Abstract
Diseases such as asthma are characterized by airway hyperresponsiveness. Enhanced airway smooth muscle (ASM) intracellular Ca2+ ([Ca2+]i) response to agonist stimulation leading to increased airway constriction has been suggested to contribute to airway hyperresponsiveness. Caveolae are flask-shaped plasma membrane invaginations that express the scaffolding protein caveolin and contain multiple proteins important in [Ca2+]i signaling (e.g., agonist receptors, ion channels). We recently demonstrated that caveolae and caveolin-1 are important in [Ca2+]i regulation in human ASM. Proinflammatory cytokines such as tumor necrosis factor (TNF)-α and interleukin (IL)-13 modulate [Ca2+]i in ASM. We hypothesized that cytokine upregulation of caveolar signaling in ASM contributes to enhanced agonist-induced [Ca2+]i in inflammation. Enzymatically dissociated human ASM cells were exposed to medium (control), 20 ng/ml TNF-α, or 50 ng/ml IL-13 for 24 h. Caveolae-enriched membrane fractions displayed substantial increase in caveolin-1 and -2 expressions by TNF-α and IL-13. Transfection with caveolin-1-mRed DNA substantially accelerated and increased plasma membrane caveolin-1 expression by TNF-α and to a lesser extent by IL-13. Caveolin-1 enhancement was inhibited by nuclear factor-κB and mitogen-activated protein kinase inhibitors. In fura 2-loaded ASM cells, [Ca2+]i responses to 1 μM ACh, 10 μM histamine, or 10 nM bradykinin were all exaggerated by TNF-α as well as IL-13 exposure. However, disruption of caveolae using caveolin-1 suppression via small-interfering RNA resulted in significant blunting of agonist-induced [Ca2+]i responses of vehicle and TNF-α-exposed cells. These functional data were correlated to the presence of TNFR1 receptor (but not the IL-4/IL-13 receptor) within caveolae. Overall, these results indicate that caveolin-1 plays an important role in airway inflammation by modulating the effect of specific cytokines on [Ca2+]i.
Keywords: caveolae, lipid raft, lung, inflammation, asthma, cytokine, mitogen-activated protein kinase, nuclear factor-κB, small-interfering ribonucleic acid
airway inflammation underlies diseases such as asthma and contributes to increased airway contractility, pathological narrowing, and increased resistance to airflow observed in these diseases. Increased airway contractility during inflammation may result from enhanced intracellular calcium ([Ca2+]i) responses of airway smooth muscle (ASM) in response to bronchoconstrictor agonists. Accordingly, factors that contribute to increased [Ca2+]i in ASM are key to understanding reactive airway diseases.
Caveolae are flask-shaped plasma membrane invaginations rich in cholesterol and sphingolipids (44, 45). They express any of three caveolin proteins (caveolin-1, caveolin-2, and/or caveolin-3) and contain agonist receptors, ion channels, and other membrane proteins. Furthermore, caveolin proteins have been recognized as organizers and facilitators of signal transduction (4, 5, 14, 44, 45). We and others have established the presence of caveolae and caveolins in ASM (8, 14, 18, 33, 35). In ASM, bronchoconstrictors such as ACh or histamine increase [Ca2+]i by both sarcoplasmic reticulum (SR) Ca2+ release and plasma membrane Ca2+ influx (20, 29, 33, 34). We and others have reported that ASM plasma membrane fractions enriched in caveolae contain several Ca2+ regulatory proteins, including agonist receptors and plasma membrane Ca2+ influx channels (8, 33). Furthermore, caveolins have been shown to regulate [Ca2+]i in ASM (8, 14, 33). In this regard, caveolin-1 appears to be particularly important, with caveolin-3 (the isoform most associated with striated muscle) being absent in ASM (at least in humans) (14, 33).
While there is evidence that caveolins regulate [Ca2+]i in ASM, their role in the inflamed airway is still not well-established. In the lung, caveolin-1 has been shown to regulate nuclear factor-κB (NF-κB) and inflammatory responses to sepsis (13). Previous studies have reported caveolin regulation of p42/44 mitogen-activated protein (MAP) kinase (16) as well as RhoA and Rho kinase signaling (40), modulating cellular proliferation, a major aspect of diseases such as asthma. In the present study, using human ASM, we hypothesized that cytokine upregulation of caveolar signaling in ASM contributes to enhanced agonist-induced [Ca2+]i in inflammation, thus placing caveolae centrally within regulation of airway hyperresponsiveness.
MATERIALS AND METHODS
Isolation of human ASM cells.
The techniques for isolation of human ASM cells have been described previously (32, 33). Briefly, ASM cells were enzymatically dissociated from normal areas of third- to sixth-generation bronchi of surgical lung samples from patients at Mayo Clinic Rochester (deidentified samples from lobectomies or pneumenectomies considered surgical waste by the pathologist following clinical diagnoses; approved and considered exempt by Mayo Institutional Review Board). Bronchial segments were dissected and placed in ice-cold Hanks' balanced salt solution (HBSS; Invitrogen, Carlsbad, CA) supplemented with 10 mM HEPES and 2 mM Ca2+. Under light microscopy, ASM tissue were separated from cartilage, epithelium, and surrounding tissues and minced, and single cells were dissociated using papain and collagenase with ovomucoid/albumin separation (Worthington Biochemical, Lakewood, NJ). Cell pellets were resuspended and plated in sterile culture flasks or eight-well glass-bottomed Lab-Tek imaging chambers (Nalge Nunc International, Rochester, NY) and maintained at 37°C (5% CO2-95% air) in phenol red-free DMEM-F-12 medium (Invitrogen) supplemented with 10% FBS until ∼80% confluent. Before experiments, cells were washed in PBS solution (Invitrogen), and medium was changed to DMEM-F-12 lacking serum for 48 h. All experiments were performed in cells from passages 1–3 of subculture. Periodic assessment of ASM phenotype (smooth muscle actin and myosin, and agonist receptors, as well as lack of fibroblast markers) was performed.
