Abstract
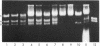
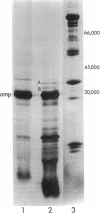
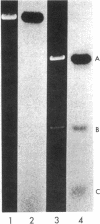
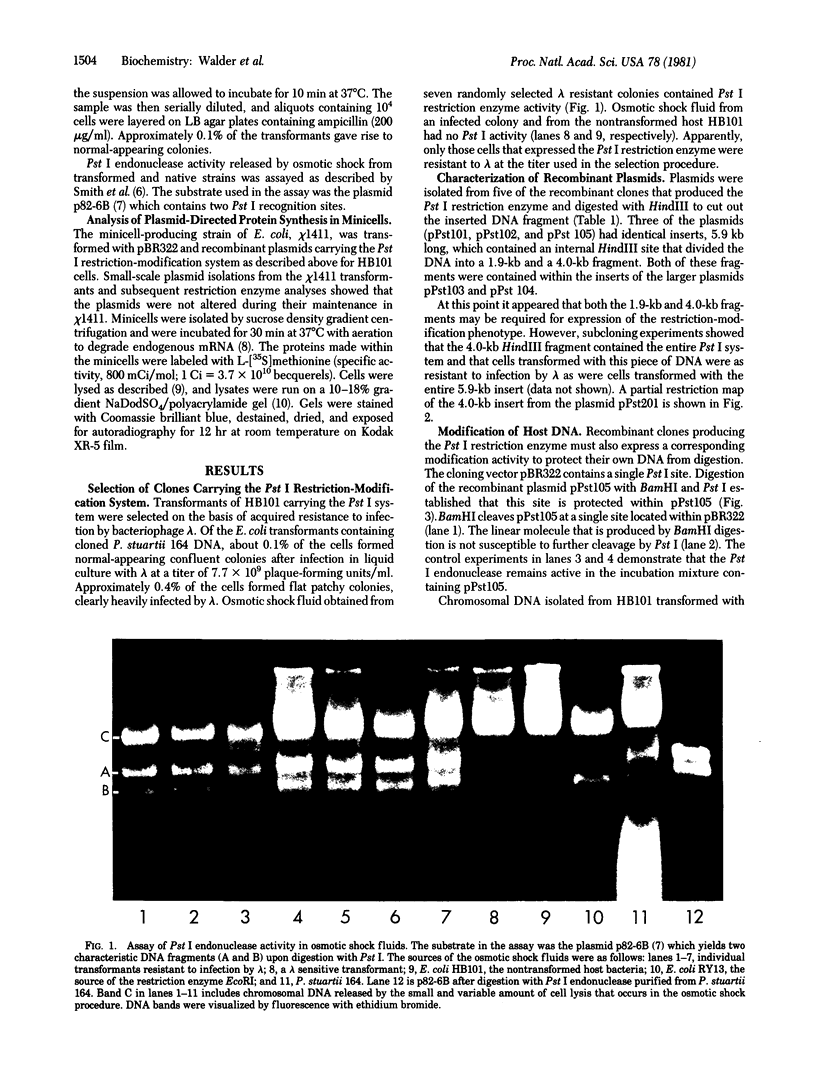
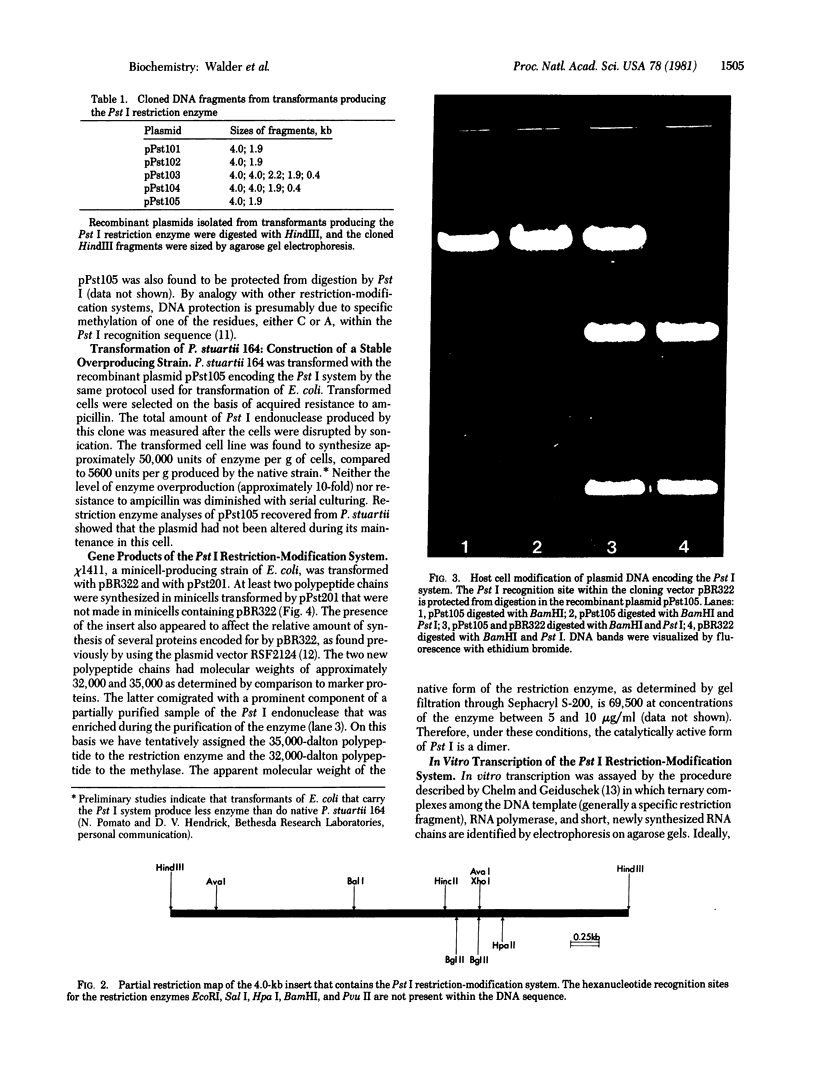
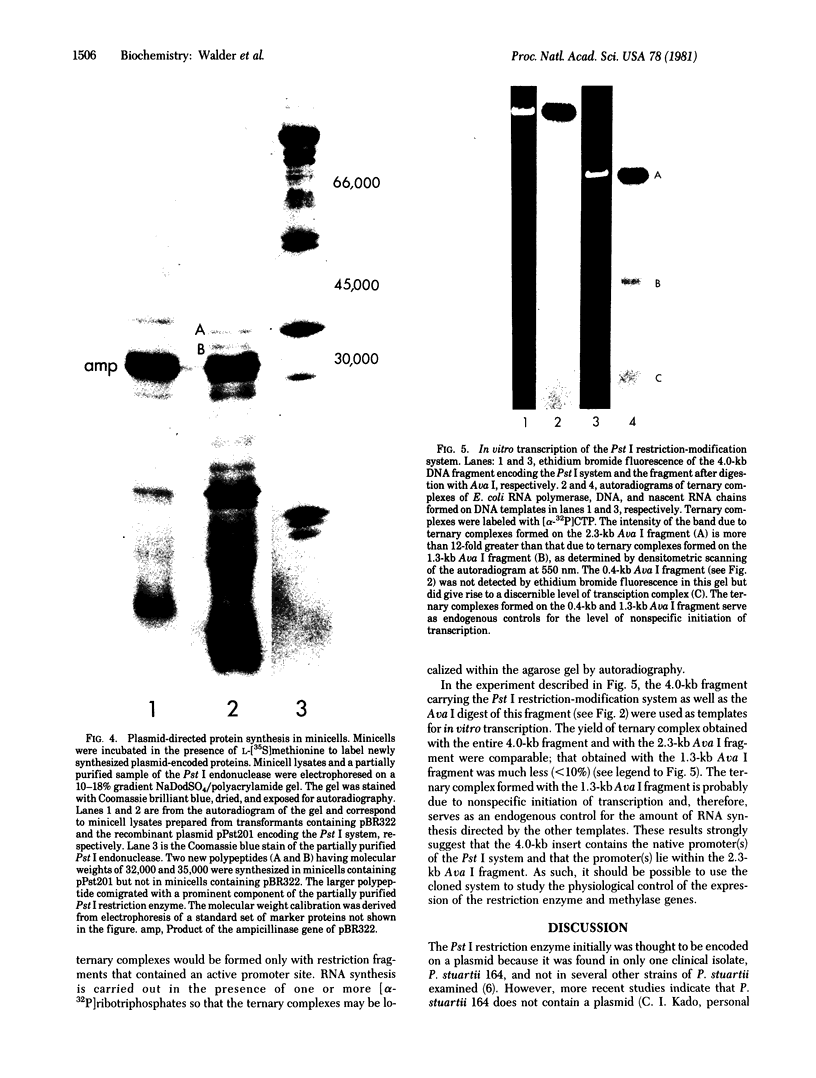
Here we report the cloning and preliminary characterization of the Pst I restriction-modification system of Providencia stuartii 164. Transformants of Escherichia coli carrying the Pst I gene system inserted into the cloning vector pBR322 were selected on the basis of acquired resistance to bacteriophage lambda infection. Pst I endonuclease was detected in osmotic shock fluid from each of the resistant clones. Plasmid and chromosomal DNA from these clones could not be digested by Pst I, indicating that the gene for the corresponding modification enzyme had also been cloned and was being expressed. The smallest recombinant plasmid encoding both activities, pPst201, contains an insert of approximately 4000 base pairs. In vitro transcription studies indicate that this DNA fragment also contains the endogenous promoter(s) of the system. When pPst201 was introduced into a minicell-producing strain of E. coli, two new proteins, 32,000 and 35,000 daltons, were synthesized. We have assigned these to the Pst I modification (methylase) and restriction enzymes, respectively. The active form of the restriction enzyme is a dimer, as determined by gel filtration. Constructed transformants of P. stuartii 164 that carry the Pst I system inserted into pBR322 produce approximately 10 times more Pst I endonuclease activity than does the native strain.