Abstract
Previously we showed that cytokine-induced neutrophil chemoattractant (CINC), but not macrophage inflammatory protein-2 (MIP-2), is detected in plasma after intratracheal challenge with LPS or the particular chemokines. To further understand the differences between CINC and MIP-2 flux from the lung, we attempted to detect the two chemokines in isolated erythrocytes and leukocytes in rats after intratracheal LPS challenge. In response to intratracheal LPS, we found both CINC and MIP-2 in isolated erythrocytes and leukocytes, suggesting that MIP-2 produced in the LPS-challenged lung entered the circulation like CINC. To assess the relative flux of CINC and MIP-2 from the intra-alveolar compartment into the blood, experiments were performed in rats implanted with vascular catheters in which both chemokines were either injected intratracheally (5 μg) or infused intravenously (20 ng/min) and subsequently measured in plasma or with the cellular elements. Both chemokines appeared in the blood following intratracheal injection, with CINC detected in plasma and cells but MIP-2 only detected in the cellular fraction of blood. Infusion of both chemokines allowed detection of MIP-2 and CINC in plasma and with the cellular elements, which allowed us to calculate clearance for each chemokine and to assess CINC and MIP-2 rates of appearance (Ra) following intratracheal injection. On the basis of plasma and whole blood clearance, CINC Ra was more than sevenfold and fourfold higher, respectively, than MIP-2 Ra. This analysis indicates that differences exist in the rate of flux of CINC and MIP-2 across the epithelial/endothelial barrier of the lung, despite similar molecular size.
Keywords: Duffy antigen receptor for chemokines, intratracheal chemokines, intratracheal LPS, chemokines
cxc chemokines are a family of related polypeptide molecules that are potent immune cell chemotactic factors (9). Members bearing a NH2-terminal ELR+ sequence upstream from the conserved cysteine residues are potent inducers of neutrophil chemotaxis (14, 29). Cytokine-induced neutrophil chemoattractant (CINC) and macrophage inflammatory protein-2 (MIP-2) are rat ELR+ CXC chemokines (functional counterparts to human CXCL8/IL-8), which have been shown to increase in lungs challenged with bacteria and to be responsible for neutrophil migration into the alveoli (4, 5, 20, 24). Antibody neutralization of either chemokine significantly attenuates neutrophil migration into the intrapulmonary compartment during infection and decreases bacterial clearance (5, 7, 28).
Previously we reported that CINC, but not MIP-2, appears in the plasma in response to intratracheal (IT) challenge with LPS despite their similar molecular size (7.8 and 7.9 kDa, respectively) and high levels of lung production of both cytokines (20). In this former study, increased CINC and MIP-2 mRNAs in response to IT LPS challenge were largely confined to lung tissue, where high levels of both proteins were found in bronchoalveolar lavage (BAL) fluid. When recombinant CINC (rCINC) and/or recombinant MIP-2 (rMIP-2) were injected IT, only CINC was detected in plasma, where it exhibited a sustained plateau for up to 4 h without induction of CINC mRNA (20). During this time, MIP-2 was not detected in plasma. Furthermore, in response to IT administration of 125I-rCINC, but not 125I-rMIP-2, plasma CINC possessed similar specific activity to that recovered in BAL fluid (20). From these results, we concluded that CINC, but not MIP-2, is selectively transported from the intrapulmonary compartment into the plasma. However, not considered in this previous work was the possibility that MIP-2 did enter the blood but was bound to cellular elements, thus preventing its detection in the plasma. CINC and MIP-2 exhibit high-affinity binding to CXCR2 on leukocytes (15) and vascular endothelial cells (21) and to Duffy antigen receptor for chemokines (DARC) on erythrocytes (3, 18) and endothelial cells (8). All chemokines bind to glycosaminoglycans and this interaction has been proposed to control the function of CXC chemokines in tissues (6). Differential binding to these CXC binding molecules could alter flux and/or plasma concentrations. For example, it is known that CXCR2 receptors possess differential affinities for each chemokine, with MIP-2 exhibiting 70-fold greater binding affinity over CINC (16). Previous research has suggested that DARC functions as a CXC chemokine sink and possibly plays a role in sequestering circulating chemokine concentrations to cellular elements (3, 18). Additionally, DARC on venular endothelium has been proposed to mediate transport of CXC chemokines from the abluminal to luminal surface through binding, internalization, and transcytosis (12).
Because of these interactions and the fact that they may vary between CINC and MIP-2, we performed an initial set of experiments to determine whether MIP-2 produced in response to intrapulmonary LPS challenge or CXC chemokines delivered into the lungs appears in the blood but is sequestered on the cellular elements of the blood, preventing its detection in plasma. Because we found substantial amounts of both CINC and MIP-2 associated with the cellular elements of blood, additional experiments were performed to analyze the relative flux of CINC and MIP-2 from the intrapulmonary compartment into the circulation in response to IT administration. In addition, plasma and whole blood clearances of CINC and MIP-2 during intravenous (IV) steady-state infusion were calculated and used to assess flux following IT administration of these CXC chemokines.
MATERIALS AND METHODS
Reagents.