Western blot analysis.
Proteins were separated by SDS-PAGE (either 15 or 4–15% gradient gels, Criterion Gel System; Bio-Rad, Hercules, CA) and then transferred to polyvinylidene fluoride membranes (Bio-Rad) for 60 min. Five percent milk in TBS containing 0.1% Tween (TBST) was used to block the membranes for 1 h. Membranes were then incubated overnight at 4°C with anti-caveolin-1, -2, or -3 (1:1,000) or antibodies against tumor necrosis factor (TNF) receptor 1 (TNFR1) or interleukin (IL)-13/IL-4 receptor (IL-4R). Following three washes with TBST, primary antibodies were detected using horseradish peroxidase-conjugated secondary antibodies and signals developed by Supersignal West Pico Chemiluminescent Substrate (Pierce Chemical, Rockford, IL).
Preparation of caveolar membranes.
The technique for preparation of caveolae-enriched membranes has been previously described by Wang et al. (42) and applied to human ASM cells (33). Briefly, ASM cells were homogenized in cold 0.25 M sucrose, 1 mM EDTA, and 20 mM Tricine, pH 7.8, layered onto a 30% Percoll gradient, and centrifuged at 84,000 g for 30 min. Plasma membrane fraction was extracted, sonicated, resuspended in a solution of OptiPrep (23%), and placed in a centrifuge tube. A linear 20–10% OptiPrep gradient was layered on top and centrifuged (52,000 g for 90 min). The caveolae-enriched upper membrane layer was collected and used for further experimentation. Purity of the caveolae-enriched fraction was verified by presence of caveolin proteins, but lack of Golgi or SR proteins.
Caveolin-1 knock down by small-interfering RNA.
As previously described (33), caveolin-1 small-interfering RNA (siRNA) duplex targeting against the open reading frame of bovine caveolin-1 mRNA (223–241 bases; 5′-CCA GAA GGA ACA CAC AGU U-dTdT-3′) and a negative control siRNA (5′-GCG CGC UUU GUA GGA UUC G-dTdT-3′) were selected for caveolin-1 knock down (Dharmacon, Lafayette, CO). Transfection of ASM cells at ∼60% confluence was achieved using 20 nM siRNA and Lipofectamine 2000 (Invitrogen) in DMEM-F-12 lacking FBS and antibiotics with a ratio of 20 pmol siRNA/μl Lipofectamine. After transfection, fresh growth medium was added after 6 h, and cells were analyzed after 48 h. In our previous study (33), we had verified the knock down efficacy (∼75%) and specificity by decreased expression of caveolin-1 (but not other isoforms) and lack of effect of the nonsense siRNA. In pilot studies, we verified such efficacy and specificity in the samples used here but do not show the data.
Caveolin-1 overexpression.
An mRed-tagged caveolin-1 construct was used, which was generated from cav-1-green fluorescent protein by removing a BamH1-HindIII fragment containing the cav-1 gene from the construct and inserting an mRed expression vector driven by a viral promoter, as described by Singh et al. (39). Upon reaching 70–90% confluence, ASM cells were transfected with the vector using Lipofectamine 2000. Cells were incubated with the transfection mix in DMEM-F-12 media without serum for 24 h before analysis. For the experiments, DMEM-F-12 medium with 10% FBS was added 4 h posttransfection and maintained for 24 h. Serum-free medium was then used for an additional 24 h to drive the cells to quiescence before further experimentation. Expression of the fluorescently tagged caveolin-1 (cav-1-mRed) was verified by fluorescence imaging.
[Ca2+]i imaging.
The techniques for [Ca2+]i imaging of ASM cells have been described previously (32, 33). ASM cells were incubated in 5 μM fura 2-AM (Invitrogen) for 60 min at room temperature and visualized using a fluorescence imaging system (MetaFluor; Universal Imaging, Downingtown, PA) on a Nikon Diaphot inverted microscope (Fryer Instruments, Edina, MN). Cells were initially perfused with HBSS (2.5 mM Ca2+ at room temperature), and baseline fluorescence levels were established. [Ca2+]i responses of 20–30 cells per chamber were obtained for individual software-defined regions of interest. Fura 2 was alternately excited at 340 and 380 nm, and fluorescence emissions were collected separately at 510 nm at 1.33 Hz with images acquired using a Photometric Cascade digital camera system (Roper Scientific, Tucson, AZ). Quantification of [Ca2+]i levels from fura 2 emissions were as previously described (2, 33). For agonist stimulation, amplitudes of [Ca2+]i responses were calculated (difference between peak Ca2+ and baseline Ca2+ before stimulation). The subsequent plateau level was measured from the same resting baseline.
Confocal fluorescence microscopy.
ASM cells grown on eight-chambered Lab-Tek chambers and transfected with cav-1-mRed were visualized using a ×100/1.3 oil immersion objective lens on an Olympus FluoView laser scanning confocal microscope and the 568-nm line of a Kr laser. Pixel images (1024 × 1024) were acquired with fixed laser and photomultiplier gain/contrast settings to allow comparison across groups.
Materials.
