizing antibody. Data shown are means of four experiments. *P < 0.05 compared with all other groups.
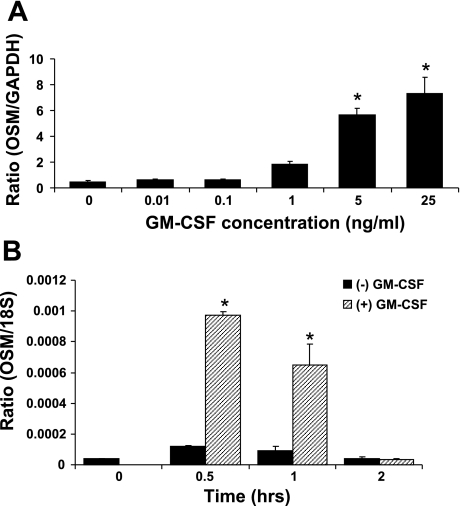
To further confirm that GM-CSF-induced OSM expression by neutrophils is due to engagement of the β2 integrins, PMNs were incubated in the presence and/or absence of GM-CSF and anti-CD18 Ab on fibrinogen-coated culture plates and compared with agarose (nonadhesive)-coated plates. Fibrinogen is a native ligand of the β2 integrins, thus this less complex setting compared with HUVEC transwell setup will aid in pinpointing the contribution of β2 integrin-mediated adhesion to GM-CSF induction of OSM in PMN. But first, we wanted to select the appropriate conditions for this study; thus we performed a dose-response study that revealed GM-CSF-induction effect on OSM expression was dose dependent with an EC50 of ∼5 ng/ml (Fig. 4A). However, OSM expression peaked at ∼30 min and then rapidly declined by 2 h to levels seen in absence of GM-CSF (Fig. 4B). Therefore, we chose to stimulate PMN in our following study with 5 ng/ml GM-CSF for 30 min only.
Fig. 4.
GM-CSF-induced OSM expression in neutrophils in a dose-dependent manner. A: dose-response of GM-CSF on OSM expression in neutrophils. Isolated nuetrophils were incubated with various concentrations of GM-CSF for 1 h at which point were analyzed for OSM expression using RT PCR (*P < 0.01 compared with control nontreated neutrophils; n = 3). B: time course of OSM mRNA expression in GM-CSF-induced neutrophils. Isolated neutrophils were incubated with 5 ng/ml GM-CSF and OSM expression was analyzed for each time point using RT-PCR (*P < 0.01 compared with zero time point; n = 3).
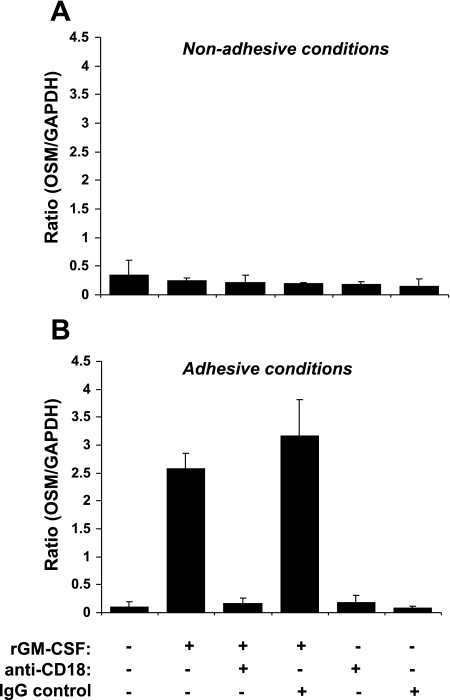
Under agarose (nonadhesive to PMN) experimental conditions, addition of rGM-CSF to isolated neutrophils did not alter OSM expression levels compared with control nonstimulated cells (Fig. 5A). However, OSM levels increased tremendously after incubation with stimulated neutrophils on fibrinogen (a Mac-1 ligand)-coated plates (Fig. 5B). Addition of anti-CD18 neutralizing antibody to PMN followed by GM-CSF stimulation in agarose-coated plates did not alter PMN OSM expression level (Fig. 5A). However, precincubation of PMN with anti-CD18 before their GM-CSF stimulation in fibrinogen-coated plates profoundly decreased OSM expression (Fig. 5B), indicating GM-CSF-mediated OSM increase is adhesion dependent. Interestingly, the levels of OSM expression in the nonstimulated PMN incubated on fibrinogen-coated plates were similar to the prestimulation levels observed under nonadhesive conditions, indicating that adherence in itself does not account for the enhanced OSM expression. Addition of control IgG isotype in the presence of GM-CSF stimulation did not affect OSM levels. However, lack of OSM expression was observed after addition of IgG in the absence of GM-CSF under adhesive or nonadhesive conditions similar to what is seen in nonstimulated cells. Similarly, addition of anti-CD18 with no stimulation had no effect on OSM levels.
