Abstract
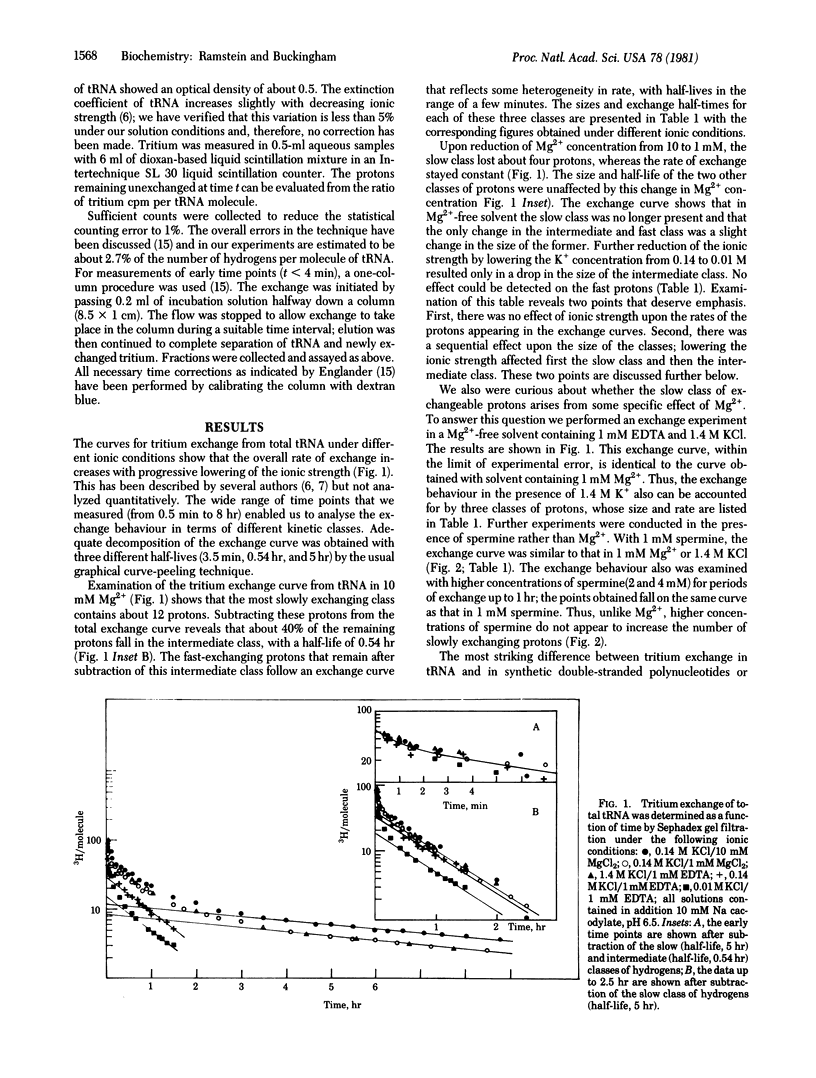
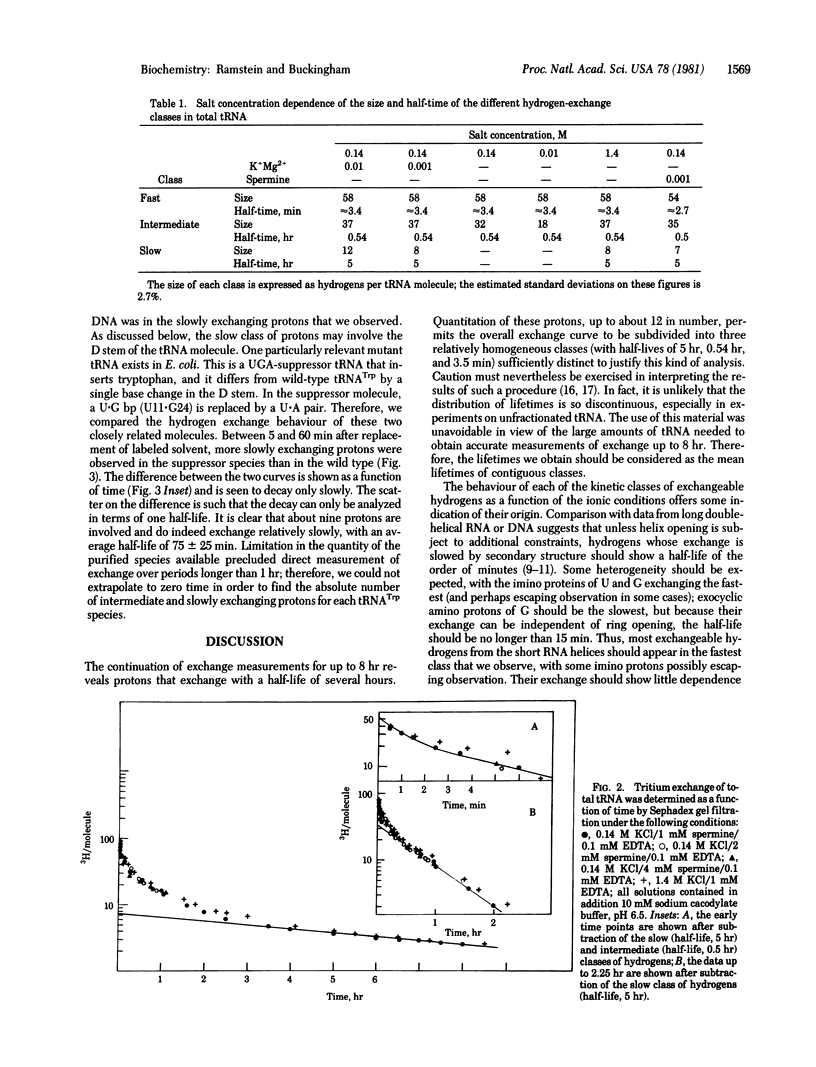
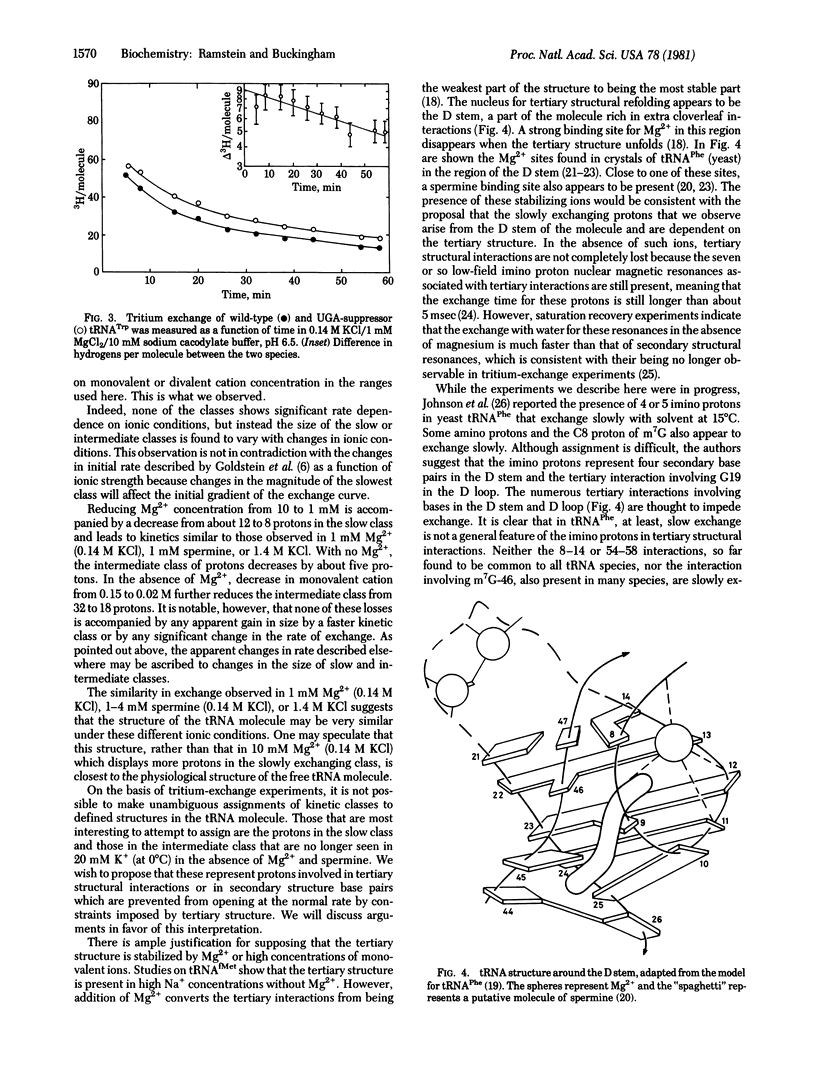
Measurements of tritium exchange on tRNA were made for periods from 0.5 min to 8 hr after separation from labeled solvent. The exchange curve was analysed in terms of three kinetic classes of exchanging protons with half-lives of 5 hr (12 protons), 0.54 hr (37 protons) and about 3.5 min (58 protons) at 0 degrees C in 0.14 M K+/10 mM Mg2+. The behaviour under varying ionic conditions of protons in the slowest exchange class and of some protons in the intermediate class suggests that they are dependent on the tertiary structure of the molecule. Moreover, in the same range of exchange times characteristic of these latter protons, about 9 more protons were observed in the case of a mutant form of tRNA Trp, the UGA-suppressor species, than in the wild-type tRNATrp. These two species differ only in base 24 in the dihydrouridine stem. This dynamic difference between the wild-type and suppressor species may be related to a functionally important difference in coupling between the conformation of the molecule and interactions at the anticodon.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Buckingham R. H. Anticodon conformation and accessibility in wild-type and suppressor tryptophan tRNA from E. coli. Nucleic Acids Res. 1976 Apr;3(4):965–975. doi: 10.1093/nar/3.4.965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckingham R. H., Kurland C. G. Codon specificity of UGA suppressor tRNATrp from Escherichia coli. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5496–5498. doi: 10.1073/pnas.74.12.5496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danchin A., Thang M. N. Multiple states in macromolecules II. Entropic behaviour of tRNA degraded by polynucleotide phosphorylase. FEBS Lett. 1972 Jan 1;19(4):297–300. doi: 10.1016/0014-5793(72)80064-4. [DOI] [PubMed] [Google Scholar]