Rat rCINC, rat rMIP-2, and Cytoscreen ELISA kits for rat MIP-2 were obtained from Biosource (Camarillo, CA). Rabbit anti-rat GRO/KC [growth-related protein/keratinocyte-derived cytokine (CINC)] capture antibody and biotinylated anti-rat CINC-1 detection antibody for CINC ELISA assay were purchased from PeproTech (Rocky Hill, NJ) and R&D Systems (Minneapolis, MN), respectively. Escherichia coli-derived LPS (serotype 026:B6), Triton X-100, methyl cellulose, and normal mouse plasma were purchased from Sigma-Aldrich (St. Louis, MO). Bovine serum albumin and fetal bovine serum were purchased from HyClone (Logan, UT). Complete protease inhibitor cocktail was purchased from Roche Applied Science (Indianapolis, IN). Ketamine and xylazine were obtained from Butler Schein Animal Health Supply (Dublin, OH). Isoflurane was purchased from Minrad (Bethlehem, PA). Gentra RBC lysis buffer was obtained from Qiagen Sciences (Valencia, CA). PBS was purchased from Life Technologies (Grand Island, NY).
Animals.
Animal experiments were submitted to and approved by Louisiana State University Health Sciences Center Institutional Animal Care and Use Committee. Male virus antibody-free Sprague-Dawley International Gold Standard rats (Charles River Laboratories, Wilmington, MA) weighing 250–330 g were housed in a controlled environment (12:12-h light-dark cycle) and had access to standard rodent chow and water for at least 1 wk prior to initiating experiments.
IT LPS administration.
One set of rats was administered 0.1 mg LPS IT in 0.5 ml saline or vehicle under isoflurane anesthesia. Rats were anesthetized with an intramuscular injection of ketamine and xylazine (50 mg/kg and 5 mg/kg, respectively) 2 h after LPS administration to obtain blood and then euthanized. Red blood cells (RBCs) and white blood cells (WBCs) were isolated for analysis of CINC and MIP-2. WBCs were isolated by adding 12 ml Gentra RBC lysis buffer per 1 ml whole blood per manufacturer's instructions and washed twice with PBS. After counting they were lysed in PBS containing 1% Triton X-100 plus Complete protease inhibitor cocktail and frozen at −80°C until assayed. RBCs were isolated by mixing 1 ml methyl cellulose (2% wt/vol in PBS at room temperature) per 2 ml blood and centrifugation. The RBC fraction was diluted 1:10 with PBS, lysed with 500 mM NaCl containing 0.1% Triton X-100 and 0.25% bovine serum albumin, and stored at −80°C until assayed.
IT and IV chemokine administration.
One day prior to experiments, catheters were implanted into a carotid artery and jugular vein of rats under ketamine and xylazine anesthesia as previously described (20). Rat rCINC and rMIP-2 (∼5 μg each) were injected IT in 0.5 ml saline to one group of rats under isoflurane anesthesia. A separate group received rCINC and rMIP-2 intravenously as an infusion (∼20 ng/min) for 4 h. As both CINC and MIP-2 are produced together in response to IT LPS, a coinjection or coinfusion of full-length rCINC and rMIP-2 proteins were used in the present set of experiments. Previously we showed similar rat plasma CINC levels whether rCINC was administered intratracheally alone or in combination with rMIP-2 (20). Also, IT rMIP-2 alone did not result in any change in plasma CINC concentrations. Likewise, plasma MIP-2 was not detected after IT injection of rMIP-2 alone or in combination with rCINC.
During experiments, arterial blood samples (0.6 ml) were drawn at selected time points over a 4-h observation period. Rats were euthanized at 4 h under isoflurane anesthesia for us to perform BAL and obtain a final blood sample. Plasma was separated from blood cells, which were washed twice with PBS. A 0.1-ml aliquot of blood cells was lysed with PBS containing 1% Triton X-100 plus Complete protease inhibitor cocktail. Plasma and cell lysis samples were stored at −80°C until assayed for CINC and MIP-2 protein concentrations. Hematocrits were determined on blood and isolated blood cells and used to normalize cytokine concentrations to 1 ml of packed blood cells.
Measurement of CINC and MIP-2.
MIP-2 was determined in all samples by using the Cytoscreen ELISA kit for MIP-2 and the procedures supplied by the manufacturer. CINC was measured by a specific ELISA using paired antibodies as previously described (32) with the exception that we used rabbit anti-rat GRO/KC (CINC) capture antibody from PeproTech. Standard CINC and cell lysis samples were prepared with a PBS dilution buffer containing 2% heat-inactivated fetal bovine serum, and standard CINC and plasma samples were prepared with a PBS dilution buffer containing 5% normal mouse plasma.
Statistical analysis.
All values were expressed as means ± SE of the number of animals noted in each figure. Statistical significance was analyzed by using Proc mixed (23) two-way with repeated-measures model (chemokine × time within subject) unless otherwise noted in the figure legend. Post hoc pairwise comparisons were performed by Tukey-Kramer range test (10). Proc mixed (23) (mixed analysis of variance) two-way model (chemokine × route) was used to analyze baseline data. Differences were considered statistically significant at P < 0.05.
RESULTS
Distribution of chemokines in blood in response to IT LPS challenge.
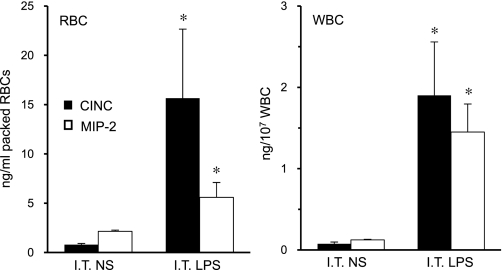
Both CINC and MIP-2 were detected with isolated erythrocytes and leukocytes following IT challenge with LPS (Fig. 1). This observation suggests that LPS-induced production of CINC and MIP-2 in the lung enters the systemic circulation. To confirm this we examined the chemokine partitioning between the cellular elements and plasma of blood following IT coinjection of rCINC and rMIP-2 (5 μg, each). Additionally, we examined the kinetics of CINC and MIP-2 flux from the lung to the blood.