Fig. 5.
Blocking β2 integrins inhibits GM-CSF-induced OSM increase in neutrophils. Human PMN were incubated in the presence and/or absence of GM-CSF and anti-CD18 Ab on fibrinogen-coated culture plates (A) and compared with agarose (nonadhesive)-coated plates (B). Results are representative of three independent experiments.
DISCUSSION
The physiological roles of EC-derived PMN activating molecules responsible for PMN transmigration have been widely explored using functional blocking antibodies and molecule-deficient mice (40). For example, IL-1 activation of ECs generates activators of neutrophils such as G-CSF and GM-CSF, which themselves could play an important role in the development of inflammatory responses in vitro and in vivo. This paper provides evidence that contact with activated ECs is sufficient to induce expression and release of OSM by neutrophils. That this phenomenon is primarily the result of endothelium-derived GM-CSF is supported by the observed presence of GM-CSF in the medium of IL-1-activated ECs and the ability of anti-GM-CSF (in contrast to anti-G-CSF or anti-IL-8) to prevent OSM expression.
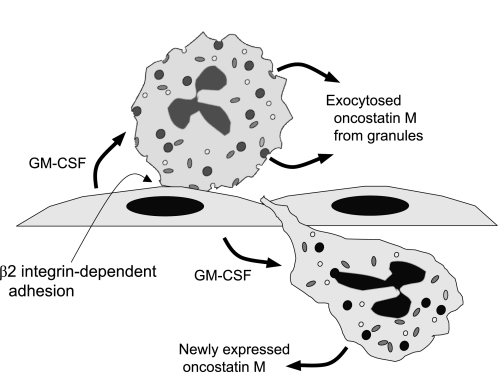
Transmigrated neutrophils exhibited, within 1 h of contacting the activated endothelial monolayer, levels of mRNA and protein release significantly greater than nontransmigrated neutrophils; though both transmigrated and nontransmigrated cells expressed more OSM than naïve neutrophils. In addition, it is known that OSM is contained in naïve neutrophils in a compartment from which it can be readily released upon stimulation (15). Thus neutrophils may deliver OSM to an inflammatory site by two mechanisms: the rapid release from the granular storage pool and new synthesis induced by endothelial GM-CSF (Fig. 6).
Fig. 6.
Proposed model of endothelial GM-CSF influence on neutrophils adherent to and transmigrating through vascular endothelial cell monolayers. GM-CSF apparently stimulates release of prestored OSM from neutrophil granules and new synthesis of OSM that requires β2 integrin-dependent adhesion of the stimulated neutrophils. Release from granules is a rapid event occurring within minutes and new synthesis is more delayed.
The finding that the process of adhesion and transmigration initiates OSM synthesis may explain other studies showing increased OSM production by exudated PMNs. Hurst et al. (18) found elevated OSM concentrations in peritoneal dialysate effluents obtained from patients with clinical peritonitis, which directly correlated with the degree of neutrophil infiltration. Similarly, Grenier et al. (16) found that OSM concentrations were significantly higher in bronchiolar lavage fluid from patients with pneumonia, and the amount of OSM correlated strongly with the absolute number of PMN in BAL fluid. Our data supports the claims of these studies as it clearly demonstrated using a transwell migration model a correlation of OSM increased levels with number of transmigrated neutrophils.
Previous studies have explored the effect of OSM as a potent modulator of the cytokines involved in the inflammatory response (2, 31). For example, it has been previously shown tht OSM inhibits IL-8 and granulocyte-macrophage colony-stimulating factor production by lung fibroblasts (29). In contrast, we sought in our report to explore the effects on OSM after blocking IL-8, GM-CSF, along with G-CSF. Only GM-CSF inhibition affected OSM levels while IL-8 and G-CSF were dispensable. Thus it can be deduced that GM-CSF plays a major role in OSM production and release by neutrophils, which is consistent with previous reports showing GM-CSF key role in mediating acute inflammation (26). Furthermore, the mechanisms of GM-CSF activation of PMN have been studied, and one report focused on GM-CSF-mediated activation of STAT proteins in nuclear extracts of human PMN (11). Results of this study showed that two known STAT5-regulated genes, encoding pim-1 and OSM proteins, failed to be induced by GM-CSF in PMN. This can be explained by our findings that GM-CSF-induced OSM increase in neutrophils is adhesion dependent. In fact, comparison of OSM expression and protein levels between transmigrated and nontransmigrated PMNs showed a synergistic effect of both adhesion and transmigration with the former being an essential part for OSM increase. This was supported by the finding that conditioned media from IL-1-activated endothelium had no effect on OSM levels in neutrophils alone. In fact, although GM-CSF level was significant in the conditioned media, however, absence of adhesive effects made GM-CSF presence alone incapable of altering PMN OSM levels.
