Abstract
Fibrotic interstitial pneumonias are more prevalent in males of advancing age, although little is known about the underlying mechanisms. To evaluate the contributions of age and sex to the development of pulmonary fibrosis, we intratracheally instilled young (8–12 wk) and aged (52–54 wk) male and female mice with bleomycin and assessed the development and severity of fibrotic lung disease by measurements of lung collagen levels, static compliance, leukocyte infiltration, and stereological quantification of fibrotic areas in histological sections. We also quantified proinflammatory and profibrotic chemokine and cytokine levels in the bronchoalveolar lavage fluid. Aged male mice developed more severe lung disease, indicated by increased mortality, increased collagen deposition, and neutrophilic alveolitis compared with aged female mice or young mice of either sex. Aged male mice also exhibited increased levels of transforming growth factor-β, IL-17A, and CXCL1 in their bronchoalveolar lavage fluid. Young male mice developed a more fibrotic disease after bleomycin instillation compared with female mice, regardless of age. There was no difference in fibrosis between young and aged female mice. Taken together, these findings suggest that the variables of advanced age and male sex contribute to the severity of pulmonary fibrosis in this model. Our findings also emphasize the importance of stratifying experimental groups on the basis of age and sex in experimental and epidemiological studies of this nature.
Keywords: aging, gender, inflammation
fibrotic interstitial lung diseases, including idiopathic pulmonary fibrosis (IPF), are more prevalent in males as they advance in age (23, 40). Although the mechanisms remain unknown, two factors have been proposed to contribute to this observation. First, in a meta-analysis of six case-controlled studies conducted since 1990, Tasker and Coultas (54) concluded that environmental exposures to cigarette smoke, livestock, wood dust, metal dust, and sand/stone dusts were significant risk factors for the development of IPF. While these environmental exposures can occur in both men and women, older men are more likely to have been lifelong smokers compared with women, and men more commonly occupy positions in the workforce associated with these types of occupational exposures (33, 38). Second, studies conducted in rodents have suggested that androgens may contribute to enhanced injury to, and fibrosis of, the lung and other organs (10, 19, 37, 51, 55). However, the interactions between age and sex have not been systematically studied in animal models of pulmonary fibrosis.
Intrapulmonary exposure to bleomycin has become a standard model of injury-associated pulmonary fibrosis in mice (1, 49). Following intratracheal instillation at sublethal doses, bleomycin promotes a rapid oxidant-dependent acute lung injury associated with apoptosis of the alveolar epithelium and an ensuing inflammatory response associated with loss of epithelial barrier function (2, 3). Subsequently, the initial inflammatory response is replaced by a fibroproliferative response, as transforming growth factor (TGF)-β and other mediators are expressed by resident and infiltrating macrophages and by the surviving and regenerating alveolar epithelium (32). The fibroproliferative phase is associated with fibrocyte recruitment, epithelial-mesenchymal transition, and resident lung fibroblast migration, proliferation, and transdifferentiation into myofibroblasts (25, 27, 34, 43). Most studies using the bleomycin model have been conducted with young mice (8–12 wk) of mixed sexes. Consequently, little is known about how age and sex affect the development of pulmonary fibrosis in mice in response to bleomycin instillation.
To address the possible contributions of age and sex in the development of bleomycin-induced pulmonary fibrosis, we investigated inflammatory cell accumulation, lung mechanics, and the development of fibrosis in young (8–12 wk) and aged (52–54 wk) male and female C57BL/6 mice. As we will show, the development of pulmonary fibrosis in bleomycin-instilled mice was specifically increased in aged male mice compared with age-matched female mice and young mice of both sexes. In addition, the increase in fibrosis in aged male mice was associated with increased mortality and increased accumulation of inflammatory cells, especially neutrophils, in the air spaces compared with aged female mice or young male or female mice.
MATERIALS AND METHODS
Animal care and intratracheal instillation.
C57BL/6J male and female mice were purchased from Jackson Laboratory. Young mice (8–10 wk) and aged mice (52–54 wk) were housed on hardwood bedding with 12:12-h light-dark cycles and fed standard rodent diet at the Center for Laboratory Animal Care at National Jewish Health. Mice were anesthetized by intraperitoneal injection of ketamine/xylazine and subsequently administered bleomycin (3 U/kg, TEVA Pharmaceuticals, North Wales, PA) in 50 μl of sterile saline or 50 μl of saline alone as a control by intratracheal instillation following direct visualization of the trachea with a mouse laryngoscope (Penn-Century, Wyndmoor, PA), as described (18, 45). Aged mice were dosed with the same amount of bleomycin as young mice, because, while their body mass increases with age, their lung volume remains stable once mature (Fig. 1A). This approach ensured that all mice received the same amount of bleomycin per lung volume and that changes in fibrosis in aged male mice were not simply due to the administration of a weight-corrected increased dose of bleomycin. All mouse procedures were conducted under a protocol approved by the National Jewish Health Institutional Animal Care and Use Committee. Five mice were harvested for each experimental point, and all experiments were repeated three times.
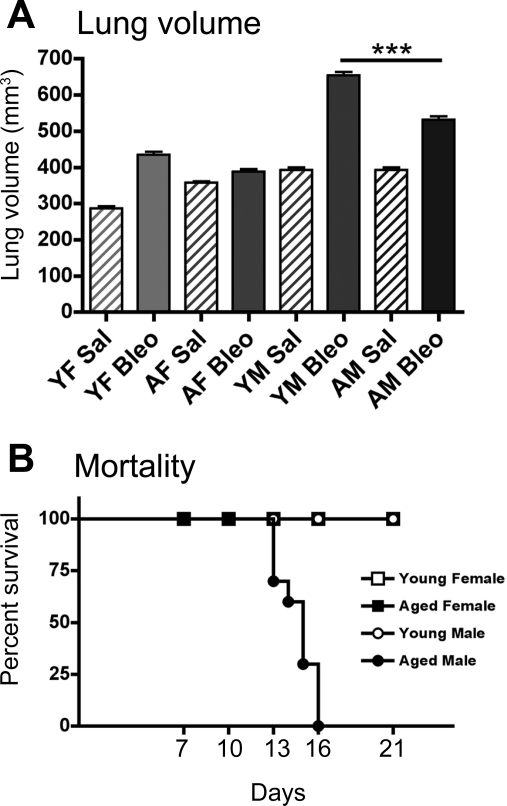
Fig. 1.
Aged male (AM) mice have increased lung tissue volume and mortality after bleomycin (Bleo) instillation. A: lung tissue volume does not change with age, but is significantly increased in young female (YF) and male (YM) mice and AM mice after Bleo (P < 0.001). AM mice have a significant increase in lung tissue volume compared with YF and aged female (AF) (***P < 0.001) mice, and a significantly lower lung tissue volume compared with YM mice (***P < 0.001). Values are means ± SE. B: AM mice exhibit increased mortality after intratracheal administration of Bleo compared with female mice and YM mice. Sal, saline.