Fig. 1.
Cellular distribution of cytokine-induced neutrophil chemoattractant (CINC) and macrophage inflammatory protein-2 (MIP-2) chemokines in blood 2 h after intratracheal (IT) LPS or vehicle [normal saline (NS)] administration. RBCs, red blood cells; WBCs, white blood cells. Values are expressed as means ± SE (n = 6). *P < 0.05 compared with saline.
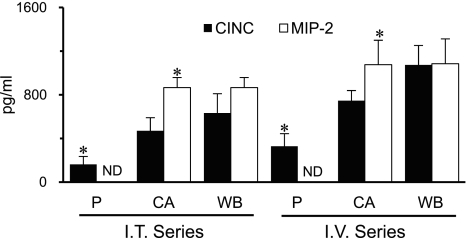
Baseline distribution of chemokines in blood.
Plasma and cell-associated CINC and MIP-2 concentrations were measured in blood obtained before IT or IV administration of rCINC and rMIP-2 (Fig. 2). Baseline blood CINC and MIP-2 concentrations were similar in rats receiving IT (633 ± 177, 866 ± 93 pg/ml, respectively) or IV (1,074 ± 179, 1,085 ± 277 pg/ml, respectively) chemokines. CINC was detected in plasma and the cellular elements of blood, and the distribution between plasma and the cellular elements did not differ between IT and IV treated groups. However, MIP-2 was only detected with the cellular elements. For all subsequent figures, the chemokine concentrations shown are above baseline values. There were no differences in hematocrit between groups over the 4-h observation period (data not shown).
Fig. 2.
Blood CINC and MIP-2 levels prior to IT or intravenous (IV) experimental series. Whole blood (WB) chemokine levels represent the sum of plasma (P) and cell-associated (CA) values adjusted for baseline hematocrits. Values are expressed as means ± SE (n = 5 or 6). ND, not detected. *P < 0.05 compared between chemokines.
Chemokine distribution in blood in response to IT coinjection of rCINC and rMIP-2.
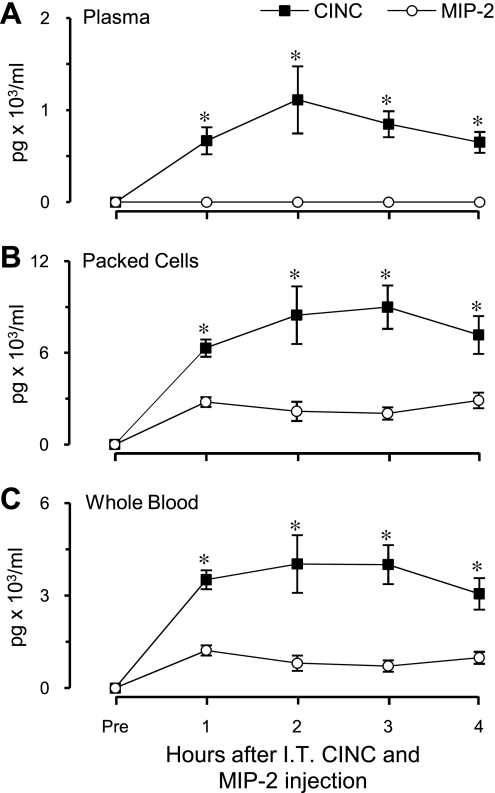
After IT coinjection of rCINC and rMIP-2 [5,054 ± 59 ng (SD) and 4,873 ± 139 ng (SD), respectively], on the basis of measured values in the injectate, plasma MIP-2 levels remained undetected, whereas CINC concentrations were significantly elevated above baseline (284 ± 128 pg/ml) throughout the entire 4-h observation period, reaching steady state at 2 h (Fig. 3A). Both cell-associated CINC and MIP-2 concentrations were significantly elevated above baseline (P < 0.05) and remained unchanged between 2 and 4 h (Fig. 3B). However, during this interval, cell-associated CINC concentrations were significantly higher (P < 0.01) than MIP-2 concentrations (8,518 ± 1,394 and 2,369 ± 386 pg/ml packed cells, respectively). From measured values in plasma and the cellular components of blood, the amount of each chemokine in 1 ml of whole blood was calculated by taking into account hematocrit. Whole blood CINC (3,698 ± 652 pg/ml) and MIP-2 (836 ± 159 pg/ml) concentrations did not change between 2 and 4 h after IT coinjection of chemokines (Fig. 3C). During this interval the whole blood MIP-2 concentration over baseline was ∼23% of that for CINC (P < 0.01).
Fig. 3.
Chemokine distribution in blood in response to IT coinjection of CINC and MIP-2. Whole blood chemokine levels represent the sum of plasma and packed cell chemokine concentrations adjusted for measured hematocrits. Values are expressed as means ± SE (n = 6). *P < 0.05 compared between chemokines at the same time point. All values significantly different compared with baseline (P < 0.05). No significant difference was observed in either chemokine concentration over a 2- to 4-h interval.