The possible physiological significance of the observed effect of GM-CSF was strengthened by the fact that PMN adhering to immobilized fibrinogen, a matrix that is functionally equivalent to fibrin, elicited the same response exhibited in transwell migration model. Specifically, GM-CSF-mediated increase of OSM levels in PMNs under adhesive conditions was abrogated upon blocking of CD18 adhesion. Thus this demonstrated that while β2 integrin-mediated adhesion might suffice to induce gene activation in human PMN, there still remains a need for the right cytokine activation and production by ECs. In this case, endothelium-derived GM-CSF appears to exert differential effects on neutrophil behavior demonstrated by increased migration and function as exemplified by increased production of OSM.
It has been previously shown that human PMN contain an intracellular pool of OSM that is rapidly released in degranulating conditions induced by phorbol myristate acetate or GM-CSF (15). The importance of this preexisting OSM pool has been implicated to be an early event in the multistep process of PMN activation. Subcellular fractionation of human neutrophils has been described previously to help investigators determine localization of neutrophil constituents (21). Neutrophil granules are an important reservoir of matrix-degrading enzymes and membrane receptors needed during neutrophil extravasation and diapedesis. In fact, this indicates that OSM itself may play a major role in further recruitment and migration of neutrophils. Indeed, it is well known that neutrophil-endothelial interactions trigger mobilization of secretory vesicles from neutrophils, which in turn enrich neutrophil surface with the β2-integrin CD11b/CD18 (3). In turn, translocated β2-integrins bind endothelial adhesion molecules of the intercellular adhesion molecule-family, which mediates firm adhesion and initiates neutrophil transmigration (5). OSM and its receptor (OSMRβ) have been previously implicated in coordinating leukocyte trafficking via regulation of inflammatory chemokine expression (17). Furthermore, OSM has been shown to control differential expression of inflammatory chemokines (10) and therefore probably facilitate additional leukocyte recruitment as part of local inflammation. It is possible that OSM release from preexisting pool and de novo OSM production could play a major role in neutrophil migration. Indeed, a previous study by Fearon et al. (12) showed OSM and IL-1β promoted production of matrix metalloproteinases indicating OSM is a key player in EC migration. Conversely, OSM has also been shown to induce a protease inhibitor in smooth muscle cells suggesting a more general role for OSM and matrix remodeling (9). Exocytosis of gelatinase granules liberating OSM could probably play a significant role in the degradation of the vascular basement membrane during neutrophil extravasation.
In summary, our results collectively offer for the first time a correlation between levels of OSM following transendothelial migration. Endothelium-derived GM-CSF exerts a stimulating effect on OSM expression and release by neutrophils that is a adhesion-dependent event. There exists a readily available pool of OSM in neutrophil granules primarily in gelatinase (MMP-9) granules that could play a role in neutrophil extravasation and migration. Overall, increased transmigration and adhesion both lead to increased production of OSM by neutrophils, which then may play a major role in inflammatory response.