Assessment of lung static compliance.
Anesthetized mice were attached by a tracheal tube to the SCIREQ small-animal ventilator to measure respiratory mechanics. Static compliance, a measurement that reflects the static elastic recoil pressure of the lungs at a volume of 0.95 ml, was determined. Female mice and young male mice were harvested 3 wk after bleomycin or saline instillation. Aged male mice were harvested after 2 wk due to high mortality by week 3 (Fig. 1B).
Bronchoalveolar lavage.
Mice were killed by lethal intraperitoneal injection of Nembutal (Ovation Pharmaceuticals, Paramus, NJ). The tracheas were cannulated, and the lungs lavaged three times with 1 ml PBS containing 0.6 mM EDTA and processed as previously described (6).
Quantification of cytokines and albumin by ELISA.
IL-17A, TGF-β, CXCL1, CXCL2 (R&D Systems, Minneapolis, MN), and albumin (Bethyl Laboratories, Montgomery, TX) levels were measured in bronchoalveolar lavage fluid (BALF) samples by ELISA following the manufacturer's protocol.
Evaluation of lung histology.
Paraffin embedded lung sections cut at 5-μm intervals were stained using Mason's Trichrome. Deposited collagen stains a bright blue color. Images were taken on an upright Olympus BX51. Final magnification of images is ×100–400. For stereological determination of total lung tissue volume, the left and postcaval lobes of the lung were cut into 2-mm sections, and tissue volume was estimated using surface area point counts by the Cavalieri method (39). Sections were randomly arranged into paraffin blocks. Blocks were cut at 5-μm intervals, three slides per mouse were taken, and a total of 15 uniformed, randomly sampled images were obtained at ×40 magnification. Images were analyzed using stereology grid-counting techniques to quantify areas of fibrotic disease. Total percentage of lung fibrosis was determined as described (30). Error was assessed using the Gunderson coefficient of error method (24) and was calculated to be <0.05. Stereological analysis was performed according to American Thoracic Society guidelines (28). Immunohistochemical staining of phospho-Smad2/3 was performed as previously described (46). Briefly, sections were deparafinized and rehydrated, followed by antigen retrieval using citrate buffer. Endogenous peroxidase activity was blocked using 3% H2O2. Nonspecific binding was reduced by incubation in 10% blocking serum (Vector, Burlingame, CA). Primany phospho-Smad2/3 antibody (Cell Signaling, Danvers, MA) was used at a 1:200 dilution, followed by incubation with a biotinylated secondary antibody (Vector), and streptavidin/peroxidase. Slides were developed in 3,3′-diaminobenzidine solution (Vector). Five images per slide and three slides per treatment group were examined for phospho-Smad2/3 staining. The final magnification of images was ×400.
Measurement of lung hydroxyproline.
Changes in lung collagen were quantified by measurements of hydroxyproline levels following homogenization in PBS and hydrolysis in an equal volume of 12 N HCl for 8 h at 120°C, as previously described (18). To allow the assessment of multiple parameters of lung inflammation and fibrosis in the same mouse, we conducted an initial comparative analysis of hydroxyproline levels in the upper right lobe and the whole left lung in mice that had been instilled with bleomycin or saline for 14 days. Both lung samples exhibited an approximately twofold increase in hydroxyproline after bleomycin (data not shown). Consequently, the upper right lobe was used to sample lung collagen levels throughout the study.
Statistics.
Data are presented as means ± SE. Differences between conditions at specific time points were examined using Student's unpaired t-test. One-way ANOVA with Newman-Keuls post hoc analysis compared results from more than two groups, with P < 0.05 considered significant.
RESULTS
Age and sex affect the development of pulmonary fibrosis in mice following bleomycin administration.
To investigate how age and sex affect the development of pulmonary fibrosis following bleomycin administration, young (8–10 wk) and aged (52 wk) male and female C57BL/6 mice were instilled intratracheally with bleomycin (3 U/kg) and followed for up to 21 days. Lung tissue volumes were significantly increased in bleomycin-instilled young female, young male, and aged male mice compared with saline-instilled control mice (P < 0.001; Fig. 1A). Young male mice exhibited the greatest increase in lung tissue volume after bleomycin (P < 0.001; Fig. 1A). Bleomycin-instilled aged male mice also had a significant increase in lung tissue volumes compared with young female (P < 0.01) and aged female (P < 0.001; Fig. 1A) (50).
Pulmonary exposure to bleomycin also affected mortality in the different groups of mice. Figure 1B shows that, while young male, young female, and aged female mice survived for 21 days, increased mortality was observed in aged male mice, with none of the mice surviving beyond 16 days. These findings suggest that the combination of age and male sex contribute to the severity of bleomycin-induced mortality. Consequently, all subsequent experiments were conducted at day 14 postbleomycin instillation, since previous studies have shown that the development of bleomycin-induce fibrosis reaches a plateau by day 14 (11, 31).