Chemicals and supplies were obtained from Sigma (St. Louis, MO) unless mentioned otherwise. Tissue culture reagents, including Dulbecco's modified Eagle's medium F-12 (DMEM-F-12) and FBS, were obtained from Invitrogen. Cav-1-mRed construct was kindly provided by Dr. Richard Pagano, Professor of Pulmonary and Critical Care, Mayo Clinic (Rochester, MN). The following antibodies were used [from Santa Cruz Biotechnology (Santa Cruz, CA) unless otherwise noted]: mouse monoclonal anti-caveolin-1, rabbit polyclonal anti-caveolin-2 (Abcam, Cambridge, MA), mouse monoclonal anti-caveolin-3 (BD Transduction Laboratories, Franklin Lakes, NJ), and rabbit polyclonal anti-IL-4R. TNF-α and IL-13 were purchased from Calbiochem (La Jolla, CA). The NF-κB inhibitors SN-50 and CAY-10512 were purchased from Calbiochem and Cayman Chemical (Ann Arbor, MI), respectively.
Statistical analysis.
Bronchial samples from at least five patients were used to obtain ASM cells, with biochemistry and molecular biology protocols being repeated a minimum of three times. [Ca2+]i experiments were performed in at least 20 cells from each bronchial sample. Not all protocols were performed in each sample obtained. Comparisons between groups or drug effects (e.g., cytokine exposures, [Ca2+]i responses) were performed using one- or two-way ANOVA, with Bonferroni correction for repeated comparisons where appropriate. Statistical significance was established at P < 0.05. All values are expressed as means ± SE.
RESULTS
Effect of inflammatory cytokines on caveolin expression in human ASM.
Western blot analysis of caveolar fractions from human ASM cells demonstrated significant increase in caveolin-1 expression following exposure to TNF-α compared with vehicle only (P < 0.05, Fig. 1). Exposure to IL-13 also significantly increased caveolin-1, but to a smaller extent than TNF-α. Caveolin-2 was expressed by human ASM cells, and the expression of caveolin-2 increased significantly with both cytokines (P < 0.05, Fig. 1). Caveolin-3 was absent in control as well as TNF-α- and IL-13-treated cells, consistent with our previous findings (33). Specificity of the caveolin-3 antibody was verified using appropriate positive controls (rat heart), as was the affinity for human proteins (human diaphragm muscle).
Fig. 1.
Effect of proinflammatory cytokines tumor necrosis factor (TNF)-α and interleukin-13 (IL-13) on caveolin-1, -2, and -3 expression in caveolae-enriched fractions of human airway smooth muscle (ASM) cells. ASM cell plasma membrane fractions enriched in caveolae were obtained as described previously (33). Exposure for 24 h to 20 ng/ml TNF-α significantly increased caveolin-1, and to a lesser extent caveolin-2, expression. Exposure to IL-13 also increased caveolin-1 and -2 expression compared with control, but to a lesser extent compared with TNF-α. Caveolin-3 was not expressed within caveolar membrane fractions of human ASM, even with cytokine exposure (positive controls such as rat heart or human diaphragm containing caveolin-3 not shown). AU, arbitrary units. Values are means ± SE. *Significant TNF-α effect compared with vehicle control. #Significant IL-13 effect compared with vehicle control. @Significant difference between TNF-α and IL-13 (P < 0.05).
Mechanisms of TNF-α-induced increase in caveolin-1.
Western blot analysis of ASM cells demonstrated significantly increased expression of caveolin-1 with TNF-α exposure (P < 0.05) but a reduced effect of TNF-α in the presence of either 2 μM PD-98059 (to inhibit ERK1/2) or one of the NF-κB inhibitors (20 μM SN-50 or 1 μM CAY-10512) (P < 0.05 for all inhibitors, Fig. 2A). Separately, these same inhibitors also substantially decreased expression of cav-1-mRed (data not shown).
Fig. 2.
Effect of TNF-α on caveolin-1 expression and intracellular Ca2+ ([Ca2+]i) responses. A: Western analysis demonstrated increased caveolin-1 with TNF-α exposure, an effect that was significantly reduced in the presence of either 2 μM PD-98059 [mitogen-activated protein (MAP) kinase inhibitor] or 20 μM SN-50 or 1 μM CAY-10512 [both nuclear factor-κB (NF-κB) inhibitors], respectively. B: [Ca2+]i responses under the same experimental conditions as shown above. While TNF-α significantly increased [Ca2+]i responses, additional exposure to PD-98059, SN-50, or CAY-10512 significantly inhibited [Ca2+]i responses, suggesting a functional role for MAP kinases and NF-κB as underlying mechanisms. Values are means ± SE. *Significant difference from control. #Significant inhibitor effect vs. TNF-α treatment (P < 0.05).
Investigating the effects on [Ca2+]i responses under the same experimental conditions as shown in Fig. 2A, exposure for 24 h to TNF-α significantly increased [Ca2+]i responses to histamine (10 μM) (P < 0.05, Fig. 2B). Additional exposure to either of the three inhibitors (PD-98059, SN-50, or CAY-10512) significantly blunted [Ca2+]i responses, suggesting a functional role for MAP kinases and NF-κB as underlying mechanisms (P < 0.05, Fig. 2B).
Effect of altered caveolin-1 expression on [Ca2+]i regulation.
Exposure to 20 ng/ml TNF-αfor 24 h increased [Ca2+]i responses to three different agonists (1 μM ACh, 10 μM histamine, and 10 nM bradykinin) compared with vehicle (culture buffer) or Lipofectamine (siRNA vehicle) controls (P < 0.05 for TNF-α effect for each agonist, Fig. 3A). Suppression of caveolin-1 expression using specific siRNA decreased [Ca2+]i responses to all three agonists compared with Lipofectamine only (P < 0.05, Fig. 3B). Nonsense siRNA transfection resulted in a nonsignificant 10–15% decrease in [Ca2+]i responses (depending on the agonist) compared with Lipofectamine controls in the absence or presence of TNF-α (data not shown). In the presence of caveolin-1 siRNA, the enhancing effect of TNF-α on [Ca2+]i responses to all three agonists was substantially and significantly reduced compared with TNF-α alone (P < 0.05 for each agonist, Fig. 3B). However, even in the presence of caveolin-1 siRNA, TNF-α still significantly increased (albeit to a smaller extent) [Ca2+]i responses to histamine and bradykinin (but not ACh).
