Abstract
Objectives
Because of the prevailing penicillin resistance in microorganisms, broad spectrum cephalosporins are used empirically specially in developing countries. The aim of this study is to determine the susceptibility pattern of different gram positive and gram negative pathogens against third generation cephalosporin-ceftriaxone to explore the existing effectiveness of this antibiotic.
Methods
180 clinical isolates of different gram positive and gram negative pathogens including P.mirabilis, S. typhi P.aeruginosa, E. coli, S. aureus and Klebsiella were collected from blood and urine samples of in-patients. 30 isolates of all species were tested against each of six brands of ceftriaxone using in vitro sensitivity tests by disc diffusion method (NCCLS criteria). The susceptibility limit was ≥21 mm zone of inhibition, while moderately susceptible was considered at 20-14 mm, and those isolates which showed >13 mm or no zone of inhibition were resistant to this antibacterial drug.
Results
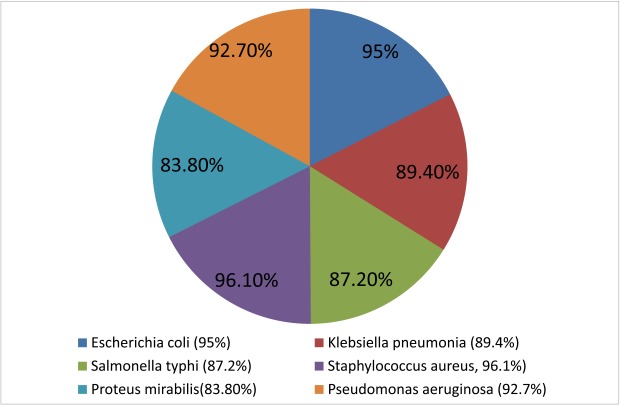
Ceftriaxone was found most effective against S. aureus. While 96.1% of the isolates showed susceptibility towards ceftriaxone, followed by E. coli (95%), P. aeruginosa (92.7%), K. pneumonia (89.4%) and S. typhi (87.2%). P. mirabilis showed lowest susceptibility amongst all the test organisms (83.8%).
Conclusion
Ceftriaxone can be used as a drug of choice in infections caused by S. aureus, E. coli, P. aurigenosa, K. pneumonia and S. typhi. However, it should be used with other antimicrobial agents in order to increase its effectiveness against P. mirabilis.
Introduction
Ceftriaxone is a broad spectrum third generation cephalo-sporin.1 It is highly effective against Gram negative as well as Gram positive organisms. Ceftriaxone is unique because of its prolonged serum half-life, which permits once- or twice-daily dosing of this member of Cephalosporin family.2 Ceftriaxone is stable to beta-lactamases, particularly those produced by Gram-negative bacteria. It has high potency against all the Enterobacteriaceae, Haemophilus influenzae, the Nisseria and most Gram-positive cocci except Enterococcal spp.3
Effectiveness of an antibiotic is compromised due to the emergence of antibiotic resistant microbes.4 Despite the constantly increasing need for new antimicrobial agents, antibiotic drug discovery and development seems to have greatly decelerated in recent years.5 Antimicrobial resistance has become a major health problem worldwide, affecting every country to some degree.2 Encountered with the significant problem of advancing antimicrobial resistance, the global scientific community has attempted to find alternative solutions; one of the most promising ones is the evaluation and use of old antibiotic compounds.5
Due to the wide spread use of antibiotics in the health care system the clinical isolates were developing resistance to different antibiotics. According to Technical Report by United States Agency for International Development, Ministry of Science and Technology, Government of Pakistan and Higher Education Commission, the tedious problem of antimicrobial resistance needs to develop resources to address the issue. This study aims to define contemporary susceptibility patterns of clinical isolates of gram positive and gram negative bacteria towards ceftriaxone. This study also gives an insight to the resistance pattern in different ceftriaxone sensitive bacteria and will help physicians, clinicians and consultants to decide its use in the most appropriate clinical infections.
Methods
180 Clinical isolates of each of six different bacterial species, namely Escherichia coli, Staphylococcus aureus, Klebsiella pneumoniae, Proteus mirabilis, Salmonella typhi and Pseudomonas aeruginosa were collected from Jinnah Post Graduate Medical Centre (JPMC), National Institute of Child Health (NICH) and National Institute of Cardiovascular Diseases (NICVD in Karachi from nosocomial infections. All the isolates were collected from the blood and urine samples of hospitalized patients.
Mueller-Hinton Agar was prepared by cooling the medium after autoclaving at the temperature of 121°C for 20 minutes. Susceptibility plates were prepared and stored at 2 - 8°C unless used. The broth culture was incubated at 37°C until it achieved the turbidity of the McFarland 0.5 standard.