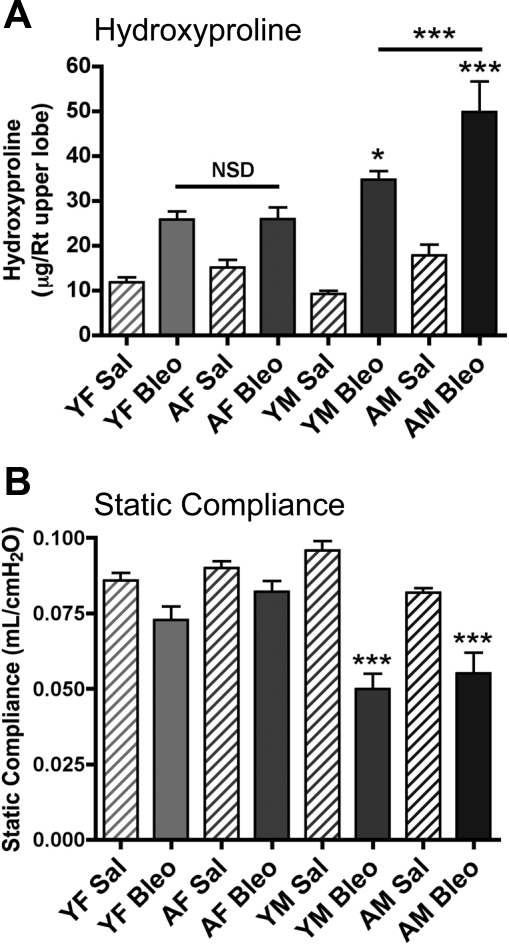
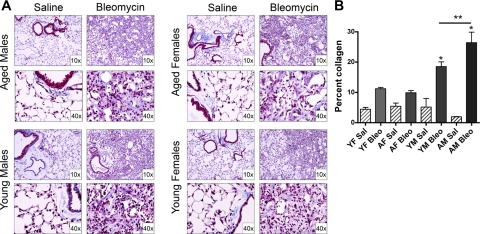
To assess the effects of age and sex on bleomycin-induced pulmonary fibrosis, we evaluated lung static compliance, lung collagen (as assessed by hydroxyproline) levels, and histopathology. Figure 2A shows that bleomycin instillation into the lungs of young and aged female mice resulted in a significant (P < 0.05) approximately twofold increase in lung collagen deposition, as reflected by increased hydroxyproline levels compared with control mice injected with saline. There was no significant difference (P > 0.1) in the amount of deposited collagen between young and aged female mice after bleomycin. In contrast, both young and aged male mice treated with bleomycin exhibited a significant increase (P < 0.05 and P < 0.001, respectively) in lung collagen levels compared with female mice. In addition, aged male mice had significantly higher lung collagen levels compared with young male mice (P < 0.001). There were no significant differences between baseline hydroxyproline levels in any of the saline-instilled mice (P > 0.05). Lung static compliance, a measurement of lung elasticity, was not significantly different (P > 0.1) in bleomycin-instilled female mice compared with saline controls. However, young and aged male mice exhibited a significant decrease (P < 0.001) in static compliance in response to bleomycin (Fig. 2B), suggesting that bleomycin-induced pulmonary fibrosis is more severe in male mice. There were no significant differences between static compliance values in any of the saline-instilled mice (P > 0.05). To confirm that the age and sex differences in static compliance were due to differences in collagen deposition, we employed stereological techniques to quantitatively assess areas of fibrosis in tissue sections stained with Masson's trichrome. All bleomycin-instilled mice had areas of fibrosis, as indicated by blue staining (Fig. 3A). Figure 3B shows quantitatively that collagen staining was increased in young and aged male mice (P < 0.05), but to a greater extent in aged males. There were no significant differences in the quantitative analysis of baseline collagen between groups in saline-instilled mice (P > 0.05). Taken together, these data indicate that both increased age and male sex contribute to the severity of bleomycin-induced pulmonary fibrosis.
Fig. 2.
AM mice have increased pulmonary fibrosis following Bleo administration. A: hydroxyproline levels were measured in upper right lobe from Sal- and Bleo-treated mice. There was no significant difference between groups of Sal-instilled mice by ANOVA (P > 0.05). All Bleo groups were significantly increased vs. Sal controls. YM mice have significantly greater hydroxyproline levels compared with females (*P < 0.05), and AMs have increased levels compared with all other groups (***P < 0.001). B: Bleo-instilled male mice have decreased static compliance measurements compared with female mice and Sal controls (***P < 0.001). There was no significant difference between groups of Sal-instilled mice by ANOVA (P > 0.05). Values are means ± SE.
Fig. 3.
Bleo induces greater collagen deposition in the lungs of male mice. A: mouse lung sections stained with Masson's trichrome. Collagen deposition (blue) is observed adjacent to airways and large vessels in Sal control mice, as well as in fibrotic areas in response to Bleo. B: quantitative assessment of collagen deposition indicates male mice have a significantly greater percentage of lung collagen compared with female mice (*P < 0.05). There was no significant difference between groups of Sal-instilled mice by ANOVA (P > 0.05). AM mice have a higher percentage of collagen vs. YM mice (**P < 0.01). YM and YF mice and AM Bleo-instilled mice have significantly more collagen them Sal-treated mice (at least P < 0.05). Values are means ± SE.
Age and sex affect lung permeability and inflammation initiated by bleomycin administration.
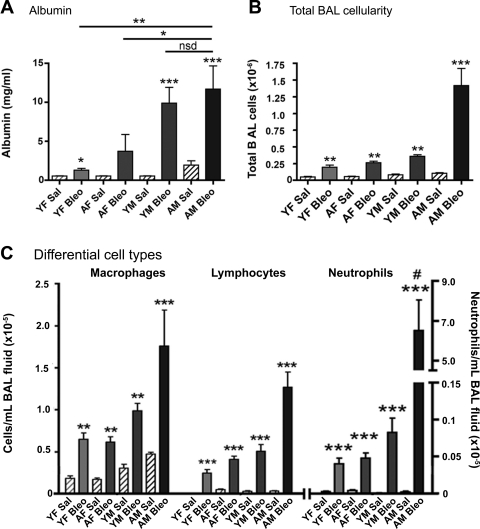
One of several potential mechanisms that may contribute to the increased collagen deposition seen in male mice, especially aged male mice, is increased injury to the alveolar-capillary units. To evaluate the effect of age and sex on lung epithelial barrier function, we subjected saline and bleomycin-instilled young and aged male and female mice to BAL and quantified albumin leakage into the air spaces by examining BALF. While bleomycin-instilled young and aged male and female mice all developed increased albumin leakage (P < 0.001 in male mice), bleomycin-instilled young and aged male mice exhibited significantly greater albumin leakage than female mice (P < 0.01), although there was no significant difference (P > 0.1) in albumin leak between young and aged male mice (Fig. 4A).
Fig. 4.
Male mice have increased epithelial cell injury and inflammatory cell infiltration after Bleo instillation. A: AM mice have a significant increase in lavaged albumin compared with YF (**P < 0.01) and AF (*P < 0.05) mice, but no significant difference (nsd) compared with YM mice (P > 0.1). Male mice have a significant increase in albumin after Bleo administration compared with Sal controls (***P < 0.001). B: total bronchoalveolar lavage (BAL) cell numbers. All Bleo-treated groups are significantly elevated compared with Sal controls (at least **P < 0.01). AMs treated with Bleo were significantly higher than all groups (***P < 0.001). C: differential BAL counts identifying macrophages, lymphocytes, and neutrophils. All mice have a significant increase in cells after Bleo instillation (**P < 0.01, ***P < 0.001) compared with Sal controls. AM mice have significantly increased neutrophil infiltration after Bleo administration compared with AF mice and young mice of either sex (#P < 0.001). Values are means ± SE.
A second possible mechanism that may contribute to the age- and male sex-associated increase in bleomycin-induced pulmonary fibrosis is differences in leukocyte recruitment to the lung. Figure 4B shows that total number of bronchoalveolar cells lavaged from the lungs was significantly increased (P < 0.01) after bleomycin administration in young and aged male and female mice compared with age- and sex-matched control mice instilled with saline. However, in response to bleomycin instillation, the total number of BAL cells was increased approximately threefold in aged male mice compared with young male and female mice and aged female mice (P < 0.001; Fig. 4B). Although differential cell counts revealed an increase in macrophages, lymphocytes, and neutrophils in all bleomycin exposed groups, bleomycin-instilled aged males mice exhibited a significant increase in pulmonary neutrophil accumulation in the BAL compared with the other groups (P < 0.001; Fig. 4C).