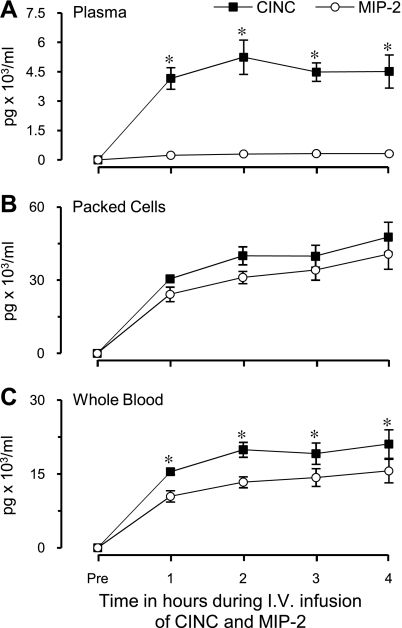
Chemokine distribution in blood in response to a constant IV coinfusion of rCINC and rMIP-2.
Constant-rate IV coinfusion of rCINC and rMIP-2 for 4 h was used to assess distribution of CINC and MIP-2 between plasma and the cell components of blood under conditions in which the rate of appearance was largely known. Infusion rates for CINC and MIP-2 were 20.8 ± 1.4 (SD) and 16.8 ± 1.6 (SD) ng/min, respectively, on the basis of measured values in the infusate. During the final 2 h, plasma CINC and MIP-2 concentrations plateau with plasma CINC concentrations ∼15-fold higher than MIP-2 levels (Fig. 4A). In contrast, cell-associated CINC and MIP-2 concentrations increased to similar concentrations (Fig. 4B) exhibiting a CINC/MIP-2 ratio of 1.27 ± 0.15. The ratio of plasma to cell-associated CINC was 0.112 compared with 0.009 for MIP-2 (P < 0.01) (Fig. 4, A and B). As with plasma, whole blood CINC and MIP-2 concentrations (sum of plasma and cell-associated chemokine concentrations adjusted for measured hematocrits) plateau between 2 and 4 h with CINC concentrations ∼1.46 ± 0.17-fold higher than MIP-2 concentrations (P < 0.05) (Fig. 4C).
Fig. 4.
Chemokine distribution in blood in response to a constant IV coinfusion of CINC and MIP-2. Whole blood chemokine levels represent the sum of plasma and packed cell chemokine concentrations adjusted for measured hematocrits. Values are expressed as means ± SE (n = 5). *P < 0.05 compared between chemokines at the same time point. All values significantly different compared with baseline (P < 0.05). No significant difference was observed in either chemokine concentration over a 2- to 4-h interval.
Rate of chemokine clearance from plasma and whole blood.
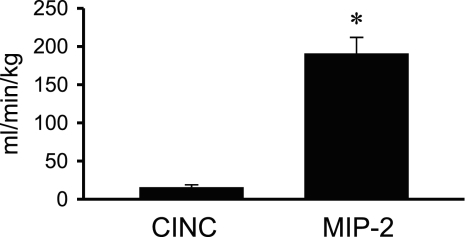
Because CINC and MIP-2 concentrations did not change between 2 and 4 h of infusion, the clearance of CINC and MIP-2 was calculated as the respective chemokine infusion rate (pg/ml) divided by the chemokine concentration in plasma and blood during the plateau period (Fig. 4, A and C). The rate of plasma chemokine clearance was significantly higher for MIP-2 (∼12-fold higher) than that for CINC (Fig. 5). However, the magnitude of whole blood chemokine clearance did not differ between MIP-2 (4.06 ± 0.37 ml·min−1·kg−1) and CINC (3.66 ± 0.51 ml·min−1·kg−1) (Fig. 6).
Fig. 5.
Plasma clearance rates for CINC and MIP-2 calculated by using steady-state plasma chemokine concentrations achieved during a constant IV coinfusion of CINC and MIP-2 (Fig. 4A). Values are expressed as means ± SE (n = 5). *P < 0.05 compared between chemokines.
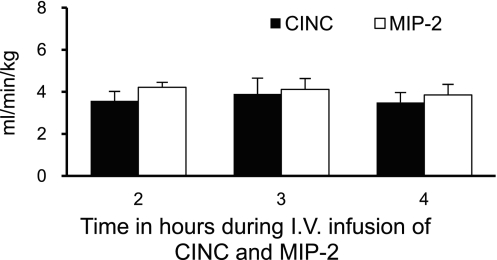
Fig. 6.
Whole blood clearance rates for CINC and MIP-2 calculated by use of steady-state whole blood chemokine concentrations (combined plasma and cell-associated chemokine concentrations adjusted for measured hematocrits), observed 2–4 h into a constant IV coinfusion of CINC and MIP-2 (Fig. 4C). Values are expressed as means ± SE (n = 5). No significant difference was observed between chemokine clearance rates at 2, 3, or 4 h of chemokine infusion.
Rate of chemokine appearance into plasma and whole blood after IT chemokine administration.
