Abstract
Objectives
Viusid is a nutritional supplement with recognised antioxidant and immunomodulatory properties which could have beneficial effects on cirrhosis-related clinical outcomes such as survival, disease progression and development of hepatocellular carcinoma (HCC). This study evaluated the efficacy and safety of viusid in patients with HCV-related decompensated cirrhosis.
Design
A randomised double-blind and placebo-controlled study was conducted in a tertiary care academic centre (National Institute of Gastroenterology, Havana, Cuba). The authors randomly assigned 100 patients with HCV-related decompensated cirrhosis to receive viusid (three oral sachets daily, n=50) or placebo (n=50) during 96 weeks. The primary outcome of the study was overall survival at 96 weeks, and the secondary outcomes included time to disease progression, time to HCC diagnosis, time to worsening of the prognostic scoring systems Child–Pugh and Model for End-Stage Liver Disease, and time to a new occurrence or relapse for each one of the main clinical complications secondary to portal hypertension at 96 weeks.
Results
Viusid led to a significant improvement in overall survival (90%) versus placebo (74%) (HR 0.27, 95% CI 0.08 to 0.92; p=0.036). A similar improvement in disease progression was seen in viusid-treated patients (28%), compared with placebo-treated patients (48%) (HR 0.47, 95% CI 0.22 to 0.89; p=0.044). However, the beneficial effects of viusid were wholly observed among patients with Child–Pugh classes B or C, but not among patients with Child–Pugh class A. The cumulative incidence of HCC was significantly reduced in patients treated with viusid (2%) as compared with placebo (12%) (HR 0.15, 95% CI 0.019 to 0.90; p=0.046). Viusid was well tolerated.
Conclusions
The results indicate that treatment with viusid leads to a notable improvement in overall clinical outcomes such as survival, disease progression and development of HCC in patients with HCV-related decompensated cirrhosis.
Trial registration number
Article summary
Article focus
Hepatitis C virus (HCV)-related decompensated cirrhotic patients have a poor therapeutic response and reduced tolerance to the current standard of care therapy.
Therapeutic goals in these patients should be directed towards reducing liver-related morbidity and mortality, and the need for liver transplantation.
Viusid is a nutritional supplement with recognised antioxidant and immunomodulatory properties that could modulate the histological pattern of CHC, especially inflammation and fibrosis, in an attempt to halt disease progression and consequently improve liver function and liver-related morbidity and mortality, and prevent development of hepatocellular carcinoma (HCC).
Key messages
The administration of viusid to HCV-related decompensated cirrhotic patients induced a significant improvement of overall survival, a significant reduction in the disease progression and development of HCC.
The benefit of viusid was also seen in the secondary end point of worsening of the prognostic scores such as Model for End-Stage Liver Disease and Child–Pugh scores.
The viusid effects on survival and disease progression were selective for patients with advanced stage of liver disease (Child–Pugh B or C).
Viusid was well tolerated, and only minor transient adverse events such as nausea and diarrhoea were reported.
Strengths and limitations of this study
The main strength of this study was to demonstrate that viusid improves overall clinical outcomes (survival, HCC and disease progression) in cirrhotic patients who have failed to achieve sustained virological response with standard of care, and these benefits appear to be more prominent in patients with poorer liver function (Child–Pugh B or C).
The study was designed with a small sample size.
Further multicentre and large-scale studies are needed to corroborate the impact of viusid on the clinical outcomes in patients with HCV-related decompensated cirrhosis.
Introduction
Chronic hepatitis C virus (HCV) infection is a leading cause of end-stage liver disease and hepatocellular carcinoma (HCC) worldwide1 and the most common indication for orthotopic liver transplantation in the western world.2 Once HCV cirrhosis has developed, the risk of clinical decompensation is about 5% per year,3–5 and the risk of mortality is considerably high with a survival rate of 50% at 5 years.6 7 Cumulative data of patients with compensated cirrhosis indicate that the 5-year risk of decompensation is estimated to be 15–28%, and the annual risk of developing HCC is 1.4–6.7%.3 4 8 Liver-related mortality increases considerably as soon as decompensation is established, and then liver transplantation is the only successful therapeutic option. Unfortunately, once the liver is grafted, disease recurrence is universal. The recurrence of the infection leads to cirrhosis in approximately 25% of the transplant recipients within 5–10 years after transplantation. The cumulative probability of decompensation 1 year after cirrhosis is in the order of 30%, and the 1-year survival is 46%.9
HCV-related cirrhotic patients have a poor therapeutic response and reduced tolerance to the current standard of care (SOC) therapy.10–15 Peginterferon (PEG-IFN) plus ribavirin (RBV) is the recommended treatment strategy for patients with compensated cirrhosis.16 However, the efficacy of antiviral therapy is in this group significantly lower than in non-cirrhotic patients, achieving the poorest rates of sustained virological response (SVR, 5–25%) in patients with genotype 1–4.17 Current evidence indicates that antiviral treatment with PEG-IFN alone or in combination with RBV reduces the rate of clinical decompensation, improves liver-related survival and decreases the development of HCC, but only in those patients who achieved SVR.18–20 However, this benefit should be balanced with severe side effects that lead to therapy discontinuation and derangement of liver function in a high proportion of patients with Child–Pugh (CP) class B–C cirrhosis.
Thus, an effective treatment is needed immediately in cirrhotic patients who have failed to achieve SVR or with advanced disease to avoid further deterioration and death.
Several studies have demonstrated an important association between increased levels of products related to oxidative stress and advanced stages of the disease.21 22 Likewise, cytokine dysregulation is thought to play a crucial role in the persistence of viral infection and as a key mediator in inflammatory and fibrogenic processes in patients with HCV infection.23 Therefore, the administration of compounds with antioxidant and immunomodulatory properties could be a plausible strategy to halt the natural course of the disease, particularly in cirrhotic patients with advanced disease.
Viusid (Catalyis laboratory, Madrid, Spain) is a nutritional supplement that contains different molecules (ascorbic acid, zinc and glycyrrhizic acid) with recognised antioxidant and immunomodulatory properties (table 1).24–26 Glycyrrhizin (0.033 g), the most important active ingredient of the supplement, is known to have various immune-modulating, antiviral and biological response-modifier activities. It demonstrated different anti-inflammatory properties (increased production of interleukin 10 (is a potent anti-inflammatory cytokine which inhibits the syntheses of many pro-inflammatory proteins)), an anti-apoptotic effect, hepatocyte proliferation and stabilisation of hepatic cellular membranes.27–30
Table 1.