Fig. 3.
Effect of caveolin-1 small-interfering RNA (siRNA) on TNF-α enhancement of [Ca2+]i responses of ASM cells to agonist stimulation. Exposure for 24 h to TNF-αsignificantly increased [Ca2+]i responses to ACh (1 μM), histamine (10 μM), and bradykinin (10 nM) compared with buffer (vehicle) control (A). In cells transfected with caveolin-1 siRNA, [Ca2+]i responses to all agonists were significantly decreased compared with transfection vehicle (Lipofectamine) controls (B). Caveolin-1 siRNA also blunted [Ca2+]i responses to agonist in the presence of TNF-α; however, in the case of histamine and bradykinin (but not ACh), there remained a residual enhancing effect of TNF-α on [Ca2+]i. Values are means ± SE. *Significant TNF-α effect. #Significant cav-1 siRNA effect compared with Lipofectamine control. @Significant cav-1 siRNA effect in the presence of TNF-α (compared to TNF-α in Lipofectamine alone) (P < 0.05).
Exposure to 50 ng/ml IL-13 also increased [Ca2+]i responses to all agonists compared with controls (culture medium or Lipofectamine; P < 0.05, Fig. 4A). Caveolin-1 suppression significantly decreased [Ca2+]i responses to all three agonists compared with Lipofectamine control (P < 0.05, Fig. 4B) and (as with TNF-α) blunted IL-13 enhancement of [Ca2+]i responses to the three agonists (P < 0.05, Fig. 4B). However, unlike TNF-α, even with caveolin-1 siRNA, IL-13 still enhanced [Ca2+]i responses to ACh (Fig. 4B). As with TNF-α, nonsense siRNA transfection resulted in a nonsignificant 10–15% decrease in [Ca2+]i responses in the absence or presence of IL-13 (data not shown). However, the blunting effect of caveolin-1 siRNA on IL-13-induced enhancement of [Ca2+]i was substantially less than the effects of the siRNA when TNF-α was used instead (P < 0.05).
Fig. 4.
Effect of caveolin-1 siRNA on IL-13 enhancement of [Ca2+]i responses to agonist stimulation. Exposure to IL-13 significantly increased [Ca2+]i responses to ACh, histamine, and bradykinin compared with control (with a greater effect on responses to ACh) (A). In cells transfected with caveolin-1 siRNA, [Ca2+]i responses to all agonists were decreased significantly compared with control (B). However, even with caveolin-1 siRNA, exposure to IL-13 increased the [Ca2+]i responses to all agonists. The effect of caveolin-1 siRNA on IL-13 enhancement of [Ca2+]i was not as pronounced as with TNF-α. Values are means ± SE. *Significant IL-13 effect. #Significant cav-1 siRNA effect compared with Lipofectamine control. @Significant cav-1 siRNA effect in the presence of IL-13 (compared with IL-13 in Lipofectamine alone) (P < 0.05).
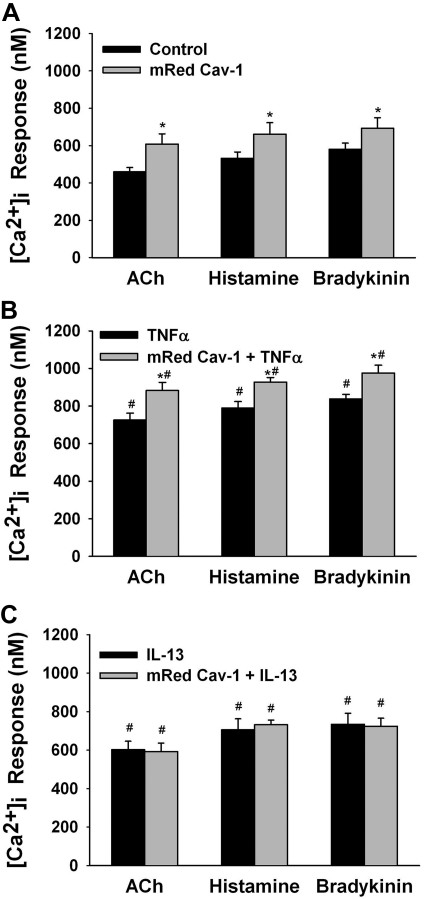
When caveolin-1 was overexpressed using the cav-1-mRed construct, [Ca2+]i responses to all three agonists were increased (P < 0.05, Fig. 5A). In the presence of overexpressed caveolin-1, 24 h exposure to TNF-α even further increased [Ca2+]i responses to agonists (P < 0.05, Fig. 5B). While IL-13 increased [Ca2+]i responses to agonists (as in Fig. 4), overexpression of caveolin-1 did not have an additional enhancing effect (Fig. 5C).
Fig. 5.
Effect of caveolin-1 overexpression on cytokine effects on [Ca2+]i responses. Overexpression of caveolin-1 using cav-1-mRed significantly increased [Ca2+]i responses to ACh, histamine, and bradykinin compared with nontransfected controls (A). Exposure to TNF-α increased [Ca2+]i responses to all agonists (B). The presence of cav-1-mRed further enhanced the effect of TNF-α on [Ca2+]i. The effect of cav-1-mRed on IL-13-induced increase in [Ca2+]i responses was minimal (C). Values are means ± SE. *Significant mRed cav-1 effect. #Significant cytokine effect compared with Lipofectamine control (P < 0.05).
Expression of TNF and IL-13 (IL-4) receptor within caveolae.