GRANTS
This work was supported by National Institutes of Health Grants DK-064233 and EY018239 and United States Department of Agriculture Grant 6250-51000-046.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
REFERENCES
- 1. Begley CG, Lopez AF, Nicola NA, Warren DJ, Vadas MA, Sanderson CJ, Metcalf D. Purified colony-stimulating factors enhance the survival of human neutrophils and eosinophils in vitro: a rapid and sensitive microassay for colony-stimulating factors. Blood 68: 162–166, 1986 [PubMed] [Google Scholar]
- 2. Boniface K, Diveu C, Morel F, Pedretti N, Froger J, Ravon E, Garcia M, Venereau E, Preisser L, Guignouard E, Guillet G, Dagregorio G, Pène J, Moles J.P, Yssel H, Chevalier S, Bernard FX, Gascan H, Lecron JC. Oncostatin M secreted by skin infiltrating T lymphocytes is a potent keratinocyte activator involved in skin inflammation. J Immunol 178: 4615–4622, 2007 [DOI] [PubMed] [Google Scholar]
- 3. Borregaard N, Kjeldsen L, Sengelov H, Diamond MS, Springer TA, Anderson HC, Kishimoto TK, Bainton DF. Changes in subcellular localization and surface expression of L-selectin, alkaline phosphatase, and Mac-1 in human neutrophils during stimulation with inflammatory mediators. J Leukoc Biol 56: 80–87, 1994 [DOI] [PubMed] [Google Scholar]
- 4. Burns AR, Walker DC, Brown ES, Thurmon LT, Bowden RA, Keese CR, Simon SI, Entman ML, Smith CW. Neutrophil transendothelial migration is independent of tight junctions and occurs preferentially at tricellular corners. J Immunol 159: 2893–2903, 1997 [PubMed] [Google Scholar]
- 5. Carlos TM, Harlan JM. Leukocyte-endothelial adhesion molecules. Blood 84: 2068–2101, 1994 [PubMed] [Google Scholar]
- 6. Chakraborty A, Hentzen ER, Seo SM, Smith CW. Granulocyte colony-stimulating factor promotes adhesion of neutrophils. Am J Physiol Cell Physiol 284: C103–C110, 2003 [DOI] [PubMed] [Google Scholar]
- 7. Coxon A, Tang T, Mayadas TN. Cytokine-activated endothelial cells delay neutrophil apoptosis in vitro and in vivo: a role for granulocyte/macrophage colony-stimulating factor. J Exp Med 190: 923–934, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Cross A, Edwards SW, Bucknall RC, Moots RJ. Secretion of oncostatin M by neutrophils in rheumatoid arthritis. Arthritis Rheum 50: 1430–1436, 2004 [DOI] [PubMed] [Google Scholar]
- 9. Demyanets S, Kaun C, Rychli K, Rega G, Pfaffenberger S, Afonyushkin T, Bochkov V.N, Maurer G, Huber K, Wojta J. The inflammatory cytokine oncostatin M induces PAI-1 in human vascular smooth muscle cells in vitro via PI 3-kinase and ERK1/2-dependent pathways. Am J Physiol Heart Circ Physiol 293: H1962–H1968, 2007 [DOI] [PubMed] [Google Scholar]
- 10. Elbjeirami WM, Truong LD, Tawil A, Wang W, Dawson S, Lan HY, Zhang P, Garcia GE, Wayne Smith C. Early differential expression of oncostatin M in obstructive nephropathy. J Interferon Cytokine Res 30: 513–23, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Epling-Burnette PK, Garcia R, Bai F, Ismail S, Loughran TP, Jr, Djeu JY, Jove R, Wei S. Carboxy-terminal truncated STAT5 is induced by interleukin-2 and GM-CSF in human neutrophils. Cell Immunol 217: 1–11, 2002 [DOI] [PubMed] [Google Scholar]
- 12. Fearon U, Mullan R, Markham T, Connolly M, Sullivan S, Poole AR, FitzGerald O, Bresnihan B, Veale DJ. Oncostatin M induces angiogenesis and cartilage degradation in rheumatoid arthritis synovial tissue and human cartilage cocultures. Arthritis Rheum 54: 3152–3162, 2006 [DOI] [PubMed] [Google Scholar]
- 13. Gonzalez A, El-Bjeirami W, West J, McIntire L, Smith W. Transendothelial migration enhances integrin-dependent human neutrophil chemokinesis. J Leukoc Biol 81: 686–695, 2007 [DOI] [PubMed] [Google Scholar]
- 14. Goren I, Kampfer H, Muller E, Schiefelbein D, Pfeilschifter J, Frank S. Oncostatin M expression is functionally connected to neutrophils in the early inflammatory phase of skin repair: implications for normal and diabetes-impaired wounds. J Invest Dermatol 126: 628–637, 2006 [DOI] [PubMed] [Google Scholar]