Age and sex affect lung cytokine levels following bleomycin administration.
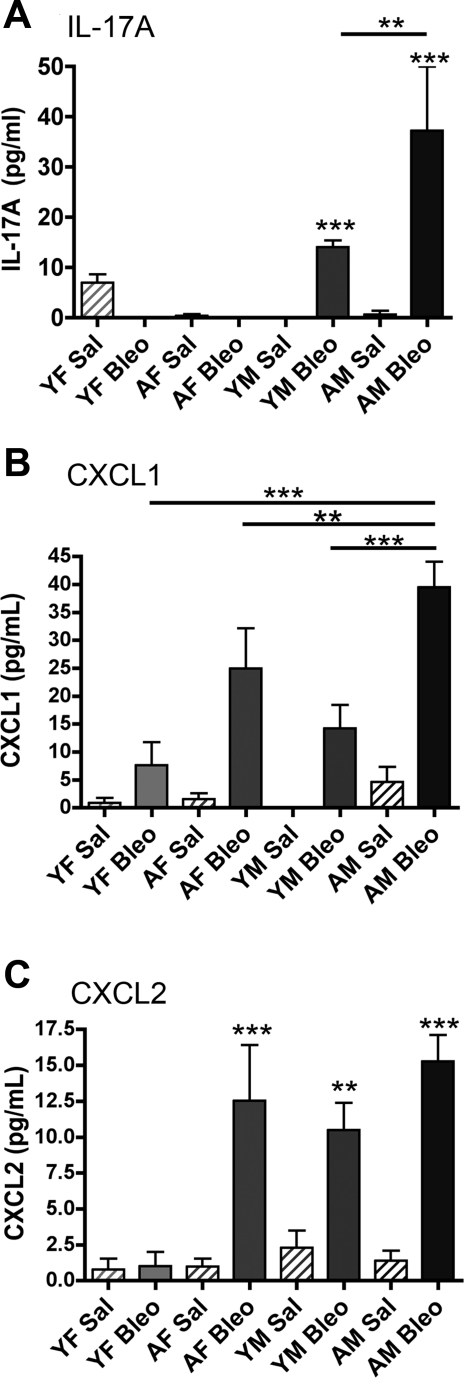
To address additional mechanisms that may contribute to the increased fibrosis and neutrophil accumulation seen in aged male mice, we quantified IL-17A, CXCL1, and CXCL2 levels in cell-free BALF obtained from bleomycin- and saline-instilled young and aged male and female mice. Figure 5A shows that IL-17A levels were significantly increased (P < 0.001) in the BALF of male mice after intratracheal instillation of bleomycin compared with saline. Importantly, bleomycin-instilled aged male mice exhibited a significantly higher (∼3-fold) level of IL-17A compared with young male mice. CXCL1 was also significantly increased in BALF after bleomycin treatment in aged female mice and young and aged male mice (P < 0.001, P < 0.01, and P < 0.001, respectively), while aged male mice had significantly greater levels of CXCL1 after bleomycin compared with all other groups (P < 0.01 aged females, P < 0.001 young females and males). There was no significant difference in CXCL1 levels between aged female mice and young male mice after bleomycin (P = 0.219) (Fig. 5B). CXCL2 levels were significantly elevated in bleomycin-instilled aged female and young and aged male mice (P < 0.001, P < 0.01, and P < 0.001, respectively) compared with saline controls (Fig. 5C). There was no significant difference in BALF CXCL2 levels between young and aged male mice (P > 0.1) and aged male and aged female mice (P > 0.5).
Fig. 5.
AM mice have increased IL-17A, CXCL1, and CXCL2 in the BAL fluid (BALF) following Bleo instillation. A: AM mice have a significant increase in lavaged IL-17A compared with YM (**P < 0.01) and YF and AF mice (***P < 0.001). Male mice have a significant increase in IL-17A after Bleo administration vs. Sal control (***P < 0.001). B: AM mice have a significant increase in CXCL1 measured in BALF compared with YM (***P < 0.001) and YF (***P < 0.001) and AF mice (**P < 0.01). C: YM and AM mice and AF mice have a significant increase in CXCL2 after treatment with Bleo (**P < 0.01 and ***P < 0.001). Values are means ± SE.
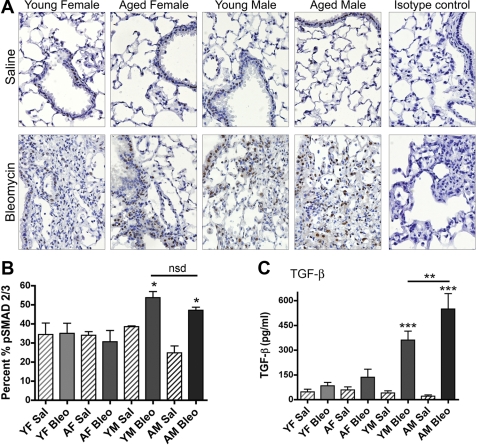
Next, to assess signaling by active TGF-β, we used an immunohistochemical approach to stain lung tissues from young and aged male and female mice for phospho-Smad2/3 using a previously validated approach (48). Figure 6A shows that there is a consistent baseline level of staining in airway and alveolar epithelium of saline-instilled mice, regardless of sex and age. There is an increase in phospho-Smad2/3 positive cells within fibrotic areas, macrophages, and in the airway epithelium after bleomycin instillation in young and aged male mice. Quantitative assessment of phospho-Smad2/3 stained indicated that ∼30–40% of total lung tissue (including conducting airways and lung parenchyma) exhibited nuclear staining of phospho-Smad2/3 in saline-instilled young and aged male and female mice, consistent with the notion of basal tonic signaling by active TGF-β in lung epithelial cells (P > 0.05) (Fig. 6B). There was no change in phospho-Smad2/3 staining in young and aged female mice after bleomycin instillation (Fig. 6B). In contrast, young and aged male mice exhibited a significant increase (P < 0.05) in phospho-Smad2/3 staining, although the level of nuclear translocation of phospho-Smad2/3 was not significantly different (P = 0.2) between bleomycin-instilled young and aged male mice (Fig. 6C). We also evaluated the effects of age and sex on BALF levels of TGF-β. Figure 6C shows that total TGF-β levels in BALF were significantly greater (P < 0.001) in bleomycin-instilled young and aged male mice compared with young and aged female mice. There was a significant difference (P < 0.01) in the level of total TGF-β in the BALF of bleomycin-instilled young and aged male mice.
Fig. 6.