To determine the reason underlying differences between TNF-α and IL-13 in the role of caveolin-1 in [Ca2+]i regulation, we determined the expression of the receptors for either cytokine within caveolae. Both TNFR1 as well as IL-4Rα were expressed in whole ASM cell lysates and significantly increased in the presence of their respective ligand (TNF-α vs. IL-13; P < 0.05, Fig. 6). However, only TNFR1 was expressed within caveolar membrane fractions (Fig. 6) and upregulated by 24 h exposure to TNF-α (P < 0.05). In contrast, IL-4Rα was not localized to caveolar membrane fractions under control conditions or with cytokine exposure (Fig. 6).
Fig. 6.
Caveolar expression of TNF receptor 1 (TNFR1) and IL-4α receptor (IL-4Rα). Both TNFR1 and IL4Rα were expressed in whole ASM cell lysates and were significantly increased when cells were exposed to TNF-α or IL-13, respectively (A). However, only TNFR1 was expressed within caveolar membrane fractions, with increased expression following TNF-α exposure (B). Such differential expression may underlie the different effects of altered caveolin-1 expression on TNF-α vs. IL-13 enhancement of [Ca2+]i. Values are means ± SE. *Significant cytokine effect vs. control (P < 0.05).
DISCUSSION
In the present study, we demonstrate that inflammatory cytokines such as TNF-α (and to a lesser extent IL-13) increase caveolar caveolin-1 expression in human ASM. This increase in caveolin-1 expression (mediated via signaling pathways such as MAP kinases and NF-κB) is associated with increased TNFR1 expression within caveolae and contributes to the enhancement of [Ca2+]i responses to agonists induced by TNF-α. Thus downregulation of caveolin-1 expression blunts [Ca2+]i responses even in the presence of TNF-α. Furthermore, the relative smaller effect of altered caveolin-1 expression on IL-13 (compared with TNF-α)-induced enhancement of [Ca2+]i is associated with the absence of IL-13 receptor within caveolae, suggesting that caveolar modulation of cytokine effects may be dependent on the cytokine in question.
Caveolae and caveolins (of which 3 isoforms have been identified) have been examined in a number of cell types. Caveolins are integral caveolar membrane proteins that can regulate other caveolar proteins as well induce and modulate intracellular signal transduction (5, 44, 45) potentially via caveolin scaffolding domains (6, 45). Thus, relevant to the present study, caveolae may be central to [Ca2+]i regulation, as demonstrated using the siRNA studies, as well as in our previous study using cyclodextrins (33). We and others (7, 10, 31, 33, 37) have previously shown that caveolae in smooth muscle cells (including human ASM) contain proteins that are important not only for [Ca2+]i signaling but also contraction and cellular proliferation, thus facilitating regulation of signaling mechanisms at the plasma membrane and beyond. In this regard, it appears that caveolin-1 may be the isoform of interest in ASM cells, since we and others (8, 16, 18, 33) have found that human and canine ASM cells and tissue express caveolin-1 and caveolin-2 (which is usually associated with caveolin-1) but not caveolin-3. The lack of caveolin-3 in the present study is thus consistent with previous work (33) and set the stage for further examination of the role of caveolin-1 in airway inflammation.
Airway inflammation and caveolae.
A hallmark symptom of clinically important diseases such as asthma is inflammation and increased airway narrowing (21, 25, 26). The two are linked by the enhancing effect of cytokines on ASM contractility (1, 38). Here, the role of cytokines such as TNF-α and IL-13 in airway diseases has been well described (3). Furthermore, ASM has been found to itself regulate inflammation by releasing inflammatory mediators and expressing a repertoire of proteins, including the receptors for proinflammatory cytokines (41). Accordingly, factors that modulate ASM structure and function in the presence of inflammation are key to understanding airway diseases.
Altered caveolin levels or function have been associated with cardiovascular diseases (11, 17). Although the presence of caveolae and caveolins in ASM of different species is now recognized (14, 18, 33), their role during ASM inflammation, or in reactive airway disease, is not well established. In this regard, the role of caveolae (specifically caveolin-1) in proinflammatory cytokine-induced airway inflammation has been barely examined. In a largely biochemical study using human ASM, Hunter and Nixon (24) demonstrated that lipid rafts are important in specific TNF-α-mediated effects. In that study, TNFR1 is localized to both lipid raft and nonraft regions of the plasma membrane, and lipid rafts are important for TNFR1-mediated activation of RhoA (but not NF-κB and MAP kinase pathways), as demonstrated using depletion of cholesterol from rafts using methyl-β-cyclodextrin. However, this study did not examine the role of caveolin-1, which necessitates techniques such as siRNA rather than the cyclodextrins, which are relatively nonspecific, or the Ca2+ regulation aspect. To the best of our knowledge, there are currently no data on the role of caveolae in IL-13 effects on ASM. A single study in ovalbumin-sensitized/challenged mice reported that caveolin-1 is necessary for IL-4-mediated enhancement of transforming growth factor (TGF)-β signaling but did not examine airway reactivity (27).