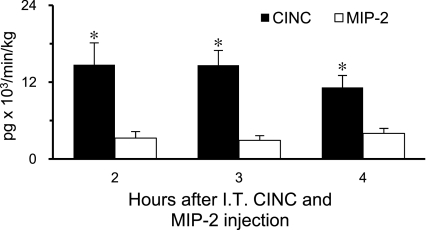
The rate of chemokine appearance (Ra) after IT coinjection of rCINC and rMIP-2 was calculated as the respective chemokine clearance rate (ml·min−1·kg−1) (Figs. 5 and 6) multiplied by the chemokine concentration in plasma and blood during the 2–4 h steady state (Fig. 3, A and C). The plasma CINC Ra was 14,812 ± 3,253 pg·min−1·kg−1. Plasma MIP-2 Ra could not be determined because plasma MIP-2 concentrations were undetectable (Fig. 3A). However, on the basis of the lowest MIP-2 assay standard (10 pg/ml) used in this study, the calculated plasma MIP-2 Ra is ≤1,932 pg·min−1·kg−1. This suggests that plasma CINC Ra was greater than sevenfold that of plasma MIP-2 Ra. In whole blood the MIP-2 Ra could be calculated because of its elevated cell-associated concentration. The whole blood CINC Ra (13,521 ± 2,384 pg·min−1·kg−1) was approximately fourfold higher than that for MIP-2 Ra (3,396 ± 648 pg·min−1·kg−1), P < 0.05, (Fig. 7).
Fig. 7.
Whole blood rates of appearance for CINC and MIP-2 calculated from previously determined chemokine clearance rates using IV data (Fig. 6) and steady-state whole blood chemokine concentrations observed 2–4 h after IT coinjection of CINC and MIP-2 (Fig. 3C). Values are expressed as means ± SE (n = 6). *P < 0.05 compared between chemokines at the same time point.
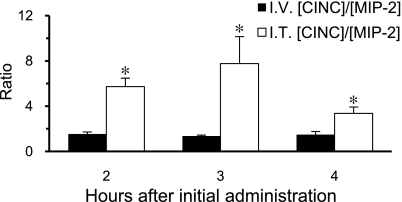
Comparison of whole blood MIP-2-to-CINC ratios observed after coadministration of chemokines by IT injection and as a constant rate of IV infusion.
To help control for any inherent assay bias when comparing different chemokines and to further assess whether the rates of CINC and MIP-2 flux from the pulmonary compartment differ, we compared whole blood CINC-to-MIP-2 ratios observed 2–4 h after chemokine administration by IV infusion and by IT injection, as previously described (Fig. 8). During the final 2 h of observation, the ratio of CINC to MIP-2 was 1.46 and 5.62 when chemokines were administered intravenously and intratracheally, respectively. Because CINC was infused at a higher rate than MIP-2 as indicated above, the difference in these ratios indicate that CINC leaves the lung at a faster rate than does MIP-2 when equal concentrations of chemokines are intratracheally coadministered, assuming that clearance is similar in the two experiments.
Fig. 8.
Whole blood CINC concentration-to-MIP-2 concentration ratios ([CINC]/[MIP-2]) after IT injection or during IV infusion of CINC and MIP-2. Values are expressed as means ± SE (n = 5 or 6). *P < 0.05 compared between chemokines at the same time point.
DISCUSSION
In contrast to our previous studies (20, 33), this study shows that MIP-2 produced in response to intrapulmonary LPS challenge or CXC chemokine delivered into the lungs appears in the blood, with plasma concentrations being maintained below the limit of detection by rapid binding to cellular elements, neutrophil CXCR2 and erythrocyte DARC. As in previous studies (20, 33), CINC, but not MIP-2, was detected in the plasma. However, CINC and MIP-2 were associated with erythrocytes and leukocytes and were substantially increased over baseline following IT LPS or chemokine challenges. Our data cannot preclude the possibility of pulmonary endothelial/epithelial- or monocyte-derived CINC and MIP-2 produced in response to IT LPS entered the circulation without first appearing within the intrapulmonary compartment. However, the finding that both chemokines increase in the blood following IT chemokine administration indicates the capability of intrapulmonary CINC and MIP-2 entering the blood compartment. This, together with the high concentrations of both chemokines recovered by BAL following IT LPS challenge, indicates the strong likelihood that some intrapulmonary-derived CXC chemokines enter the blood following intrapulmonary infections. This is further supported by our inability to detect LPS in the circulation following IT LPS challenge (33).
In the present study, we did not use radiolabeled chemokines to verify flux of these chemokines from the lung into the blood. However, it is highly unlikely that the substantial increase of CINC in the plasma and CINC and MIP-2 associated with blood cells came from an endogenous source after either IV or IT administration of these chemokines. In our previous study (20) we showed that 1) CINC is detected in plasma within 5 min of IT CINC administration; 2) plasma CINC specific activity following IT 125I-CINC injection is similar to that recovered by BAL, indicating that CINC readily moves from the intrapulmonary compartment to the blood, accounting for the CINC found in the plasma; and 3) CINC mRNA was low to nondetectable and did not increase in lung and selected extrapulmonary tissues following IT CINC. Because CINC and MIP-2 signal through CXCR2, it is reasonable to assume that neither IT CINC nor MIP-2 elicits MIP-2 mRNA production as well. Furthermore, in the present study, similar kinetics and distribution between plasma and the blood cellular elements were observed for CINC and MIP-2 infused intravenously, albeit somewhat higher concentrations, compared with IT injection.
When rats were administered both chemokines by IT injection, more CINC than MIP-2 was found in the blood. The greater amount of CINC compared with MIP-2 detected in blood after IT CXC chemokine challenge is due to either decreased CINC clearance and/or increased CINC flux from the lungs compared with MIP-2. Although MIP-2 plasma clearance was substantially greater than CINC, differences were not statistically different when considering whole blood clearance (Figs. 5 and 6). Therefore, differences in CINC and MIP-2 flux from the lungs to blood is in part responsible for the greater amount of CINC compared with MIP-2 detected in blood after IT CXC chemokine challenge. In this regard, calculated CINC Ra from the lung into blood was significantly higher compared with MIP-2 Ra. This difference in flux may potentially be attributed to differential chemokine binding to glycosaminoglycans within the lung. Given the caveat of different species, murine MIP-2 exhibits slower association and dissociation kinetics to heparin and diffuses more slowly across an in vitro extracellular matrix containing heparan sulfate, compared with mouse KC (CINC) (26). Similar differences in rat plasma CINC and MIP-2 levels following IT injection have been observed for the murine CXC chemokines KC (CINC) and MIP-2 (26). Also, murine KC and MIP-2 and rat CINC and MIP-2 share considerable identity in their putative heparin-binding domain (Table 1).