Susceptibility plates were inoculated and allowed to dry for approximately 5 minutes, but no longer than 15 minutes, before adding the antibiotic disk. Six different brands of 1g ceftriaxone (injectable) available in the local market of Karachi were used to determine bacterial susceptibility. Antimicrobial susceptibility test was carried out using the disk diffusion technique. 30 μg Ceftriaxone discs were prepared by serial dilution.6 Ceftriaxone containing discs were applied to inoculated Muller-Hinton agar plates. After placement of ceftriaxone containing discs, the susceptibility plates were placed at 37°C in incubator for 16-18 hours. The procedure was repeated for all six brands of ceftriaxone. The breakpoints for susceptible, intermediate, resistant isolates were set as ≥21 mm (susceptible: S), 20-14 mm (intermediate resistant: IR) and >13 mm or no zone of inhibition (resistant: R). After the incubation period, zone of inhibition appeared around the antibiotic discs. The diameter of the zone of inhibition including the diameter of the disk was measured, using sliding callipers held on the back of the inverted Petri plate. Sensitivity, intermediate resistance and resistances were determined by the zone of complete growth inhibition around each disk.
Results
The present study was designed to identify the susceptibility profile of different clinical isolates from nosocomial infections. The zones of inhibition around every disc were calculated in millimetre for all six types of bacteria against six different brands of ceftriaxone. The frequency of susceptible (with zone of inhibition ³ to 21 mm), intermediate resistant (with zone of inhibition 20-14 mm) and resistant (with zone of inhibition ≤ 13 mm) are shown in Table 1. Susceptibility of these bacteria towards all of the six different brands of ceftriaxone is given cumulatively in Fig. 1.
Table 1. Susceptibility Pattern of Different Bacteria Towards Ceftriaxone.
| Ceftriaxone brand code | Cef -1 | Cef -2 | Cef -3 | Cef -4 | Cef -5 | Cef -6 | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Name of bacteria | Frequency (n) | |||||||||||||||||
| S | IR | R | S | IR | R | S | IR | R | S | IR | R | S | IR | R | S | IR | R | |
| Staphylococcus aureus | 30 | - | - | 30 | - | - | 28 | - | 2 | 27 | - | 3 | 28 | - | 2 | 30 | - | - |
| Escherichia coli | 30 | - | - | 30 | - | - | 29 | - | 1 | 29 | - | 1 | 27 | - | 3 | 26 | - | 4 |
| Pseudomonas aeruginosa | 27 | - | 3 | 30 | - | - | 30 | - | - | 26 | 1 | 3 | 27 | 1 | 2 | 27 | - | 3 |
| Klebsiella pneumonia | 27 | - | 3 | 25 | 2 | 3 | 26 | - | 4 | 26 | 2 | 2 | 27 | - | 3 | 27 | - | 3 |
| Salmonella typhi | 26 | - | 4 | 26 | - | 4 | 26 | - | 4 | 27 | - | 3 | 26 | - | 4 | 26 | - | 4 |
| Proteus mirabilis | 24 | 1 | 5 | 22 | 4 | 5 | 24 | 1 | 5 | 23 | 5 | 3 | 24 | 1 | 5 | 24 | 1 | 5 |
Cef 1-6: different brands of ceftriaxone, S= susceptible, IR=intermediate susceptible, R=resistance, n = 1080
Figure 1.
Susceptibility of different bacteria towards Ceftriaxone
Discussion
Microbial drug resistance is an inescapable consequence of the use of antimicrobial agents. The rate at which resistance occurs among microbial populations is often driven by the overuse and abuse of antimicrobial agents in many clinical settings.7 Differences in susceptibility to antibiotics by microorganisms has become a major factor in drug choice and success of treatment. Great concerns have been raised regarding emerging antimicrobial resistance among bacteria that may result in unpredictable antimicrobial susceptibility and failure of therapy.8,9
Some trends in the antimicrobial resistance patterns and rates are universal, while others appear to be unique for specific regions. The Ceftriaxone, cephalosporin of the third generation is a potent antibiotic used in the treatment of life threatening infections. Since the rising trends of highly resistant organisms stresses the increasing importance of continuous surveillance system and stewardship of antibiotics.10 This study was conducted to determine the present sensitivity profile of different gram positive and gram negative bacteria (Escherichia coli, Staphylococcus aureus, Klebsiella pneumonia, Proteus mirabilis, Salmonella typhi and Pseudomonas aeruginosa) in Karachi.