Male mice exhibit increased phospho-Smad2/3 lung tissue staining and total transforming growth factor (TGF)-β in their BALF following Bleo instillation. A: Sal-instilled mice have positive nuclear staining of phospho-Smad2/3 in the airway and alveolar epithelium. YM and AM mice have increased staining in the airway epithelium, macrophages, and fibrotic areas following Bleo instillation compared with female mice. Final magnification of images is ×400. B: quantitative analysis of positive phospho-Smad2/3 nuclei indicates that male mice have a significant increase in staining after Bleo instillation compared with female mice (*P < 0.05). Sal instilled mice from all groups have a baseline level of staining that is not significantly different by ANOVA (P > 0.05). C: AM mice have a significant increase in total TGF-β in the BALF compared with all other groups treated with Sal and Bleo (**P < 0.01). YM mice have a significant increase in total TGF-β compared with Sal control (***P < 0.001). Values are means ± SE.
DISCUSSION
Increasing age and male sex are associated with a dramatic increase in the prevalence of fibrosis in patients with idiopathic interstitial pneumonias. In IPF, the overall prevalence increases 50-fold when comparing subjects less than 35 yr of age with those over 75 yr of age (13, 44, 47, 56). The goal of the work presented herein was to systematically investigate the roles of age and sex in the development of pulmonary fibrosis in response to intratracheal delivery of bleomycin. Here, we show for the first time that aged male C57BL/6 mice develop a more severe form of bleomycin-induced pulmonary fibrosis than aged-matched female mice or young mice of either sex. The lung disease in aged male mice was associated with increased neutrophil recruitment into the air spaces and substantially increased mortality. Although studies in human subjects have largely been unable to differentiate life-long, male-associated environmental, smoking, and occupational exposures from the independent effects of male sex and age, our findings suggest that aging and male sex augment pulmonary inflammation and fibrosis independently of environmental exposures. Our findings also emphasize the importance of stratifying experimental groups on the basis of age and sex in experimental and epidemiological studies of this nature.
Seeking to determine how aging and male sex augment the development of bleomycin-induced pulmonary fibrosis, the BALF was evaluated for neutrophil chemoattractants (IL-17A, CXCL1, and CXCL2), lung epithelial barrier function (albumin leak), and the fibrotic cytokine, TGF-β (36, 41, 42, 53). Neutrophil recruitment into the air spaces occurred in all groups instilled with bleomycin and correlated with increased levels of CXCL1 and CXCL2. However, the increased neutrophil infiltration and fibrosis in aged male mice were associated with higher BALF levels of IL-17A, consistent with a recent study emphasizing the importance of IL-17A in the development of bleomycin-induced pulmonary fibrosis (58). While TGF-β was detected in the BALF of saline-instilled mice, and all mice exposed to bleomycin, TGF-β levels were considerably higher in young and aged male mice exposed to bleomycin. Analysis of signaling by active TGF-β via quantification of nuclear localization of Smad2/3 phosphorylation revealed substantial TGF-β-dependent tonic signaling in alveolar epithelial cells and macrophages, consistent with previous reports (46, 48). However, the level of phospho-Smad2/3 nuclear localization was increased in bleomycin-instilled young and aged male mice compared with female mice. We speculate that these combined differences in neutrophil infiltration, chemokine, and cytokine signaling contribute to the increased mortality and severity of pulmonary fibrosis seen in male mice, especially aged male mice.
In studies aimed at investigating how aging and sex affect lung pathology, physiology, and function, Aoshiba and Nagai (5) interrogated the basal transcriptomes of young (12 wk) and aged (104 wk) female BALB/c mice and showed that eight inflammation-related genes, including CXCR3, IL-8RB, and CD8, were increased in the lungs of aged mice. They also observed increased numbers of CD4+ and CD8+ T cells, B cells, and macrophages in the lungs of aged mice, suggesting that, in the absence of experimental lung exposures, aged mice display minor increases in inflammatory cell recruitment. Using an LPS-induced model of sepsis, Gomez et al. (21) reported that the lungs of aged (72–80 wk) female BALB/c mice exhibit an approximately fivefold increase in neutrophil accumulation in lung tissues associated with increased BAL levels of the neutrophil chemokines, CXCL1 and CXCL2, compared with young mice (8–12 wk). Starr and colleagues (52) recently confirmed these findings and suggested that the increased accumulation of neutrophils arose as a consequence of decreased anti-oxidant defenses, especially extracellular superoxide dismutase. Similarly, Gould et al. (22) reported that aging promotes lung inflammation consequential to decreased pulmonary glutathione adaptive responses in a cigarette smoke exposure model. Although none of these studies have systematically evaluated the consequences of age and sex together in the same study, their collective results suggest that aging and male sex may impact the inflammatory response in multiple ways, contributing to the neutrophil-dominated response observed following bleomycin instillation into the lungs of aged male mice. Interestingly, while these in vivo studies have shown that inflammatory responses increase during aging, mechanistic studies in vitro have paradoxically shown that the production of proinflammatory cytokines, oxidants, and other mediators by macrophages is reduced as a consequence of aging (8, 12). Furthermore, the reduced production of proinflammatory cytokines has been linked to reduced signaling via the MAPK and NF-κB pathways (8, 12). In seeking to reconcile these findings, Gomez and colleagues (20) have hypothesized that local microenvironmental factors may alter macrophage programming in vitro. Alternatively, other cell types e.g., epithelial cells, may become more responsive to inflammatory stimuli during aging. Clearly, based on the aging and male sex-associated phenotype observed herein, future studies should be directed at identifying the broad cell lineages, e.g., hematopoietic vs. stromal cells, involved in generating the more proinflammatory and profibrotic phenotype seen in bleomycin-exposed aged male mice.
Although the prevalence of IPF is greater in men (40), previous studies have raised the possibility that sex bias is related to the higher prevalence of cigarette smoking and other habitual, occupational, or environmental exposures in predominately male-associated occupations (7, 54). Mice are constantly exposed to bedding materials, ammonia, and other environmental agents; these exposures were the same when comparing the pulmonary responses in aged male and female mice or young male and female mice. The aged mice obviously had a longer exposure to their cage environment than the young mice. However, in a recent study, Xu and colleagues (59) showed that senescence-accelerated prone mice were also more susceptible to bleomycin-induced pulmonary fibrosis than age-matched senescence-accelerated resistant mice, suggesting that aging rather than environmental exposure over time play a dominant role in the augmented fibrotic response. With these caveats in mind, it is plausible that the observed differences in fibrosis, pulmonary inflammatory cell accumulation, and mortality between young male and female mice and aged male and female mice are reflective of authentic age- and sex-dependent differences. Consistent with this conclusion, Voltz et al. (55) reported that young male mice display a greater decline in static lung compliance compared with young female mice following bleomycin instillation. Castrated male mice exhibited a similar response to female mice, whereas female mice that were virilized with testosterone showed a similar decline in static lung compliance to male mice. These findings suggest that androgens are responsible for the more restrictive lung physiology seen in young male mice. In contrast to our study, Voltz et al. (55) did not observe sex-associated differences in lung hydroxyproline levels. However, in a similar study, Brass and colleagues (9) reported that young male mice develop more extensive lung fibrosis than female mice in a model of silica-induced pulmonary fibrosis. Interestingly, female mice were seen to develop a more striking alveolitis than male mice following silica instillation, an effect not seen in ovariectomized mice (9). Taken together, these findings suggest that both estrogens and androgens affect the pattern of lung inflammation and fibrosis in different settings. Furthermore, age-related changes in androgens or estrogen levels, especially in which androgen levels have been shown to diminish in aging mice (17), may differentially affect patterns of lung inflammation and fibrosis.