Ingredients of viusid
| Malic acid | 0.666 g |
| Glycyrrhizic acid | 0.033 g |
| Glucosamine | 0.666 g |
| Arginine | 0.666 g |
| Glycine | 0.333 g |
| Calcium pantothenate | 0.002 g |
| Ascorbic acid | 0.020 g |
| Folic acid | 66 μg |
| Cyanocobalamine | 0.3 μg |
| Zinc sulfate | 0.005 g |
| Pyridoxal | 0.6 mg |
Encouraging effects of viusid on liver histology have been reported in patients with non-alcoholic fatty liver disease and chronic hepatitis C.31 32 The authors reported that the addition of viusid to the conventional interferon/ribavirin therapy was associated with significant histological and biochemical improvements, especially in patients without a sustained virological response. In another study, the same authors showed that the administration of viusid combined with a lifestyle modification based on a hypocaloric diet and exercise during 6 months was associated with marked histological improvements in steatosis, lobular inflammation, ballooning and NAFLD activity score in patients with non-alcoholic fatty liver disease. No significant clinical and laboratory adverse events (AEs) have been reported with the use of viusid in previous trials.
Recent data suggest that viusid improves oxidative stress through reduction of lipid peroxidation products and has an immunomodulatory effect on cytokine secretion via increased production of IFN-γ and interleukin (IL)-10, decreased production of IL-1α and stabilised tumour necrosis factor-α secretion in patients with HCV who have failed previous antiviral treatment.33
All of these effects could modulate the histological pattern of CHC, especially inflammation and fibrosis in an attempt to halt disease progression and consequently improve liver function and liver-related morbidity and mortality, and prevent the development of HCC.
Thus, a randomised double-blind and placebo-controlled study was conducted to evaluate whether viusid may have a beneficial effect on survival, time to disease progression and time to diagnosis of HCC in HCV-related cirrhotic patients with decompensated disease.
Materials and methods
Participants
We recruited 100 patients with HCV liver-related cirrhosis at a tertiary care academic centre (National Institute of Gastroenterology, Havana, Cuba) between May 2005 and June 2007, and who fulfilled the following inclusion criteria: male and female patients of 18–70 years of age, clinical or histological diagnosis of cirrhosis, naïve patients or non-responders to previous treatment with PEG-IFN plus RBV with decompensated cirrhosis, defined as a Child–Pugh score ≥7 or clinical evidence or history of ascites, encephalopathy, upper-gastrointestinal bleeding and/or impaired hepatic synthetic function, who had contraindicated the antiviral treatment, absence of active alcoholism (alcohol abstinence was monitored at each clinic visit in the course of patient interview) and ability to provide informed consent. Patients were excluded if they had presence of other causes of liver disease, uncontrollable clinical or biochemical complications related to severe liver failure (hepatic encephalopathy, hepatorenal syndrome, gastrointestinal bleeding, serum total bilirubin >85 mmol/l (5 mg/dl), international normalised ratio >2.5), serum creatinine >180 mmol/l (2 mg/dl), positive screening for viral hepatitis A and B and HIV, pregnancy or lactation, concomitant disease with reduced life expectancy, severe psychiatric conditions, drug dependence, and if they had presence of other causes of liver diseases at entry into the study on the basis of ultrasonography and α-fetoprotein levels higher than 200 ng/l.
Ethics
The study was conducted in compliance with the Declaration of Helsinki and approved by the ethics committee and the institutional review board of the National Institute of Gastroenterology. All patients provided written informed consent for participation.
Interventions
After initial evaluation, all patients who met the eligibility criteria were consecutively enrolled in the study. They were randomly assigned to receive: viusid (three oral sachets daily, n=50) or placebo (three oral sachets daily, n=50) for 96 weeks.
Randomisation was conducted by blocks of four (block randomisation) and performed by a health worker experienced in randomisation techniques and not involved in the evaluation or treatment of the participants. The physicians, study coordinators and patients did not have access to the randomisation scheme.
The researchers, study coordinators and patients were blinded as to the treatment administered. When the patients were allocated, they brought their entry code to the pharmacy which was provided with the randomised list. The code was revealed to the researchers at the end of the study protocol. Catalysis, Madrid, Spain provided the viusid and placebo sachets. There was no difference in appearance, smell and flavour between viusid and placebo.
Treatment started 4 weeks after the clinical evidence of decompensation had been treated and controlled with appropriate therapy.
Clinical and laboratory assessment
All patients were closely monitored for clinical, biochemical and haematological assessment at baseline, weekly for the first 8 weeks, and every 8 weeks thereafter until the end of the study.
Clinical assessment included physical examination along with compliance with the study medication (verified through sachet count). Biochemical and haematological evaluations included complete blood count, liver tests, glucose, coagulation and renal-function tests.
We defined overweight as a BMI of 25 to 30 kg/m2 and defined obesity as a BMI of >30 kg/m2. Patient's data with diagnosis of diabetes mellitus at baseline, elevated fasting glucose levels (>6.1 mmol/l), a positive glucose tolerance test and antidiabetic medication used were recorded.
Liver ultrasonography and serum α-fetoprotein determinations were carried out at baseline and every 24 weeks during the study to screen for HCC.
An upper-digestive endoscopy was performed before admission.
The HCV-RNA level was quantified by PCR assay (Amplicor Monitor HCV v.2.0; Roche Molecular System; lower limit of detection, 600 IU/ml). HCV genotyping was performed by reverse hybridisation (Inno-LiPA HCV; Innogenetics, Ghent, Belgium).
Definition of outcomes
The terminology to define outcomes in this study was slightly modified as compared with the original protocol because it is more precise and less subjective to assess time-dependent clinical complications in cirrhotic patients. Additionally, primary and secondary outcomes are in accordance with standardised terminology used in the majority of the trials evaluating the impact of treatments on patients with HCV-related cirrhosis.