The present study demonstrates two novel aspects of interplay between caveolae and cytokines (especially TNF-α) in ASM inflammation. First is the finding that TNF-α (and to a lesser extent IL-13) increases caveolin-1 expression in human ASM. As our experiments using cav-1-mRed demonstrate, increased caveolin-1 expression alone has the potential to enhance [Ca2+]i regulation following agonist exposure (with the converse finding that caveolin-1 suppression reduces [Ca2+]i), placing caveolae and caveolin-1 centrally in regulation of [Ca2+]i. Enhancement of [Ca2+]i can result from increased caveolar expression of agonist receptors [e.g., muscarinic or histaminergic receptors known to be in caveolae (33)] or of Ca2+ influx mechanisms such as transient receptor potential channels involved in store-operated Ca2+ entry (33). Previous studies, including our own, have already reported that cytokines such as TNF-α can enhance muscarinic receptor activation as well as Ca2+ influx mechanisms (23, 28, 43). Now, the blunting effect of suppressed caveolin-1 on TNF-α enhancement of [Ca2+]i, and conversely the potentiating effect of cav-1-mRed on TNF-α-induced modulation of [Ca2+]i, highlight a role of caveolar signaling. Here, the second novel finding is the expression and upregulation of caveolar TNFR1, suggesting that caveolae are not only a target of TNF-α signaling (which increases caveolin-1 expression) but are also a source of TNF-α effects. These findings again place caveolae centrally within [Ca2+]i regulation of ASM. By virtue of the presence of TNFR1 and other [Ca2+]i regulatory proteins, caveolae (and caveolin-1) may alter [Ca2+]i responses to agonist both in the absence and presence of cytokines such as TNF-α, thus contributing to enhanced [Ca2+]i in airway inflammation. Here, TNF-α-induced increase in caveolin-1 and caveolae may further increase the availability of these regulatory proteins and thus promote enhancement of [Ca2+]i.
In contrast to a link between TNF-α effects and caveolae, the significantly smaller effect of altered caveolin-1 expression on IL-13 enhancement of [Ca2+]i is interesting. There is currently no information on IL-13 regulation of caveolin-1. Here, our novel data show a small upregulation of caveolin-1 expression by IL-13. Separately, as shown by other studies, we also found that [Ca2+]i responses to agonists are increased by IL-13 exposure (9, 12, 34). However, unlike TNF-α, suppression of caveolin-1 had smaller effects on IL-13 enhancement of [Ca2+]i, and cav-1-mRed did not potentiate IL-13 effects, suggesting a smaller role for caveolin-1 or caveolae in the signaling of this cytokine. This may be partly due to the fact that the IL-4Rα is not expressed within caveolae (even with cytokine stimulation). Nonetheless, the smaller effect of caveolin-1 siRNA on IL-13 effects may be mediated by cytokine-induced changes in caveolar expression of agonist receptors, for example.
The mechanisms by which cytokines such as TNF-α can modulate caveolin-1 expression in ASM have not been previously examined. In our study, we found that exposure to TNF-α increased production of the cav-1-mRed protein and accelerated its insertion into the plasma membrane. Such increased caveolin-1 expression was significantly blunted in the presence of inhibitors of MAP kinases and NF-κB, suggesting involvement of these pathways, which are already known to be important in mediating the effects of TNF-α (1, 22). In addition to being important in modulating caveolin-1 expression, these pathways may also be relevant to mediating caveolin-1 effects themselves. Studies in different cell types (including some in ASM) have shown that caveolin-1 can modulate signaling cascades relevant to [Ca2+]i, force, and cell proliferation (16, 19, 30, 33, 36). For example, Gosens et al. (16) reported in human ASM cells that disruption of caveolae using cyclodextrins or caveolin-1 siRNA promotes p42/p44 MAP kinase activation and increases cell proliferation. While we did not examine caveolin-1 effects on MAP kinases in the presence of TNF-α signaling, the previous data in combination with our present results suggest another potential mechanism of interplay between cytokines and caveolae. TNF-α can increase ASM contractility as well as cell proliferation via multiple signaling mechanisms besides MAP kinases (1, 22).
Furthermore, increased caveolin-1 may also increase [Ca2+]i (as shown here), RhoA (24), and other mechanisms that increase contractility. Indeed, we interpret increased caveolin-1 as having a role in enhancing the contractile phenotype of ASM. Interestingly, Gosens et al. (15) recently published a study demonstrating that caveolin-1 is required for TGF-β-induced promotion of ASM cells of contractile phenotype, although [Ca2+]i or contractility per se was not examined. Nonetheless, caveolin-1 appears to contribute to both major aspects of reactive airway diseases.
In conclusion, the results of the present study demonstrate that inflammatory cytokines such as TNF-α increase caveolin-1 expression via mechanisms such as MAP kinase and NF-κB. Increased caveolin-1 expression in turn contributes to increased [Ca2+]i. Furthermore, increased caveolin-1 allows for greater expression of TNFR1 within caveolae, which would further facilitate enhancement of [Ca2+]i. In contrast to TNF-α, IL-13 does increase caveolin-1 expression in ASM, but the lack of IL-4Rα within caveolae, and lack of enhancement of IL-13 effects on [Ca2+]i by caveolin-1 overexpression, would suggest that this cytokine and caveolin-1 are not functionally linked in terms of regulation of [Ca2+]i.
GRANTS
This study was supported by National Heart, Lung, and Blood Institute Grants HL-090595 and HL-090595-S2 (C. M. Pabelick) and HL-088029 (Y. S. Prakash).
DISCLOSURES
No conflicts of interest are declared by the authors.