- Ehrenberg M., Rigler R., Wintermeyer W. On the structure and conformational dynamics of yeast phenylalanine-accepting transfer ribonucleic acid in solution. Biochemistry. 1979 Oct 16;18(21):4588–4599. doi: 10.1021/bi00588a020. [DOI] [PubMed] [Google Scholar]
- Englander J. J., Kallenbach N. R., Englander S. W. Hydrogen exchange study of some polynucleotides and transfer RNA. J Mol Biol. 1972 Jan 14;63(1):153–169. doi: 10.1016/0022-2836(72)90527-x. [DOI] [PubMed] [Google Scholar]
- Englander S. W., Englander J. J. Hydrogen-tritium exchange. Methods Enzymol. 1972;26:406–413. doi: 10.1016/s0076-6879(72)26021-9. [DOI] [PubMed] [Google Scholar]
- Goldstein R. N., Stefanovic S., Kallenbach N. R. On the conformation of transfer RNA in solution: dependence of denaturation temperature and structural parameters of mixed and formylmethionyl Escherichia coli transfer RNA on sodium ion concentration. J Mol Biol. 1972 Aug 21;69(2):217–236. doi: 10.1016/0022-2836(72)90227-6. [DOI] [PubMed] [Google Scholar]
- Hingerty B., Brown R. S., Jack A. Further refinement of the structure of yeast tRNAPhe. J Mol Biol. 1978 Sep 25;124(3):523–534. doi: 10.1016/0022-2836(78)90185-7. [DOI] [PubMed] [Google Scholar]
- Hirsh D., Gold L. Translation of the UGA triplet in vitro by tryptophan transfer RNA's. J Mol Biol. 1971 Jun 14;58(2):459–468. doi: 10.1016/0022-2836(71)90363-9. [DOI] [PubMed] [Google Scholar]
- Holbrook S. R., Sussman J. L., Warrant R. W., Church G. M., Kim S. H. RNA-ligant interactions. (I) Magnesium binding sites in yeast tRNAPhe. Nucleic Acids Res. 1977 Aug;4(8):2811–2820. doi: 10.1093/nar/4.8.2811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jack A., Ladner J. E., Rhodes D., Brown R. S., Klug A. A crystallographic study of metal-binding to yeast phenylalanine transfer RNA. J Mol Biol. 1977 Apr 15;111(3):315–328. doi: 10.1016/s0022-2836(77)80054-5. [DOI] [PubMed] [Google Scholar]