Table 1.
Amino acid sequence of COOH-terminal region of human, mouse, and rat CXC chemokines
| Chemokine | Amino Acid Sequence | Reference | ||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Human CXCL8 | C | L | D | P | K | E | N | W | V | Q | R | V | V | E | K | F | L | K | R | A | E | N | S | 11,*13 |
| Rat MIP-2 | C | L | N | P | E | A | P | L | V | Q | R | I | V | Q | K | I | L | N | K | G | K | A | N | 17 |
| Murine MIP-2 | C | L | D | P | E | A | P | L | V | Q | K | I | I | Q | K | I | L | N | K | G | K | A | N | 27 |
| Rat CINC | C | L | D | P | E | A | P | M | V | Q | K | I | V | Q | K | M | L | K | G | V | P | K | 30 | |
| Murine KC | C | L | D | P | E | A | P | L | V | Q | K | I | V | Q | K | M | L | K | G | V | P | K | 1 | |
The COOH-terminal regions of the chemokines have been aligned on the last cysteine; the positively charged amino acids arginine (R) and lysine (K) are in bold type; and the 4 amino acids of the GAG-binding domain found in the COOH-terminus of human CXCL8 are italicized. The glycosaminoglycan-binding domain of human CXCL8 consists of the 5 positively charged amino acids, K20 (not shown), R60, K64, K67, and R68.
MIP-2, macrophage inflammatory protein-2; CINC, cytokine-induced neutrophil chemoattractant; KC, keratinocyte-derived cytokine.
Mutations in K64 and R68 caused the largest decrease in heparin binding (11).
The substantial clearance of MIP-2 from plasma compared with CINC may also be attributed to selective chemokine binding to CXCR2, DARC, and/or glycosaminoglycans. Although neutrophil CXCR2 has a 70-fold greater affinity for MIP-2 than CINC (16) and degradation may have occurred after binding to CXCR2, as has been shown to occur for CXCL8 (22), it is unlikely given the relatively low numbers of leukocytes expressing CXCR2 (neutrophils) compared with other cell types (erythrocytes and other leukocytes). Additionally, we found that CINC and MIP-2 were not appreciably degraded when added to whole blood during a 90-min incubation period at 37°C (data not shown). Preferential binding to erythrocytes via DARC does not account for this clearance difference because when the two chemokines are infused intravenously cell-associated CINC and MIP-2 concentrations did not differ and exhibited the same CINC-to-MIP-2 ratio as infusate, suggesting that DARC exhibits similar binding affinity for MIP-2 and CINC. These findings exclude selective chemokine clearance by the cellular elements of blood as a mechanism accounting for this difference and suggest that plasma clearance of chemokines potentially involves differential flux into extravascular compartments, perhaps through preferential binding to vascular endothelium CXCR2, to heparin-like molecules of vascular endothelium, and to DARC expressed on postcapillary venules. These potential mechanisms require further investigation.
Determining that the difference between CINC and MIP-2 flux was statistically significant depended on performing statistical analysis between CINC and MIP-2 concentrations, clearances, and rates of appearances. The validity for this somewhat depends on the respective assays for the two chemokines to be similarly quantitative for both plasma and cellular extracts. To avoid this problem, we compared ratios of CINC to MIP-2 in whole blood from rats administered the two chemokines by IT injection or IV infusion. This comparison controls for differences in assay accuracy assuming that the accuracy was the same in both series of experiments. That the ratio after IT administration of chemokines was 380% of the ratio observed following IV infusion of chemokines indicates that the flux of MIP-2 from the lung into the circulation is impeded compared with CINC. This is in agreement with our finding of higher whole blood CINC Ra compared with MIP-2 Ra.
In this study, we used full-length rCINC and rMIP-2 proteins devoid of posttranslational modification, which might alter chemokine flux. Three types of posttranslational modification of chemokines are possible: glycosylation, citrullination, and proteolysis. Other studies have shown that such modifications can increase or decrease chemokines' known functions. For example, citrullination of CXCL8 and proteolysis of CXCL11 decreased the ability of these two chemokines to bind to glycosaminoglycans (2, 19). Citrullination and proteolysis also alter the ability of chemokines to promote tissue inflammation and in vitro chemotactic activity (2, 19, 15). However, these studies also showed that these endogenous chemokines existed in different posttranslational forms. Furthermore, the rCINC and rMIP-2 used in the present study are well characterized in vitro and in the lungs of rats, where they are shown to have neutrophil chemotactic activity. Whether posttranslational modifications of rat CINC and/or MIP-2 alter their flux from the lungs is not known and will require further study.