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blobel G. Intracellular protein topogenesis. Proc Natl Acad Sci U S A. 1980 Mar;77(3):1496–1500. doi: 10.1073/pnas.77.3.1496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolivar F., Backman K. Plasmids of Escherichia coli as cloning vectors. Methods Enzymol. 1979;68:245–267. doi: 10.1016/0076-6879(79)68018-7. [DOI] [PubMed] [Google Scholar]
- Clewell D. B., Helinski D. R. Supercoiled circular DNA-protein complex in Escherichia coli: purification and induced conversion to an opern circular DNA form. Proc Natl Acad Sci U S A. 1969 Apr;62(4):1159–1166. doi: 10.1073/pnas.62.4.1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartley J. L., Donelson J. E. Nucleotide sequence of the yeast plasmid. Nature. 1980 Aug 28;286(5776):860–865. doi: 10.1038/286860a0. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Mann M. B., Rao R. N., Smith H. O. Cloning of restriction and modification genes in E. coli: the HbaII system from Haemophilus haemolyticus. Gene. 1978 Apr;3(2):97–112. doi: 10.1016/0378-1119(78)90054-9. [DOI] [PubMed] [Google Scholar]
- Miller D. L., Andreone T. L., Donelson J. E. Translation in Escherichia coli mini-cells containing hamster mitochondrial DNA-Co1E1 - Ampr recombinant plasmids. Biochim Biophys Acta. 1977 Aug 16;477(4):323–333. doi: 10.1016/0005-2787(77)90251-9. [DOI] [PubMed] [Google Scholar]