Bleomycin-induced pulmonary inflammation and fibrosis in young mice is widely acknowledged to be a reliable model of acute lung injury-associated fibrosis (31). However, because of its transient nature and lack of fibroblastic foci, cuboidal alveolar epithelial cells, and other histopathological features, it does not fully recapitulate the features of IPF (14, 15, 49). Nevertheless, intratracheal delivery of bleomycin has led to significant advances in our understanding of the fundamental mechanisms that regulate lung injury, repair, and fibrosis. The role of inflammation, especially neutrophilic alveolitis, in the development of IPF has been controversial. In a study reported by Haslam and colleagues (4), it was suggested that BAL neutrophilia predicted increased deterioration of lung function (26), while a similar study by Watters et al. (57), found no predictive value of BAL neutrophilia. However, these studies were conducted prior to the American Thoracic Society/European Respiratory Society reclassification of the idiopathic interstitial pneumonias in 2002. Based on an appropriately powered analysis with a cohort of surgical lung biopsy-proven IPF patients, Kinder and colleagues (35) recently reported that BAL neutrophilia is an independent predictor of early mortality. In addition, other recent studies aimed at shedding new light on the natural history of IPF have suggested that, while many patients exhibit a progressive and steady decline in lung function, a subset of patients develop acute exacerbations, possibly reflective of a period of disease acceleration (16, 29). With regard to the current findings of increased neutrophilic alveolitis in aged male mice, acute IPF exacerbations are also associated with BAL neutrophilia. Thus, while the bleomycin model has its limitations when used to study the development of fibrosis in young mice, we speculate that aged male mice may be an appropriate model in which to recapitulate the acute exacerbations seen in IPF patients.
In summary, our studies reveal previously underappreciated effects of age and male sex on the development of lung inflammation and fibrosis in mice exposed to bleomycin. In addition, our studies suggest that the increased inflammation and fibrosis seen in aged male mice arise independently of additional environmental exposures. We suggest that future studies in this field need to be stratified and analyzed with appropriate consideration of the effects of age and sex on the pathophysiological pulmonary responses to bleomycin.
GRANTS
This work was supported by Public Health Service grants HL068628 and HL055549 (D. W. H. Riches), HL095363 (S. Faubel), HL090669 (G. P. Downey), and HL081151 (P. M. Henson) from the National Heart, Lung, and Blood Institute (NHLBI) of the National Institutes of Health. E. F. Redente was supported by Ruth L. Kirschstein F32 National Research Service Award HL095274 from the NHLBI and a Viola Vestal Coulter Scholarship from National Jewish Health.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
ACKNOWLEDGMENTS
We acknowledge outstanding technical assistance from Linda Remigio and Christopher Altmann.
REFERENCES
- 1. Adamson IY, Bowden DH. The pathogenesis of bloemycin-induced pulmonary fibrosis in mice. Am J Pathol 77: 185–197, 1974 [PMC free article] [PubMed] [Google Scholar]
- 2. Adamson IY, Hedgecock C. Patterns of particle deposition and retention after instillation to mouse lung during acute injury and fibrotic repair. Exp Lung Res 21: 695–709, 1995 [DOI] [PubMed] [Google Scholar]
- 3. Adamson IY, Hedgecock C, Bowden DH. Epithelial cell-fibroblast interactions in lung injury and repair. Am J Pathol 137: 385–392, 1990 [PMC free article] [PubMed] [Google Scholar]
- 4. American Thoracic Society/European Respiratory Society International Multidisciplinary Consensus Classification of the Idiopathic Interstitial Pneumonias This joint statement of the American Thoracic Society (ATS), and the European Respiratory Society (ERS) was adopted by the ATS board of directors, June 2001 and by the ERS Executive Committee, June 2001. Am J Respir Crit Care Med 165: 277–304, 2002 [DOI] [PubMed] [Google Scholar]
- 5. Aoshiba K, Nagai A. Chronic lung inflammation in aging mice. FEBS Lett 581: 3512–3516, 2007 [DOI] [PubMed] [Google Scholar]
- 6. Bauer AK, Dwyer-Nield LD, Keil K, Koski K, Malkinson AM. Butylated hydroxytoluene (BHT) induction of pulmonary inflammation: a role in tumor promotion. Exp Lung Res 27: 197–216, 2001 [DOI] [PubMed] [Google Scholar]
- 7. Baumgartner KB, Samet JM, Coultas DB, Stidley CA, Hunt WC, Colby TV, Waldron JA. Occupational and environmental risk factors for idiopathic pulmonary fibrosis: a multicenter case-control study. Collaborating Centers Am J Epidemiol 152: 307–315, 2000 [DOI] [PubMed] [Google Scholar]
- 8. Boehmer ED, Goral J, Faunce DE, Kovacs EJ. Age-dependent decrease in Toll-like receptor 4-mediated proinflammatory cytokine production and mitogen-activated protein kinase expression. J Leukoc Biol 75: 342–349, 2004 [DOI] [PubMed] [Google Scholar]
- 9. Brass DM, McGee SP, Dunkel MK, Reilly SM, Tobolewski JM, Sabo-Attwood T, Fattman CL. Gender influences the response to experimental silica-induced lung fibrosis in mice. Am J Physiol Lung Cell Mol Physiol 299: L664–L671, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Card JW, Carey MA, Bradbury JA, DeGraff LM, Morgan DL, Moorman MP, Flake GP, Zeldin DC. Gender differences in murine airway responsiveness and lipopolysaccharide-induced inflammation. J Immunol 177: 621–630, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Chaudhary NI, Schnapp A, Park JE. Pharmacologic differentiation of inflammation and fibrosis in the rat bleomycin model. Am J Respir Crit Care Med 173: 769–776, 2006 [DOI] [PubMed] [Google Scholar]