Selective partitioning of CINC and MIP-2 in whole blood and differences in flux of these chemokines from the lung may be a potential means by which effective chemokine gradients are manufactured, enabling relatively higher chemokine concentrations at local sites of inflammation and infection. By sequestering MIP-2 from plasma, cell migration toward areas of higher MIP-2 concentration (i.e., lung) could be promoted, as well as lessening desensitization of neutrophil responses to subsequent CXC chemokine exposure, as a result of previous MIP-2-CXCR2 binding (25). Liberated but effective concentrations of plasma CINC may enhance neutrophil recruitment to sites where needed (20) or potentially coordinate with the cytokine granulocyte colony-stimulating factor to mobilize neutrophils from the bone marrow (31). Possibly, the bioavailability of CINC but not MIP-2 may allow sufficient marrow mobilization while maintaining local gradients toward MIP-2.
In conclusion, our findings of differences in the rate of flux of CINC and MIP-2 across the epithelial/endothelial barrier of the lung and drastic differences in whole blood distribution further support that CINC and MIP-2 are not redundant chemokines and that novel mechanisms exist that control the differential flux of chemokines from lungs into the circulation.
GRANTS
This work was supported in part by National Institutes of Health Grant AA07577.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
ACKNOWLEDGMENTS
We thank Amy B. Weinberg, Rhonda R. Martinez, Jane A. Schexnayder, and Howard L. Blakesley for expert technical assistance.
REFERENCES
- 1. Bozic CR, Kolakowski LF, Jr, Gerard NP, Garcia-Rodriguez C, von Uexkull-Guldenband C, Conklyn MJ, Breslow R, Showell HJ, Gerard C. Expression and biologic characterization of the murine chemokine KC. J Immunol 154: 6048–6057, 1995 [PubMed] [Google Scholar]
- 2. Cox JH, Dean RA, Roberts CR, Overall CM. Matrix metalloproteinase processing of CXCL11/I-TAC results in loss of chemoattractant activity and altered glycosaminoglycan binding. J Biol Chem 283: 19389–19399, 2008 [DOI] [PubMed] [Google Scholar]
- 3. Darbonne WC, Rice GC, Mohler MA, Apple T, Hébert CA, Valente AJ, Baker JB. Red blood cells are a sink for interleukin 8, a leukocyte chemotaxin. J Clin Invest 88: 1362–1369, 1991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Frevert CW, Farone A, Danaee H, Paulauskis JD, Kobzik L. Functional characterization of rat chemokine macrophage inflammatory protein-2. Inflammation 19: 133–142, 1995 [DOI] [PubMed] [Google Scholar]
- 5. Frevert CW, Huang S, Danaee H, Paulauskis JD, Kobzik L. Functional characterization of the rat chemokine KC and its importance in neutrophil recruitment in a rat model of pulmonary inflammation. J Immunol 154: 335–344, 1995 [PubMed] [Google Scholar]
- 6. Gill S, Wight TN, Frevert CW. Proteoglycans: key regulators of pulmonary inflammation and the innate immune response to lung infection. Anat Rec (Hoboken) 293: 968–981, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Greenberger MJ, Strieter RM, Kunkel SL, Danforth JM, Laichalk LL, McGillicuddy DC, Standiford TJ. Neutralization of macrophage inflammatory protein-2 attenuates neutrophil recruitment and bacterial clearance in murine Klebsiella pneumonia. J Infect Dis 173: 159–165, 1996 [DOI] [PubMed] [Google Scholar]
- 8. Hadley TJ, Lu ZH, Wasniowska K, Martin AW, Peiper SC, Hesselgesser J, Horuk R. Postcapillary venule endothelial cells in kidney express a multispecific chemokine receptor that is structurally and functionally identical to the erythroid isoform, which is the Duffy blood group antigen. J Clin Invest 94: 985–991, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Keane MP, Strieter RM. Chemokine signaling in inflammation. Crit Care Med 28: N13–N26, 2000 [DOI] [PubMed] [Google Scholar]
- 10. Kramer CY. Extension of multiple range tests to group means with unequal numbers of replications. Biometrics 12: 307–310, 1956 [Google Scholar]
- 11. Kuschert GS, Hoogewerf AJ, Proudfoot AE, Chung CW, Cooke RM, Hubbard RE, Wells TN, Sanderson PN. Identification of a glycosaminoglycan binding surface on human interleukin-8. Biochemistry 37: 11193–11201, 1998 [DOI] [PubMed] [Google Scholar]
- 12. Lee JS, Frevert CW, Wurfel MM, Peiper SC, Wong VA, Ballman KK, Ruzinski JT, Rhim JS, Martin TR, Goodman RB. Duffy antigen facilitates movement of chemokine across the endothelium in vitro and promotes neutrophil transmigration in vitro and in vivo. J Immunol 170: 5244–5251, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Matsushima K, Morishita K, Yoshimura T, Lavu S, Kobayashi Y, Lew W, Appella E, Kung HF, Leonard EJ, Oppenheim JJ. Molecular cloning of a human monocyte-derived neutrophil chemotactic factor (MDNCF) and the induction of MDNCF mRNA by interleukin 1 and tumor necrosis factor. J Exp Med 167: 1883–1893, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Miller MD, Krangel MS. Biology and biochemistry of the chemokines: a family of chemotactic and inflammatory cytokines. Crit Rev Immunol 12: 17–46, 1992 [PubMed] [Google Scholar]