The use of ceftriaxone has increased for most infection causing organisms of gram positive and gram negative types as well as for aerobic and anaerobic organism.11-21 This in vitro study of ceftriaxone shows that it is still effective against all these bacteria. All six different organisms showed susceptibility towards ceftriaxone. However, Staphylococcus aureus showed highest susceptibility against ceftriaxone. The result also favours another study conducted in Karachi in 2001, which found that ceftriaxone inhibited nearly 92% strains of Staphylococcus aureus and it is the most active β-lactam agent against this bacterium.22 Escherichia coli is a gram negative bacterium commonly harmless, but some can cause serious food poisoning in humans, and is occasionally responsible for costly product recalls and simulating blood and urinary infections.23,24 As gram-negative organisms, E. coli are resistant to many antibiotics that are effective against Staphylococus aureus (a gram positive bacteria), and gram negatives such as Klebsiella, Salmonella typhi and Pseudomonas aeruginos.(24)
In this study, ceftriaxone proved to be highly active against Escherichia coli and it can be safely used in infections caused by Escherichia coli.
Over the past decade, the pathogenesis of infectious keratitis involving the opportunistic pathogen Pseudomonas aeruginosa has been investigated.25 There has been rising trends of resistance noted in 2002 in Pseudomonas aeruginosa against third generation cephalosporin.26 In the current study, 92.7% of the organisms showed susceptibility towards third generation cephalosporin-ceftriaxone which highlights the significance of this antibacterial agent in infections caused by Pseudomonas aeruginosa.
A prospective study heralds that E. coli and Klebsiella are the most common organisms causing UTI in the metropolitan city of Karachi.27 In this study, Klebsiella showed lesser sensitivity towards ceftriaxone compared to Escherichia coli, but still 89.4% of the isolates were susceptible. Only 10.6% of the Klebseilla isolates showed resistance as the antibiotic did not develop any zone of inhibition. Klebsiella was found to be more sensitive against meropenem and imipenem, amikacin, gentamicin and tobramycin, Cefipime and Aztreonam.28 But the current findings prove that ceftriaxone is still effective against klebseilla as 87.7% showed susceptibility. In a study conducted in 1992 in Karachi the Salmonella typhi responsible for typhoid fever were found sensitive to ceftriaxone.22 However, in the current study, Salmonella typhi showed 87.2% susceptibility (12.8% resistance) towards ceftriaxone, which means that ceftriaxone can still be used effectively against S. typhi. While Proteus mirabilis, a gram-negative, facultative anaerobic bacterium causing 90% of all ‘Proteus’ infections in humans. The study conducted in 2006, in Karachi, 70.4% of Proteus mirabilis were sensitive to ceftriaxone,29 and this current study demonstrates that 78.3% of the Proteus mirabilis used in the invitro sensitivity test were susceptible to the ceftriaxone.
The present study proves that all six different brands of ceftriaxone are effective therapy for the treatment of infections caused by Staphylococcus aureus, E. Coli, Ps. aeruginosa, K. pneumonia, Salmonella typhi and Proteus mirabilis. This report shall be helpful for physicians, prescribers and consultants to prescribe alternative antibiotic with cost effectiveness. There is need for appropriate national guidelines for the use of this antibacterial agent to avoid the development of resistance by sensitive organisms.
Conclusion
In reality, the extensive use of β-lactam antibiotics in hospitals and communities has created major evolutionary pressures in bacteria to evolve towards resistance. The β-lactamase antibiotics are the largest and currently most widely used antibacterial agents. Therefore, their resistance means a level of antimicrobial activity associated with a high likelihood of therapeutic failure.
The antibiotic policy guidelines preparation is the need of the hour to avoid any bitter experience of misuse of this class of drug therapy. The team efforts of the members comprising consultants, prescribing physicians, and members of health services team should participate in producing an effective plan to make better decisions in this regards. In addition, surveillance programs should be conducted periodically to evaluate the sensitivity and susceptibility of these bacteria against prescribed antibiotics. Since the emergence of resistance remains silent for periods and takes time to become apparent, these antibiotics should be prescribed with cautions so that the bitter experience of antibiotic resistance can be avoided. These in vitro sensitivity studies also help in cost-effective prescribing of different brands of the antibiotic to the different patients with varying socioeconomic backgrounds. Overall, resistance patterns in different regions should be conducted so that the profiles of the specific region are maintained and monitored accordingly. This will therefore act as a guide towards the formulation of a comprehensive monitoring program of chemotherapy.
Acknowledgements
The authors reported no conflict of interest and no funding was received on this work.