The primary outcome of the study was overall survival (OS), which was measured from the date of randomisation until the date of death (related to liver disease). Patients with liver-unrelated death or lost to follow-up were censored at the time of death or discontinuation, and patients undergoing liver transplantation were censored at the transplant date.
Secondary outcomes included the time to disease progression, time to diagnosis of HCC, time to worsening of the prognostic scoring systems Child–Pugh and Model for End-Stage Liver Disease (MELD), time to a new occurrence or relapse for each of the main clinical complications secondary to portal hypertension and safety.
The time to disease progression was reflected as the time between random assignment and disease progression, defined as the incidence of liver-related death, the development of HCC or the first occurrence or relapse (only for those patients with a previous history of clinical decompensation) of at least one of the following clinical conditions: ascites, hepatic encephalopathy, spontaneous bacterial peritonitis, hepatorenal syndrome or upper-gastrointestinal bleeding secondary to portal hypertension.
The time to diagnosis of HCC was calculated from the date of randomisation to the date of occurrence of HCC. Diagnosis of HCC was implemented using currently accepted diagnostic criteria for HCC.34–36
The time to worsening of the prognostic scores was defined as the time from randomisation to worsening of the Child–Pugh score in at least two points and the MELD score in at least four points on the basis of independent clinical evaluation on two consecutive study visits. The Child–Pugh and MELD scores are measures of the severity of liver disease, with higher numbers indicating greater decompensation.
The time to a new occurrence or relapse for each of the main clinical complications secondary to portal hypertension was defined from the date of randomisation to the date of a new occurrence or relapse (only for those patients with a previous history of clinical complications) of the following clinical conditions: ascites, hepatic encephalopathy, upper-gastrointestinal bleeding, hepatorenal syndrome and spontaneous bacterial peritonitis.
The evidence for each end point was verified and confirmed by two blinded independent hepatologists.
Safety was assessed by dynamic reports of AEs, clinical laboratory test (haematological and biochemical analysis), physical examination and measurement of vital signs. The presence of sepsis and hospitalisation were included in the safety reports. Episodes of sepsis were recorded, and they were diagnosed and treated according to recommended guidelines. Sepsis was graded as severe if requiring hospitalisation or treatment discontinuation.
Statistical methods
The baseline characteristics were summarised in percentage for categorical variables and as mean±SD for continuous variables. The χ2 test was applied to categorical variables. The two-sample t test was used to compare means, and the Mann–Whitney U test if they were not normally distributed. Outcome measurements included all patients who were randomised and received at least one dose of study medication (intention-to-treat analysis). The safety analysis included all treated patients who had at least one safety evaluation after baseline.
Both primary and secondary outcomes were analysed by the Kaplan–Meier method, and differences were compared using Cox proportional hazard models adjusted by sex and age, baseline CP and MELD scores, previous history of clinical decompensation, and current use of diuretics and propranolol.
We defined OS time at 96 weeks as a primary end point to compute sample size. The study was designed to have a statistical power of 80% to detect an absolute difference of 25% in the survival rates at 96 weeks (95% in the experimental group vs 70% in the control group). Considering a type I error of 0.05 and a type II error of 0.20, 43 patients per arm were needed to reach statistical significance. After considering patient loss as a result of dropout, we set the target number of patients at 50 per arm, or 100 in total.
All CIs, significance tests and resulting p values were two-sided, with an α level of 0.05.
Statistical analyses were performed using STATA software, release 11.
The study was designed by Catalysis Laboratory in conjunction with the principal investigator. The data were collected and analysed by the investigators. All authors had access to the data.
Results
Patients
Between May 2005 and June 2007, 124 patients were screened. One hundred of these patients met the eligibility criteria and were randomly assigned to the viusid (n=50) and the placebo arms (n=50). These patients were all included in the intention-to-treat analysis. Twenty-four patients were excluded from the study during the screening period because they did not meet the inclusion criteria, met one or more of the exclusion criteria or withdrew their consent. The flow of the participants through the trial is presented in figure 1. None of the patients received co-interventions during the trial that could have affected the outcomes. One death secondary to myocardial infarction occurred in each group of treatment during the study. Four of the seven patients with HCC were not discontinued and completed the study because diagnosis was made only at the end of the treatment.
Figure 1.
Flow of patients through the study. *Four patients with hepatocellular carcinoma (HCC) were not discontinued because diagnosis was made at the end of the treatment.
Demographic and baseline disease characteristics of the ITT population were generally well balanced between treatment arms (table 2). The patients' mean age was 57.5 years, and 60% were women. All patients had genotype 1 infection. The mean CP and MELD scores at baseline were 6.32 and 12.94, respectively.
Table 2.