REFERENCES
- 1. Amrani Y, Chen H, Panettieri RA., Jr Activation of tumor necrosis factor receptor 1 in airway smooth muscle: a potential pathway that modulates bronchial hyper-responsiveness in asthma? Respir Res 1: 49–53, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Ay B, Prakash YS, Pabelick CM, Sieck GC. Store-operated Ca2+ entry in porcine airway smooth muscle. Am J Physiol Lung Cell Mol Physiol 286: L909–L917, 2004 [DOI] [PubMed] [Google Scholar]
- 3. Barnes PJ. Cytokine-directed therapies for asthma. J Allergy Clin Immunol 108: S72–S76, 2001 [DOI] [PubMed] [Google Scholar]
- 4. Chidlow JH, Sessa WC. Caveolae, caveolins, and cavins: complex control of cellular signalling and inflammation. Cardiovasc Res 86: 219–225, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Cohen AW, Hnasko R, Schubert W, Lisanti MP. Role of caveolae and caveolins in health and disease. Physiol Rev 84: 1341–1379, 2004 [DOI] [PubMed] [Google Scholar]
- 6. Couet J, Belanger MM, Roussel E, Drolet MC. Cell biology of caveolae and caveolin. Adv Drug Deliv Rev 49: 223–235, 2001 [DOI] [PubMed] [Google Scholar]
- 7. Daniel EE, Eteraf T, Sommer B, Cho WJ, Elyazbi A. The role of caveolae and caveolin 1 in calcium handling in pacing and contraction of mouse intestine. J Cell Mol Med 13: 352–364, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Darby PJ, Kwan CY, Daniel EE. Caveolae from canine airway smooth muscle contain the necessary components for a role in Ca2+ handling. Am J Physiol Lung Cell Mol Physiol 279: L1226–L1235, 2000 [DOI] [PubMed] [Google Scholar]
- 9. Deshpande DA, Dogan S, Walseth TF, Miller SM, Amrani Y, Panettieri RA, Kannan MS. Modulation of calcium signaling by interleukin-13 in human airway smooth muscle: role of CD38/cyclic adenosine diphosphate ribose pathway. Am J Respir Cell Mol Biol 31: 36–42, 2004 [DOI] [PubMed] [Google Scholar]
- 10. El-Yazbi AF, Cho WJ, Schulz R, Daniel EE. Calcium extrusion by plasma membrane calcium pump is impaired in caveolin-1 knockout mouse small intestine. Eur J Pharmacol 591: 80–87, 2008 [DOI] [PubMed] [Google Scholar]
- 11. Frank PG, Lisanti MP. Caveolin-1 and caveolae in atherosclerosis: differential roles in fatty streak formation and neointimal hyperplasia. Curr Opin Lipidol 15: 523–529, 2004 [DOI] [PubMed] [Google Scholar]
- 12. Gao YD, Zou JJ, Zheng JW, Shang M, Chen X, Geng S, Yang J. Promoting effects of IL-13 on Ca2+ release and store-operated Ca2+ entry in airway smooth muscle cells. Pulm Pharmacol Ther 23: 182–189, 2010 [DOI] [PubMed] [Google Scholar]
- 13. Garrean S, Gao XP, Brovkovych V, Shimizu J, Zhao YY, Vogel SM, Malik AB. Caveolin-1 regulates NF-kappaB activation and lung inflammatory response to sepsis induced by lipopolysaccharide. J Immunol 177: 4853–4860, 2006 [DOI] [PubMed] [Google Scholar]
- 14. Gosens R, Mutawe M, Martin S, Basu S, Bos ST, Tran T, Halayko AJ. Caveolae and caveolins in the respiratory system. Curr Mol Med 8: 741–753, 2008 [DOI] [PubMed] [Google Scholar]
- 15. Gosens R, Stelmack GL, Bos ST, Dueck G, Mutawe MM, Schaafsma D, Unruh H, Gerthoffer WT, Zaagsma J, Meurs H, Halayko AJ. Caveolin-1 is required for contractile phenotype expression by airway smooth muscle cells. J Cell Mol Med In press [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Gosens R, Stelmack GL, Dueck G, McNeill KD, Yamasaki A, Gerthoffer WT, Unruh H, Gounni AS, Zaagsma J, Halayko AJ. Role of caveolin-1 in p42/p44 MAP kinase activation and proliferation of human airway smooth muscle. Am J Physiol Lung Cell Mol Physiol 291: L523–L534, 2006 [DOI] [PubMed] [Google Scholar]
- 17. Gratton JP, Bernatchez P, Sessa WC. Caveolae and caveolins in the cardiovascular system. Circ Res 94: 1408–1417, 2004 [DOI] [PubMed] [Google Scholar]
- 18. Halayko AJ, Stelmack GL. The association of caveolae, actin, and the dystrophin-glycoprotein complex: a role in smooth muscle phenotype and function? Can J Physiol Pharmacol 83: 877–891, 2005 [DOI] [PubMed] [Google Scholar]
- 19. Hassan GS, Williams TM, Frank PG, Lisanti MP. Caveolin-1-deficient aortic smooth muscle cells show cell autonomous abnormalities in proliferation, migration, and endothelin-based signal transduction. Am J Physiol Heart Circ Physiol 290: H2393–H2401, 2006 [DOI] [PubMed] [Google Scholar]
- 20. Hirota S, Helli P, Janssen LJ. Ionic mechanisms and Ca2+ handling in airway smooth muscle. Eur Respir J 30: 114–133, 2007 [DOI] [PubMed] [Google Scholar]
- 21. Hirota S, Helli PB, Catalli A, Chew A, Janssen LJ. Airway smooth muscle excitation-contraction coupling and airway hyperresponsiveness. Can J Physiol Pharmacol 83: 725–732, 2005 [DOI] [PubMed] [Google Scholar]
- 22. Holgate ST, Holloway J, Wilson S, Howarth PH, Haitchi HM, Babu S, Davies DE. Understanding the pathophysiology of severe asthma to generate new therapeutic opportunities. J Allergy Clin Immunol 117: 496–507, 2006 [DOI] [PubMed] [Google Scholar]
- 23. Hotta K, Emala CW, Hirshman CA. TNF-alpha upregulates Gialpha and Gqalpha protein expression and function in human airway smooth muscle cells. Am J Physiol Lung Cell Mol Physiol 276: L405–L411, 1999 [DOI] [PubMed] [Google Scholar]