References
- 1.Nath SK, Foster GA, Mandell LA, Rotstein C. Antimicrobial activity of ceftriaxone versus cefotaxime: negative effect of serum albumin binding of ceftriaxone. J Antimicrob Chemother 1994. Jun;33(6):1239-1243. 10.1093/jac/33.6.1239 [DOI] [PubMed] [Google Scholar]
- 2.Masood H, Naqvi SB, Aslam N. Cost effective analysis of different brands of ceftriaxone available in Karachi Pakistan. Pak J Pharmacol 2008;25:13-19. [Google Scholar]
- 3.Hwang KP, Tang YF, Shen YH. Activity of ertapenem, ciprofloxacin, ceftriaxone, piperacillin-tazobactam, and ampicillin-sulbactam against 12 common clinical isolates of community-acquired bacteremia. J Microbiol Immunol Infect 2009. Oct;42(5):433-438. [PubMed] [Google Scholar]
- 4.Supp DM, Gardner J, Klingenberg JM, Neely AN. Antibiotic resistance in clinical isolates of Acinetobacter baumannii, Pseudomonas aeruginosa, and Staphylococcus aureus does not impact sensitivity to human beta defensin 4. Burns 2009. Nov;35(7):949-955. Published online 6 Jun 2009. 10.1016/j.burns.2009.02.016 [DOI] [PubMed] [Google Scholar]
- 5.Liaqat I, Sumbal F, Sabri AN. Tetracycline and chloramphenicol efficiency against selected biofilm forming bacteria. Curr Microbiol 2009. Aug;59(2):212-220. 10.1007/s00284-009-9424-9 [DOI] [PubMed] [Google Scholar]
- 6.Bell JM, Turnidge JD, Gales AC, Pfaller MA, Jones RN, Sentry APAC Study Group . Prevalence of extended spectrum β-lactamase (ESBL)-producing clinical isolates in the Asia-Pacific region and South Africa: regional results from SENTRY Antimicrobial Surveillance Program (1998-99). Diagn Microbiol Infect Dis 2002. Mar;42(3):193-198. 10.1016/S0732-8893(01)00353-4 [DOI] [PubMed] [Google Scholar]
- 7.Khan S, Gupta DK, Khan DN. Comparative study of three antimicrobial drugs protocol (Ceftriaxone, Gentamicin/Amikacin and Metronidazole) versus two antimicrobial drugs protocol (Ceftriaxone and Metronidazole) in cases of intra-abdominal sepsis. Kathmandu Univ Med J (KUMJ) 2005. Jan-Mar;3(1):55-63. KUMJ. [PubMed] [Google Scholar]
- 8.Huang TM, Lin TL, Wu CC. Antimicrobial susceptibility and resistance of chicken Escherichia coli, Salmonella spp., and Pasteurella multocida isolates. Avian Dis 2009. Mar;53(1):89-93. 10.1637/8268-021608-Reg.1 [DOI] [PubMed] [Google Scholar]
- 9.Khameneh ZR, Afshar AT. Antimicrobial susceptibility pattern of urinary tract pathogens. Saudi J Kidney Dis Transpl 2009. Mar;20(2):251-253. [PubMed] [Google Scholar]
- 10.Irfan S, Idrees F, Mehraj V, Habib F, Adil S, Hasan R. Emergence of Carbapenem resistant Gram negative and vancomycin resistant Gram positive organisms in bacteremic isolates of febrile neutropenic patients: a descriptive study. BMC Infect Dis 2008;8:80. 10.1186/1471-2334-8-80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zarakolu P, Sakizligil B, Unal S. [Antimicrobial resistance of Neisseria gonorrhoeae strains isolated from sex workers in Ankara]. Mikrobiyol Bul 2006. Jan-Apr;40(1-2):69-73. [PubMed] [Google Scholar]
- 12.King A, Bethune L, Phillips I. In vitro activity of MDL 62,879 (GE2270 A) against aerobic gram-positive and anaerobic bacteria. Antimicrob Agents Chemother 1993. Apr;37(4):746-749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Madhulika U, Harish BN, Parija SC. Current pattern in antimicrobial susceptibility of Salmonella Typhi isolates in Pondicherry. Indian J Med Res 2004. Aug;120(2):111-114. [PubMed] [Google Scholar]
- 14.Francioli P, Etienne J, Hoigné R, Thys JP, Gerber A. Treatment of streptococcal endocarditis with a single daily dose of ceftriaxone sodium for 4 weeks. Efficacy and outpatient treatment feasibility. JAMA 1992. Jan;267(2):264-267. 10.1001/jama.267.2.264 [DOI] [PubMed] [Google Scholar]