Baseline characteristics
| Variable | Viusid (n=50) | Placebo (n=50) | p Value* |
| Age (years) | 58.5±8.9 | 56.6±8.4 | 0.29 |
| Sex, n (%) | |||
| Male | 22 (44%) | 18 (36%) | 0.41 |
| Female | 28 (56%) | 32 (64%) | |
| BMI (kg/m2) | 25.4±4.6 | 26.7±4.5 | 0.16 |
| BMI >25 (kg/m2), n (%) | 28 (56%) | 31 (62%) | 0.54 |
| Hepatitis C virus RNA >600 000 IU/ml | 42 (84%) | 38 (76%) | 0.45 |
| Genotype 1, n (%) | 50 (100%) | 50 (100%) | 1.0 |
| Clinical scores | |||
| Child–Pugh Class A | 32 (64%) | 29 (58%) | |
| Child–Pugh Class B | 15 (30%) | 15 (30%) | 0.56 |
| Child–Pugh Class C | 3 (6%) | 6 (12%) | |
| Model for End-Stage Liver Disease | 12.5±3.7 | 13.3±4.7 | 0.46 |
| History of diabetes or fasting glucose ≥7 (mmol/l), n (%) | 17 (34) | 21 (42) | 0.41 |
| Previous history of clinical decompensation, n (%)† | |||
| Ascites | 22 (44) | 14 (32) | 0.10 |
| Upper-gastrointestinal bleeding | 9 (18) | 5 (10) | 0.25 |
| Spontaneous bacterial peritonitis | 2 (4) | 2 (4) | 1.00 |
| Hepatic encephalopathy | 4 (8) | 3 (6) | 0.69 |
| Evidence of esophageal varices | 23 (46) | 18 (36) | 0.31 |
| Current propranolol use, n (%) | 13 (26) | 10 (20) | 0.65 |
| Mean doses | 70±17.7 | 80±37.7 | 0.37 |
| Current spironolactone use, n (%) | 21 (42) | 12 (24) | 0.09 |
| Mean doses | 84.5±39.1 | 111±40 | 0.14 |
| Current furosemide use, n (%) | 4 (8) | 5 (10) | 1.00 |
| Mean doses | 40±10 | 64±22 | 0.19 |
| Alanine aminotransferase (U/l) | 92.2±76.6 | 82.7±49.5 | 0.86 |
| Aspartate aminotransferase (U/l) | 105±80.2 | 94.1±56.6 | 0.72 |
| Fasting plasma glucose (mmol/l) | 4.9±1.2 | 5.1±1.3 | 0.70 |
| Alkaline phosphatase (mmol/l) | 290.4±108 | 281±78.8 | 0.96 |
| Creatinine (mmol/l) | 1±0.3 | 1±0.3 | 0.88 |
| Haemoglobin (g/l) | 125.8±13.8 | 129.5±17.6 | 0.32 |
| Cholesterol (mmol/l) | 3.85±0.9 | 3.85±1 | 0.50 |
| Total bilirubin (mmol/l) | 24.3±17.6 | 23.9±17.7 | 0.98 |
| Albumin (g/l) | 38.9±4.3 | 38.9±4.3 | 0.52 |
| Partial thromboplastin time (s) | 38.4±9.7 | 39.3±12.3 | 0.73 |
| Prothrombin time (s) | 4.7±2.5 | 5.5±3.7 | 0.38 |
| International normalized ratio | 1.49±0.3 | 1.58±0.4 | 0.38 |
| White blood cells (×103/μl) | 6.1±1.9 | 5.9±1.7 | 0.70 |
| Platelets (×103/μl) | 133.7±57.9 | 130.6±65.7 | 0.48 |
| Platelets <100×103/μl | 20 (40) | 24 (48) | 0.42 |
| α-Fetoprotein (ng/ml) | 11±16.9 | 10±12.7 | 0.25 |
Plus–minus values are means±SD. For all laboratory measures and for continuous demographics: p value Mann–Whitney U test. Proportions: percentage, p value χ2. The Child–Pugh and Model for End-Stage Liver Disease scores are measures of the severity of liver disease. Prothrombin time (s): value is expressed in seconds upper the utilised control. Partial thromboplastin time (s): value in seconds. To convert mmol/l of bilirubin to mg/dl, multiply by 0.0585. To convert mmol/l of creatinine to mg/dl, multiply by 0.01131.
p Values are for the comparison between viusid and placebo.
Previous history of clinical decompensation within 1 year before enrolment.
All patients with a previous history of hepatic decompensation were controlled and treated with appropriate therapy before trial admission.
At study entry, none of the patients had any evidence of HCC, ascites, hepatic encephalopathy, renal failure, upper-gastrointestinal bleeding or spontaneous bacterial peritonitis.
Efficacy (primary end point)
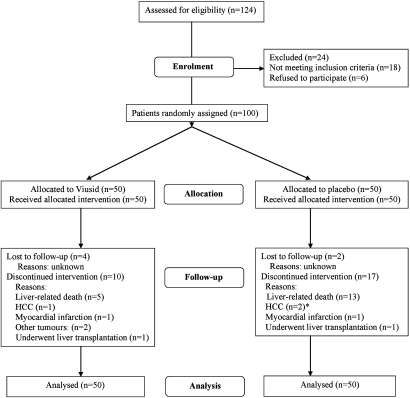
OS at 96 weeks was significantly higher in the patients assigned to the nutritional supplement (90% with a 95% CI 75 to 95) as compared with the patients assigned to placebo (74% with a 95% CI 56 to 83; HR 0.27; 95% CI 0.08 to 0.92; p=0.036; table 3). However, the beneficial effects of viusid on survival appear to be selective for patients with poor hepatic reserve (Child–Pugh B or C) (figure 2A). Survival in patients with CP classes B or C was significantly higher in the experimental group than in the placebo group (80% vs 48%; HR in the viusid group, 0.36; 95% CI 0.10 to 0.91; p=0.041).
Table 3.
Summary of outcome measures
| Variable | Viusid (N=50) | Placebo (N=50) | HR* (95% CI) | p Value |
| No of patients (%) | ||||
| Primary outcomes, no (%) | ||||
| Overall survival | 45 (90) | 37 (74) | 0.27 (0.08 to 0.92) | 0.036 |
| Secondary outcomes, no (%) | ||||
| Time to disease progression | 14 (28) | 24 (48) | 0.47 (0.22 to 0.89) | 0.044 |
| Time to diagnosis of hepatocellular carcinoma† | 1 (2) | 6 (12) | 0.15 (0.019 to 0.90) | 0.046 |
| Worsening of Child–Pugh score in at least two points | 7 (14) | 19 (38) | 0.34 (0.14 to 0.81) | 0.015 |
| Worsening of Model for End-Stage Liver Disease score in at least four points | 6 (12) | 15 (30) | 0.39 (0.15 to 0.92) | 0.042 |
| Ascites | 7 (14) | 16 (32) | 0.32 (0.11 to 0.90) | 0.031 |
| Hepatic encephalopathy | 1 (2) | 5 (10) | 0.20 (0.10 to 1.7) | 0.10 |
| Spontaneous bacterial peritonitis | 1 (2) | 5 (10) | 0.20 (0.13 to 1.7) | 0.09 |
| Upper-gastrointestinal bleeding | 8 (16) | 10 (20) | 0.78 (0.31 to 1.99) | 0.67 |
HRs were computed using the Cox proportional hazard model adjusted for sex and age, baseline Child–Pugh and Model for End-Stage Liver Disease scores, previous history of clinical decompensation, and current use of diuretics and propranolol. CI denotes the CI for the HR.
All cases of hepatocellular carcinoma were diagnosed during the second year of treatment.
Figure 2.