- 12. Chelvarajan RL, Liu Y, Popa D, Getchell ML, Getchell TV, Stromberg AJ, Bondada S. Molecular basis of age-associated cytokine dysregulation in LPS-stimulated macrophages. J Leukoc Biol 79: 1314–1327, 2006 [DOI] [PubMed] [Google Scholar]
- 13. Chibbar R, Shih F, Baga M, Torlakovic E, Ramlall K, Skomro R, Cockcroft DW, Lemire EG. Nonspecific interstitial pneumonia and usual interstitial pneumonia with mutation in surfactant protein C in familial pulmonary fibrosis. Mod Pathol 17: 973–980, 2004 [DOI] [PubMed] [Google Scholar]
- 14. Chua F, Gauldie J, Laurent GJ. Pulmonary fibrosis: searching for model answers. Am J Respir Cell Mol Biol 33: 9–13, 2005 [DOI] [PubMed] [Google Scholar]
- 15. Chung MP, Monick MM, Hamzeh NY, Butler NS, Powers LS, Hunninghake GW. Role of repeated lung injury and genetic background in bleomycin-induced fibrosis. Am J Respir Cell Mol Biol 29: 375–380, 2003 [DOI] [PubMed] [Google Scholar]
- 16. Collard HR, Moore BB, Flaherty KR, Brown KK, Kaner RJ, King TE, Jr, Lasky JA, Loyd JE, Noth I, Olman MA, Raghu G, Roman J, Ryu JH, Zisman DA, Hunninghake GW, Colby TV, Egan JJ, Hansell DM, Johkoh T, Kaminski N, Kim DS, Kondoh Y, Lynch DA, Muller-Quernheim J, Myers JL, Nicholson AG, Selman M, Toews GB, Wells AU, Martinez FJ. Acute exacerbations of idiopathic pulmonary fibrosis. Am J Respir Crit Care Med 176: 636–643, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Coquelin A, Desjardins C. Luteinizing hormone and testosterone secretion in young and old male mice. Am J Physiol Endocrinol Metab 243: E257–E263, 1982 [DOI] [PubMed] [Google Scholar]
- 18. Frankel SK, Moats-Staats BM, Cool CD, Wynes MW, Stiles AD, Riches DW. Human insulin-like growth factor-IA expression in transgenic mice promotes adenomatous hyperplasia but not pulmonary fibrosis. Am J Physiol Lung Cell Mol Physiol 288: L805–L812, 2005 [DOI] [PubMed] [Google Scholar]
- 19. Gharaee-Kermani M, Hatano K, Nozaki Y, Phan SH. Gender-based differences in bleomycin-induced pulmonary fibrosis. Am J Pathol 166: 1593–1606, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Gomez CR, Boehmer ED, Kovacs EJ. The aging innate immune system. Curr Opin Immunol 17: 457–462, 2005 [DOI] [PubMed] [Google Scholar]
- 21. Gomez CR, Hirano S, Cutro BT, Birjandi S, Baila H, Nomellini V, Kovacs EJ. Advanced age exacerbates the pulmonary inflammatory response after lipopolysaccharide exposure. Crit Care Med 35: 246–251, 2007 [DOI] [PubMed] [Google Scholar]
- 22. Gould NS, Min E, Gauthier S, Chu HW, Martin R, Day BJ. Aging adversely affects the cigarette smoke-induced glutathione adaptive response in the lung. Am J Respir Crit Care Med 182: 1114–1122, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Gribbin J, Hubbard RB, Le Jeune I, Smith CJ, West J, Tata LJ. Incidence and mortality of idiopathic pulmonary fibrosis and sarcoidosis in the UK. Thorax 61: 980–985, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Gundersen HJ, Jensen EB, Kieu K, Nielsen J. The efficiency of systematic sampling in stereology–reconsidered. J Microsc 193: 199–211, 1999 [DOI] [PubMed] [Google Scholar]
- 25. Hashimoto N, Jin H, Liu T, Chensue SW, Phan SH. Bone marrow-derived progenitor cells in pulmonary fibrosis. J Clin Invest 113: 243–252, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Haslam PL, Turton CW, Lukoszek A, Salsbury AJ, Dewar A, Collins JV, Turner-Warwick M. Bronchoalveolar lavage fluid cell counts in cryptogenic fibrosing alveolitis and their relation to therapy. Thorax 35: 328–339, 1980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Hoyles RK, Derrett-Smith EC, Khan K, Shiwen X, Howat SL, Wells AU, Abraham DJ, Denton CP. An essential role for resident fibroblasts in experimental lung fibrosis is defined by lineage-specific deletion of high-affinity type II transforming growth factor beta receptor. Am J Respir Crit Care Med 183: 249–261, 2011 [DOI] [PubMed] [Google Scholar]
- 28. Hsia CC, Hyde DM, Ochs M, Weibel ER. An official research policy statement of the American Thoracic Society/European Respiratory Society: standards for quantitative assessment of lung structure. Am J Respir Crit Care Med 181: 394–418, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Huie TJ, Olson AL, Cosgrove GP, Janssen WJ, Lara AR, Lynch DA, Groshong SD, Moss M, Schwarz MI, Brown KK, Frankel SK. A detailed evaluation of acute respiratory decline in patients with fibrotic lung disease: aetiology and outcomes. Respirology 15: 909–917, 2010 [DOI] [PubMed] [Google Scholar]
- 30. Hyde DM, Tyler NK, Plopper CG. Morphometry of the respiratory tract: avoiding the sampling, size, orientation, and reference traps. Toxicol Pathol 35: 41–48, 2007 [DOI] [PubMed] [Google Scholar]
- 31. Izbicki G, Segel MJ, Christensen TG, Conner MW, Breuer R. Time course of bleomycin-induced lung fibrosis. Int J Exp Pathol 83: 111–119, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Khalil N, Bereznay O, Sporn M, Greenberg AH. Macrophage production of transforming growth factor beta and fibroblast collagen synthesis in chronic pulmonary inflammation. J Exp Med 170: 727–737, 1989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Kim DS, Collard HR, King TE., Jr Classification and natural history of the idiopathic interstitial pneumonias. Proc Am Thorac Soc 3: 285–292, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Kim KK, Kugler MC, Wolters PJ, Robillard L, Galvez MG, Brumwell AN, Sheppard D, Chapman HA. Alveolar epithelial cell mesenchymal transition develops in vivo during pulmonary fibrosis and is regulated by the extracellular matrix. Proc Natl Acad Sci U S A 103: 13180–13185, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Kinder BW, Brown KK, Schwarz MI, Ix JH, Kervitsky A, King TE., Jr Baseline BAL neutrophilia predicts early mortality in idiopathic pulmonary fibrosis. Chest 133: 226–232, 2008 [DOI] [PubMed] [Google Scholar]