- Johnston P. D., Figueroa N., Redfield A. G. Real-time solvent exchange studies of the imino and amino protons of yeast phenylalanine transfer RNA by Fourier transform NMR. Proc Natl Acad Sci U S A. 1979 Jul;76(7):3130–3134. doi: 10.1073/pnas.76.7.3130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston P. D., Redfield A. G. An NMR study of the exchange rates for protons involved in the secondary and tertiary structure of yeast tRNA Phe. Nucleic Acids Res. 1977 Oct;4(10):3599–3615. doi: 10.1093/nar/4.10.3599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knox D. G., Rosenberg A. Fluctuations of protein structure as expressed in the distribution of hydrogen exchange rate constants. Biopolymers. 1980 May;19(5):1049–1068. doi: 10.1002/bip.1980.360190509. [DOI] [PubMed] [Google Scholar]
- Laiken S. L., Printz M. P. Kinetic class analysis of hydrogen-exchange data. Biochemistry. 1970 Mar 31;9(7):1547–1553. doi: 10.1021/bi00809a011. [DOI] [PubMed] [Google Scholar]
- Mandal C., Kallenbach N. R., Englander S. W. Base-pair opening and closing reactions in the double helix. A stopped-flow hydrogen exchange study in poly(rA).poly(rU). J Mol Biol. 1979 Dec 5;135(2):391–411. doi: 10.1016/0022-2836(79)90443-1. [DOI] [PubMed] [Google Scholar]
- Quigley G. J., Teeter M. M., Rich A. Structural analysis of spermine and magnesium ion binding to yeast phenylalanine transfer RNA. Proc Natl Acad Sci U S A. 1978 Jan;75(1):64–68. doi: 10.1073/pnas.75.1.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reid B. R., McCollum L., Ribeiro N. S., Abbate J., Hurd R. E. Identification of tertiary base pair resonances in the nuclear magnetic resonance spectra of transfer ribonucleic acid. Biochemistry. 1979 Sep 4;18(18):3996–4005. doi: 10.1021/bi00585a024. [DOI] [PubMed] [Google Scholar]