Kaplan–Meier curves for (A) survival and (B) time to disease progression according to Child–Pugh (CP) classes (A vs B or C). Time to disease progression was defined as the incidence of liver-related death, the development of hepatocellular carcinoma or the first occurrence or relapse (only for those patients with a previous history of hepatic decompensation) of at least one of the following clinical conditions: ascites, hepatic encephalopathy, spontaneous bacterial peritonitis, hepatorenal syndrome or upper-gastrointestinal bleeding secondary to portal hypertension. The CP score is a measure of the severity of liver disease. Numbers in parentheses show the number of events.
Efficacy (secondary end points)
The Kaplan–Meier estimates of the proportion of patients with disease progression at 96 weeks (table 3) were 28% (95% CI 19 to 45) in the experimental group and 48% (95% CI 38 to 66) in the control group. The HR for the viusid arm was 0.47 (95% CI 0.22 to 0.89; p=0.044). Nevertheless, this effect was seen among patients classified as Child–Pugh B or C, but not among patients with CP classes A (figure 2B). Among patients with CP scores B or C, the disease progression rates were lower in patients treated with the experimental intervention (47%) and were progressively higher in patients assigned to placebo arm (80%) (HR in the viusid arm, 0.51; 95% CI 0.23 to 0.96; p=0.047; figure 2B).
The cumulative incidence of HCC at 96 weeks was 2% (95% CI 0.3 to 15) in the active product-treated patients and 12% (95% CI 6 to 33) in the placebo group, with a HR for the group assigned to active product of 0.15 (95% CI 0.019 to 0.90; p=0.046) (table 3). All patients with HCC were diagnosed during the second year after randomisation. Two of the seven patients with HCC were eligible for liver transplantation, and three had transarterial chemoembolisation.
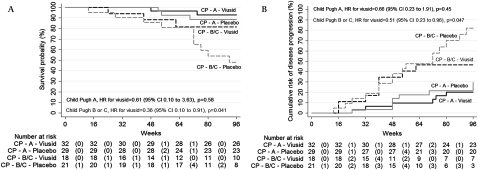
An increase in the CP score (figure 3A) occurred in seven patients (14%; 95% CI 8 to 32) allocated to the nutritional supplement group as compared with 19 patients (38%; 95% CI 30 to 59) allocated to the placebo group. The HR for the viusid arm was 0.34 (95% CI 0.14 to 0.81; p=0.015). Likewise, a significant worsening in the MELD score (figure 3B) was observed in 15 individuals (30%; 95% CI 21 to 49) assigned to placebo as compared with six individuals (12%; 95% CI 6 to 26) assigned to nutritional supplement, with an HR for the viusid group of 0.39 (95% CI 0.15 to 0.92, p=0.042).
Figure 3.
Kaplan–Meier estimates of the time to worsening of the (A) Child–Pugh (CP) and (B) Model for End-Stage Liver Disease (MELD) scores during the treatment. Numbers in parentheses show the number of events. The MELD and CP scores are measures of the severity of liver disease.
The cumulative incidence of ascites at 96 weeks was significantly higher in the patients assigned to placebo (32%; 95% CI 14 to 39) than in the patients assigned to viusid (14%, 95% CI 7 to 28). The HR for the viusid arm was 0.32 (95% CI 0.11 to 0.90; p=0.031), but the differences were not statistically significant for hepatic encephalopathy, upper-gastrointestinal bleeding, and spontaneous bacterial peritonitis. Type 2 hepatorenal syndrome was reported in one patient of each group of treatment. The primary and secondary outcome measures are summarised in table 3.
Safety
Cramps (33%), asthenia (32%), sepsis (27%), predominantly bacterial infections and muscle pain (24%) were the most frequent AEs. The main causes of sepsis were urinary infection (11%), SBP (6%), pneumonia (5%) and lymphangitis (3%). None of the patients had infections related to leucopenia or neutropenia.
A smaller proportion of patients treated with the nutritional product than treated with placebo had fatigue (experimental group, 10%; placebo, 26%; p=0.04), cramps (experimental group, 22%; placebo, 44%; p=0.02) and sepsis (experimental group, 14%; placebo, 40%; p<0.01), respectively.
A high percentage of patients (24%) were hospitalised during the study secondary to episodes of hepatic decompensation or severe sepsis; however, there was no difference between the treatment groups. A summary of AEs is given in table 4. There were no significant laboratory abnormalities in the two study groups. Neither was there any incidence of viusid discontinuation or dose modification secondary to AEs.
Table 4.
Incidence of adverse events*
| Variable | Viusid (n=50) | Placebo (n=50) | p Value† |
| No (%) | No (%) | ||
| Asthenia | 12 (24) | 20 (40) | 0.08 |
| Fatigue or malaise | 5 (10) | 13 (26) | 0.04 |
| Muscle pain | 8 (16) | 16 (32) | 0.06 |
| Anorexia | 5 (10) | 9 (18) | 0.24 |
| Cramps | 11 (22) | 22 (44) | 0.02 |
| Discomfort on the right upper quadrant | 7 (14) | 13 (26) | 0.13 |
| Gingival bleeding | 5 (10) | 10 (20) | 0.16 |
| Epistaxis | 5 (10) | 10 (20) | 0.16 |
| Nausea | 5 (10) | 1 (2) | 0.12 |
| Diarrhoea | 5 (5) | 1 (2) | 0.12 |
| Sepsis | 7 (14) | 20 (40) | <0.01 |
| Hospitalisation | 9 (18) | 15 (30) | 0.24 |
The adverse events listed are those recorded in at least 5% of the patients in either study group.
p Values were calculated on the basis of the two-sided χ2.
Discussion
HCV-related cirrhotic patients represent an important population with increased morbidity and mortalities. Unfortunately, current antiviral therapy, especially for patients with decompensated disease, is generally limited by side effects, and early discontinuation is common. Therefore, liver transplantation is the most appropriate therapeutic option for these patients. Recent studies have demonstrated encouraging SVR rates and, consequently, clinical outcome improvements (OS, HCC and hepatic decompensation) in decompensated cirrhotic patients, but this was only achieved in a minority of patients (SVR, 5–25%) infected with genotype 1–4.17 Therefore, there is a critical need to explore new therapeutic options for patients with HCV-related end-stage liver disease who are never listed for liver transplant and could receive a beneficial impact on their clinical outcomes.