- Miller D. L., Gubbins E. J., Pegg E. W., 3rd, Donelson J. E. Transcription and translation of cloned Drosophila DNA fragments in Escherichia coli. Biochemistry. 1977 Mar 22;16(6):1031–1038. doi: 10.1021/bi00625a001. [DOI] [PubMed] [Google Scholar]
- Reeve J. Use of minicells for bacteriophage-directed polypeptide synthesis. Methods Enzymol. 1979;68:493–503. doi: 10.1016/0076-6879(79)68038-2. [DOI] [PubMed] [Google Scholar]
- Smith D. I., Blattner F. R., Davies J. The isolation and partial characterization of a new restriction endonuclease from Providencia stuartii. Nucleic Acids Res. 1976 Feb;3(2):343–353. doi: 10.1093/nar/3.2.343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith H. O. Nucleotide sequence specificity of restriction endonucleases. Science. 1979 Aug 3;205(4405):455–462. doi: 10.1126/science.377492. [DOI] [PubMed] [Google Scholar]
- Talmadge K., Stahl S., Gilbert W. Eukaryotic signal sequence transports insulin antigen in Escherichia coli. Proc Natl Acad Sci U S A. 1980 Jun;77(6):3369–3373. doi: 10.1073/pnas.77.6.3369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wensink P. C., Finnegan D. J., Donelson J. E., Hogness D. S. A system for mapping DNA sequences in the chromosomes of Drosophila melanogaster. Cell. 1974 Dec;3(4):315–325. doi: 10.1016/0092-8674(74)90045-2. [DOI] [PubMed] [Google Scholar]