- 15.Mamishi S, Pourakbari B, Ashtiani MH, Hashemi FB. Frequency of isolation and antimicrobial susceptibility of bacteria isolated from bloodstream infections at Children’s Medical Center, Tehran, Iran, 1996-2000. Int J Antimicrob Agents 2005. Nov;26(5):373-379. 10.1016/j.ijantimicag.2005.08.004 [DOI] [PubMed] [Google Scholar]
- 16.Dunne EF, Fey PD, Kludt P, Reporter R, Mostashari F, Shillam P, et al. Emergence of domestically acquired ceftriaxone-resistant Salmonella infections associated with AmpC beta-lactamase. JAMA 2000. Dec;284(24):3151-3156. 10.1001/jama.284.24.3151 [DOI] [PubMed] [Google Scholar]
- 17.Hsueh PR, Chen WH, Luh KT. Relationships between antimicrobial use and antimicrobial resistance in Gram-negative bacteria causing nosocomial infections from 1991-2003 at a university hospital in Taiwan. Int J Antimicrob Agents 2005. Dec;26(6):463-472. 10.1016/j.ijantimicag.2005.08.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Karlowsky A, Jones ME, Mayfield DC, Thornsberry C, SahmDF. Ceftriaxone activity against Gram-positive and Gram-negative bacteria isolated in US clinical microbiology laboratories from 1996 to 2000: results from The Surveillance Network Database-USA International Journal of Antimicrobial Agents 2002;19 (5): 2002, 413-426. [DOI] [PubMed]
- 19.Lee SC, Huang SS, Lee CW, Fung CP, Lee N, Shieh WB, et al. Comparative antimicrobial susceptibility of aerobic and facultative bacteria from community-acquired bacteremia to ertapenem in Taiwan. BMC Infect Dis 2007;7:79. 10.1186/1471-2334-7-79 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Boyanova L, Kolarov R, Gergova G, Deliverska E, Madjarov J, Marinov M, et al. Anaerobic bacteria in 118 patients with deep-space head and neck infections from the University Hospital of Maxillofacial Surgery, Sofia, Bulgaria. J Med Microbiol 2006. Sep;55(9):1285-1289. 10.1099/jmm.0.46512-0 [DOI] [PubMed] [Google Scholar]
- 21.George AK. Archiva SWl, Catherine, Monserrat, Carlyn C. Use of the National Committee for Clinical Laboratory Standards Guide Lies for Disk Diffusion Susceptibility Testing in New York State Laboratories. J Clin Microbiol 2000;38(9):3348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shoaib MH. Naqvi SB, Shiekh D, Hashmi HK. Cephalosporin Resistance And B-Lactamase Production In Clinical Isolates Of Staphylococcus Aureus In Karachi. Pak J Pharm Sci 2001;14(2):23-32. [PubMed] [Google Scholar]
- 23.Vogt RL, Dippold L. Escherichia coli O157:H7 outbreak associated with consumption of ground beef, June-July 2002. Public Health Rep 2005. Mar-Apr;120(2):174-178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Abdul Jalil, 2Shafiq Ahmad Tariq, 2Iftikhar Ud Din Niazi. Comparative susceptibilities of Escherichia coli to ceftriaxone alone and in combination with clavulanic acid. J. Med. Sci. (Peshawar, Print) January 2009, Vol. 17, No. 1: 12-15
- 25.Fleiszig SM, Evans DJ. The pathogenesis of bacterial keratitis: studies with Pseudomonas aeruginosa. Clin Exp Optom 2002. Sep;85(5):271-278. 10.1111/j.1444-0938.2002.tb03082.x [DOI] [PubMed] [Google Scholar]
- 26.Irfan S, Idrees F, Mehraj V, Habib F, Adil S, Hasan R. Emergence of Carbapenem resistant Gram negative and vancomycin resistant Gram positive organisms in bacteremic isolates of febrile neutropenic patients: a descriptive study. BMC Infect Dis 2008;8:80. 10.1186/1471-2334-8-80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Farooqi BJ, Shareeq F, Rizvi QK, Qureshi HS, Ashfaq MK. Changing pattern of antimicrobial susceptibility of organisms causing community acquired urinary tract infections. J Pak Med Assoc 2000. Nov;50(11):369-373. [PubMed] [Google Scholar]
- 28.Naeem I, Naqvi BS, Hashmi K, Gauhar S. Paediatric nosocomial infections: resistance pattern of clinical isolates. Pak J Pharm Sci 2006. Jan;19(1):52-57. [PubMed] [Google Scholar]
- 29.Iqbal SM, Udaipurwala IH, Shafiq AH, Mughal S. Chronic suppurative otitis media: disease pattern and drug sensitivity. J Surg Pak 2006;11(1):17-19. [Google Scholar]