The study was designed to evaluate the efficacy and safety of viusid in a particular population of older cirrhotic patients who had a previous history or current evidence of clinical hepatic decompensation and genotype 1 infection, and therefore the poorest chance of achieving SVR and elevated probabilities of adverse clinical outcome in their next years of follow-up.
In the current study, we demonstrated that administration of a nutritional supplement to HCV-related decompensated cirrhotic patients induced a significant improvement in OS, compared with placebo. Similarly, a significant reduction in the disease progression, defined as the presence of liver-related death, the development of HCC or a first occurrence or relapse of at least one of the main portal hypertension-related clinical complications, was observed in patients treated with viusid in comparison with those patients treated with placebo. However, the effect of viusid on survival and disease progression was irrelevant for patients with CP classes A, in contrast with those patients with CP classes B or C. Interestingly, the cumulative incidence of HCC was notably reduced in those patients assigned to experimental arm, compared with placebo arm. However, a stratified analysis according to Child–Pugh classes was not performed, owing to a small proportion of patients with presence of HCC, which could generate a bias in the interpretation of the results.
In the present study, we found increased rates of mortality, disease progression and cumulative incidence of HCC in the placebo group than previously reported rates in a large, prospective and multicentre trial.37 The most likely explanation for the disparity between these rates appears to be related to the difference in the study design. Our study was designed to include a large proportion of patients who had a previous history or current evidence of clinical hepatic decompensation (poor hepatic reserve), subjects who were excluded from the Hepatitis C Antiviral Long-term Treatment against Cirrhosis (HALT-C) Trial.37 A recent controlled study has validated the efficacy and safety of IFN-based therapy for HCV-related decompensated cirrhotic patients.14 One of the main advantages of the study was to include a group of untreated patients (controls) with decompensated events who were enrolled to define survival and progression disease during 30 months of follow-up. The results obtained in this study show that this group of patients have a poor chance to survive (68%) and increased rates of hepatic decompensation (88%) and HCC (10%). These results suggest that the natural history of HCV-related cirrhotic patients is more accelerated in patients with a previous history or current evidence of clinical hepatic decompensation.
Data from the HALT-C study show an increased annual risk of HCC in patients with a low platelet count and the presence of esophageal varices. This could be another reasonable theory to explain the increased risk of HCC in our study. In the current study, an elevated percentage of patients (∼50%) had evidence of esophageal varices and/or thrombocytopenia (<100×103/μl).38
Finally, an increased prevalence of diabetes was reported in our study (42% in placebo group and 34% in viusid group), which has been associated with the development of HCC and accelerated disease progression.39
In the current study, the rate of new occurrence or relapse of overall clinical outcome secondary to portal hypertension was statistically reduced in the patients assigned to viusid in comparison with those allocated to placebo. The cumulative incidence of ascites was the only remarkable clinical condition reduced in the patients treated with viusid as compared with placebo. In contrast, no differences were observed between the treatment groups for hepatic encephalopathy, spontaneous bacterial peritonitis, hepatorenal syndrome and upper-gastrointestinal bleeding.
The benefit of viusid was also seen in the secondary end point of worsening of the prognostic scores. A significant increase in the CP and MELD scores was observed in the placebo-treated patients compared with the experimental group.
During the viusid therapy, the risk of bacterial infections decreased independently from neutropenia, which could suggest an improvement in the qualitative neutrophil function, but this effect should be additionally studied.
Viusid was well tolerated, and only minor transient AEs such as nausea and diarrhoea were reported.
The mechanisms responsible for the beneficial effects of viusid on the clinical outcomes such as survival, development of HCC and disease progression have not yet been fully studied. However, there are several reasons to understand why its administration might improve overall clinical end points.
A recent trial has suggested that viusid therapy combined with SOC in patients with chronic hepatitis C may reduce inflammation and fibrosis, irrespective of virological response.32 Another recently published study has reported a dual role to explain possible mechanisms of action of viusid on liver histology.33 The authors found that MDA and 4-hydroxyalkenal levels were significantly reduced in patients treated with viusid, indicating an important effect on lipid peroxidation products. Furthermore, viusid provided immunomodulatory effects on cytokine secretion via increased production of anti-inflammatory cytokines (IL-10) and decreased or stabilised production of pro-inflammatory cytokines (IL-1α and tumour necrosis factor-α). Current studies are focusing on the biological effects of viusid on hepatic stellate cell apoptosis as a critical step to clarify the potential mechanism of viusid in liver fibrogenesis. On the other hand, it would be important to evaluate whether the viusid effects on the clinical outcomes are directly related to the significant reduction in portal pressure in cirrhotic patients. Further studies should be addressed to answer this concern.
Recently, the HALT-C study was designed to determine whether low-dose peginterferon α 2a maintenance therapy over 3.5 years could reduce hepatic decompensation, HCC and mortality in patients with advanced fibrosis or cirrhosis who failed to achieve SVR with SOC.37 Unfortunately, no overall reduction in any of these clinical end points was achieved. Like the HALT-C study, two other studies (COPILOT and EPIC) failed to demonstrate any overall benefit on clinical outcomes in HCV-related cirrhotic patients.40 41 A recent analysis of the HALT-C trial has demonstrated that benefits on clinical outcomes could only be reached in patients with profound viral suppression obtained with full-dose peginterferon and ribavirin.19
The main strength of this study was to demonstrate that viusid improves overall clinical outcomes (survival, HCC, and disease progression) in cirrhotic patients who have failed to achieve SVR with SOC, and these benefits appear not to be associated with viral suppression rates.32
Our study was designed with a small sample size. Therefore, further multicentre and large-scale studies are needed to corroborate the impact of viusid on the clinical outcomes in patients with HCV-related decompensated cirrhosis.
In conclusion, the study supports the use of viusid in patients with HCV-related decompensated cirrhosis who have failed to achieve SVR, with full-dose peginterferon and ribavirin in an attempt to prevent disease progression and improve OS. However, additional studies are required to confirm the long-term effect of viusid in patients with poorer liver function (Child–Pugh B or C).