- 36. Laan M, Cui ZH, Hoshino H, Lotvall J, Sjostrand M, Gruenert DC, Skoogh BE, Linden A. Neutrophil recruitment by human IL-17 via C-X-C chemokine release in the airways. J Immunol 162: 2347–2352, 1999 [PubMed] [Google Scholar]
- 37. Li Y, Kishimoto I, Saito Y, Harada M, Kuwahara K, Izumi T, Hamanaka I, Takahashi N, Kawakami R, Tanimoto K, Nakagawa Y, Nakanishi M, Adachi Y, Garbers DL, Fukamizu A, Nakao K. Androgen contributes to gender-related cardiac hypertrophy and fibrosis in mice lacking the gene encoding guanylyl cyclase-A. Endocrinology 145: 951–958, 2004 [DOI] [PubMed] [Google Scholar]
- 38. Myers JL, Katzenstein AL. Beyond a consensus classification for idiopathic interstitial pneumonias: progress and controversies. Histopathology 54: 90–103, 2009 [DOI] [PubMed] [Google Scholar]
- 39. Ogbuihi S, Cruz-Orive LM. Estimating the total number of lymphatic valves in infant lungs with the fractionators. J Microsc 158: 19–30, 1990 [DOI] [PubMed] [Google Scholar]
- 40. Olson AL, Swigris JJ, Lezotte DC, Norris JM, Wilson CG, Brown KK. Mortality from pulmonary fibrosis increased in the United States from 1992 to 2003. Am J Respir Crit Care Med 176: 277–284, 2007 [DOI] [PubMed] [Google Scholar]
- 41. Pan LH, Yamauchi K, Uzuki M, Nakanishi T, Takigawa M, Inoue H, Sawai T. Type II alveolar epithelial cells and interstitial fibroblasts express connective tissue growth factor in IPF. Eur Respir J 17: 1220–1227, 2001 [DOI] [PubMed] [Google Scholar]
- 42. Phan SH, Kunkel SL. Lung cytokine production in bleomycin-induced pulmonary fibrosis. Exp Lung Res 18: 29–43, 1992 [DOI] [PubMed] [Google Scholar]
- 43. Phillips RJ, Burdick MD, Hong K, Lutz MA, Murray LA, Xue YY, Belperio JA, Keane MP, Strieter RM. Circulating fibrocytes traffic to the lungs in response to CXCL12 and mediate fibrosis. J Clin Invest 114: 438–446, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Raghu G, Weycker D, Edelsberg J, Bradford WZ, Oster G. Incidence and prevalence of idiopathic pulmonary fibrosis. Am J Respir Crit Care Med 174: 810–816, 2006 [DOI] [PubMed] [Google Scholar]
- 45. Redente EF, Higgins DM, Dwyer-Nield LD, Orme IM, Gonzalez-Juarrero M, Malkinson AM. Differential polarization of alveolar macrophages and bone marrow-derived monocytes following chemically and pathogen-induced chronic lung inflammation. J Leukoc Biol 88: 159–168, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Richter A, Yeager ME, Zaiman A, Cool CD, Voelkel NF, Tuder RM. Impaired transforming growth factor-beta signaling in idiopathic pulmonary arterial hypertension. Am J Respir Crit Care Med 170: 1340–1348, 2004 [DOI] [PubMed] [Google Scholar]
- 47. Rosas IO, Ren P, Avila NA, Chow CK, Franks TJ, Travis WD, McCoy JP, Jr, May RM, Wu HP, Nguyen DM, Arcos-Burgos M, MacDonald SD, Gochuico BR. Early interstitial lung disease in familial pulmonary fibrosis. Am J Respir Crit Care Med 176: 698–705, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Rosendahl A, Checchin D, Fehniger TE, ten Dijke P, Heldin CH, Sideras P. Activation of the TGF-beta/activin-Smad2 pathway during allergic airway inflammation. Am J Respir Cell Mol Biol 25: 60–68, 2001 [DOI] [PubMed] [Google Scholar]
- 49. Scotton CJ, Chambers RC. Bleomycin revisited: towards a more representative model of IPF? Am J Physiol Lung Cell Mol Physiol 299: L439–L441, 2010 [DOI] [PubMed] [Google Scholar]
- 50. Shofer S, Badea C, Qi Y, Potts E, Foster WM, Johnson GA. A micro-CT analysis of murine lung recruitment in bleomycin-induced lung injury. J Appl Physiol 105: 669–677, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Speyer CL, Rancilio NJ, McClintock SD, Crawford JD, Gao H, Sarma JV, Ward PA. Regulatory effects of estrogen on acute lung inflammation in mice. Am J Physiol Cell Physiol 288: C881–C890, 2005 [DOI] [PubMed] [Google Scholar]
- 52. Starr ME, Ueda J, Yamamoto S, Evers BM, Saito H. The effects of aging on pulmonary oxidative damage, protein nitration, and extracellular superoxide dismutase down-regulation during systemic inflammation. Free Radic Biol Med 50: 371–380, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Tanino Y, Makita H, Miyamoto K, Betsuyaku T, Ohtsuka Y, Nishihira J, Nishimura M. Role of macrophage migration inhibitory factor in bleomycin-induced lung injury and fibrosis in mice. Am J Physiol Lung Cell Mol Physiol 283: L156–L162, 2002 [DOI] [PubMed] [Google Scholar]
- 54. Taskar VS, Coultas DB. Is idiopathic pulmonary fibrosis an environmental disease? Proc Am Thorac Soc 3: 293–298, 2006 [DOI] [PubMed] [Google Scholar]
- 55. Voltz JW, Card JW, Carey MA, Degraff LM, Ferguson CD, Flake GP, Bonner JC, Korach KS, Zeldin DC. Male sex hormones exacerbate lung function impairment after bleomycin-induced pulmonary fibrosis. Am J Respir Cell Mol Biol 39: 45–52, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Vourlekis JS, Schwarz MI, Cherniack RM, Curran-Everett D, Cool CD, Tuder RM, King TE, Jr, Brown KK. The effect of pulmonary fibrosis on survival in patients with hypersensitivity pneumonitis. Am J Med 116: 662–668, 2004 [DOI] [PubMed] [Google Scholar]
- 57. Watters LC, Schwarz MI, Cherniack RM, Waldron JA, Dunn TL, Stanford RE, King TE. Idiopathic pulmonary fibrosis. Pretreatment bronchoalveolar lavage cellular constituents and their relationships with lung histopathology and clinical response to therapy. Am Rev Respir Dis 135: 696–704, 1987 [DOI] [PubMed] [Google Scholar]
- 58. Wilson MS, Madala SK, Ramalingam TR, Gochuico BR, Rosas IO, Cheever AW, Wynn TA. Bleomycin and IL-1beta-mediated pulmonary fibrosis is IL-17A dependent. J Exp Med 207: 535–552, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Xu J, Gonzalez ET, Iyer SS, Mac V, Mora AL, Sutliff RL, Reed A, Brigham KL, Kelly P, Rojas M. Use of senescence-accelerated mouse model in bleomycin-induced lung injury suggests that bone marrow-derived cells can alter the outcome of lung injury in aged mice. J Gerontol A Biol Sci Med Sci 64: 731–739, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]