- 15. Grenier A, Dehoux M, Boutten A, Arce-Vicioso M, Durand G, Gougerot-Pocidalo MA, Chollet-Martin S. Oncostatin M production and regulation by human polymorphonuclear neutrophils. Blood 93: 1413–1421, 1999 [PubMed] [Google Scholar]
- 16. Grenier A, Combaux D, Chastre J, Gougerot-Pocidalo MA, Gibert C, Dehoux M, Chollet-Martin S. Oncostatin M production by blood and alveolar neutrophils during acute lung injury. Lab Invest 81: 133–41, 2001 [DOI] [PubMed] [Google Scholar]
- 17. Hams E, Colmont CS, Dioszeghy V, Hammond VJ, Fielding CA, Williams AS, Tanaka M, Miyajima A, Taylor PR, Topley N, Jones SA. Oncostatin M receptor-β signaling limits monocytic cell recruitment in acute inflammation. J Immunol 181: 2174–2180, 2008 [DOI] [PubMed] [Google Scholar]
- 18. Hurst SM, McLoughlin RM, Monslow J, Owens S, Morgan L, Fuller GM, Topley N, Jones SA. Secretion of onostatin M by ifiltrating neutrophils: regulation of IL-6 and chemokine expression in human mesothelial cells. J Immunol 169: 5244–5251, 2002 [DOI] [PubMed] [Google Scholar]
- 19. Kerfoot SM, Raharjo E, Ho M, Kaur J, Serirom S, McCafferty DM, Burns AR, Patel KD, Kubes P. Exclusive neutrophil recruitment with oncostatin M in a human system. Am J Pathol 159: 1531–1539, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kjeldsen L, Bjerrum O.W, Askaa J, Borregaard N. Subcellular localization and release of human neutrophil gelatinase, confirming the existence of separate gelatinase-containing granules. Biochem J 287: 603–610, 1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kjeldsen L, Sengelov H, Borregaard N. Subcellular fractionation of human neutrophils on percoll density gradients. J Immunol Methods 232: 131–143, 1999 [DOI] [PubMed] [Google Scholar]
- 22. Kjeldsen L, Sengelov H, Lollike K, Nielsen MH, Borregaard N. Isolation and characterization of gelatinase granules from human neutrophils. Blood 83: 1640–1649, 1994 [PubMed] [Google Scholar]
- 23. Lafontant PJ, Burns AR, Donnachie E, Haudek SB, Smith CW, Entman ML. Oncostatin M differentially regulates CXC chemokines in mouse dardiac fibroblasts. Am J Physiol Cell Physiol 291: C18–C26, 2006 [DOI] [PubMed] [Google Scholar]
- 24. Langdon C, Kerr C, Tong L, Richards CD. Oncostatin M regulates eotaxin expression in fibroblasts and eosinophilic inflammation in C57BL/6 Mice. J Immunol 170: 548–555, 2003 [DOI] [PubMed] [Google Scholar]
- 25. Langdon C, Leith J, Smith F, Richards CD. Oncostatin M stimulates monocyte chemoattractant protein-1- and interleukin-1-induced matrix metalloproteinase-1 production by human synovial fibroblasts in vitro. Arthritis Rheum 40: 2139–2146, 1997 [DOI] [PubMed] [Google Scholar]
- 26. Lawlor KE, Wong PK, Campbell IK, van Rooijen N, Wicks IP. Acute CD4+ T lymphocyte-dependent interleukin-1-driven arthritis selectively requires interleukin-2 and interleukin-4, joint macrophages, granulocyte-macrophage colony-stimulating factor, interleukin-6, andleukemia inhibitory factor. Arthritis Rheum 52: 3749–3754, 2005 [DOI] [PubMed] [Google Scholar]
- 27. Modur V, Feldhaus MJ, Weyrich AS, Jicha DL, Prescott SM, Zimmerman GA, McIntyre TM. Oncostatin M is a proinflammatory mediator. In vivo effects correlate with endothelial cell expression of inflammatory cytokines and adhesion molecules. J Clin Invest 100: 158–168, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Mollinedo F, Nakajima M, Llorens A, Barbosa E, Callejo S, Gajate C, Fabra A. Major co-localization of the extracellular-matrix degradative enzymes heparanase and gelatinase in tertiary granules of human neutrophils. Biochem J 327: 917–923, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Richards CD, Langdon C, Botelho F, Brown TJ, Agro A. Oncostatin M inhibits IL-1 induced expression of IL-8 and granulocyte-macrophage colony-stimulating factor by synovial and lung fibroblasts. J Immunol 156: 343–349, 1996 [PubMed] [Google Scholar]