Supplementary Material
Footnotes
To cite: Vilar Gomez E, Sanchez Rodriguez Y, Torres Gonzalez A, et al. Viusid, a nutritional supplement, increases survival and reduces disease progression in HCV-related decompensated cirrhosis: a randomised and controlled trial. BMJ Open 2011;2:e000140. doi:10.1136/bmjopen-2011-000140
Funding: This work was supported in part by a grant from Catalysis Laboratories, Spain.
Competing interests: None.
Ethics approval: Ethics approval was provided by the ethics committee and the institutional review board of the National Institute of Gastroenterology.
Contributions: The authors were collectively responsible for the study design, data collection, statistical analysis and interpretation of data, the writing of the manuscript and the decision to submit the manuscript for publication. EVG was involved in the study concept and design, acquisition of data, analysis and interpretation of data, drafting of the manuscript, critical revision of the manuscript for important intellectual content and statistical analysis, and obtained funding. YSR was involved in the study concept and design, acquisition of data, analysis and interpretation of data, critical revision of the manuscript for important intellectual content and drafting of the manuscript. ATG was involved in the study concept and design, acquisition of data, analysis and interpretation of data, and drafting of the manuscript. LCB was involved in the study concept and design, acquisition of data, analysis and interpretation of data, drafting of the manuscript, and critical revision of the manuscript for important intellectual content. EAS was involved in the study concept and design, critical revision of the manuscript for important intellectual content and drafting of the manuscript. YMP was involved in the study concept and design, acquisition of data, analysis and interpretation of data, and drafting of the manuscript. AYG was involved in the study concept and design, acquisition of data, analysis and interpretation of data, drafting of the manuscript and critical revision of the manuscript for important intellectual content. MdRAV was involved in the study concept and design, acquisition of data, analysis and interpretation of data, drafting of the manuscript, critical revision of the manuscript for important intellectual content and statistical analysis. All authors approved the final draft submitted.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data sharing statement: The permission to share data of this clinical trial was denied by the ethic committee of the National Institute of Gastroenterology.
References
- 1.Thomas DL, Seeff LB. Natural history of hepatitis C. Clin Liver Dis 2005;9:383–98, vi. [DOI] [PubMed] [Google Scholar]
- 2.Shepard CW, Finelli L, Alter MJ. Global epidemiology of hepatitis C virus infection. Lancet Infect Dis 2005;5:558–67 [DOI] [PubMed] [Google Scholar]
- 3.Fattovich G, Pantalena M, Zagni I, et al. Effect of hepatitis B and C virus infections on the natural history of compensated cirrhosis: a cohort study of 297 patients. Am J Gastroenterol 2002;97:2886–95 [DOI] [PubMed] [Google Scholar]
- 4.Sangiovanni A, Prati GM, Fasani P, et al. The natural history of compensated cirrhosis due to hepatitis C virus: A 17-year cohort study of 214 patients. Hepatology 2006;43:1303–10 [DOI] [PubMed] [Google Scholar]
- 5.Benvegnu L, Gios M, Boccato S, et al. Natural history of compensated viral cirrhosis: a prospective study on the incidence and hierarchy of major complications. Gut 2004;53:744–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fattovich G, Giustina G, Degos F, et al. Effectiveness of interferon alfa on incidence of hepatocellular carcinoma and decompensation in cirrhosis type C. European Concerted Action on Viral Hepatitis (EUROHEP). J Hepatol 1997;27:201–5 [DOI] [PubMed] [Google Scholar]
- 7.Alazawi W, Cunningham M, Dearden J, et al. Systematic review: outcome of compensated cirrhosis due to chronic hepatitis C infection. Aliment Pharmacol Ther 2010;32:344–55 [DOI] [PubMed] [Google Scholar]
- 8.Hu KQ, Tong MJ. The long-term outcomes of patients with compensated hepatitis C virus-related cirrhosis and history of parenteral exposure in the United States. Hepatology 1999;29:1311–16 [DOI] [PubMed] [Google Scholar]
- 9.Firpi RJ, Clark V, Soldevila-Pico C, et al. The natural history of hepatitis C cirrhosis after liver transplantation. Liver Transpl 2009;15:1063–71 [DOI] [PubMed] [Google Scholar]
- 10.Syed E, Rahbin N, Weiland O, et al. Pegylated interferon and ribavirin combination therapy for chronic hepatitis C virus infection in patients with Child–Pugh Class A liver cirrhosis. Scand J Gastroenterol 2008;43:1378–86 [DOI] [PubMed] [Google Scholar]
- 11.Crippin JS, McCashland T, Terrault N, et al. A pilot study of the tolerability and efficacy of antiviral therapy in hepatitis C virus-infected patients awaiting liver transplantation. Liver Transpl 2002;8:350–5 [DOI] [PubMed] [Google Scholar]
- 12.Forns X, Garcia-Retortillo M, Serrano T, et al. Antiviral therapy of patients with decompensated cirrhosis to prevent recurrence of hepatitis C after liver transplantation. J Hepatol 2003;39:389–96 [DOI] [PubMed] [Google Scholar]
- 13.Everson GT, Trotter J, Forman L, et al. Treatment of advanced hepatitis C with a low accelerating dosage regimen of antiviral therapy. Hepatology 2005;42:255–62 [DOI] [PubMed] [Google Scholar]
- 14.Iacobellis A, Siciliano M, Perri F, et al. Peginterferon alfa-2b and ribavirin in patients with hepatitis C virus and decompensated cirrhosis: a controlled study. J Hepatol 2007;46:206–12 [DOI] [PubMed] [Google Scholar]
- 15.Tekin F, Gunsar F, Karasu Z, et al. Safety, tolerability, and efficacy of pegylated-interferon alfa-2a plus ribavirin in HCV-related decompensated cirrhotics. Aliment Pharmacol Ther 2008;27:1081–5 [DOI] [PubMed] [Google Scholar]