- 24. Hunter I, Nixon GF. Spatial compartmentalization of TNFR1-dependent signaling pathways in human airway smooth muscle cells: lipid rafts are essential for TNF-alpha-mediated activation of RhoA but dispensable for the activation of the NF-kappa B and MAPK pathways. J Biol Chem 281: 34705–34715, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Joubert P, Hamid Q. Role of airway smooth muscle in airway remodeling. J Allergy Clin Immunol 116: 713–716, 2005 [DOI] [PubMed] [Google Scholar]
- 26. Lazaar AL, Panettieri RA., Jr Airway smooth muscle: a modulator of airway remodeling in asthma. J Allergy Clin Immunol 116: 488–495, 2005 [DOI] [PubMed] [Google Scholar]
- 27. Le Saux CJ, Teeters K, Miyasato SK, Hoffmann PR, Bollt O, Douet V, Shohet RV, Broide DH, Tam EK. Down-regulation of caveolin-1, an inhibitor of transforming growth factor-beta signaling, in acute allergen-induced airway remodeling. J Biol Chem 283: 5760–5768, 2008 [DOI] [PubMed] [Google Scholar]
- 28. Oenema TA, Kolahian S, Nanninga JE, Rieks D, Hiemstra PS, Zuyderduyn S, Halayko AJ, Meurs H, Gosens R. Pro-inflammatory mechanisms of muscarinic receptor stimulation in airway smooth muscle (Abstract). Respir Res 11: 130, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Pabelick CM, Sieck GC, Prakash YS. Invited review: significance of spatial and temporal heterogeneity of calcium transients in smooth muscle. J Appl Physiol 91: 488–496, 2001 [DOI] [PubMed] [Google Scholar]
- 30. Patel HH, Murray F, Insel PA. Caveolae as organizers of pharmacologically relevant signal transduction molecules. Annu Rev Pharmacol Toxicol 48: 359–391, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Pojoga LH, Romero JR, Yao TM, Loutraris P, Ricchiuti V, Coutinho P, Guo C, Lapointe N, Stone JR, Adler GK, Williams GH. Caveolin-1 ablation reduces the adverse cardiovascular effects of N-omega-nitro-l-arginine methyl ester and angiotensin II. Endocrinology 151: 1236–1246, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Prakash YS, Iyanoye A, Ay B, Mantilla CB, Pabelick CM. Neurotrophin effects on intracellular Ca2+ and force in airway smooth muscle. Am J Physiol Lung Cell Mol Physiol 291: L447–L456, 2006 [DOI] [PubMed] [Google Scholar]
- 33. Prakash YS, Thompson MA, Vaa B, Matabdin I, Peterson TE, He T, Pabelick CM. Caveolins and intracellular calcium regulation in human airway smooth muscle. Am J Physiol Lung Cell Mol Physiol 293: L1118–L1126, 2007 [DOI] [PubMed] [Google Scholar]
- 34. Sathish V, Thompson MA, Bailey JP, Pabelick CM, Prakash YS, Sieck GC. Effect of proinflammatory cytokines on regulation of sarcoplasmic reticulum Ca2+ reuptake in human airway smooth muscle. Am J Physiol Lung Cell Mol Physiol 297: L26–L34, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Sathish V, Yang B, Meuchel L, VanOosten S, Ryu A, Thompson MA, Prakash YS, Pabelick CM. Caveolin-1 and force regulation in airway smooth muscle. Am J Physiol Lung Cell Mol Physiol 300: L920–L926, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Sedding DG, Braun-Dullaeus RC. Caveolin-1: dual role for proliferation of vascular smooth muscle cells. Trends Cardiovasc Med 16: 50–55, 2006 [DOI] [PubMed] [Google Scholar]
- 37. Shakirova Y, Mori M, Ekman M, Erjefalt J, Uvelius B, Sward K. Human urinary bladder smooth muscle is dependent on membrane cholesterol for cholinergic activation. Eur J Pharmacol 634: 142–148, 2010 [DOI] [PubMed] [Google Scholar]
- 38. Shore SA, Moore PE. Effects of cytokines on contractile and dilator responses of airway smooth muscle. Clin Exp Pharmacol Physiol 29: 859–866, 2002 [DOI] [PubMed] [Google Scholar]
- 39. Singh RD, Puri V, Valiyaveettil JT, Marks DL, Bittman R, Pagano RE. Selective caveolin-1-dependent endocytosis of glycosphingolipids. Mol Biol Cell 14: 3254–3265, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Sommer B, Montano LM, Carbajal V, Flores-Soto E, Ortega A, Ramirez-Oseguera R, Irles C, El-Yazbi AF, Cho WJ, Daniel EE. Extraction of membrane cholesterol disrupts caveolae and impairs serotonergic (5-HT2A) and histaminergic (H1) responses in bovine airway smooth muscle: role of Rho-kinase. Can J Physiol Pharmacol 87: 180–195, 2009 [DOI] [PubMed] [Google Scholar]
- 41. Tliba O, Amrani Y. Airway smooth muscle cell as an inflammatory cell: lessons learned from interferon signaling pathways. Proc Am Thorac Soc 5: 106–112, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Wang PY, Kitchens RL, Munford RS. Bacterial lipopolysaccharide binds to CD14 in low-density domains of the monocyte-macrophage plasma membrane. J Inflamm 47: 126–137, 1995 [PubMed] [Google Scholar]
- 43. White TA, Xue A, Chini EN, Thompson M, Sieck GC, Wylam ME. Role of transient receptor potential C3 in TNF-alpha-enhanced calcium influx in human airway myocytes. Am J Respir Cell Mol Biol 35: 243–251, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Williams TM, Lisanti MP. The Caveolin genes: from cell biology to medicine. Ann Med 36: 584–595, 2004 [DOI] [PubMed] [Google Scholar]
- 45. Williams TM, Lisanti MP. The caveolin proteins (Abstract). Genome Biol 5: 214, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]