- 15. Mortier A, Loos T, Gouwy M, Ronsse I, Van Damme J, Proost P. Posttranslational modification of the NH2-terminal region of CXCL5 by proteases or peptidylarginine deiminases (PAD) differently affects its biological activity. J Biol Chem 285: 29750–29759, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Murakami K, Shibata F, al-Mokdad M, Nakagawa H, Ueno A, Kondo T. Identification and characterization of receptor for cytokine-induced neutrophil chemoattractant-3 on rat neutrophils. Biochem Biophys Res Commun 232: 562–567, 1997 [DOI] [PubMed] [Google Scholar]
- 17. Nakagawa H, Komorita N, Shibata F, Ikesue A, Konishi K, Fujioka M, Kato H. Identification of cytokine-induced neutrophil chemoattractants (CINC), rat GRO/CINC-2 α and CINC-2 β, produced by granulation tissue in culture: purification, complete amino acid sequences and characterization. Biochem J 301: 545–550, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Neote K, Darbonne W, Ogez J, Horuk R, Schall TJ. Identification of a promiscuous inflammatory peptide receptor on the surface of red blood cells. J Biol Chem 268: 12247–12249, 1993 [PubMed] [Google Scholar]
- 19. Proost P, Loos T, Mortier A, Schutyser E, Gouwy M, Noppen S, Dillen C, Ronsse I, Conings R, Struyf S, Opdenakker G, Maudgal PC, Van Damme J. Citrullination of CXCL8 by peptidylarginine deiminase alters receptor usage, prevents proteolysis, and dampens tissue inflammation. J Exp Med 205: 2085–2097, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Quinton LJ, Nelson S, Zhang P, Boe DM, Happel KI, Pan W, Bagby GJ. Selective transport of cytokine-induced neutrophil chemoattractant from the lung to the blood facilitates pulmonary neutrophil recruitment. Am J Physiol Lung Cell Mol Physiol 286: L465–L472, 2004 [DOI] [PubMed] [Google Scholar]
- 21. Reutershan J, Morris MA, Burcin TL, Smith DF, Chang D, Saprito MS, Ley K. Critical role of endothelial CXCR2 in LPS-induced neutrophil migration into the lung. J Clin Invest 116: 695–702, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Samanta AK, Oppenheim JJ, Matsushima K. Interleukin 8 (monocyte-derived neutrophil chemotactic factor) dynamically regulates its own receptor expression on human neutrophils. J Biol Chem 265: 183–189, 1990 [PubMed] [Google Scholar]
- 23. SAS Institute. SAS OnlineDoc 9.1.3. Cary, NC: SAS Institute, 2004 [Google Scholar]
- 24. Schmal H, Shanley TP, Jones ML, Friedl HP, Ward PA. Role for macrophage inflammatory protein-2 in lipopolysaccharide-induced lung injury in rats. J Immunol 156: 1963–1972, 1996 [PubMed] [Google Scholar]
- 25. Shibata F, Konishi K, Kato H, Komorita N, al-Mokdad M, Fujioka M, Nakagawa H. Recombinant production and biological properties of rat cytokine-induced neutrophil chemoattractants, GRO/CINC-2 α, CINC-2 β and CINC-3. Eur J Biochem 231: 306–311, 1995 [DOI] [PubMed] [Google Scholar]
- 26. Tanino Y, Coombe DR, Gill SE, Kett WC, Kajikawa O, Proudfoot AE, Wells TN, Parks WC, Wight TN, Martin TR, Frevert CW. Kinetics of chemokine-glycosaminoglycan interactions control neutrophil migration into the airspaces of the lungs. J Immunol 184: 2677–2685, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Tekamp-Olson P, Gallegos C, Bauer D, McClain J, Sherry B, Fabre M, van Deventer S, Cerami A. Cloning and characterization of cDNAs for murine macrophage inflammatory protein 2 and its human homologues. J Exp Med 172: 911–919, 1990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Ulich TR, Howard SC, Remick DG, Wittwer A, Yi ES, Yin S, Guo K, Welply JK, Williams JH. Intratracheal administration of endotoxin and cytokines VI. Antiserum to CINC inhibits acute inflammation. Am J Physiol Lung Cell Mol Physiol 268: L245–L250, 1995 [DOI] [PubMed] [Google Scholar]
- 29. Watanabe K, Koizumi F, Kurashige Y, Tsurufuji S, Nakagawa H. Rat CINC, a member of the interleukin-8 family, is a neutrophil-specific chemoattractant in vivo. Exp Mol Pathol 55: 30–37, 1991 [DOI] [PubMed] [Google Scholar]
- 30. Watanable K, Konishi K, Fujioka M, Kinoshita S, Nakagawa H. The neutrophil chemoattractant produced by the rat kidney epitheliod cell line NRK-52E is a protein related to the KC/gro protein. J Biol Chem 264: 19559–19563, 1989 [PubMed] [Google Scholar]
- 31. Wengner AM, Pitchford SC, Furze RC, Rankin SM. The coordinated action of G-CSF and ELR + CXC chemokines in neutrophil mobilization during acute inflammation. Blood 111: 42–49, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Zang P, Bagby GJ, Kolls JK, Welsh DA, Summer WR, Andresen J, Nelson S. The effects of granulocyte colony-stimulating factor and neutrophil recruitment on the pulmonary chemokine response to intratracheal endotoxin. J Immunol 166: 458–465, 2001 [DOI] [PubMed] [Google Scholar]
- 33. Zhang P, Nelson S, Holmes MC, Summer WR, Bagby GJ. Compartmentalization of macrophage inflammatory protein-2, but not cytokine-induced neutrophil chemoattractant, in rats challenged with intratracheal endotoxin. Shock 17: 104–108, 2002 [DOI] [PubMed] [Google Scholar]