- 16.Ghany MG, Strader DB, Thomas DL, et al. ; American Association for the Study of Liver Diseases Diagnosis, management, and treatment of hepatitis C: an update. Hepatology 2009;49:1335–74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Iacobellis A, Andriulli A. Antiviral therapy in compensated and decompensated cirrhotic patients with chronic HCV infection. Expert Opin Pharmacother 2009;10:1929–38 [DOI] [PubMed] [Google Scholar]
- 18.Bruno S, Stroffolini T, Colombo M, et al. ; Italian Association of the Study of the Liver Disease (AISF) Sustained virological response to interferon-alpha is associated with improved outcome in HCV-related cirrhosis: a retrospective study. Hepatology 2007;45:579–87 [DOI] [PubMed] [Google Scholar]
- 19.Shiffman ML, Morishima C, Dienstag JL, et al. Effect of HCV RNA suppression during peginterferon alfa-2a maintenance therapy on clinical outcomes in the HALT-C trial. Gastroenterology 2009;137:1986–94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Veldt BJ, Saracco G, Boyer N, et al. Long term clinical outcome of chronic hepatitis C patients with sustained virological response to interferon monotherapy. Gut 2004;53:1504–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.De Maria N, Colantoni A, Fagiuoli S, et al. Association between reactive oxygen species and disease activity in chronic hepatitis C. Free Radic Biol Med 1996;21:291–5 [DOI] [PubMed] [Google Scholar]
- 22.Boya P, de la Pena A, Beloqui O, et al. Antioxidant status and glutathione metabolism in peripheral blood mononuclear cells from patients with chronic hepatitis C. J Hepatol 1999;31:808–14 [DOI] [PubMed] [Google Scholar]
- 23.Gramenzi A, Andreone P, Loggi E, et al. Cytokine profile of peripheral blood mononuclear cells from patients with different outcomes of hepatitis C virus infection. J Viral Hepat 2005;12:525–30 [DOI] [PubMed] [Google Scholar]
- 24.Duarte TL, Lunec J. Review: When is an antioxidant not an antioxidant? A review of novel actions and reactions of vitamin C. Free Radic Res 2005;39:671–86 [DOI] [PubMed] [Google Scholar]
- 25.Lee CH, Park SW, Kim YS, et al. Protective mechanism of glycyrrhizin on acute liver injury induced by carbon tetrachloride in mice. Biol Pharm Bull 2007;30:1898–904 [DOI] [PubMed] [Google Scholar]
- 26.Kang YJ, Zhou Z. Zinc prevention and treatment of alcoholic liver disease. Mol Aspects Med 2005;26:391–404 [DOI] [PubMed] [Google Scholar]
- 27.Abe M, Akbar F, Hasebe A, et al. Glycyrrhizin enhances interleukin-10 production by liver dendritic cells in mice with hepatitis. J Gastroenterol 2003;38:962–7 [DOI] [PubMed] [Google Scholar]
- 28.Yoshikawa M, Toyohara M, Ueda S, et al. Glycyrrhizin inhibits TNF-induced, but not Fas-mediated, apoptosis in the human hepatoblastoma line HepG2. Biol Pharm Bull 1999;22:951–5 [DOI] [PubMed] [Google Scholar]
- 29.Kimura M, Inoue H, Hirabayashi K, et al. Glycyrrhizin and some analogues induce growth of primary cultured adult rat hepatocytes via epidermal growth factor receptors. Eur J Pharmacol 2001;431:151–61 [DOI] [PubMed] [Google Scholar]
- 30.Shimoyama Y, Sakamoto R, Akaboshi T, et al. Characterization of secretory type IIA phospholipase A2 (sPLA2-IIA) as a glycyrrhizin (GL)-binding protein and the GL-induced inhibition of the CK-II-mediated stimulation of sPLA2-IIA activity in vitro. Biol Pharm Bull 2001;24:1004–8 [DOI] [PubMed] [Google Scholar]
- 31.Vilar Gomez E, Rodriguez De Miranda A, Gra Oramas B, et al. Clinical trial: a nutritional supplement Viusid, in combination with diet and exercise, in patients with nonalcoholic fatty liver disease. Aliment Pharmacol Ther 2009;30:999–1009 [DOI] [PubMed] [Google Scholar]
- 32.Vilar Gomez E, Gra Oramas B, Soler E, et al. Viusid, a nutritional supplement, in combination with interferon alpha-2b and ribavirin in patients with chronic hepatitis C. Liver Int 2007;27:247–59 [DOI] [PubMed] [Google Scholar]
- 33.Gomez EV, Perez YM, Sanchez HV, et al. Antioxidant and immunomodulatory effects of Viusid in patients with chronic hepatitis C. World J Gastroenterol 2010;16:2638–47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bruix J, Sherman M; Practice Guidelines Committee, American Association for the Study of Liver Diseases Management of hepatocellular carcinoma. Hepatology 2005;42:1208–36 [DOI] [PubMed] [Google Scholar]
- 35.Bruix J, Sherman M, Llovet JM, et al. Clinical management of hepatocellular carcinoma. Conclusions of the Barcelona-2000 EASL conference. European Association for the Study of the Liver. J Hepatol 2001;35:421–30 [DOI] [PubMed] [Google Scholar]
- 36.Befeler AS, Hayashi PH, Di Bisceglie AM. Liver transplantation for hepatocellular carcinoma. Gastroenterology 2005;128:1752–64 [DOI] [PubMed] [Google Scholar]
- 37.Di Bisceglie AM, Shiffman ML, Everson GT, et al. ; HALT-C Trial Investigators Prolonged therapy of advanced chronic hepatitis C with low-dose peginterferon. N Engl J Med 2008;359:2429–41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lok AS, Seeff LB, Morgan TR, et al. ; HALT-C Trial Group Incidence of hepatocellular carcinoma and associated risk factors in hepatitis C-related advanced liver disease. Gastroenterology 2009;136:138–48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Veldt BJ, Chen W, Heathcote EJ, et al. Increased risk of hepatocellular carcinoma among patients with hepatitis C cirrhosis and diabetes mellitus. Hepatology 2008;47:1856–62 [DOI] [PubMed] [Google Scholar]
- 40.Afdhal NH, Levine R, Brown R, et al. Colchicine versus peginterferon alfa 2b long term therapy: results of the 4 year copilot trial. J Hepatol 2008;48(Suppl 2):S4 [Google Scholar]
- 41.Bruix J, Poynard T, Colombo M, et al. Pegintron maintenance therapy in cirrhotic (Metavir F4) HCV patients, who failed to respond to interferon/ribavirin therapy: final results of the EPIC3 cirrhosis maintenance trial. J Hepatol 2009;50(Suppl 1):S22 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.