- 30. Rose TM, Bruce AG. Oncostatin M is a member of a cytokine family that includes leukemia-inhibitory factor, granulocyte colony-stimulating factor, and interleukin 6. Proc Natl Acad Sci USA 88: 8641–8645, 1991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Rychli K, Kaun C, Hohensinner PJ, Rega G, Pfaffenberger S, Vyskocil E, Breuss JM, Furnkranz A, Uhrin P, Zaujec J, Niessner A, Maurer G, Huber K, Wojta J. The inflammatory mediator oncostatin M induces angiopoietin 2 expression in endothelial cells in vitro and in vivo. J Thromb Haemost 8: 596–604, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Senaldi G, Varnum B.C, Sarmiento U, Starnes C, Lile J, Scully S, Guo J, Elliott G, McNinch J, Shaklee CL, Freeman D, Manu F, Simonet WS, Boone T, Chang MS. Novel neurotrophin-1/B cell-stimulating factor-3: a cytokine of the IL-6 family. Proc Natl Acad Sci USA 96: 11458–11463, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Sengelov H, Boulay F, Kjeldsen L, Borregaard N. Subcellular localization and translocation of the receptor for N-formylmethionyl-leucyl-phenylalanine in human neutrophils. Biochem J 299: 473–479, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Sengelov H, Follin P, Kjeldsen L, Lollike K, Dahlgren C, Borregaard N. Mobilization of granules and secretory vesicles during in vivo exudation of human neutrophils. J Immunol 154: 4157–4165, 1995 [PubMed] [Google Scholar]
- 35. Smith CW, Rothlein R, Hughes BJ, Mariscalco MM, Schmalstieg FC, Anderson DC. Recognition of an endothelial determinant for CD18-dependent human neutrophil adherence and transendothelial migration. J Clin Invest 82: 1746–1756, 1988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Sullivan R, Griffin JD, Simons ER, Schafer AI, Meshulam T, Fredette JP, Maas AK, Gadenne AS, Leavitt JL, Melnick DA. Effects of recombinant human granulocyte and macrophage colony-stimulating factors on signal transduction pathways in human granulocytes. J Immunol 139: 3422–3430, 1987 [PubMed] [Google Scholar]
- 37. Takahashi T, Hato F, Yamane T, Fukumasu H, Suzuki K, Ogita S, Nishizawa Y, Kitagawa S. Activation of human neutrophil by cytokine-activated endothelial cells. Circ Res 88: 422–429, 2001 [DOI] [PubMed] [Google Scholar]
- 38. Tanaka M, Miyajima A. Oncostatin M, a multifunctional cytokine. Rev Physiol Biochem Pharmacol 149: 39–52, 2003 [DOI] [PubMed] [Google Scholar]
- 39. Wagner JG, Roth RA. Neutrophil migration mechanisms, with an emphasis on the pulmonary vasculature. Pharmacol Rev 52: 349–374, 2000 [PubMed] [Google Scholar]
- 40. Wang Q, Doerschuk CM. The signaling pathways induced by neutrophil-endothelial cell adhesion. Antioxid Redox Signal 4: 39–47, 2002 [DOI] [PubMed] [Google Scholar]
- 41. Welte K, Platzer E, Lu L, Gabrilove JL, Levi E, Mertelsmann R, Moore MA. Purification and biochemical characterization of human pluripotent hematopoietic colony-stimulating factor. Proc Natl Acad Sci USA 82: 1526–1530, 1985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Whitlock BB, Gardai S, Fadok V, Bratton D, Henson PM. Differential roles for alpha(M)beta(2) integrin clustering or activation in the control of apoptosis via regulation of Akt and ERK survival mechanisms. J Cell Biol 151: 1305–1320, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Xu S, Hoglund M, Venge P. The Effect of granulocyte colony-stimulating factor (G-CSF) on the degranulation of secondary granule proteins from human neutrophils in vivo may be indirect. Br J Haematol 93: 558–568, 1996 [DOI] [PubMed] [Google Scholar]
- 44. Yong KL. Granulocyte colony-stimulating factor (G-CSF) increases neutrophil migration across vascular endothelium independent of an effect on adhesion: comparison with granulocyte-macrophage colony-stimulating factor (GM-CSF). Br J Haemtol 94: 40–47, 1996 [DOI] [PubMed] [Google Scholar]
- 45. Zhang XG, Gu JJ, Lu ZY, Yasukawa K, Yancopoulos GD, Turner K, Shoyab M, Taga T, Kishimoto T, Bataille R. Ciliary neurotropic factor, interleukin 11, leukemia inhibitory factor, and oncostatin m are growth factors for human myeloma cell lines using the interleukin 6 signal transducer Gp130. J Exp Med 179: 1337–1342, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Yao L, Pan L, Setiadi H, Patel KD, McEver RP. Interleukin 4 or Oncostatin M induces a prolonged increase in p-selectin mRNA and protein in human endothelial cells. J Exp Med 184: 81–92, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]