- Rich A., RajBhandary U. L. Transfer RNA: molecular structure, sequence, and properties. Annu Rev Biochem. 1976;45:805–860. doi: 10.1146/annurev.bi.45.070176.004105. [DOI] [PubMed] [Google Scholar]
- Schwarz U., Menzel H. M., Gassen H. G. Codon-dependent rearrangement of the three-dimensional structure of phenylalanine tRNA, exposing the T-psi-C-G sequence for binding to the 50S ribosomal subunit. Biochemistry. 1976 Jun 1;15(11):2484–2490. doi: 10.1021/bi00656a035. [DOI] [PubMed] [Google Scholar]
- Stein A., Crothers D. M. Conformational changes of transfer RNA. The role of magnesium(II). Biochemistry. 1976 Jan 13;15(1):160–168. doi: 10.1021/bi00646a025. [DOI] [PubMed] [Google Scholar]
- Sussman J. L., Kim S. Three-dimensional structure of a transfer rna in two crystal forms. Science. 1976 May 28;192(4242):853–858. doi: 10.1126/science.775636. [DOI] [PubMed] [Google Scholar]
- Teitelbaum H., Englander S. W. Open states in native polynucleotides. I. Hydrogen-exchange study of adenine-containing double helices. J Mol Biol. 1975 Feb 15;92(1):55–78. doi: 10.1016/0022-2836(75)90091-1. [DOI] [PubMed] [Google Scholar]
- Teitelbaum H., Englander S. W. Open states in native polynucleotides. II. Hydrogen-exchange study of cytosine-containing double helices. J Mol Biol. 1975 Feb 15;92(1):79–92. doi: 10.1016/0022-2836(75)90092-3. [DOI] [PubMed] [Google Scholar]
- Thang M. N., Beltchev B., Grunberg-Manago M. Phosphorolysis of tRNA. Multiple conformational states of the tRNA in solution. Eur J Biochem. 1971 Mar 11;19(2):184–193. doi: 10.1111/j.1432-1033.1971.tb01303.x. [DOI] [PubMed] [Google Scholar]
- Webb P. K., Fresco J. R. Tritium exchange studies of transfer RNA in native and denaturated conformations. J Mol Biol. 1973 Mar 5;74(3):387–402. doi: 10.1016/0022-2836(73)90379-3. [DOI] [PubMed] [Google Scholar]



